Abstract
Psoriasis is an inflammatory skin disease characterized by hyperproliferation of keratinocytes, impaired barrier function, and pronounced infiltration of inflammatory cells. Tight junctions (TJs) are cell-cell junctions that form paracellular barriers for solutes and inflammatory cells. Altered localization of TJ proteins in the epidermis was described in plaque-type psoriasis. Here we show that localization of TJ proteins is already altered in early-stage psoriasis. Occludin, ZO-1, and claudin-4 are found in more layers than in normal epidermis, and claudin-1 and -7 are down-regulated in the basal and in the uppermost layers. In plaque-type psoriasis, the staining patterns of occludin and ZO-1 do not change, whereas the claudins are further down-regulated. Near transmigrating granulocytes, all TJ proteins except for junctional adhesion molecule-A are down-regulated. Treatment of cultured keratinocytes with interleukin-1β and tumor necrosis factor-α, which are present at elevated levels in psoriatic skin, results in an increase of transepithelial resistance at early time points and a decrease at later time points. Injection of interleukin-1β into an ex vivo skin model leads to an up-regulation of occludin and ZO-1, resembling TJ protein alteration in early psoriasis. Our results show for the first time that alteration of TJ proteins is an early event in psoriasis and is not the consequence of the more profound changes found in plaque-type psoriasis. Our data indicate that cytokines are involved in alterations of TJ proteins observed in psoriasis.
Tight junctions (TJs) are cell-cell junctions that seal the intercellular space between neighboring cells. They consist of a variety of TJ transmembrane proteins, eg, claudins (Cldns), occludin (Occl), and junctional adhesion molecules (JAMs) as well as of TJ-plaque proteins, eg, ZO-1 to -3, symplekin, and cingulin (reviewed in Refs. 1,2). In simple epithelia and endothelia it was shown that TJs are important for the establishment and maintenance of a barrier to the paracellular passage of molecules and inflammatory cells. In addition, TJ-associated proteins were demonstrated to be involved, eg, in cell proliferation and differentiation as well as in vesicle transport (reviewed in Refs. 2,3). In mammalian epidermis, typical TJ structures are localized in the stratum granulosum, whereas the distribution patterns of TJ proteins are more widespread (reviewed in Ref. 4). For instance, Occl is restricted to the stratum granulosum, ZO-1 and Cldn-4 are found in the upper layers of the epidermis, and Cldn-1, Cldn-7, and JAM-A are found in all layers. TJ proteins are involved in (murine) inside-out barrier function of the epidermis, which was shown in several knockout mouse models, especially Cldn-1-deficient mice, which die shortly after birth because of a tremendous transepidermal water loss and which are characterized by leaky TJs5 (reviewed in Refs. 4,6).
Psoriasis is an inflammatory skin disease affecting approximately 2% of the Western population. It is characterized by hyperproliferation of keratinocytes, impaired barrier function, and pronounced infiltration of inflammatory cells into dermis and epidermis (reviewed in Refs. 7,8). Histopathologically, psoriasis is a dynamic process. Early psoriasis is characterized by dilation of vessels and immigration of mononuclear cells and granulocytes into the dermis and shortly thereafter into the epidermis as well as by spongiosis of the epithelium. Later stages show variable parakeratosis, epidermotropic neutrophil immigration forming subcorneal pustules, acanthosis, and dilated vessels in the papillary dermis with a moderate mononuclear infiltrate.
Altered localization of TJ proteins in the epidermis has been described in plaque-type psoriasis. TJ proteins that are normally restricted to the stratum granulosum (Occl) or stratum granulosum and upper stratum spinosum (ZO-1 and Cldn-4) exhibit a broader localization pattern.9,10,11 This broader expression is not found in nonlesional skin and is reversed in healed psoriatic plaques except for Cldn-4.12 In contrast, TJ proteins that are normally localized in all layers of the epidermis, ie, Cldn-1, are down-regulated.9,13 Watson et al13 suggested that interleukin (IL)-1β, which is produced by keratinocytes and monocytes/macrophages, plays a role in down-regulation of Cldns.
To further elucidate the molecular causes for alterations of TJ proteins observed in psoriasis we investigated early-stage in comparison with plaque-type psoriasis. In addition, we analyzed the influence of IL-1β and tumor necrosis factor (TNF)-α, two important cytokines involved in the pathogenesis of psoriasis, on TJ functionality in keratinocytes.
Here we demonstrate for the first time that alteration of TJ proteins is already found in early-stage psoriasis and is therefore not (only) a consequence of epidermal changes manifested in plaque-type psoriasis. TJ localization is affected by inflammatory cells, and IL-1β is able to influence TJ expression and functionality in a time- and dose-dependent manner. In addition, we show that TNFα also alters TJ functionality in keratinocytes.
Materials and Methods
Tissues, Antibodies, Cytokines, and Primers
The samples we chose as representative for early-stage psoriasis (n = 5) were characterized by a discrete spongiosis of epidermal keratinocytes and immigration of mononuclear cells and granulocytes into the dermis and epidermis. Fully developed plaque-type psoriasis (n = 5) was characterized by parakeratosis, acanthosis with elongated rete ridges, and the presence of epidermotropic neutrophils and dermal lymphocytes. The samples were used after diagnostic procedures had been completed and in agreement with the local medical ethics committee of Hamburg (WF08/08). All patients gave their informed consent.
Antibodies specific for Cldn-1 (MH25), Cldn-4 (ZMD.306), Cldn-7 (ZMD.241), ZO-1 (Z-R1), and Occl (Z-T22) were purchased from Zymed Laboratories (San Francisco, CA). Antibodies directed to JAM-A (AF1103) were purchased from R&D Systems (Minneapolis, MN). Antibodies specific for CD1a (O10), CD15 (C3D-1), CD68 (PG-M1), and Ki-67 (7240) were purchased from DAKO (Hamburg, Germany), antibodies specific for involucrin (MS-126-P0) were from Lab Vision Products (Fremont, CA), and antibodies specific for filaggrin (060600302) were from Quartett (Berlin, Germany). TNFα and IL-1β were purchased from PeproTech (Rocky Hill, NJ). FAM dye-labeled real-time (rt)-PCR TaqMan MGB probes for Cldn-1 (Hs01076359_m1), Cldn-4 (Hs00533616_s1), Cldn-7 (Hs00600772_m1), ZO-1 (Hs01551876_m1), Occl (Hs00170162_m1), JAM-A (Hs00170991_m1), and 18S RNA (Hs99999901_s1) were purchased from Applied Biosystems (Carlsbad, CA).
Treatment of Ex Vivo Skin Organ Models with Cytokines
Ex vivo skin organ models were obtained from porcine ears. Directly after cleaning and disinfection of the pig ears (approximately 1 hour after slaughtering), 6-mm punch biopsies were taken and placed dermis down on sterile gauze in culture dishes and immersed in medium in such a way that the dermis only was in contact with the medium, whereas the epidermis remained exposed to air (“air-liquid-interphase”). The medium consisted of Dulbecco’s modified Eagle’s medium supplemented with hydrocortisone, 2% fetal calf serum, penicillin, and streptomycin. Next 50 μl of cytokines (100 ng/ml and 1 μg/ml, diluted in PBS) was directly injected into the dermis, and the ex vivo skin organ model was incubated in ambient air with 10% CO2 at 37°C for 30 minutes, 60 minutes, 6 hours, and 24 hours. For controls the same amount of PBS was injected.
Human skin samples were obtained as surgical margins during routine clinical procedures; the samples were localized at least 2 cm from the suspect lesions. Subsequently human skin organ models were produced in the same way as porcine models.
Cell Culture
Primary human keratinocytes were isolated from foreskin and cultured in serum-free keratinocyte growth medium (medium 154 containing 70 μmol/L Ca2+; Cascade Biologics, Portland, OR) as described.14 For calcium switch experiments confluent cells were transferred to high-calcium medium containing 1.8 mmol/L Ca2+.
Immunofluorescence Microscopy
Cryosections (6 μm) of skin organ models were fixed in acetone at −20°C for 10 minutes. Primary antibodies were diluted in PBS (Cldn-1, 1:150; ZO-1, 1:100; Occl, 1:100, Ki-67, 1:50; involucrin, 1:200; and filaggrin, 1:300) and applied to the sections for 30 minutes at room temperature. For ZO-1, Occl, and involucrin a blocking step (2% normal goat serum and 0.1% Triton X-100; 15 minutes at room temperature) preceded the antibody incubation. The samples were subsequently washed for 2 × 10 minutes in PBS.
Paraffin sections (5 μm) of formaldehyde-fixed tissues were deparaffinated and rehydrated. Antigen retrieval was performed by microwave oven heating (4 × 5 minutes, 600 W) in Tris-EDTA-citrate buffer (pH 7.8), followed by 0.001% trypsin for 10 minutes at 37°C. Unspecific-binding sites of the tissues were blocked with a Protein Block (DAKO). Primary antibodies were diluted in PBS (Cldn-1, 1:150; Cldn-4, 1:50; Cldn-7, 1:10; ZO-1, 1:80; Occl, 1:20; JAM-A, 1:30; CD1a, 1:400; CD15, 1:75; CD68, 1:100, Ki-67, 1:50; involucrin, 1:100; and filaggrin, 1:200) and applied to the sections overnight at 4°C, followed by washing of the samples for 3 × 3 minutes in Tris-buffered saline and Tween 20 (TBST).
Cultured cells grown on coverslips were fixed in cold methanol (−20°C, 5 minutes) and acetone (−20°C, 15 seconds). Primary antibodies were diluted in PBS (Cldn-1, 1:100; Cldn-4, 1:100; ZO-1, 1:100; and Occl, 1:100) and applied to the coverslips for 30 minutes at room temperature. A blocking step (2% normal goat serum and 0.1% Triton X-100; 15 minutes at room temperature) preceded the antibody incubation. The samples were subsequently washed for 2 × 10 minutes in PBS.
Then Alexa 488- and Alexa 594- or Cy3-labeled secondary antibodies (Jackson ImmunoResearch, Suffolk, UK) were applied for 30 minutes at room temperature, followed by washing in PBS and a subsequent incubation with 4,6-diamidino-2-phenylindole (1:5000 in PBS). Finally, the slides or coverslips were washed again with PBS followed by aquadest and mounted with Fluoromount (Southern Biotechnology Associates, Inc. Birmingham, AL). Isotype-matched antibodies were used for negative controls. An Axiophot II microscope (Zeiss, Göttingen, Germany) and the software Openlab 2.0.4 (Improvision, Coventry, UK) were used for the evaluation of stainings. All images of stainings from a series of experiments were acquired and processed at the same settings, and representative areas were photographed.
Measurement of Transepithelial Resistance
For transepithelial resistance (TER) experiments, keratinocytes were trypsinized and plated onto Transwells of 0.4-μm pore size (Millipore, Bedford, MA) with a cell density of 100,000 cells/well. When cells reached confluence they were transferred to high-calcium medium containing 1.8 mmol/L Ca2+ and 1 or 100 ng/ml cytokines, respectively. TER was measured at different time points (0, 24, 48, 72, and 96 hours) after the calcium switch (t = 0 hours) by using a Millicell-ERS epithelial voltohmmeter (Millipore). Cytokines were applied at t = 0 hours and every 48 hours. In a further approach cytokines were applied 48 hours after the calcium switch, when TER was already present (>350 Ω · cm2). TER values were corrected by subtracting the blank value (no cells) and are expressed in Ω · cm2.
Western Blot Analysis
Ex vivo skin organ models were frozen in liquid nitrogen and lysed in RIPA buffer (1% Nonidet P-40, 0.5% deoxycholate, 0.1% SDS, 5 mmol/L EDTA, 150 mmol/L NaCl, and 50 mmol/L Tris, pH 8.0) and a cocktail of protease inhibitors (Sigma, Munich, Germany). The same lysis buffer was applied to the primary keratinocytes. Total protein (30 μg) was separated by SDS-polyacrylamide gel electrophoresis and transferred to nitrocellulose. After blocking for 1 hour with 5% dry milk powder in TBST, membranes were probed with the appropriate antibodies also used in immunofluorescence microscopy overnight at 4°C. Subsequently membranes were washed for 3 × 5 minutes with TBST and incubated for 30 minutes with the appropriate secondary antibodies coupled with horseradish peroxidase (Jackson ImmunoResearch). After another washing for 2 × 5 minutes with TBST immunoreactions were visualized by the ECL system (Amersham, Buckinghamshire, UK).
Real-Time-PCR Analysis
Total mRNA was isolated from 3-mm punch biopsies from three patients with psoriasis and three samples of healthy skin as well as from cultured keratinocytes by using an RNeasy mini kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Three micrograms of total RNA was used for first-strand cDNA synthesis with the iScript cDNA synthesis kit (Bio-Rad, Munich, Germany) as suggested by the manufacturer. Two microliters of the cDNA was used as a template in real-time-PCR analysis with the FAM dye-labeled TaqMan MGB probes for Cldn-1, Cldn-4, Cldn-7, ZO-1, Occl, JAM-A, and 18S RNA in an iCycler iQ thermal cycler (Bio-Rad) under the conditions recommended by Applied Biosystems. All real-time-PCR analyses were performed in triplicate for three independent experiments. Relative transcriptional levels within distinct experiments were determined by using the 2−ΔΔCt method.15
Cell Proliferation Assay
Cell proliferation of keratinocytes with and without IL-1β or TNFα was investigated by using the Quick Cell Proliferation Assay Kit II (Biocat, Heidelberg, Germany). Keratinocytes were incubated for 5, 24, 48, 72, and 96 hours in high-calcium medium with cytokines (100 ng/ml) or PBS. Subsequently the tetrazolium salt, WST-1, was added and the cells were further incubated for 4 hours at 37°C. The assay is based on the cleavage of the tetrazolium salt to formazan by cellular mitochondrial dehydrogenase. The amount of the dye generated by activity of dehydrogenase is directly proportional to the number of living cells. The absorbance was measured at 440 nm by using a microplate autoreader.
Evaluation of the Proliferative Index (Ki-67-Positive Cells) in Skin Organ Models
Ki-67-positive cells in skin organ models were detected and counted by using the above-mentioned immunofluorescence microscope. The cells highlighted by Ki-67 were correlated to the total amount of cells in the basal cell layer (4,6-diamidino-2-phenylindole-positive cells). Three visual fields were evaluated per sample.
Statistics
Statistical analysis was performed with Student’s t-test. Values are shown as mean ± SEM.
Results
Localization of TJ Proteins in Early-Stage and Plaque-Type Psoriasis
To clarify whether the broadened expression of distinct TJ proteins as well as the down-regulation of other TJ proteins observed in plaque-type psoriasis are early or late events we investigated the distribution of TJ proteins in early-stage compared with plaque-type psoriasis. From the variety of Cldns we chose two different Cldns that are localized in all layers of normal epidermis (Cldn-1 and Cldn-7) to investigate whether these proteins show similar alterations. Further, we studied Cldn-4, which is restricted to the upper layers of the epidermis to elucidate whether this Cldn shows changes comparable to those of the other Cldns or whether its fate resembles that of other TJ proteins that are restricted to the upper layers of the epidermis, ie, Occl and ZO-1.
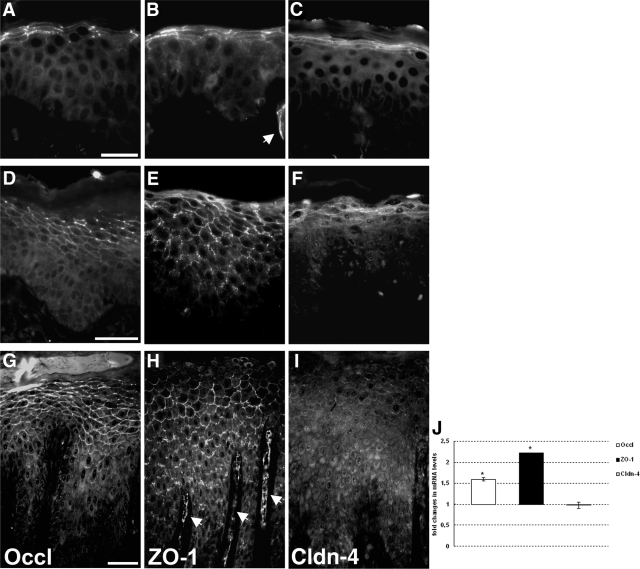
Occludin in normal skin is mainly restricted to the lateral plasma membranes of the granular cells. Occasionally, a faint staining is also seen at the entire plasma membrane (Figure 1A). ZO-1 and Cldn-4 are enriched at the lateral plasma membranes but are also observed at the entire membranes of the upper layers of the epidermis (Figure 1, B and C). In early-stage as well as plaque-type psoriasis we observed expression of Occl, ZO-1, and Cldn-4 in more epidermal layers compared with those for normal skin (Figure 1; Table 1; Supplemental Figure S1, see http://ajp.amjpathol.org). Occl and ZO-1 are concentrated at the lateral plasma membranes but can also be seen at the entire plasma membrane and, especially in plaque-type psoriasis, in the cytoplasm (Figure 1, D, E, G, and H). Cldn-4 is mainly found in the cytoplasm (Figure 1, F and I). Real-time PCR revealed increased levels of Occl and ZO-1 mRNAs, whereas there was no change for Cldn-4 mRNA in psoriatic skin compared with normal skin (Figure 1J).
Figure 1.
Localization and expression of Occl, ZO-1, and Cldn-4. Immunofluorescence localization of Occl (A, D, and G), ZO-1 (B, E, and H), and Cldn-4 (C, F, and I) in normal skin (A, B, and C) and in early-stage (D, E, and F) and plaque-type (G, H, and I) psoriasis. Epifluorescence pictures. Note the broadened localization of Occl and ZO-1 in psoriasis. Arrows denote blood vessels positive for TJ proteins. Scale bars: 20 μm (A–C); 50 μm (D–I). J: Real-time PCR analysis of Occl, ZO-1, and Cldn-4 expression levels. Values are denoted as fold changes in psoriatic skin compared with normal skin (means ± SEM; n = 3). *P < 0.05.
Table 1.
Comparison of the Staining Patterns of the Various TJ Proteins in Psoriatic Skin and Skin Models and Keratinocytes Treated with IL-1β
| TJ protein | IL-1β injection in ex vivo skin organ models
|
Psoriasis
|
Cultured keratinocytes with IL-1β
|
|||||
|---|---|---|---|---|---|---|---|---|
| 30 minutes | 60 minutes | 6 hours | 24 hours | Early-stage | Plaque-type | 24 hours | 96 hours | |
| Occl | (↑) | ↑ | ↑ | ↑ (↑) | ↑ (↑) | ↑↑* | (↑) | ↓↓ |
| ZO-1 | (↑) | ↑ | ↑ | ↑ (↑) | ↑ (↑) | ↑↑* | (↑) | ↓ |
| Cldn-4 | — | — | — | — | ↑* | † | † | † |
| Cldn-1 | (↓)‡ | (↓)‡ | (↓)‡ | (↓)‡ or ↓ | ↓‡ | ↓‡ or ↓↓ | ↑ | ↓↓ |
| Cldn-7 | nt | nt | nt | nt | ↓‡ | ↓‡ or ↓↓ | nt | nt |
| JAM-A | nt | nt | nt | nt | (↓)§ | (↓)§ | nt | nt |
↑, broadened expression/increased staining intensity; ↓, down-regulation; (↑) to ↑↑ reflect different degrees of changes; —, no changes; nt, not tested.
Cell-cell borders and cytoplasm.
Mainly cytoplasm and plasma membrane.
Uppermost and lowermost layers.
Uppermost layers.
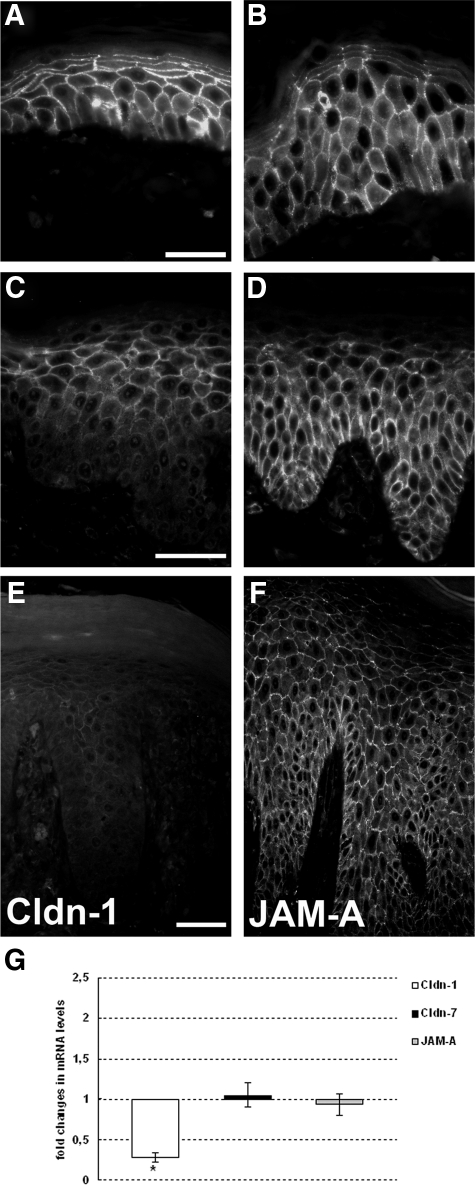
Cldn-1 and Cldn-7, which are normally found in all layers of the epidermis (for Cldn-7 with a more intensive staining in the upper layers), were observed at decreased levels, especially in the lowermost but also in the uppermost layers of the epidermis in early-stage psoriasis (Figure 2, A and C, for Cldn-1, Cldn-7 data not shown; Table 1; Supplemental Figure S2, see http://ajp.amjpathol.org). In plaque-type psoriasis, an overall down-regulation was found in many cases (Figure 2E; Table 1; Supplemental Figure S2, see http://ajp.amjpathol.org), whereas in other cases Cldn-1 staining was still quite intense except for the uppermost and lowermost layers.
Figure 2.
Expression and localization of Cldn-1, Cldn-7, and JAM-A. Immunofluorescence localization of Cldn-1 (A, C, and E) and JAM-A (B, D, and F) in normal skin (A and B), early-stage (C and D), and plaque-type (E and F) psoriasis. Epifluorescence pictures. Note the down-regulation of Cldn-1 in the lower and uppermost layers in early-stage psoriasis and a further down-regulation in plaque-type psoriasis. Scale bars: 20 μm (A and B); 50 μm (C–F). G: Real-time PCR analysis of Cldn-1, Cldn-7, and JAM-A expression levels. Values are denoted as fold changes in psoriatic skin compared with normal skin (means ± SEM; n = 3). *P < 0.05.
Of note, there was no alteration of JAM-A, a TJ protein also normally localized in all layers of the epidermis (Figure 2B), aside from a slight down-regulation in the uppermost layers (Figure 2, D and F; Table 1; Supplemental Figure S2, see http://ajp.amjpathol.org). Cldn-1, Cldn-7, and JAM-A were uniformly distributed at the plasma membranes in healthy as well as in psoriatic skin; an increase of staining intensity at the lateral plasma membranes was rarely observed except for JAM-A in the upper layers (Figure 2 for Cldn-1 and JAM-A; data not shown for Cldn-7). Real-time PCR revealed a decreased level of Cldn-1 mRNA in psoriatic skin compared with that in normal skin, whereas there was no change in the levels of Cldn-7 and JAM-A (Figure 2G).
Down-Regulation of TJ Proteins near Inflammatory Cells
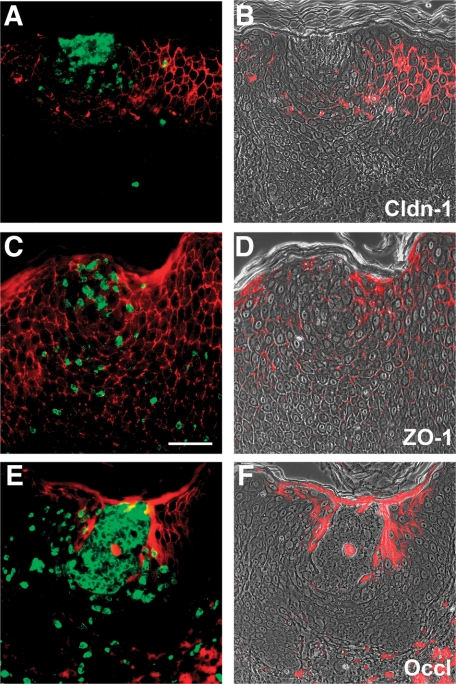
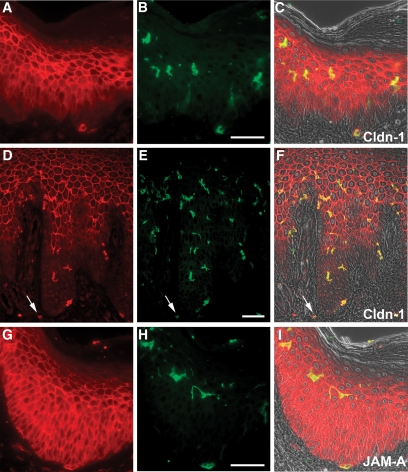
Because psoriasis is also characterized by epithelial transmigration of granulocytes forming subsequently discrete subcorneal pustules, we also examined the expression of TJ proteins near these leukocytes. We observed a down-regulation of most TJ proteins near granulocytes, which were detected by CD15, particularly near pustules (Figure 3, A–F, for Cldn-1, ZO-1, and Occl). However, there also existed a colocalization of CD15-positive cells and TJ proteins. Again, JAM-A was not down-regulated (data not shown). We did not observe a down-regulation of TJ proteins in the proximity of CD68-positive macrophages in the epidermis even though a down-regulation of Cldn-1 was sometimes observed in epidermis adjacent to dermal CD68-positive cells (data not shown). There was no down-regulation of TJ proteins near or at Langerhans cells. On the contrary, Langerhans cells were, as was shown before in normal epidermis,16 positive for Cldn-1 (Figure 4, A–F). Interestingly, Langerhans cells were also positive for Cldn-1 in epidermal areas in which keratinocytes were negative for Cldn-1 (Figure 4, A–F). In addition, dermal CD1a-positive cells were also positive for Cldn-1 (Figure 4B, arrow). Of note, we also observed JAM-A staining in epidermal and dermal CD1a-positive cells (Figure 4, G–I).
Figure 3.
Colocalization by double-staining of granulocytes and TJ proteins. Localization of Cldn-1 (red; A and B), ZO-1 (red; C and D), and Occl (red; E and F) as well as CD15 (green; A, C and E) in psoriasis. A, C, and E: overlay of red and green epifluorescence pictures. B, D, and F: overlay of red epifluorescence and phase-contrast pictures. Note the down-regulation of TJ proteins near granulocytes as well as colocalization with granulocytes. Scale bar = 50 μm.
Figure 4.
Colocalization of Cldn-1 as well as JAM-A and Langerhans cells. Immunofluorescence localization of Cldn-1 (red; A, C, D, and F), JAM-A (red; G, and I), and CD1a (green; B, C, E, F, H, and I) in early-stage (A–C and G–I) and plaque-type (D–F) psoriasis. Note the colocalization of CD1a with Cldn-1 and JAM-A. Arrow, dermal CD1a positive cell. Scale bar = 50 μm.
Influence of IL-1β and TNFα on TJ Protein Expression and Function in Keratinocytes
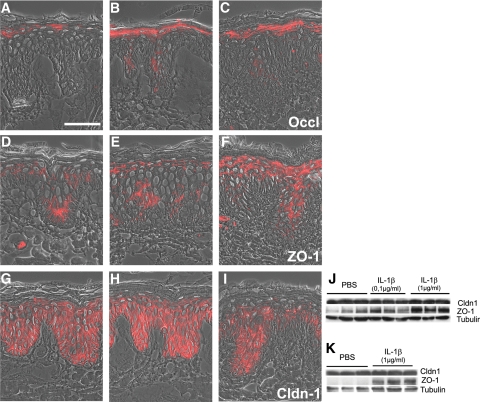
It is known that in psoriatic skin keratinocytes as well as infiltrating inflammatory cells produce elevated levels of IL-1β.17 In addition, results obtained by Watson et al13 hinted that IL-1β may have a putative role in the regulation of Cldns in plaque-type psoriasis. Therefore, we examined whether broadened expression as well as down-regulation of distinct TJ proteins might be influenced by this cytokine. Injection of 50 μl of 100 ng/ml and 1 μg/ml IL-1β into ex vivo porcine (Figure 5; Table 1) and human (data not shown) skin organ models for 24 hours resulted in a broadened localization of Occl as well as of ZO-1 (Figure 5, A–F). Injection of 100 ng/ml IL-1β did not change Cldn-1 staining intensity (Figure 5, G and H), whereas 1 μg/ml resulted in a down-regulation in some sections (Figure 5I) and in other sections staining intensity remained unaltered (data not shown). Western blot analysis of porcine and human models confirmed the up-regulation of ZO-1; but levels of Cldn-1 remained unchanged (Figure 5, J and K). The broadened localization of Occl and ZO-1 was already observed 30 minutes (faintly) and 60 minutes (more intense then after 30 minutes but less intensive than after 24 hours) after injection of 100 ng/ml and 1 μg/ml IL-1β (Table 1; Supplemental Figure S3, see http://ajp.amjpathol.org).
Figure 5.
Influence of IL-1β on localization and protein amount of TJ proteins 24 hours after injection in ex vivo skin organ models. Same amounts of PBS (A, D, and G), 0.1 μg/ml IL-1β (B, E, and H), and 1 μg/ml IL-1β (C, F, and I) were injected into porcine ex vivo skin organ models (n = 9). A–I: Immunofluorescence staining of the models after 24 hours of cytokine treatment (A–C: Occl; D–F: ZO-1; G–I: Cldn-1) and Western blot analysis (J: porcine samples; K: human samples). Scale bar = 50 μm.
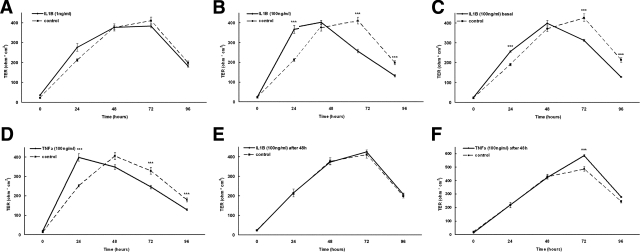
To investigate the influence of IL-1β on TJ functionality we incubated primary human keratinocytes with IL-1β and observed a dose- and time-dependent up- and down-regulation of transepithelial resistance (Figure 6, A–C). Twenty-four hours after the calcium switch and application of IL-1β an up-regulation of TER was observed that was more pronounced with 100 ng/ml IL-1β than with 1 ng/ml. At later time points (72 and 96 hours) TER was significantly reduced by the influence of IL-1β. Again, this result was more obvious with 100 than with 1 ng/ml. There was no difference between the applications of IL-1β to the apical or basal part of the cells (Figure 6, B and C).
Figure 6.
Influence of IL-1β and TNFα on TJ functionality. Transepithelial resistance of cultured keratinocytes after the calcium switch and incubation with 1 ng/ml IL-1β (A), 100 ng/ml IL-1β applied to the apical side of the membrane (B), 100 ng/ml IL-1β applied to the basal side (C), and 100 ng/ml TNFα (D). E and F: IL-1β (E) and TNFα (F) were applied 48 hours after the calcium switch when TER was fully established. Note the time-dependent influence of IL-1β and TNFα and the dose-dependent influence of IL-1β in cells directly after calcium switch whereas only TNFα is effective in cells 48 hours after calcium switch. ***P < 0.05 between control and IL-1β- or TNFα-treated cells (n = 9).
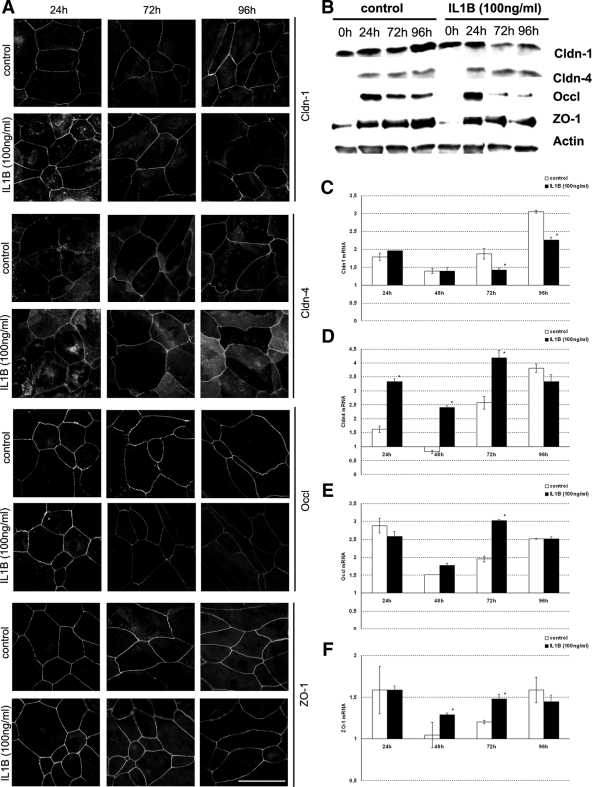
Immunofluorescence staining of the keratinocytes revealed an increased staining intensity for Cldn-1 and Cldn-4 in the cytoplasm and increased and more continuous staining at the cell-cell borders 24 hours after the calcium switch and application of IL-1β. A slight increase at the cell-cell borders and more continuous staining was also observed for ZO-1 and Occl (Figure 7A; Table 1). Therefore, increased TER goes along with increased staining intensity for Cldns, ZO-1, and Occl. The increase in staining intensity was not reflected by a change in protein amount (Figure 7B). Decreased staining intensity compared with the control was observed 72 hours after the calcium switch and application of IL-1β for Occl and 96 hours for Occl, ZO-1, and Cldn-1 (Figure 7A; Table 1). For Occl and Cldn-1 a decrease in the protein amount was observed at 72 and 96 hours (Figure 7B). Therefore, decreased TER goes along with decreased staining intensity and protein amounts for Cldn-1 and Occl and, in some respects, for ZO-1.
Figure 7.
Influence of IL-1β on TJ protein localization and expression in cultured keratinocytes. A: Immunofluorescence localization of TJ proteins in cultured keratinocytes at the indicated time points after the calcium switch and application of IL-1β. Scale bar = 20 μm. B: Western blot analysis of keratinocytes that were harvested 24, 72, and 96 hours after the calcium switch without (left, control) and with (right) application of 100 ng/ml IL-1β. Equal amounts of proteins were separated by SDS-polyacrylamide gel electrophoresis and immunoblotted with antibodies specific for the proteins indicated. Actin was used as gel loading control. C–F: Transcriptional differences of Cldn-1 (C), Cldn-4 (D), Occl (E), and ZO-1 (F) between cells with and without treatment with IL-1β at the indicated time points. Values denote fold changes compared with 0 hours and are shown as mean ± SEM. *P < 0.05 (comparison between IL-1β-treated and nontreated cells).
Regarding RNA level we observed a significant increase of Cldn-4 mRNA 24 hours after IL-1β application, whereas there were minor changes for the other proteins. At 72 hours after application there was a significant increase of Cldn-4, Occl, and ZO-1, but a significant decrease for Cldn-1. At 96 hours after application, there was only a significant decrease for Cldn-1 (Figure 7, C–F).
Because it is also known that TNFα plays an important role in psoriasis18 and it is found in elevated levels in psoriatic plaques,19 we applied TNFα to cultured human keratinocytes. Application of 100 ng/ml of TNFα showed results analogous to those for IL-1β concerning the up- and down-regulation of TER (Figure 6D).
When adding IL-1β to fully developed tight junctions 48 hours after the calcium switch (characterized by a TER >350 Ω · cm2), we observed no increase in TER. However, there was a significant increase in TER after application of TNFα (Figure 6, E and F).
Correlation of TJ Alteration with Keratinocyte Differentiation and Proliferation
Psoriatic plaques are characterized by alteration of differentiation markers as well as increased proliferative index of keratinocytes. The question arises, whether alteration of TJ protein expression and localization observed in psoriasis and after application of IL-1β might be a consequence of alteration of differentiation and proliferation. Therefore, we studied the localization of the differentiation markers involucrin and filaggrin as well as cell proliferation in psoriasis and in skin models injected with IL-1β. We observed an alteration of expression of involucrin and filaggrin in early-stage psoriasis (Supplemental Figure S4, D and E, see http://ajp.amjpathol.org) that was even more pronounced in plaque-type psoriasis (Supplemental Figure S4, G and H, see http://ajp.amjpathol.org), compared with normal skin (Supplemental Figure S4, A and B, see http://ajp.amjpathol.org). The proliferative index was increased in both stages of psoriasis (Supplemental Figure S5, C, F, and I, see http://ajp.amjpathol.org). Therefore, early-stage psoriasis shows an alteration of TJ protein expression, epidermal differentiation, and the proliferative index. However, immunohistological stainings of ex vivo skin models 30 minutes, 60 minutes, 6 hours, and 24 hours after injection of IL-1β did not show alterations of involucrin and filaggrin localization and staining intensity or of the proliferative index (Supplemental Figure S5, A–G, see http://ajp.amjpathol.org) even though we observed an alteration of TJ protein expression (Supplemental Figure S3, see http://ajp.amjpathol.org). In addition, in cultured keratinocytes we did not observe an alteration in the proliferative index after application of IL-1β nor TNFα (Supplemental Figure 5H, see http://ajp.amjpathol.org) even though an alterations in TJ protein expression, localization, and function were observed (Figure 7). Therefore, alterations in TJ expression and localization do not seem to depend on changes of differentiation and proliferation.
Discussion
Plaque-type psoriasis was described as being associated with an alteration in TJ proteins, ie, a broadened expression of normally restricted TJ proteins as well as a down-regulation of Cldns.9,10,11,12,13 We demonstrate here for the first time that altered localization of TJ proteins can already be seen in the early stages of psoriasis. Our data strongly suggest that broadened expression of Occl and ZO-1 is an early event in psoriasis that continues as psoriasis progresses. It is thus not (only) a consequence of the more profound changes found in plaque-type psoriasis such as acanthosis of the epithelium. Down-regulation of Cldns is also already seen in early-stage psoriasis but is much more restricted than in plaque-type psoriasis.
Of note, localization of ZO-1 and Occl as well as of Cldns and JAM-A was found in addition to the lateral plasma membranes in the basal and apical plasma membranes and in the cytoplasm. This means that the localization of these proteins is not restricted to TJs. Therefore, the alterations of TJ proteins seen in psoriatic skin might have many different consequences, including some that are independent of TJ structures.
It is known that ZO-1 plays a role in the formation of adherens junctions and gap junctions as well as in cell proliferation and differentiation.1,2,3 Because an increase in the proliferative index as well as an alteration of differentiation markers is already seen in early-stage psoriasis, the question arises whether ZO-1 alteration precedes these events. IL-1β, which is produced by keratinocytes and macrophages/monocytes, was demonstrated at elevated levels in psoriatic skin13,20 and might thus be a possible cause for the observed alterations of TJ proteins. To mimic this situation we injected human and porcine ex vivo skin organ models with IL-1β. Twenty-four hours after injection of various concentrations of IL-1β we observed a broadened localization of Occl and ZO-1. This localization pattern in our experiments resembles that of early-stage psoriasis, indicating that IL-1β is involved in the altered expression of ZO-1 and Occl in psoriasis, at least in early stages. Detailed investigation of our skin models at various time points (30 minutes, 60 minutes, 6 hours, and 24 hours) revealed that the broadened expression of Occl and ZO-1 was already seen at early time points, whereas the differentiation and proliferative index remained unaltered. This finding suggests that changes of Occl and ZO-1 precede alterations of differentiation and proliferation.
Occludin has been shown to be involved in the barrier as well as in the fence function of TJs.2,21 Increased expression of Occl in epithelial cells was shown to be associated with increased functionality of TJs, ie, transepithelial resistance.22 Occl hallmarks the areas in the epidermis where dermally injected extracellular tracer stops and TJ structures can be seen in electron microscopy.5 Whether the up-regulation of Occl in the epidermis is accompanied by an increase in barrier function is not clear as yet. Broadened expression of Occl leads to more cell layers with co-expression of Cldn-1, Occl, ZO-1, and Cldn-7, at least in early-stage psoriasis. As was shown in healthy murine skin, functional TJs are only found in the stratum granulosum where all these proteins colocalize; thus, it is tempting to speculate that a broadened localization might result in more epidermal layers with functional TJs. This suggests that the broadened expression of TJ proteins in early-stage psoriasis might be an attempt to keep up the barrier function of the epidermis. However, this hypothesis has to be proven in future experiments.
In contrast with the broadened localization of Occl and ZO-,1 a down-regulation of claudins at the plasma membranes was observed, which was restricted to the uppermost and lower layers in early-stage psoriasis and, in many cases, was more prominent in plaque-type psoriasis. Comparable to the results described here a down-regulation of Cldn-1 in lower layers of the epidermis was also observed in a mouse skin tumorigenesis model characterized by hyperplasia, T-cell infiltration, and finally the formation of squamous cell carcinoma.23 As psoriasis and the tumorigenesis model are characterized by hyperproliferation and infiltration of inflammatory cells, both might be linked to alterations in Cldn expression. On the other hand, Cldn-1 has been shown to be important for inside-out barrier function in murine newborn skin (reviewed in Refs. 4,6). The loss of Cldns in all layers of plaque-type psoriasis might therefore reflect an impairment of TJ barrier function at this stage.
In our ex vivo experiments we observed a down-regulation of Cldn-1 in the uppermost layers after injection of 100 ng/ml and 1 μg/ml IL-1β. An overall down-regulation was not observed with 100 ng/ml but occasionally was seen in stainings of models with 1 μg/ml, putatively at injection sites with locally very high concentrations. Normal human skin contains 175 pg/ml IL-1β, and psoriatic skin contains 600 pg/ml.20 Because this is an overall value for skin biopsy specimens that might not reflect the value at special areas of the skin, we chose for our ex vivo experiments approximately 200-fold (100 ng/ml) and approximately 2000-fold (1 μg/ml) higher concentrations. However, the correct concentrations that might resemble the in vivo situation can only be estimated and will also be inhomogeneous in the tissue as there may be, eg, concentration gradients near cytokine-producing cells. These gradients might explain why we observe an overall down-regulation of Cldn-1 in plaque-type psoriasis in many but not all sections. Interestingly, Watson et al13 showed a down-regulation of Cldn-1 after injection of 50 μl of >107 IU/mg IL-1β into healthy volunteers and uninvolved skin of patients with psoriasis.
Investigation of TJ functionality in cell culture experiments revealed that the application of IL-1β influences the TER in keratinocytes in a dose- and time-dependent manner. Whereas the up-regulation of TER at early time points seems to be associated with an increased staining intensity of TJ proteins at the cell-cell borders but not an increase in the amount of protein, the down-regulation of TER at later time points seems to correlate with a decrease in staining intensity as well as a decrease in protein amount at least for some TJ proteins. Therefore, up-regulation of TER may be a consequence of relocalization of TJ proteins and other changes at the TJs, eg, phosphorylation, whereas the down-regulation is a consequence of loss of the proteins from the cell borders and degradation. A redistribution of TJ proteins has already been described to be a consequence of various stimuli, for instance, bacterial infection.24 Interestingly, there is an increase of mRNA for Occl and ZO-1 at 72 hours that might be a compensatory effect, whereas there is a decrease for Cldn-1 mRNA.
TNFα, which is known to play an important role in psoriasis18 and which was shown to be present at elevated levels in psoriatic plaques,19 is also able to influence TJ functionality in a time-dependent manner. This is a hint for a role of both IL-1β and TNFα in alteration of TJs in psoriasis. However, this theory has to be confirmed in further experiments.
Interestingly, TNFα also increased TER when the cytokine was applied to fully developed TJs (characterized by a TER >350 Ω · cm2), whereas IL-1β only changed TER when it was directly applied after the calcium switch to induce formation of TJs. It is tempting to speculate that IL-1β is (only) effective on TJ functionality in skin with an active turnover, which might be the case for psoriasis, whereas TNFα influences also skin in normal conditions. On the other hand, the effects observed could also reflect different levels of TJ remodeling.
A down-regulation of all TJ proteins except for JAM-A was observed near transmigrating granulocytes. This might be necessary for granulocytes to migrate through the epidermis. It is not clear as yet which factors result in the down-regulation of TJ proteins. Candidate molecules are proteases and cytokines produced by granulocytes, eg, IL-8.25,26,27 JAM-A was shown before to be important for leukocyte migration through blood vessels.28
Interestingly, we found a colocalization of antibodies recognizing JAM-A with antibodies specific for CD1a (Langerhans cells) in the epidermis. JAM-A-deficient mice show increased dendritic cell trafficking to lymph nodes and contact hypersensitivity.29 Therefore, JAM-A could be involved in the anchorage of Langerhans cells in the epidermis.
To summarize our results, we showed that alteration of TJ proteins is an early event in psoriasis. Further we demonstrated that expression and localization of TJ proteins as well as TJ functionality in keratinocytes are influenced by IL-1β. In addition, TJ functionality is also influenced by TNFα. Because these cytokines are important in psoriasis and because we observed similar changes in early psoriatic lesions and in skin injected with IL-1β, these cytokines might play a central role in the regulation of TJs in psoriasis.
Supplementary Material
Footnotes
Address reprint requests to Johanna Brandner, Ph.D., Department of Dermatology and Venerology, University Hospital Hamburg-Eppendorf, Martinistrasse 52, 20246 Hamburg. E-mail: brandner@uke.uni-hamburg.de.
Supplemental material for this article can be found on http://ajp.amjpathol.org.
References
- Aijaz S, Balda MS, Matter K. Tight junctions: molecular architecture and function. Int Rev Cytol. 2006;248:261–298. doi: 10.1016/S0074-7696(06)48005-0. [DOI] [PubMed] [Google Scholar]
- Schneeberger EE, Lynch RD. The tight junction: a multifunctional complex. Am J Physiol Cell Physiol. 2004;286:C1213–C1228. doi: 10.1152/ajpcell.00558.2003. [DOI] [PubMed] [Google Scholar]
- Matter K, Aijaz S, Tsapara A, Balda MS. Mammalian tight junctions in the regulation of epithelial differentiation and proliferation. Curr Opin Cell Biol. 2005;17:453–458. doi: 10.1016/j.ceb.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Brandner JM. Tight junctions and tight junction proteins in mammalian epidermis. Eur J Pharm Biopharm. 2009;72:289–294.. doi: 10.1016/j.ejpb.2008.08.007. [DOI] [PubMed] [Google Scholar]
- Furuse M, Hata M, Furuse K, Yoshida Y, Haratake A, Sugitani Y, Noda T, Kubo A, Tsukita S. Claudin-based tight junctions are crucial for the mammalian epidermal barrier: a lesson from claudin-1-deficient mice. J Cell Biology. 2002;156:1099–1111. doi: 10.1083/jcb.200110122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niessen CM. Tight junctions/adherens junctions: basic structure and function. J Invest Dermatol. 2007;127:2525–2532. doi: 10.1038/sj.jid.5700865. [DOI] [PubMed] [Google Scholar]
- Sabat R, Philipp S, Hoflich C, Kreutzer S, Wallace E, Asadullah K, Volk HD, Sterry W, Wolk K. Immunopathogenesis of psoriasis. Exp Dermatol. 2007;16:779–798. doi: 10.1111/j.1600-0625.2007.00629.x. [DOI] [PubMed] [Google Scholar]
- Segre JA. Epidermal barrier formation and recovery in skin disorders. J Clin Invest. 2006;116:1150–1158. doi: 10.1172/JCI28521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandner JM, Kief S, Wladykowski E, Houdek P, Moll I. Tight junction proteins in the skin. Skin Pharmacol Physiol. 2006;19:71–77. doi: 10.1159/000091973. [DOI] [PubMed] [Google Scholar]
- Pummi K, Malminen M, Aho H, Karvonen SL, Peltonen J, Peltonen S. Epidermal tight junctions: ZO-1 and occludin are expressed in mature, developing, and affected skin and in vitro differentiating keratinocytes. J Invest Dermatol. 2001;117:1050–1058. doi: 10.1046/j.0022-202x.2001.01493.x. [DOI] [PubMed] [Google Scholar]
- Yoshida Y, Morita K, Mizoguchi A, Ide C, Miyachi Y. Altered expression of occludin and tight junction formation in psoriasis. Arch Dermatol Res. 2001;293:239–244. doi: 10.1007/s004030100221. [DOI] [PubMed] [Google Scholar]
- Peltonen S, Riehokainen J, Pummi K, Peltonen J. Tight junction components occludin. ZO-1, and claudin-1, -4 and -5 in active and healing psoriasis. Br J Dermatol. 2007;156:466–472. doi: 10.1111/j.1365-2133.2006.07642.x. [DOI] [PubMed] [Google Scholar]
- Watson RE, Poddar R, Walker JM, McGuill I, Hoare LM, Griffiths CE, O'Neill CA. Altered claudin expression is a feature of chronic plaque psoriasis. J Pathol. 2007;212:450–458. doi: 10.1002/path.2200. [DOI] [PubMed] [Google Scholar]
- Moll I, Houdek P, Schmidt H, Moll R. Characterization of epidermal wound healing in a human skin organ culture model: acceleration by transplanted keratinocytes. J Invest Dermatol. 1998;111:251–258. doi: 10.1046/j.1523-1747.1998.00265.x. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Zimmerli SC, Kerl K, Hadj-Rabia S, Hohl D, Hauser C. Human epidermal Langerhans cells express the tight junction protein claudin-1 and are present in human genetic claudin-1 deficiency (NISCH syndrome). Exp Dermatol. 2008;17:20–23. doi: 10.1111/j.1600-0625.2007.00624.x. [DOI] [PubMed] [Google Scholar]
- Yoshinaga Y, Higaki M, Terajima S, Ohkubo E, Nogita T, Miyasaka N, Kawashima M. Detection of inflammatory cytokines in psoriatic skin. Arch Dermatol Res. 1995;287:158–164. doi: 10.1007/BF01262325. [DOI] [PubMed] [Google Scholar]
- Schottelius AJ, Moldawer LL, Dinarello CA, Asadullah K, Sterry W, Edwards CK., 3rd Biology of tumor necrosis factor-α—implications for psoriasis. Exp Dermatol. 2004;13:193–222. doi: 10.1111/j.0906-6705.2004.00205.x. [DOI] [PubMed] [Google Scholar]
- Ettehadi P, Greaves MW, Wallach D, Aderka D, Camp RD. Elevated tumour necrosis factor-α (TNF-α) biological activity in psoriatic skin lesions. Clin Exp Immunol. 1994;96:146–151. doi: 10.1111/j.1365-2249.1994.tb06244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper KD, Hammerberg C, Baadsgaard O, Elder JT, Chan LS, Taylor RS, Voorhees JJ, Fisher G. Interleukin-1 in human skin: dysregulation in psoriasis. J Invest Dermatol. 1990;95:24S–26S. doi: 10.1111/1523-1747.ep12505698. [DOI] [PubMed] [Google Scholar]
- Leyvraz C, Charles RP, Rubera I, Guitard M, Rotman S, Breiden B, Sandhoff K, Hummler E. The epidermal barrier function is dependent on the serine protease CAP1/Prss8. J Cell Biol. 2005;170:487–496. doi: 10.1083/jcb.200501038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy KM, Skare IB, Stankewich MC, Furuse M, Tsukita S, Rogers RA, Lynch RD, Schneeberger EE. Occludin is a functional component of the tight junction. J Cell Sci. 1996;109(pt 9):2287–2298. doi: 10.1242/jcs.109.9.2287. [DOI] [PubMed] [Google Scholar]
- Arabzadeh A, Troy TC, Turksen K. Changes in the distribution pattern of Claudin tight junction proteins during the progression of mouse skin tumorigenesis. BMC Cancer. 2007;7:196. doi: 10.1186/1471-2407-7-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muza-Moons MM, Schneeberger EE, Hecht GA. Enteropathogenic Escherichia coli infection leads to appearance of aberrant tight junctions strands in the lateral membrane of intestinal epithelial cells. Cell Microbiol. 2004;6:783–793. doi: 10.1111/j.1462-5822.2004.00404.x. [DOI] [PubMed] [Google Scholar]
- Bazzoni F, Cassatella MA, Rossi F, Ceska M, Dewald B, Baggiolini M. Phagocytosing neutrophils produce and release high amounts of the neutrophil-activating peptide 1/interleukin 8. J Exp Med. 1991;173:771–774. doi: 10.1084/jem.173.3.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassatella MA, Bazzoni F, Ceska M, Ferro I, Baggiolini M, Berton G. IL-8 production by human polymorphonuclear leukocytes: the chemoattractant formyl-methionyl-leucyl-phenylalanine induces the gene expression and release of IL-8 through a pertussis toxin-sensitive pathway. J Immunol. 1992;148:3216–3220. [PubMed] [Google Scholar]
- von den Driesch P. Polymorphonuclears: structure, function, and mechanisms of involvement in skin diseases. Clin Dermatol. 2000;18:233–244. doi: 10.1016/s0738-081x(99)00116-9. [DOI] [PubMed] [Google Scholar]
- Weber C, Fraemohs L, Dejana E. The role of junctional adhesion molecules in vascular inflammation. Nat Rev Immunol. 2007;7:467–477. doi: 10.1038/nri2096. [DOI] [PubMed] [Google Scholar]
- Cera MR, Del Prete A, Vecchi A, Corada M, Martin-Padura I, Motoike T, Tonetti P, Bazzoni G, Vermi W, Gentili F, Bernasconi S, Sato TN, Mantovani A, Dejana E. Increased DC trafficking to lymph nodes and contact hypersensitivity in junctional adhesion molecule-A-deficient mice. J Clin Invest. 2004;114:729–738. doi: 10.1172/JCI21231. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.