Abstract
The accumulation of bile acids during obstructive cholestasis causes liver injury and fibrosis, which is at least partly mediated by the death receptors Tumor necrosis factor-related apoptosis-inducing ligand, Tumor necrosis factor-α, and Fas. The BH3-interacting domain death agonist Bid is a critical mediator of death receptor-induced apoptosis in hepatocytes. Our aim for this study was, therefore, to elucidate whether Bid also mediates death receptor-induced liver injury in obstructive cholestasis. Overall, survival and various aspects of liver injury were analyzed in wild-type and Bid−/− mice after bile duct ligation (BDL), a commonly used model to study obstructive cholestasis in mice. Liver injury was examined at 3, 7, and 14 days after BDL. Loss of Bid did not affect the number of bile infarcts, serum aspartate aminotransferase values, or animal survival. Processing of procaspase-3 and procaspase-9, and caspase-3 enzyme activities, were not detectable in either group, and Bid−/− mice displayed the same pattern of terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling positive hepatocytes as wild-type controls following BDL. In contrast to Fas-receptor deficient lpr mice, hepatic fibrosis and the inflammatory response was not affected by loss of Bid. Together, these data suggest that Bid is not a downstream target of death receptors in obstructive cholestasis and does not significantly contribute to bile acid induced liver injury and fibrosis.
Cholestasis is defined as an impairment of bile flow and is frequently seen in human liver diseases such as primary biliary cirrhosis, primary sclerosing cholangitis, and other forms of chronic biliary injury. During cholestasis, toxic bile acids accumulate within the liver, leading to structural changes, inflammatory responses and, ultimately, to liver cirrhosis and liver failure. Therapeutic options for the treatment of cholestatic liver injury are currently limited, and a better understanding of the mechanisms leading to cellular injury may help to develop new therapeutic strategies.
Increasing evidence is now available that illustrates how bile acids induce hepatocyte and liver injury. Multiple studies have shown that bile acids induce Fas-dependent hepatocyte apoptosis in vitro providing a cellular mechanism for bile acid induced liver injury.1,2,3,4,5,6,7 Additionally, it has been shown that death receptor mediated apoptosis does not only cause hepatocyte death in vivo but also contributes to stellate cell activation and liver fibrogenesis in cholestatic liver injury.8,9,10,11 On ligand binding, activated death receptors engage the Fas associated death domain adaptor protein. The Fas associated death domain adaptor protein, in turn, recruits caspase-8 via a homophilic death effector domain interaction forming the death-inducing signaling complex leading to activation of caspase-8. In type-II cells such as hepatocytes, activation of caspase-8 at the death-inducing signaling complex alone is insufficient to efficiently trigger cell death and an amplification loop via the mitochondria is required.12 Previous studies have shown that this link between the death receptor and the mitochondria is dependent on cleavage and translocation to the mitochondria of the proapoptotic Bcl-2 family member Bid.13,14 During the apoptotic process, Bid activates Bax or Bak to initiate mitochondrial dysfunction, release of cytochrome C, and activation of effector caspases such as caspase-3.15 The role of death receptors in cholestatic liver injury is supported by studies showing that mice deficient in Fas-receptor develop less liver injury after bile duct ligation (BDL),9,16 a widely used rodent model of cholestasis. Interestingly, reduced liver injury in BDL lpr mice correlated with a less severe inflammatory response.16 Likewise, liver histology, the number of bile infarcts, serum aspartate aminotransferase (AST) values, hepatic fibrosis, and animal survival were significantly improved in BDL tumor necrosis factor-related apoptosis-inducing ligand−/− Trail−/− mice as compared with wild-type controls.17,18 Finally, a very recent study revealed that Tumor necrosis factor-α (Tnf-α) also promotes hepatotoxicity in the BDL model and that Tnf-α−/− mice develop significantly less liver fibrosis than littermate controls.19 Together, these studies suggest that all three members of the death receptor family contribute to bile acid induced liver injury and fibrosis. However, it is still unclear which down-stream effectors of the death receptors mediate the deleterious effects in murine livers after BDL. Previously, it has been shown that Bid antisense molecules reduce the acute liver injury after obstructive cholestasis suggesting that Bid might be an important down-stream target of death receptors in obstructive cholestasis.1 The overall objective of this study was, therefore, to examine the role of Bid in cholestatic liver injury by using a genetic model. We were specifically interested to determine to which extent Bid affects overall survival, liver injury, and hepatic fibrogenesis and compared the outcome of wild-type and Bid−/− mice after BDL.
Materials and Methods
Mice
C57Bl/6 mice were purchased from the Jackson Laboratory (Bar Harbor, ME). Bid−/− mice were provided by S. Korsmeyer. Floxed c-jun mice were provided by E. Wagner, c-junf/f mice were crossed with transgenic mice expressing Mx-Cre. Mx-Cre c-junf/f mice were injected twice with poly(I/C) 10 days before the experiment (15 mg/kg i.p.; Amersham Biosciences, Piscataway, NJ) to obtain mice lacking c-jun. Fas receptor deficient lpr mice and age-matched C57BL/6J wild-type mice were purchased from the Jackson Laboratory. Animal experiments were approved by the district government of Lower Saxony, Germany, and the Institutional Animal Care and Use Committee of the University of Kansas Medical Center. BDLs were performed as previously described.7 Some mice were injected intraperitoneally with 0.35 μg/g mouse Fas antibody (Jo-2) (BD Pharmingen, San Diego, CA) as positive controls for apoptotic cell death and were sacrificed after 6 hours.
Histology, Immunohistochemistry, and Terminal Deoxynucleotidyl Transferase-Mediated dUTP Nick-End Labeling Assay
Liver tissues were fixed in 10% phosphate-buffered formalin, pH 7.4, dehydrated in 100% ethanol, and embedded in paraffin wax at 58°C. Five-micron sections were rehydrated and stained with H&E. Terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) staining was performed by using the in situ cell death detection Kit (Roche, Mannheim, Germany) according to the instructions of the manufacturer. Frozen sections were used for immunohistochemical evaluation of activated caspase-3. Slides were incubated for 2 hours at room temperature with a polyclonal antibody (1:100 dilution; Cell Signaling, Beverly, MA) that specifically recognizes the large fragment (17/19 kDa) of activated, but not full length, caspase-3. After washes, slides were incubated with a prediluted Alexa Fluor 488 goat anti-rabbit IgG antibody for 1 hour. Sirius red staining and Masson’s trichrome was performed on frozen sections or on formalin fixed sections by using routine protocols.
Hepatic Neutrophil Sequestration
Neutrophil accumulation in the liver was assessed by staining tissue sections for chloroacetate esterase, a specific marker for neutrophils, by using the Naphthol-ASD chloroacetate esterase kit (Sigma, St. Louis, MO) and for CD11b, also known as Mac-1 antigen, found on mouse macrophages and granulocytes.
Aminotransferase Determinations
For aminotransferase analysis, animal blood was drawn into heparinized syringes. After centrifugation at 200 g, plasma was recovered and stored at −80°C until use. AST activity was measured by using the automated biochemistry analyzer Olympus AU 400 (Olympus Diagnostica GMBH, Hamburg, Germany) and Olympus AU reagents.
Caspase Activity
Liver lysates were prepared by homogenization in hypotonic buffer (25 mmol/L HEPES, pH 7.5, 5 mmol/L MgCl2, 1 mmol/L EGTA, 1 mmol/L phenylmethylsulfonyl fluoride). Homogenates were centrifuged at 15,000 rpm for 15 minutes, and extracted proteins (50 μg) were tested in triplicate experiments by measuring the proteolytic cleavage of specific fluorogenic substrate for caspase-3 (CaspACE Assay System; Promega, Madison, WI).
Western Blot Analysis
Protein extracts were resolved by SDS-polyacrylamide gel electrophoresis and transferred onto polyvinylidene difluoride membrane (Millipore, Bedford, MA). Coomassie staining was used to demonstrate equal protein loading. Western blotting was performed as recently described.20 Detection of immunolabeled proteins was done by using the chemiluminescence kit (BioRad, Hercules, CA) and Hyperfilm enhanced chemiluminescence film (Amersham Biosciences, Piscataway, NJ).
Real-Time PCR
Total RNA was isolated from frozen liver tissues by using the RNeasy Mini Kit (Qiagen, Hilden, Germany). RNA samples of four mice in each group were pooled and 4 μg of RNA from every pool was used to synthesize cDNA with the SuperScript II first-strand synthesis kit (Invitrogen, Carlsbad, CA). Twenty nanograms of cDNA were amplified in a total reaction volume of 25 μl in an Applied Biosystems 7300 (Darmstadt, Germany).
Measurement of Hepatic Hydroxyproline Content
Total hepatic hydroxyproline levels at different time points after BDL were determined in the hydrolysates of liver samples. Briefly, precisely weighed liver tissue samples (30 to 40 mg) were homogenized in distilled H2O. The homogenates were hydrolyzed in 10 N HCl by incubation at 110°C for 18 hours. The hydrolysates were dried by speed vacuum centrifugation over 3 to 5 hours and redissolved in a buffer containing 0.2 mol/L citric acid, 0.2 mol/L glacial acetic acid, 0.4 mol/L sodium acetate, and 0.85 mol/L sodium hydroxide, pH 6.0. Hydroxyproline levels in the hydrolysates were biochemically measured according to the procedures previously described.21
Statistical Analysis
Data are expressed as mean ± SD determined by one-way analysis of variance followed by Student’s t-test to determine significance. A P value below 0.05 was considered significant. Four to six mice in each group were analyzed.
Results
Loss of Bid Does Not Affect Liver Injury and Overall Survival of Mice after BDL
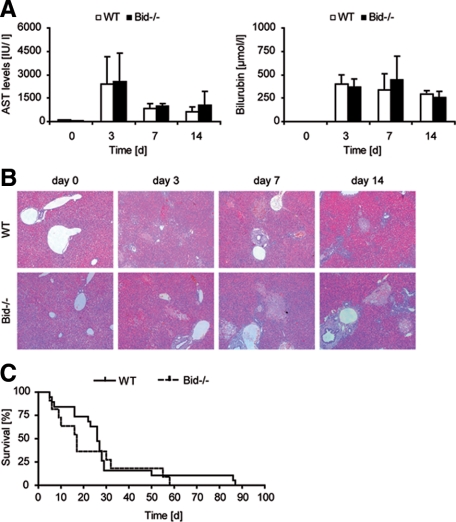
Cholestasis was induced in a cohort of experimental mice by surgical BDL. In wild-type mice, serum AST and bilirubin levels rapidly increased after BDL. AST levels peaked on day 2 to 3 and declined thereafter. Bilirubin levels remained highly elevated after BDL (Figure 1A). In contrast to Fas-receptor deficient lpr mice,9,16 a similar increase in plasma AST activities and bilirubin levels was evident in Bid−/− mice at all time points analyzed (Figure 1A). Concomitant to biochemical liver injury parameters, multiple bile infarcts were evident in livers of wild-type and Bid−/− mice 3 days after BDL (Figure 1B). Hepatocytes in these foci exhibited characteristics of oncotic cell death as previously described.16,22 Fewer bile infarcts were detectable in livers of both groups at later time points paralleling declining AST levels. In agreement with the biochemical and histological data, survival curves did not reveal a significant difference between wild-type and Bid−/− mice (Figure 1C).
Figure 1.
A: Wild-type (WT) and Bid−/− mice underwent BDL and were euthanized after 3, 7, and 14 days. Serum levels of AST and bilirubin as markers of hepatic damage were measured at indicated time points. Mean values and SD are presented for all time points (n = five in each group). B: H&E staining revealed profuse liver damage and multiple oncotic bile infarcts in BDL mice. C: Survival of WT and Bid−/− mice was recorded after BDL (n = 20 mice in each group).
Loss of Bid Does Not Affect Apoptosis of Hepatocytes after BDL Mice
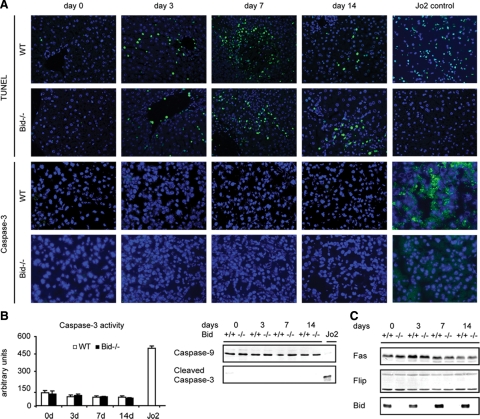
In agreement with previous studies, oncotic foci also stained positive with the TUNEL assay (Figure 2A). Additionally, numerous TUNEL positive cells were disseminated throughout the lobules of BDL livers. However, positive immunoreactivity for cleaved caspase-3 indicative of apoptosis was rarely seen. Consistent with these findings, there was no significant increase in caspase-3 activity in wild-type mice before or after BDL (Figure 2B). Importantly, the same pattern of TUNEL positive cells and cleaved caspase-3 negative cells was evident in Bid−/− mice after BDL. In agreement with the enzyme activity data, no significant difference in protein levels of full-length caspase-9 was seen between both groups (Figure 2B). Furthermore, the cleaved and active fragment of procaspase-3 was not detectable in any BDL group. In contrast, there were multiple TUNEL and caspase-3 positive hepatocytes in livers of Jo-2 treated wild-type mice (Figure 2A). Additionally, hepatic caspase-3 activity and processing of caspase-9 and caspase-3 was clearly evident in these apoptosis control mice (Figure 2B). Protein levels of the Fas receptor were not significantly changed after BDL (Figure 2C). Additionally, levels of c-Flips/L, a specific inhibitor of caspase-8,23 remained unchanged in BDL mice. As anticipated, Bid was not detectable in the livers of Bid−/− mice.
Figure 2.
A: TUNEL and cleaved caspase-3 staining of liver sections is shown from wild-type (WT) and Bid−/− mice after BDL and in mice injected with the Fas mAb Jo-2 (original magnification, ×200, n = four in each group). B: Hepatic caspase-3 activity was measured by using the CaspACE Assay kit and processing of caspase-9 (loss of full length caspase-9) and caspase-3 (detection of the cleaved fragment) was analyzed by Western blot for all time points before and after BDL and in Jo-2 injected mice as a positive control (+). C: Liver tissues of control and BDL mice were analyzed for total cellular levels of Fas, Flip, and Bid (n = 5, pooled samples).
Loss of Bid Does Not Affect Proliferation of Hepatocyte after BDL
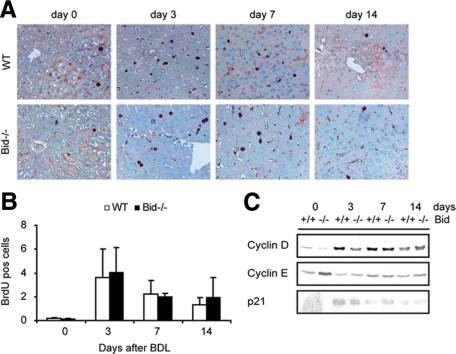
Cell death of hepatocytes after BDL induces a regenerative response in the liver.26 To study whether the loss of Bid affects proliferation of hepatocytes during cholestatic liver injury, the number of bromodeoxyuridine (BrdU)-positive hepatocytes was determined 3, 7, and 14 days after BDL (Figure 3A). Semiquantitative analysis of BrdU-labeled hepatocytes revealed an increase of positive cells with a peak at day 3 (Figure 3B). A similar number of proliferating hepatocytes was detectable in wild-type and Bid−/− mice. Next, the expression of three key regulators of hepatocellular proliferation was analyzed by Western blotting. Levels of cyclin D rapidly increased in both groups compared with sham-operated controls. In contrast, protein levels of cyclin E remained almost unchanged at these early time points after BDL (Figure 3C).
Figure 3.
A, B: Hepatocyte proliferation was assessed with BrdU immunostaining and BrdU positive cells were quantified (10 microscope fields at ×200 magnification, wild-type (WT) mice are represented as open bars and Bid−/− mice as closed bars, n = 5). C: Immunoblots were performed for cyclin D and E and p21 (n = 5, pooled samples).
Loss of Bid Does Not Attenuate Fibrogenesis after BDL
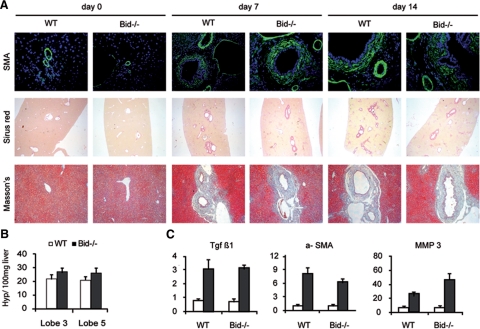
Complete obstruction of the common bile duct results in the rapid development of liver fibrosis. After BDL, fibrotic lesions mainly occur around the bile ducts. To investigate the role of Bid in liver fibrogenesis, Sirius red and Masson’s trichrome staining was performed with liver sections 3, 7, and 14 days after BDL. In wild-type BDL mice extensive peribiliary and slight interstitial collagen deposition was evident (Figure 4A). Several lines of evidence suggest that α-smooth muscle actin (α-SMA)-positive myofibroblasts are the main effector cells responsible for the overproduction of matrix components in fibrotic liver. Accordingly, multiple α-SMA positive cells became evident shortly after BDL (Figure 4B). Overall, however, there was no obvious difference for collagen deposition and stellate cell activation between wild-type and Bid−/− mice at any time point analyzed. The degree of fibrosis was further quantified by measuring the content of hydroxyproline, which is almost exclusively found in collagen in animal tissues, in hydrolysates extracted from two liver lobes of mice 7 days after BDL. Hydroxyproline content was significantly increased in liver extracts of BDL mice compared with controls (wild-type day 0: 14.36 + 4.6 (lobe 3), 14.53 + 3.2 (lobe 5); wild-type day 7: 22.66 + 3.1 [lobe 3; P = 0.0052]; 20.52 + 3.1 [lobe 5; P = 0.01]). Interestingly, hydroxyproline content was slightly higher in Bid−/− mice (Figure 4B). Next, mRNA levels of genes implicated in liver fibrosis were quantified by quantitative RT-PCR to determine whether other markers of stellate cell activation were affected by loss of Bid. Transforming growth factor-β, a key fibrogenic cytokine in the liver, α-SMA and matrix metalloproteinase-3 were significantly increased 3 days after BDL in wild-type mice. Loss of Bid did not significantly affect the transcriptional regulation of any gene analyzed (Figure 4C).
Figure 4.
A: Liver fibrosis was evaluated by α-SMA, Sirius red, and Masson’s trichrome staining 3, 7, and 14 days after BDL (Original magnification, ×200). B: Hydroxyproline content measured in the right median (#3) and the right lateral (#5) liver lobes 7 days after BDL. C: mRNA levels of transforming growth factor-β, α-SMA, and matrix metalloproteinase-3 were quantified by real-time RT PCR (sham operated mice are represented with open bars and BDL mice with closed bars).
Loss of Bid Does Not Affect the Inflammatory Response after BDL
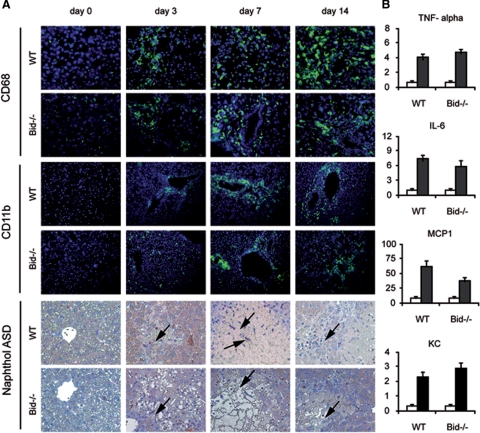
One important feature of cholestatic liver disease in both humans and rodents is hepatic inflammation. Most prominent among the inflammatory cells are neutrophils, which are recruited within hours into the liver after BDL.24 Kupffer cells, resident macrophages of the liver, and other macrophages recruited to the liver, are the major source of inflammatory cytokines and CXC chemokines in the liver that recruit neutrophils to the hepatic parenchyma.25 To evaluate the role of Bid for the activation of these inflammatory cells, liver sections were stained with CD68 to label Kupffer cells, and with CD11b and Naphthol ASD to detect neutrophils. In agreement with previous studies multiple neutrophils were observed in the parenchymal tissue of wild-type mice after BDL (Figure 5A). Additionally, more Kupffer cells were evident in BDL mice compared with controls. Analysis of Bid−/− BDL livers revealed a similar number of neutrophils and Kupffer cells as in BDL wild-type mice suggesting that Bid does not affect the recruitment of these cells to the liver. Next, mRNA levels of TNF-α, interleukin-6, monocyte chemotactic protein-1, and the neutrophil chemokine KC were analyzed by RT-PCR. In agreement with previous studies, mRNA levels of TNF-α and interleukin-6 were significantly induced in wild-type mice 3 days after BDL (Figure 5B). Furthermore, mRNA levels of KC and monocyte chemotactic protein-1, potent neutrophil and monocyte chemoattractants, were significantly induced in BDL mice compared with sham-operated controls. In contrast to lpr mice, in which the inflammatory response is significantly attenuated,17 a similar increase was seen in BDL Bid−/− mice.
Figure 5.
A: Liver sections of control and BDL mice were analyzed for CD68 expression and CD11b expression (Original magnification, ×200). Infiltrating neutrophils were also detected by (arrows) Naphthol ASD staining. B: Quantitative RT-PCR was performed by using pooled RNA extracted from wild-type (WT) and Bid−/− mice before (open bars) and after BDL (closed bars) (n = 4). Specific cDNAs of KC, monocyte chemotactic protein-1, interleukin-6, and TNF-α were amplified.
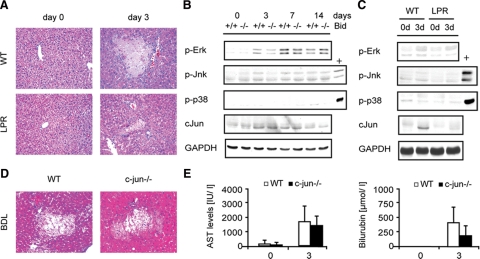
Bid−/− and lpr Mice Display Similar Activation of Stress Kinase Pathways after BDL
The data presented above strongly suggest that Bid does not contribute to BDL induced liver injury during cholestasis. However, bile acid induced liver injury was significantly reduced in lpr mice compared with wild-type and Bid−/− mice as previously reported (Figure 6A). We, therefore, wondered which other pathways down-stream of Fas-R might play a role in cholestatic liver injury. Recently, it has been shown that bile acids activate various cell signaling cascades including protein kinase C, Erk, Jnk, p38, and PI3 kinase that potentially affect survival of hepatocytes.4,27,28,29 To investigate the contribution of mitogen activated protein kinases in the inflammatory response triggered by Fas, activation of Erk, Jnk, p38, and c-jun was analyzed in wild-type and Bid−/− mice at different time points after BDL. Additionally, activation of these pathways was analyzed in wild-type and lpr mice 3 days after BDL. BDL caused a slight activation of Erk, whereas a sustained activation of Jnk and p38 was not detectable (Figure 6B). There was, however, no significant difference between Bid−/− and lpr mice and their respective littermate control mice. Interestingly, however, loss of Fas-R markedly reduced the up-regulation of c-jun in livers of BDL mice (Figure 6C). To specifically analyze the role of c-jun in cholestatic liver injury, BDL was performed with conditional c-jun−/− mice. In contrast to lpr mice, however, a similar number of oncotic foci and similar transaminase levels were evident in c-jun−/− mice suggesting that c-jun is not a direct downstream effector of Fas-R in BDL induced liver injury (n = six per group) (Figure 6, D and E).
Figure 6.
A: Wild-type (WT) and Lpr mice underwent BDL and were euthanized after 3 days (n = 4). Liver injury was analyzed by H&E staining. B: Western blot of p-Erk, p-Jnk, p-p38, and c-jun in WT and Bid−/− mice before and 3, 7, and 14 days after BDL. C: Analysis of p-Erk, p-Jnk, p-p38, and c-jun in WT and Lpr mice before and 3 days after BDL (positive control (+) for p-Jnk: WT mouse injected with Jo-2; positive control (+) for p-p38: Fah−/− mouse injected with homogentisic acid). D: Representative H&E staining of control and c-jun−/− mice 3 days after BDL. E: Serum AST and bilirubin levels were determined before and 3 days after BDL.
Discussion
Liver injury and inflammation are common complications of cholestatic liver diseases, which can culminate in liver fibrosis and failure. Previously, it has been shown that liver injury in obstructive cholestasis is at least partially mediated by death receptors. Additionally, several in vitro and in vivo studies provided evidence that Bid might be an important downstream effector of death receptors.2 In vivo, Bid antisence oligonucleotides reduce liver injury after BDL. Furthermore, toxic bile acids such deoxy cholic acid induce cleavage of Bid and translocation of the activated fragment of Bid to the mitochondria even in the absence of Fas in vitro.1 The primary objective of our study was, therefore, to analyze whether Bid affects liver injury and overall survival of mice after BDL by using a genetic model.
In agreement with previous studies, multiple TUNEL positive cells were observed in livers of BDL mice. However, Bid−/− mice displayed the same pattern of TUNEL positive hepatocytes as wild-type controls after BDL. Furthermore, AST levels, as a biochemical marker for oncosis, the degree of tissue necrosis, and the overall survival was similar in wild-type and Bid−/− mice. Together, these data suggest that loss of Bid does not ameliorate BDL induced liver injury in contrast to its role in Jo-2-induced liver failure. These observations raise the following question: why are pan-caspase inhibitors protective in BDL induced liver injury?27 Interestingly, one very recent study revealed that tumor necrosis factor-related apoptosis-inducing ligand/DR-5 mediated apoptosis of cholangiocytes is an important determinant in BDL induced liver injury, which induces an inflammatory response within the liver and subsequently hepatocyte oncosis.18 The mitochondrial pathway of apoptosis plays only a minor role in DR-5 mediated apoptosis of cholangiocytes, which might explain why the loss of Bid did not affect BDL induced liver injury while caspase inhibitors are protective.
A secondary objective of our study was to determine whether Bid affects any other aspect of bile acid-induced liver injury. There is increasing evidence that Bcl-2 like proteins do not only regulate apoptosis but also proliferation. Previously, it has been shown that deletion of Bid impedes hepatocyte proliferation during chemical induced hepatocarcinogenesis and following partial hepatectomy.29 The loss of Bid, however, did not affect hepatocyte proliferation during cholestatic liver injury. Next, the contribution of Bid to hepatic fibrogenesis was analyzed. Fas ligand induces proliferation of quiescent stellate cells30; on the other hand, stellate cells are increasingly sensitive to Fas-mediated apoptosis after activation and apoptosis of activated stellate cells has been implicated in the resolution phase of hepatic fibrogenesis.31 Bid could, therefore, have divergent effects on hepatic fibrogenesis if it is important for Fas-mediated signaling in stellate cells. Our results, however, do not support a role for Bid in the activation or pertubation of stellate cell activation. Expression of markers for stellate cell activation such as α-SMA and transforming growth factor-β were similarly expressed in wild-type and Bid−/− mice. Additionally, a similar degree of fibrosis at the protein levels was detected by Sirius red staining and hydroxy proline measurement.
In contrast to Bid−/− mice, Fas receptor-deficient lpr mice have significantly less liver injury after BDL. Interestingly, lpr mice display a reduced expression of chemokines/cytokines and hepatic neutrophil infiltration after BDL.28 Similarly, activation of Fas receptor by Jo-2 induces CXC chemokines MIP-2 and KC causing an active recruitment of neutrophils to the hepatic parenchyma.32 The mechanism by which Fas-R promotes inflammation is so far not completely understood. Previously it has been suggested that the Jo-2-mediated inflammatory response in the liver was independent on NFkB, but requires activation of caspase-3 and nuclear translocation of AP-1.32 Here, chemokine expression after BDL occurred without any significant caspase activation. Additionally, activation of stress kinase pathways was not significantly different between wild-type and lpr mice. Interestingly, c-jun was differently expressed in livers of lpr mice, however c-jun−/− mice displayed a similar number of oncotic foci as wild-type mice suggesting that the reduced c-jun protein levels in lpr mice are a consequence and not a cause of the reduced liver injury in BDL mice. Therefore, an alternative way to explain the reduced liver injury in BDL mice could be that the entire effect is independent of the Fas-R expression on hepatocytes. There is evidence that lpr mice have fewer NKT cells and thus may generate fewer inflammatory mediators.16 In support of this argument, it was shown that NKT cell depletion protects against BDL-induced liver injury.17
Together, our data suggest that Bid-mediated signaling does not play a role for death receptor-mediated liver injury in obstructive cholestasis. Our findings strongly argue against selecting Bid as a therapeutic target in cholestasis and should, therefore, have implications for the development of new treatment strategies during bile acid induced liver injury.
Footnotes
Address reprint requests to Arndt Vogel, M.D., Department of Hepatology and Gastroenterology, and Endocrinology Hannover Medical School, Carl Neu-berg str.1, 30625, Hannover, Germany. E-mail: vogela@me.com.
Supported by the Deutsche Forschungsgemeinschaft (Vo 959/3-1 to A.V.) and by National Institutes of Health Grant R01 AA12916 (H.J.).
References
- Higuchi H, Miyoshi H, Bronk SF, Zhang H, Dean N, Gores GJ. Bid antisense attenuates bile acid-induced apoptosis and cholestatic liver injury. J Pharmacol Exp Ther. 2001;299:866–873. [PubMed] [Google Scholar]
- Takikawa Y, Miyoshi H, Rust C, Roberts P, Siegel R, Mandal PK, Millikan RE, Gores GJ. The bile acid-activated phosphatidylinositol 3-kinase pathway inhibits Fas apoptosis upstream of bid in rodent hepatocytes. Gastroenterology. 2001;120:1810–1817. doi: 10.1053/gast.2001.24835. [DOI] [PubMed] [Google Scholar]
- Yerushalmi B, Dahl R, Devereaux MW, Gumpricht E, Sokol RJ. Bile acid-induced rat hepatocyte apoptosis is inhibited by antioxidants and blockers of the mitochondrial permeability transition. Hepatology. 2001;33:616–626. doi: 10.1053/jhep.2001.22702. [DOI] [PubMed] [Google Scholar]
- Reinehr R, Graf D, Haussinger D. Bile salt-induced hepatocyte apoptosis involves epidermal growth factor receptor-dependent CD95 tyrosine phosphorylation. Gastroenterology. 2003;125:839–853. doi: 10.1016/s0016-5085(03)01055-2. [DOI] [PubMed] [Google Scholar]
- Lo Ten, Foe JR, Kwee ML, Rooimans MA, Oostra AB, Veerman AJ, van Weel M, Pauli RM, Shahidi NT, Dokal I, Roberts I, Altay C, Gluckman E, Gibson RA, Mathew CG, Arwert F, Joenje H. Somatic mosaicism in Fanconi anemia: molecular basis and clinical significance. Eur J Hum Genet. 1997;5:137–148. [PubMed] [Google Scholar]
- Gumpricht E, Devereaux MW, Dahl RH, Sokol RJ. Glutathione status of isolated rat hepatocytes affects bile acid-induced cellular necrosis but not apoptosis. Toxicol Appl Pharmacol. 2000;164:102–111. doi: 10.1006/taap.2000.8894. [DOI] [PubMed] [Google Scholar]
- Faubion WA, Guicciardi ME, Miyoshi H, Bronk SF, Roberts PJ, Svingen PA, Kaufmann SH, Gores GJ. Toxic bile salts induce rodent hepatocyte apoptosis via direct activation of Fas. J Clin Invest. 1999;103:137–145. doi: 10.1172/JCI4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoemaker MH, Gommans WM, de la Rosa LC, Homan M, Klok P, Trautwein C, van Goor H, Poelstra K, Haisma HJ, Jansen PL, Moshage H. Resistance of rat hepatocytes against bile acid-induced apoptosis in cholestatic liver injury is due to nuclear factor-kappa B activation. J Hepatol. 2003;39:153–161. doi: 10.1016/s0168-8278(03)00214-9. [DOI] [PubMed] [Google Scholar]
- Miyoshi H, Rust C, Roberts PJ, Burgart LJ, Gores GJ. Hepatocyte apoptosis after bile duct ligation in the mouse involves Fas. Gastroenterology. 1999;117:669–677. doi: 10.1016/s0016-5085(99)70461-0. [DOI] [PubMed] [Google Scholar]
- Canbay A, Higuchi H, Bronk SF, Taniai M, Sebo TJ, Gores GJ. Fas enhances fibrogenesis in the bile duct ligated mouse: a link between apoptosis and fibrosis. Gastroenterology. 2002;123:1323–1330. doi: 10.1053/gast.2002.35953. [DOI] [PubMed] [Google Scholar]
- Sodeman T, Bronk SF, Roberts PJ, Miyoshi H, Gores GJ. Bile salts mediate hepatocyte apoptosis by increasing cell surface trafficking of Fas. Am J Physiol Gastrointest Liver Physiol. 2000;278:G992–G999. doi: 10.1152/ajpgi.2000.278.6.G992. [DOI] [PubMed] [Google Scholar]
- Scaffidi C, Fulda S, Srinivasan A, Friesen C, Li F, Tomaselli KJ, Debatin KM, Krammer PH, Peter ME. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 1998;17:1675–1687. doi: 10.1093/emboj/17.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Zhu H, Xu CJ, Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94:491–501. doi: 10.1016/s0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- Luo X, Budihardjo I, Zou H, Slaughter C, Wang X. Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell. 1998;94:481–490. doi: 10.1016/s0092-8674(00)81589-5. [DOI] [PubMed] [Google Scholar]
- Acehan D, Jiang X, Morgan DG, Heuser JE, Wang X, Akey CW. Three-dimensional structure of the apoptosome: implications for assembly, procaspase-9 binding, and activation. Mol Cell. 2002;9:423–432. doi: 10.1016/s1097-2765(02)00442-2. [DOI] [PubMed] [Google Scholar]
- Gujral JS, Liu J, Farhood A, Jaeschke H. Reduced oncotic necrosis in Fas receptor-deficient C57BL/6J-lpr mice after bile duct ligation. Hepatology. 2004;40:998–1007. doi: 10.1002/hep.20380. [DOI] [PubMed] [Google Scholar]
- Kahraman A, Barreyro FJ, Bronk SF, Werneburg NW, Mott JL, Akazawa Y, Masuoka HC, Howe CL, Gores GJ. TRAIL mediates liver injury by the innate immune system in the bile duct-ligated mouse. Hepatology. 2008;47:1317–1330. doi: 10.1002/hep.22136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K, Kojima Y, Ikejima K, Harada K, Yamashina S, Okumura K, Aoyama T, Frese S, Ikeda H, Haynes NM, Cretney E, Yagita H, Sueyoshi N, Sato N, Nakanuma Y, Smyth MJ. Death receptor 5 mediated-apoptosis contributes to cholestatic liver disease. Proc Natl Acad Sci USA. 2008;105:10895–10900. doi: 10.1073/pnas.0802702105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabele E, Froh M, Arteel GE, Uesugi T, Hellerbrand C, Scholmerich J, Brenner DA, Thurman RG, Rippe RA. TNF-α is required for cholestasis-induced liver fibrosis in the mouse. Biochem Biophys Res Commun. 2009;378:348–353. doi: 10.1016/j.bbrc.2008.10.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel A, van Den Berg IE, Al-Dhalimy M, Groopman J, Ou CN, Ryabinina O, Iordanov MS, Finegold M, Grompe M. Chronic liver disease in murine hereditary tyrosinemia type 1 induces resistance to cell death. Hepatology. 2004;39:433–443. doi: 10.1002/hep.20077. [DOI] [PubMed] [Google Scholar]
- Popov Y, Patsenker E, Fickert P, Trauner M, Schuppan D. Mdr2 (Abcb4)-/- mice spontaneously develop severe biliary fibrosis via massive dysregulation of pro- and antifibrogenic genes. J Hepatol. 2005;43:1045–1054. doi: 10.1016/j.jhep.2005.06.025. [DOI] [PubMed] [Google Scholar]
- Fickert P, Trauner M, Fuchsbichler A, Zollner G, Wagner M, Marschall HU, Zatloukal K, Denk H. Oncosis represents the main type of cell death in mouse models of cholestasis. J Hepatol. 2005;42:378–385. doi: 10.1016/j.jhep.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Krueger A, Schmitz I, Baumann S, Krammer PH, Kirchhoff S. Cellular FLICE-inhibitory protein splice variants inhibit different steps of caspase-8 activation at the CD95 death-inducing signaling complex. J Biol Chem. 2001;276:20633–20640. doi: 10.1074/jbc.M101780200. [DOI] [PubMed] [Google Scholar]
- Saito JM, Maher JJ. Bile duct ligation in rats induces biliary expression of cytokine-induced neutrophil chemoattractant. Gastroenterology. 2000;118:1157–1168. doi: 10.1016/s0016-5085(00)70369-6. [DOI] [PubMed] [Google Scholar]
- Canbay A, Feldstein AE, Higuchi H, Werneburg N, Grambihler A, Bronk SF, Gores GJ. Kupffer cell engulfment of apoptotic bodies stimulates death ligand and cytokine expression. Hepatology. 2003;38:1188–1198. doi: 10.1053/jhep.2003.50472. [DOI] [PubMed] [Google Scholar]
- Grambihler A, Higuchi H, Bronk SF, Gores GJ. cFLIP-L inhibits p38 MAPK activation: an additional anti-apoptotic mechanism in bile acid-mediated apoptosis. J Biol Chem. 2003;278:26831–26837. doi: 10.1074/jbc.M303229200. [DOI] [PubMed] [Google Scholar]
- Canbay A, Feldstein A, Baskin-Bey E, Bronk SF, Gores GJ. The caspase inhibitor IDN-6556 attenuates hepatic injury and fibrosis in the bile duct ligated mouse. J Pharmacol Exp Ther. 2004;308:1191–1196. doi: 10.1124/jpet.103.060129. [DOI] [PubMed] [Google Scholar]
- Gujral JS, Farhood A, Bajt ML, Jaeschke H. Neutrophils aggravate acute liver injury during obstructive cholestasis in bile duct-ligated mice. Hepatology. 2003;38:355–363. doi: 10.1053/jhep.2003.50341. [DOI] [PubMed] [Google Scholar]
- Bai L, Ni HM, Chen X, DiFrancesca D, Yin XM. Deletion of Bid impedes cell proliferation and hepatic carcinogenesis. Am J Pathol. 2005;166:1523–1532. doi: 10.1016/S0002-9440(10)62368-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinehr R, Sommerfeld A, Haussinger D. CD95 ligand is a proliferative and antiapoptotic signal in quiescent hepatic stellate cells. Gastroenterology. 2008;134:1494–1506. doi: 10.1053/j.gastro.2008.02.021. [DOI] [PubMed] [Google Scholar]
- Gong W, Pecci A, Roth S, Lahme B, Beato M, Gressner AM. Transformation-dependent susceptibility of rat hepatic stellate cells to apoptosis induced by soluble Fas ligand. Hepatology. 1998;28:492–502. doi: 10.1002/hep.510280229. [DOI] [PubMed] [Google Scholar]
- Faouzi S, Burckhardt BE, Hanson JC, Campe CB, Schrum LW, Rippe RA, Maher JJ. Anti-Fas induces hepatic chemokines and promotes inflammation by an NF-kappa B-independent, caspase-3-dependent pathway. J Biol Chem. 2001;276:49077–49082. doi: 10.1074/jbc.M109791200. [DOI] [PubMed] [Google Scholar]