Abstract
The prevalence of human immunodeficiency virus (HIV)-associated neurocognitive disorders (HAND) that result from HIV infection of the central nervous system is increasing. Macrophages, the primary target for HIV within the central nervous system, play a central role in HIV-induced neuropathogenesis. Drug abuse exacerbates HAND, but the mechanism(s) by which this increased neuropathology results in more severe forms of HAND in HIV-infected drug abusers is unclear. The addictive and reinforcing effects of many drugs of abuse, such as cocaine and methamphetamine, are mediated by increased extracellular dopamine in the brain. We propose a novel mechanism by which drugs of abuse intensify HIV neuropathogenesis through direct effects of the neurotransmitter dopamine on HIV infection of macrophages. We found that macrophages express dopamine receptors 1 and 2, and dopamine activates macrophages by increasing ERK 1 phosphorylation. Our results demonstrate for the first time that dopamine increases HIV replication in human macrophages and that the mechanism by which dopamine mediates this change is by increasing the total number of HIV-infected macrophages. This increase in HIV replication is mediated by activation of dopamine receptor 2. These findings suggest a common mechanism by which drugs of abuse enhance HIV replication in macrophages and indicate that the drug abuse-heightened levels of central nervous system dopamine could increase viral replication, thereby accelerating the development of HAND.
Human immunodeficiency virus (HIV) enters the central nervous system (CNS) soon after initial infection,1 resulting in ongoing inflammation and neurological damage that leads to the development of HIV-associated neurocognitive disorders (HAND) in as many as 50% of infected individuals.2,3 The prevalence of these complications is increasing despite the advent of antiretroviral therapy, due to the longer lifespan of infected individuals on antiretroviral therapies4 and the poor ability of most antiretroviral drugs to penetrate the blood-brain barrier.5,6 HIV is thought to enter the brain through the transmigration of infected monocytes across the blood-brain barrier.7,8,9,10 Within the CNS, macrophages are the primary source of HIV and the virus spreads primarily through infection of brain macrophages and microglia.11,12 Infected macrophages produce numerous factors that are neurotoxic and contribute to the neurological damage that occurs in HIV-infected individuals.13,14,15 Thus, HIV infection and replication within CNS macrophages plays a central role in the development of HANDs.
The incidence and severity of HAND are exacerbated by drugs of abuse, such as the psychostimulants cocaine and methamphetamine,16,17,18,19 which have been shown to increase both HIV neuropathogenesis and viral replication.20,21,22,23,24 However, the mechanism(s) by which drugs of abuse enhance HIV-related neuropathologies are not well understood. Dopamine (DA), a neurotransmitter involved in the control of locomotion, cognition, positive reinforcement, and neuroendocrine secretion,25 is central to the action of drugs of abuse. Psychostimulants such as cocaine and methamphetamine exert addictive and reinforcing effects through elevation of extracellular DA levels in the CNS.26,27,28,29,30 The use of both cocaine and methamphetamine generates extracellular CNS dopamine levels far higher than those found in the brains of non-drug-abusers.26,31,32,33,34,35,36
Dopamine acts through dopamine receptors, which are members of the G-protein coupled seven transmembrane domain family of receptors. Dopamine receptors (DRs) are divided into two subtypes designated D1-like DRs, comprised of dopamine receptor 1 (D1R) and D5R, and D2-like DRs, comprised of D2R, D3R, and D4R.25 Classically, DRs have been studied on neurons, but DR expression has also been reported in several types of peripheral blood leukocytes, including T lymphocytes and monocytes.37,38,39 Dopamine receptors have been shown to modulate the immune function of T lymphocytes.25,40,41 A recent study showed D1R on human macrophages,24 but the expression of other DRs and the functions of DRs in this cell type have not been well characterized.
In studies with simian immunodeficiency virus-infected macaques, injection with or oral administration of L-DOPA, a DA precursor that crosses the blood-brain barrier, or selegiline, a blocker of DA breakdown by monoamine oxidase, resulted in increased levels of simian immunodeficiency virus in the CNS.42,43 In addition, infected macaques exhibited an increased incidence of simian immunodeficiency virus encephalitis and induction of a spongiform polioencephalopathy in dopaminergic regions of the CNS.42,43 These studies suggest that the enhanced extracellular DA elicited by use of drugs like cocaine and methamphetamine could be sufficient to increase HIV replication in the CNS and exacerbate HIV neuropathogenesis. Macrophages play a central role in the development of HIV-induced neuropathology. Thus, examination of DA modulation of HIV infection of macrophages, as well as the characterization of intracellular signaling pathways that are involved in the DA-mediated increase in HIV infectivity, are important to the identification of mechanisms by which drugs of abuse enhance the development of HAND.
This report demonstrates that primary human monocyte-derived macrophages (MDMs) inoculated with HIV in the presence of DA exhibit increased levels of viral replication when compared with MDMs inoculated with HIV in the absence of DA. The DA-induced increase in viral replication correlated with an increase in the percentage of MDMs infected with HIV. HIV infection in the presence of the D2R agonist, quinpirole, increased viral replication similarly to DA, while infection in the presence of the D1R agonist, SKF 82958, did not alter HIV replication, suggesting that D2R is involved in the DA-mediated increase in HIV replication. The data also confirm that uninfected MDMs expressed both D1R and D2R on the cell surface and show that endogenous macrophage D2R was active by demonstrating that DA induced extracellular signal regulated kinase 1 (ERK 1) phosphorylation in macrophages through D2R. These results suggest that dopamine-induced increases in HIV replication in macrophages may be an important mechanism by which specific drugs of abuse, characterized by their ability to increase extracellular DA levels in the CNS, exacerbate the neuropathogenesis of HIV infection.
Materials and Methods
Reagents
Dopamine hydrochloride, ascorbic acid [(+)-sodium L-ascorbate], SKF 82958, quinpirole, HEPES, fish gelatin, Ig-free bovine serum albumin, and horse serum were from Sigma (St. Louis, MO). RPMI 1640 and penicillin/streptomycin (P/S) were from Invitrogen (Carlsbad, CA). Fetal calf serum and human AB serum were from Lonza (Basel, Switzerland). Macrophage colony-stimulating factor was from Peprotech (Rocky Hill, NJ). CEM-SS cells were obtained from the National Institutes of Health (NIH) AIDS and Reference Reagent Program (Germantown, MD). Antibodies used were: rabbit polyclonal anti-dopamine receptor 1 (D1R, Sigma); rabbit polyclonal anti-dopamine receptor 2 (D2R, Chemicon, Billerica, MA); mouse monoclonal anti-phospho-ERK1/2 (Thr202/Tyr204) and rabbit polyclonal anti-ERK1/2 (Cell Signaling Technology, Danvers, MA); purified mouse IgG1 myeloma protein (Cappel Pharmaceuticals, Solon, OH); mouse anti-HIV-1 p24Gag ([39/6.14], Abcam, Cambridge, MA); and mouse anti α-tubulin, fluorescein isothiocyanate (FITC)-conjugated anti-mouse secondary and FITC-conjugated anti-rabbit secondary antibodies (Sigma). Other reagents used were antifade with DAPI and phalloidin conjugate to Texas red (Molecular Probes-Invitrogen, Carlsbad, CA).
Viral Stocks
Human peripheral blood mononuclear cells or CEM-SS cells were infected with HIVADA, an R5 molecular clone derived from blood, or with HIVYU2, an R5 molecular clone derived from the brain (NIH AIDS and Reference Reagent Program). Cell-free supernatants were collected daily for 2 to 30 days from infected cultures and stored at −80°C for use as viral stocks. Stock concentration of p24/ml was quantified by HIV p24 enzyme-linked immunosorbent assay (Perkin-Elmer, Waltham, MA).
Culture and HIV Infection of Primary Human Macrophages
Human peripheral blood mononuclear cells were separated from human blood obtained from uninfected donors (New York Blood Center, LIC, New York) by Ficoll-Paque (GE Health Care, Piscataway, NJ) gradient centrifugation. MDMs were isolated from peripheral blood mononuclear cells using CD14 magnetic beads (MidiMACS separation system, Miltenyi Biotec, Auburn, CA). The CD14+ cells were cultured in RPMI 1640 with 10% fetal calf serum, 5% human AB serum, 10 mmol/L HEPES, 1% P/S, and macrophage colony-stimulating factor (10 ng/ml) for 3 days, washed, and cultured for another 3 days, after which the cells are considered MDMs.
MDMs cultured in 48-well tissue culture plates (BD, Franklin Lakes, NJ, 1 × 105 cells/well) or eight-well chamber slides (Lab-Tek, Nunc, Rochester, NY, 1.07 × 105 cells/well) were inoculated for 24 hours at 37°C with different concentrations of either HIVADA or HIVYU2. HIV infections in 48-well plates were performed in six replicate wells, except experiments infecting MDMs with 20 ng/ml HIVADA, which were performed in three replicate wells. Infections in eight-well chamber slides were performed in four replicate chambers. Concurrent with HIV inoculation, some MDMs were treated with DA (20 μmol/L), the D2R agonist quinpirole (1 or 10 μmol/L) or the D1R agonist SKF 82958 (1 or 10 μmol/L). Agonist concentrations were based on previous studies.44,45 All MDMs (both infected and control cells) were treated with 200 μmol/L ascorbic acid to prevent the formation of reactive oxygen species.46
After 24 hours, MDMs were washed to remove viral inoculum, as well as DA, any agonists and ascorbic acid, and fed with fresh macrophage media. Infections were maintained in culture for 6 days. MDMs were given fresh media and supernatants were collected from each well every 24 hours starting 2 days postinoculation. Viral replication was determined by measuring the concentration of HIV capsid protein p24Gag (p24) in the supernatant by enzyme-linked immunosorbent assay (Perkin-Elmer), as the p24 in the supernatant directly corresponds to production of HIV virions. Results were pooled to determine the mean p24 concentration at each time point. In the analysis of all infections with six replicate wells, the wells with the highest and lowest concentrations of p24 were removed from final analysis.
Immunofluorescence
MDMs cultured on chamber slides or 35-mm dishes with embedded glass slides (MatTek, Ashland, MA) were fixed and permeabilized in cold 70% ethanol (EtOH) for 30 minutes at 4°C. Cells were washed in phosphate-buffered saline (PBS), incubated in block (0.5 mol/L EDTA, 1% human AB serum, 2% fish gelatin, 1% Ig-free bovine serum albumin, 1% horse serum in H2O) for 30 minutes at room temperature and then in primary antibodies (anti-p24, D1R, D2R, or isotype-matched controls, all 1:50) overnight at 4°C. Cells were washed with PBS and incubated for 1 hour at room temperature with the appropriate secondary antibody conjugated to FITC (1:250) as well as phalloidin-conjugated Texas Red (1:200). Chamber slides and dishes were mounted using Prolong Gold Antifade reagent with DAPI (Molecular Probes-Invitrogen) and stored at 4°C. MDMs on 35-mm dishes were examined by confocal microscopy using a Leica microscope (Wetzlar, Germany) to determine surface expression of DRs.
To examine the change in the number of MDMs infected with HIV as a result of inoculation in the presence of DA, MDMs cultured in eight-well chamber slides were infected with 20 ng/ml HIVADA in the presence (four chambers per slide) or absence (four chambers per slide) of 20 μmol/L DA. A single chamber slide was fixed on days 3, 4, 5, and 6 postinoculation, stained for p24, actin, and cell nuclei. Two chambers on each slide were stained with an isotype-matched negative control antibody to determine background immunofluorescence. Day 2 postinoculation was not examined because viral replication levels were generally not high enough to detect infection by immunofluorescence. Immunofluorescence of p24 was examined using a semimotorized Zeiss Axio.D1 Observer microscope (Carl Zeiss IMT Corporation, Germany). Fluorescent images of six randomly chosen fields from each chamber were analyzed using Axiovision (Zeiss) and Adobe Photoshop. The number of total and infected MDMs were quantified and used to determine the percentage of infected MDMs in chambers infected in the presence of DA relative to chambers infected in the absence of DA.
Western Blot Analyses
Human MDMs were lysed with mammalian protein extraction reagent (M-PER, Pierce, Rockford, IL) containing protease (Halt protease inhibitor cocktail, Pierce) and phosphatase inhibitors (Halt phosphatase inhibitor cocktail, Pierce). Lysates were prepared using two methods. For cell signaling analyses, lysates were sonicated, centrifuged at 14,000 × g, and denatured by boiling. For detection of DA receptors, lysates were incubated in M-PER for 1 hour at 4°C with rocking, centrifuged for 30 minutes at 14,000 × g at 4°C, and denatured for 30 minutes by incubation in loading buffer at room temperature. These conditions allowed for correct electrophoretic mobility of DA receptors.47 Western blot analyses were performed using 10% Nupage polyacrylamide gels (Invitrogen). Proteins were transferred to 0.45-μm Invitrolon membranes (Invitrogen) and blots were blocked in TBS-T (TBS with 1.2% Tween 20, Sigma) containing 5% bovine serum albumin (ERK 1/2 blots) or 3% bovine serum albumin/5% powdered milk (DR blots). Blots were incubated overnight in antibodies to D1R (1:250), D2R (1:200), or phospho-ERK1/2 (Thr202/Tyr204, 1:400). Specificity of antibodies to D1R and D2R was confirmed by Western blot analyses of lysates from HEK 293 cells stably expressing human D1R or D2R. Anti-total ERK1/2 (1:1000) and anti α-tubulin (1:10,000) were used as loading controls. Antibody complexes were developed using Western Lightning chemiluminescence reagent (Perkin-Elmer). Densitometric analyses were performed using UN-SCAN-IT gel digitizing software (Silk Scientific, Utah).
Biotinylation
MDMs grown in 100-mm dishes were placed at 4°C for 15 minutes, washed with cold PBS (pH 8.0), and incubated in biotin-PBS (1 mg/ml EZ-Link Sulfo-NHS Biotin, Pierce) for 30 minutes at 4°C. Cells were washed three times with 100 mmol/L glycine in cold PBS and lysed by incubation in M-PER at 4°C for 1 hour. Lysate supernatant, obtained after centrifugation for 20 minutes at 4°C, was incubated overnight at 4°C with streptavidin-agarose beads. After centrifugation, supernatant was saved as the nonbiotinylated fraction. Immunoprecipitated biotinylated proteins were washed several times in M-PER, dried, and resuspended in loading buffer. Biotinylated proteins were analyzed by Western blot as described, using α-tubulin to confirm the absence of non-surface proteins in the biotinylated fraction.
Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
Total RNA was isolated from MDMs using RNeasy kit (Qiagen, Valencia, CA). Purity and concentration were determined using a Nanodrop spectrophotometer (Nanodrop Technologies, Wilmington, DE). Gene-specific primers for a 373-bp fragment of human D1R (D1RFor2 3′-TTGGAGGAGCGAGAAGACAT-5′ and D1RRev2 3′-AGCAGGGAATAGGGGTCAGT-5′) and a 670-bp fragment of human D2R (D2RFor 3′-TCAGGGGCAGCTCATAGAGT-5′ and D2RRev 3′-AAGGGAAGGGAAACAGGAGA-5′) were synthesized by Invitrogen Custom Oligonucleotides service. SuperScript III RT system (Invitrogen) was used to generate cDNA, which was amplified by nested PCR using the Platinum TaqDNA polymerase high fidelity (Invitrogen). Samples were electrophoresed on 1.5% agarose gel and visualized on a Flurochem 8800 camera system (α Innotech Corporation, San Leandro, CA).
Cell Signaling Analysis to Demonstrate Macrophage DRs Are Functional
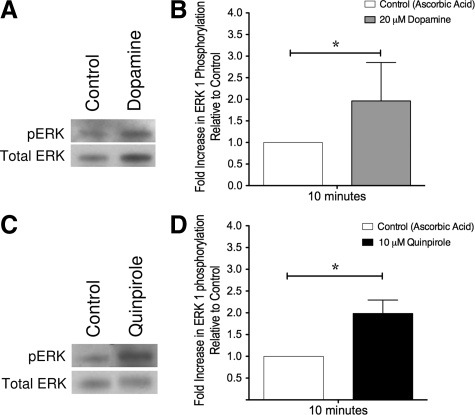
MDMs were cultured in six-well tissue culture plates or 60-mm tissue culture dishes for 6 days, and then placed in macrophage media containing no serum for 6 hours. MDMs were treated with either 20 μmol/L DA or 10 μmol/L D2R agonist quinpirole. All cells were also treated with 200 μmol/L ascorbic acid. After 10 minutes of treatment with DA or quinpirole, cells were washed with PBS and lysed for analysis by Western blotting for ERK 1/2 as described.
Statistics
Student’s one-tailed, paired t-test was used to determine significance with P < 0.05 considered significant. Analyses were performed using Microsoft Excel (Microsoft, Redmond, WA) and GraphPad Prism 4.0 (GraphPad Software, La Jolla, CA).
Results
Dopamine Increases HIV Replication in Human Macrophages
To examine DA modulation of HIV infection in MDMs, macrophages were infected with HIV in the presence and absence of DA. HIV replication was evaluated by measuring the concentration of the HIV capsid protein p24Gag (p24) in the supernatant every 24 hours for 6 days postinfection, starting on day 2 postinoculation, as described in Materials and Methods. Starting 2 days after inoculation with HIV, all infected MDM cultures exhibited significant levels of HIV replication each day through day 6 postinoculation. The amount of viral replication varied somewhat between experiments, but most commonly HIV replication increased on days 2, 3, and 4 postinoculation and either remained constant or decreased slightly on days 5 and 6 postinoculation. MDMs in all experiments were treated with 200 μmol/L ascorbic acid to prevent the formation of reactive oxygen species through DA oxidation.47 Treatment with ascorbic acid alone had no effect on HIV replication in MDMs, as viral replication was similar in the presence or absence of ascorbic acid (data not shown).
To determine the optimal concentration of DA for this model, HIV inoculations were performed in the presence of 5, 20, and 80 μmol/L DA. These concentrations of DA were chosen to approximate the large amount of DA that can be produced in a brain exposed to psychostimulants such as cocaine or methamphetamine.26,31,32,33,34,35,36 Inoculation with HIV in the presence of 5 μmol/L DA induced a significant increase in HIV replication, but at lower levels of replication than did inoculation in the presence of 20 and 80 μmol/L DA, which both induced similar, significant increases in HIV replication (data for 20 μmol/L DA in Figure 1, data for 5 μmol/L and 80 μmol/L not shown). Thus, 20 μmol/L DA was chosen as the concentration to be used in further experiments.
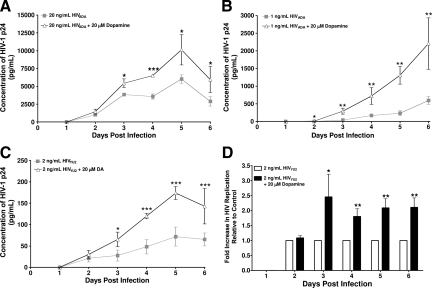
Figure 1.
HIV replication is significantly increased in macrophages exposed to DA during infection. The presence of DA during HIV inoculation significantly increased viral replication in MDMs infected with 20 ng/ml HIVADA (A, *P < 0.05 vs. 20 ng/mL HIVADA; ***P < 0.001 vs. 20 ng/mL HIVADA), 1 ng/ml HIVADA (B), and 2 ng/ml HIVYU2 (C). D shows the fold increase in HIV replication in infections of MDMs from eight different donors with 2 ng/ml HIVYU2 ± DA. MDMs were inoculated with HIV in three (A) or six (B, *P < 0.05 vs. 1 ng/mL HIVADA; **P < 0.01 vs. 1 ng/mL HIVADA; C, *P < 0.05 vs. 2 ng/mL HIVYU2; ***P < 0.001 vs. 2 ng/mL HIVYU2; D) replicate wells for 24 hours. DA (20 μmol/L) was added to designated cultures concurrently with HIV. Supernatants were collected every 24 hours and assayed for HIV p24 protein by enzyme-linked immunosorbent assay. In A–C, each data point represents the mean p24 concentration on each day from a single experiment. D shows the mean pooled fold change in p24 due to HIV infection in the presence of DA relative to HIV replication induced by infection with HIV alone, which was set to 1 (D, *P < 0.05 vs. 2 ng/mL HIVYU2; **P < 0.01 vs. 2 ng/mL HIVYU2, n = 8 independent donors).
MDMs obtained from three separate donors were each inoculated with 20 ng/ml HIVADA, an R5 strain of HIV, in the presence or absence of 20 μmol/L DA as described in Materials and Methods. HIV replication in MDMs from one donor inoculated with HIV in the presence of DA was significantly increased on days 3 through 6 postinoculation when compared with MDMs inoculated with HIVADA in the absence of DA (Figure 1A). Infections of MDMs isolated from the two additional donors using 20 ng/ml HIVADA showed similar significant increases at days 3, 5, and 6 postinfection (data not shown). To examine the effects of DA on viral replication in cultures infected with a lower concentration of HIV, MDMs obtained from three separate donors were each inoculated with 1 ng/ml HIVADA in the presence or absence of 20 μmol/L DA. A representative experiment showing infection of MDMs from a single donor with this lower concentration of HIVADA demonstrated a significant DA-induced increase in HIV replication on days 2 through 6 postinfection (Figure 1B). These data indicate that HIV replication was significantly increased by inoculation in the presence of DA and that the effect of DA was independent of the concentration of the inoculating virus.
To demonstrate that the effect of DA on HIV replication in MDMs was not dependent on the strain of HIV used for inoculation, MDMs obtained from eight separate donors were each inoculated with 2 ng/ml HIVYU2, another R5 strain of HIV, in the presence or absence of 20 μmol/L DA as described in Materials and Methods. Analysis of p24 in culture supernatant by enzyme-linked immunosorbent assay demonstrated similar results to those seen with HIVADA infection, that DA treatment of HIVYU2 infected MDMs induced significant increases in viral replication on days 3 through 6 postinfection (Figure 1, C and D) as compared with MDMs inoculated with HIV in the absence of DA. Figure 1C shows a representative infection of MDMs obtained from a single donor with HIVYU2 in the presence or absence of DA. Figure 1D shows the combined fold increase in HIV replication induced by infection in the presence of 20 μmol/L DA pooled from HIVYU2 infection of MDMs from eight separate donors. Each column represents the mean of the pooled fold increase in HIV replication in the presence of DA, as compared with the fold increase in HIV replication resulting from infection in the absence of DA, which has been set to 1. These data indicate that HIVYU2 infection of MDMs in the presence of DA significantly (*P < 0.05) increased HIV replication by approximately twofold on days 3 through 6 postinoculation. Thus, inoculation of primary human MDMs in the presence of DA significantly increased HIV replication and this effect was independent of donor, HIV strain, or the concentration of the inoculating virus.
Dopamine Increases the Number of HIV-Infected Macrophages
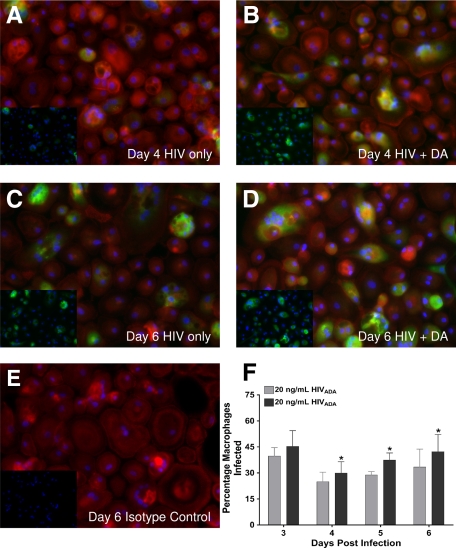
To examine the mechanism(s) by which DA increased HIV replication in MDMs, experiments were performed to examine whether DA was increasing the total number of HIV infected macrophages or modulating the level of viral replication in individual infected cells. MDMs obtained from four separate donors were each cultured in eight-chamber slides and infected with 20 ng/ml HIVADA in the presence or absence of 20 μmol/L DA. The number of HIV infected and total MDMs was quantified by immunofluorescence at days 3, 4, 5, and 6 postinfection and used to calculate the percentage of MDMs infected with HIV. In Figure 2, A–D, p24 is green (FITC), actin is red (Texas Red), and cell nuclei are blue (DAPI), with insets showing only DAPI and p24 staining. On day 4 (24.9% infection in MDMs inoculated with HIV alone versus 30% infection in MDMs inoculated with HIV + DA, *P < 0.05, Figure 2, A and B), day 5 (28.8% HIV alone versus 37.4% HIV + DA, *P < 0.05), and day 6 (33.5% HIV alone versus 42.3% HIV + DA, *P < 0.05, Figure 2, C and D) postinfection, there was a significant increase in the percentage of HIV infected MDMs in cultures inoculated with HIV in the presence of DA relative to MDM cultures inoculated in the absence of DA (Figure 2F). Nonspecific staining was not observed in HIV-infected MDMs treated with an isotype-matched negative control antibody (Figure 2E). Similar results were seen in infections of MDMs from the three additional donors, as HIV inoculation in the presence of DA increased the percentage of HIV infected cells in each experiment (data not shown), although the percentage of infected cells varied between donors. These data show that HIV inoculation in the presence of DA leads to infection of a larger number of MDMs, suggesting that a DA mediated increase in the number of HIV-infected macrophages is one mechanism by which DA enhances HIV replication.
Figure 2.
DA increases the number of macrophages infected by HIV. The presence of DA during HIV inoculation significantly increased the number of MDMs infected with HIV at days 4, 5, and 6 postinfection. MDMs were inoculated for 24 hours with 20 ng/ml HIVADA ± 20 μmol/L DA. On days 3, 4, 5, and 6 postinfection, a single slide was stained for p24 (FITC, green), actin (Texas Red) and cell nuclei (DAPI, blue). A–D show MDMs infected with HIV ± DA at days 4 (A, HIV only, B, HIV + DA) and 6 postinfection (C, HIV only, D, HIV + DA). E shows MDMs stained with an isotype matched control antibody (HIV + DA, day 6 control). A–E, magnification ×20. Insets show DAPI and p24 staining without actin staining. The number of total and infected MDMs on each day is quantified and expressed as percentage of macrophages infected (F). *P < 0.05 vs. 20 ng/mL HIVADA.
Dopamine Receptor 2 Agonist Increases HIV Replication
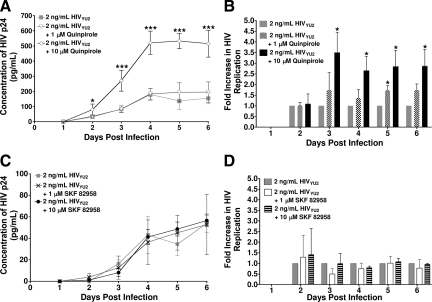
To identify the DRs that mediate DA-enhanced HIV replication in human macrophages, MDMs were infected with HIVYU2 in the presence and absence of the D2R agonist, quinpirole, or the D1R agonist SKF 82958. MDMs obtained from three separate donors were each inoculated with 2 ng/ml HIVYU2 in the presence of 1 or 10 μmol/L quinpirole. MDMs obtained from two additional donors were also inoculated with HIVYU2 in the presence of 10 μmol/L quinpirole, for a total of five donors inoculated in the presence of 10 μmol/L quinpirole. In a representative experiment (Figure 3A), MDMs inoculated with HIVYU2 in the presence of 10 μmol/L quinpirole demonstrated significantly increased HIV replication on days 2 through 6 postinfection relative to MDMs inoculated in the absence of quinpirole. The presence of 1 μmol/L quinpirole during inoculation had no significant effect on viral replication. Pooled data of MDMs from five donors inoculated with HIVYU2 in the presence of 10 μmol/L quinpirole show a threefold increase (*P < 0.05) in HIV replication on days 3 through 6 postinfection relative to MDMs inoculated with HIV alone (Figure 3B, 10 μmol/L quinpirole, n = 5). The pooled fold increase in HIV replication in the presence of 1 μmol/L quinpirole only showed a significant increase in replication on day 5 post infection (Figure 3B, 1 μmol/L quinpirole, n = 3). Each column represents the mean of the pooled fold increase in HIV replication in the presence of either 1 μmol/L or 10 μmol/L quinpirole, as compared with the fold increase in viral replication resulting from infection with HIV alone, which has been set to 1.
Figure 3.
HIV Infection of macrophages in the presence of D2R agonist increases HIV replication. The presence of the D2R agonist quinpirole significantly increased HIV replication in MDMs while the D1R agonist SKF 82958 has no effect. MDMs were inoculated in sextuplet with 2 ng/ml HIVYU2 for 24 hours in the presence of 1 or 10 μmol/L quinpirole (A, B) or SKF 82958 at 1 or 10 μmol/L (C, D). Agonists were added concurrently with HIV. Supernatant was collected every 24 hours and assayed for HIV p24 protein by enzyme-linked immunosorbent assay. In A and C, each data point represents the mean p24 concentration on each day in a single experiment. B and D show the mean of the pooled fold change in p24 due to HIV infection in the presence of agonists relative to HIV replication induced by infection with HIV alone, which was set to 1 (B, n = 3 independent donors with 1 μmol/L quinpirole or n = 5 independent donors with 10 μmol/L quinpirole and D, n = 3 independent donors with 1 μmol/L and 10 μmol/L SKF 82958). *P < 0.05 vs. 2 ng/mL HIVYU2; ***P < 0.001 vs. 2 ng/mL HIVYU2.
In contrast, MDMs inoculated with HIV in the presence of the D1R agonist SKF 82958 did not show increased HIV replication at any point up to 6 days postinoculation when compared with MDMs inoculated with HIV alone. MDMs from three separate donors were each inoculated with 2 ng/ml HIVYU2 in the presence of 1 μmol/L or 10 μmol/L of SKF 82958. Figure 3C shows a representative infection of MDMs from a single donor inoculated with HIVYU2 in the presence of 1 and 10 μmol/L SKF 82958. The fold increase in HIV replication pooled from infection of MDMs from three separate donors also demonstrated that neither 1 μmol/L nor 10 μmol/L SKF 82958 increased HIV replication (Figure 3D). Each column represents the mean of the pooled fold increase in HIV replication in the presence of either 1 or 10 μmol/L SKF 82958, as compared with the fold increase in viral replication resulting from HIV infection alone, which has been set to 1. Thus, HIV inoculation of macrophages in the presence of a D2R agonist, but not a D1R agonist, induced a significant increase in HIV replication similar to that caused by HIV inoculation in the presence of DA. These data indicate that the DA-induced increase in HIV replication is mediated, at least in part, through D2R.
Human Macrophages Express Functional DRs on the Cell Surface
The above data indicate that treatment of MDMs with DA or a D2R agonist, but not a D1R agonist, increased HIV replication. Although a number of studies demonstrated DRs in rodent macrophages,48,49,50,51 and there is some evidence for the expression of DRs on human monocytes,37 very few studies examined the presence of DRs on primary human macrophages,24 and none specifically determined the activity of D2R on this cell type. To confirm that MDMs express the DRs involved in the DA-induced enhancement in HIV replication, uninfected macrophages were examined using confocal microscopy, Western blot analyses of biotinylated surface proteins, and RT-PCR. The specificity of the antibodies used to detect D2R and D1R was confirmed by Western blot analyses of lysates derived from HEK 293 cells stably transfected with human D1R or D2R or untransfected HEK293 expressing no DA receptors (data not shown).
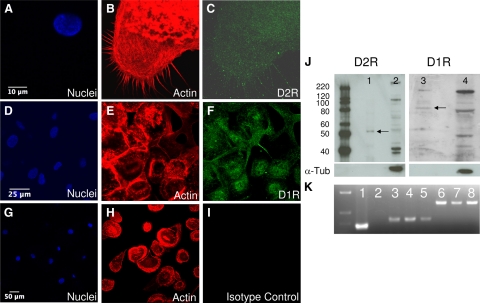
Immunofluorescence and subsequent confocal microscopy demonstrated that MDMs express both D2R and D1R. Figure 4, A–C, shows D2R immunofluorescence in MDMs, both on the surface and in the cytoplasm, with cell nuclei labeled blue (4A, DAPI), actin red (4B, Texas Red) and D2R labeled green (4C, FITC). D1R is shown in Figure 4, D–F, showing cell nuclei (4D, DAPI), actin (4E, Texas Red) and D1R labeled green (4F, FITC). Isotype-matched control antibodies had minimal reactivity, demonstrated in Figure 4, G–I, showing cell nuclei (G, DAPI), actin (B, Texas Red) and an isotype-matched control antibody (I, FITC). Surface expression of both D2R and D1R on MDMs was confirmed by Western blot analyses of biotinylated MDM membrane proteins immunoprecipitated using streptavidin beads. Both D2R (Figure 4J, lane 1, ∼55-kd band) and D1R (Figure 4J, lane 3, ∼80-kd band) were detected in the biotinylated membrane fraction. These Western blots were stripped and reprobed for the cytoplasmic protein α-tubulin (α-Tub), which was only detected in the non-membrane fractions (Figure 4J, lanes 2 and 4), confirming the specificity of the biotinylated membrane protein isolation. D1R and D2R mRNAs were also expressed in MDMs as detected by RT-PCR (Figure 4K). Primers specific for human D1R and D2R were used to generate amplicons of 373 bp corresponding to D1R (K, lanes 4 and 5) and amplicons of 670 bp corresponding to D2R (K, lanes 7 and 8) in MDM mRNA derived from two separate donors. Similar results were seen in MDM mRNA derived from six additional donors (data not shown). As positive controls, these primers were used to generate specific amplicons of D1R (K, lane 3) and D2R (K, lane 6) from HEK 293 cells stably expressing human D1R or D2R. Positive (K, lane 1) and negative (K, lane 2) PCR controls are also shown. Taken together, these data demonstrate that human MDMs express DRs, and that they are present on the cell surface.
Figure 4.
Human macrophages express DRs. MDMs express D2R and D1R on the cell surface. Surface expression of both D2R and D1R was shown in MDMs stained for D2R (A–C), D1R (D–F), or with an isotype-matched control antibody (G–I). Cell nuclei are blue, actin is red, and D2R, D1R, and the isotype control antibodies are green. Scale bars represent 10 μm (A–C), 25 μm (D–F), 50 μm (G–I). Western blot analysis of biotinylated MDM surface proteins (J) confirmed surface expression of both D2R (lane 1, ∼55 kd) and D1R (lane 3, ∼80 kd). Nonbiotinylated proteins were run in lanes 2 and 4 (J). Specificity of membrane protein isolation was shown by lack of α-tubulin (α-Tub) reactivity in the biotinylated fraction (lanes 1 and 3). RT-PCR (K) detected mRNA for D1R (lanes 4 and 5, 370 bp) and D2R (lanes 7 and 8, 670 bp) in MDMs from two different donors. Positive controls for D1R (lane 3) and D2R (lane 6) and positive (lane 1) and negative (lane 2) PCR controls were also shown.
The functions of DRs on primary human macrophages are not well defined. Our data demonstrate MDMs treated with quinpirole, a D2R agonist, during HIV infection showed increased levels of viral replication, while MDMs treated with SKF 82958, a D1R agonist, did not. To demonstrate that D2R is active on human macrophages, DA and quinpirole mediated phosphorylation of the signaling protein ERK1/2 were examined. ERK 1/2 were examined because D2-like DR activation of these proteins has been shown in other non-neuronal cell types.44,52
MDMs were treated with either 20 μmol/L DA or 10 μmol/L quinpirole for 10 minutes, lysed, and examined for changes in ERK 1/2 phosphorylation by Western blot analyses. As with all experiments, MDMs were treated with 200 μmol/L ascorbic acid to prevent DA oxidation. MDMs treated with 20 μmol/L DA for 10 minutes exhibited increased ERK 1 phosphorylation (Figure 5A). Densitometric analysis of the DA-induced fold increase in ERK 1 phosphorylation pooled from experiments with MDMs isolated from five different donors showed a significant increase in ERK 1 phosphorylation (Figure 5B, *P < 0.05). Similar to DA, treatment of MDMs with 10 μmol/L of the D2R agonist quinpirole for 10 minutes (Figure 5C) also caused a significant increase in ERK 1 phosphorylation. Densitometric analysis of the quinpirole-induced fold increase in ERK 1 phosphorylation from pooled experiments shows a significant increase in ERK 1 phosphorylation (Figure 5D, *P < 0.05). Experiments treating MDMs with 10 μmol/L of the D1R agonist SKF 82958 for 10 minutes showed no significant difference in ERK 1 phosphorylation when compared with untreated MDMs (n = 3, data not shown), suggesting that DA-induced phosphorylation of ERK 1 is specifically mediated through D2R. The quinpirole mediated activation of ERK 1 demonstrates that the D2R expressed on the surface of MDMs are active.
Figure 5.
DA modulates ERK 1 phosphorylation in human MDMs demonstrating that D2R is functional. Treatment of macrophages with DA or a D2R agonist significantly increased the phosphorylation of ERK 1 after 10 minutes. MDMs were exposed to DA (A) or the D2R agonist quinpirole (C) for 10 minutes, then lysed and examined by Western blot analysis. Densitometric analysis of the pooled mean fold change in ERK 1 phosphorylation in response to treatment with 20 μmol/L DA (B, n = 5 independent donors) or 10 μmol/L quinpirole (D, n = 3 independent donors) showed that treatment with both DA and quinpirole significantly increased ERK 1 phosphorylation. *P < 0.05.
Discussion
HIV-infected individuals are living longer as a result of antiretroviral therapy, and the prevalence of HAND is increasing. The use of psychostimulants like cocaine and methamphetamine is a major comorbidity in the development of HAND. These drugs act in concert with HIV to accelerate neuropathogenesis by disrupting the blood-brain barrier, promoting neuroinflammation and altering proliferation and cytokine production in CNS cells.17,18,53,54,55 The mechanism(s) by which drugs of abuse mediate these effects are not well understood, but one major commonality among psychostimulants is that their use increases the concentration of extracellular of DA within the CNS.27,28,56
Dopamine is a major catecholamine neurotransmitter in the CNS and is involved in diverse functions including control of locomotion, cognition, neuroendocrine secretion, and positive reinforcement.25 The molecular actions of DA are mediated by five subtypes of receptors. DRs are seven transmembrane domain G-protein coupled receptors and are divided into two subgroups, D1-like (D1R and D5R) and D2-like (D2R, D3R, and D4R). DRs are differentially expressed on dopaminergic neurons throughout the brain and can also be found on a number of other cell types, including immune cells and glia.25,37,38,57 DA plays an important role in many neurological disorders such as Parkinson’s disease but has not been well examined in the context of HIV-induced neuropathology. In simian immunodeficiency virus-infected macaques, a model for neuroAIDS, administration of the DA-enhancing substances L-DOPA and selegiline increased CNS viral load and accelerated the development of neuropathology.42,43 These studies suggest that increased extracellular DA may also contribute to the development of HIV-induced neurological disease, but the mechanism by which DA modulates these alterations remains unclear.
Studies have demonstrated that DA-rich areas of the brain, such as the basal ganglia and substantia nigra, are particularly susceptible to neurological damage during HIV infection.58,59,60 In the CNS of HIV-infected individuals, the basal ganglia had elevated viral loads61 and higher concentrations of HIV-infected macrophages/microglia as compared with other areas of the brain.62 Macrophages and other cells of the monocytic lineage are the major targets for HIV infection in the CNS.11,12 Both infected and uninfected macrophages contribute to HIV-induced neuropathogenesis through elaboration of neurotoxic factors such as tumor necrosis factor-α, interleukin-1β, chemokines, quinolinic acid, nitric oxide, and through production of viral proteins such as Tat.14,15,63,64,65 Tat has also been shown to synergize with drugs of abuse such as methamphetamine to increase further neuroinflammation and neuronal damage.66,67,68 The high numbers of infected macrophages in DA-rich areas of the CNS, in conjunction with the demonstrated vulnerability of those areas to HIV-related neurological damage, suggest that increases in CNS DA levels play an important role in macrophage-mediated neuropathology resulting from HIV infection.
Our findings support an important role for DA in HIV infection of macrophages, demonstrating that MDMs inoculated with HIV in the presence of DA generate increased levels of viral replication relative to MDMs inoculated in the absence of DA. In our infection model, MDMs were exposed to 20 μmol/L DA during the initial 24-hour inoculation with HIV and the significant increase in HIV replication induced by DA was seen on days 3, 4, 5, and 6 after the initial infection. These DA-induced increases in HIV replication were shown in infections of MDMs derived from different donors with different concentrations of two isolates of HIV, indicating that the effects of DA are independent of donor, viral strain, and the concentration of the inoculating virus.
There are a number of possible mechanisms by which DA could mediate this increase in HIV replication. DA treatment could increase the number of macrophages initially infected by HIV, resulting in higher levels of viral replication due to virion production from a greater number of infected cells. Our results demonstrate that on days 4, 5, and 6 postinoculation, there is a significant increase in the number of HIV infected MDMs when HIV inoculation is performed in the presence of DA. These data suggest that the increase in viral replication is due, at least in part, to an increase in the number of macrophages infected by HIV in the presence of DA. Additionally, DA could modulate production of HIV particles, enabling cells treated with DA to produce more virions per cell. The mechanisms mediating the DA-induced increase in HIV replication in MDMs are likely multifactorial and are the subject of ongoing research.
While the effect of DA on HIV infection of MDMs, as well as on other macrophage functions, is a new area of study, research has shown that dopamine modulates HIV infection in other cell types. DA treatment was found to increase HIV replication in both peripheral blood mononuclear cells and Jurkat T cells transfected with a proviral HIV genome through activation of NF-κB sites in the HIV-1 long terminal repeat.69 Exposure to DA was also shown to enhance HIV replication in chronically infected ACH-2 T lymphoblasts, although this effect was abrogated in the presence of the antioxidant glutathione, suggesting that the enhancement was a product of oxidative stress.70 Recent studies also demonstrated that treatment of MDMs with cocaine23 or methamphetamine increased HIV replication and that D1R antagonists were able to abrogate the effects of methamphetamine treatment.24 Neither study addressed the direct role of dopamine on HIV infection of macrophages.
Dopamine mediates its effects through activation of DRs, so the role of each DR subtype, D1-like DR or D2-like DR, in the modulation of HIV replication was examined by infecting MDMs with HIV in the presence of either a D1R or a D2R agonist. Infection of MDMs with HIV in the presence of 10 μmol/L of the D2R agonist quinpirole induced significantly increased levels of HIV replication, as compared with MDMs infected with HIV alone or in the presence of the D1R agonist SKF 82958. This effect was dose-dependent, as 1 μmol/L quinpirole had no significant effect on viral replication. These data suggest that the DR through which DA is mediating the increase in HIV replication is D2R.
It is important to note that because of the similarities between some DR subtypes, DR agonists often lack a high degree of specificity for distinguishing between DRs of the same subtype; however, they do distinguish very well between the D1-like and D2-like classes of DRs. Quinpirole is widely used as a specific D2R agonist,71 and has a higher affinity for D2R than other D2-like DRs,72 but studies have shown it to have activity against other D2-like DRs.44 Similarly, SKF 82958 acts on D1-like receptors, primarily against D1R, but there are no agonists that distinguish well between D1R and D5R.73 Another caveat is that despite the D2-like specificity of the agonists involved in the increased HIV replication, the potency of both quinpirole and DA is lower than expected. Given the apparently relatively low levels of D2R in macrophages, it is possible the majority of D2R on the cell surface might be present as heterodimers with another receptor that allosterically modulates the potency of signaling by D2R agonists. There is an increasing amount of data showing that DRs can participate in G protein-coupled receptor heterodimers, resulting in modulation of their pharmacological properties (Han Y, Moreira I, Urizar E, Weinstein H, and Javitch JA, Nature Chemical Biology, in press).74,75 Therefore, it is possible that the lower potency of both DA and quinpirole as agonists is due to modulation of D2R by heterodimer partners. This is an ongoing area of study.
These data have significant implications for HIV and drug abuse. We hypothesize that a common mechanism by which drugs of abuse, such as methamphetamine and cocaine, could intensify HIV infection in the brain is through the ability of these drugs to increase extracellular DA levels in the CNS. Our data suggest that increased extracellular DA could induce increased HIV infection of CNS monocytes/macrophages. This hypothesis is supported by the enhancement of HIV replication in MDMs by selective activation of a D2-like DR by the agonist quinpirole and by the presence of active D2R on the surface of MDMs. The D2-like DR-mediated increase in macrophage infection would then lead to higher viral loads and greater production of neurotoxic factors, thereby accelerating and increasing the severity of HIV-mediated neurological damage. The enhanced neuropathology would be particularly pronounced in DA-rich regions of the brain, such as the basal ganglia, providing an explanation for the higher levels of neurological damage seen in these regions during HIV infection.18,59 The high levels of extracellular DA produced by drug abuse could also potentiate the development of HIV-induced neurological damage by modulating intracellular signaling and contributing to the functional dysregulation of both infected and uninfected macrophages.
Our data also pose a concern for HIV-positive individuals using prescribed DA-enhancing substances as treatment for a variety of other conditions such as depression and Parkinson’s disease. The increased DA levels induced by drugs designed for treatment of non-HIV-related conditions could also induce increased viral replication in the CNS, accelerating the development of neuropathologies in those infected with HIV. Additionally, the negative effects of DA enhancing drugs may not be limited to the CNS, as increases in DA concentration in peripheral regions such as the kidneys and lungs could increase viral replication and accelerate the development of HIV-related pathologies in these organs.
As the growing epidemics of drug abuse and HIV become even more interconnected, it has become increasingly important to understand the influence of drugs of abuse in concert with HIV on the development of HAND. Our results suggest that macrophages, and particularly macrophage DA receptors, may provide targets for intervention that might slow the development of neurological disease in HIV positive individuals using DA-enhancing substances. Overall, these findings provide new avenues of investigation of macrophage functions as well as potential therapeutic targets to delay or prevent the acceleration of HAND and other HIV-related pathologies enhanced by drug abuse.
Acknowledgments
We thank the Center for AIDS Research and the analytical imaging facilities at The Albert Einstein College of Medicine.
Footnotes
Address reprint requests to Joan W. Berman, Ph.D., Department of Pathology, Albert Einstein College of Medicine, 1300 Morris Park Avenue, Bronx, NY 10461. E-mail: berman@aecom.yu.edu.
Supported by National Institutes of Mental Health grants MH083497 (to J.W.B.), MH05679 (to J.W.B. and T.M.C.), MH076679 (to E.A.E.), and MH054137 (to J.A.J); National Institutes of Drug Abuse grants F32DA024965 (to P.J.G.), DA025567 (to J.W.B. and T.M.C.), and DA022413 (to J.A.J); NIH Centers for AIDS research grant CFAR AI-051519, especially the Immunology/Pathology Core; and NIH experimental neuropathology training grant NS07098 (to P.J.G.).
A guest editor acted as editor-in-chief for this manuscript. No person at Thomas Jefferson University or Albert Einstein College of Medicine was involved in the peer review process or final disposition for this article.
References
- Davis LE, Hjelle BL, Miller VE, Palmer DL, Llewellyn AL, Merlin TL, Young SA, Mills RG, Wachsman W, Wiley CA. Early viral brain invasion in iatrogenic human immunodeficiency virus infection. Neurology. 1992;42:1736–1739. doi: 10.1212/wnl.42.9.1736. [DOI] [PubMed] [Google Scholar]
- Gartner S, Liu Y. Insights into the role of immune activation in HIV neuropathogenesis. J Neurovirol. 2002;8:69–75. doi: 10.1080/13550280290049525. [DOI] [PubMed] [Google Scholar]
- Williams KC, Hickey WF. Central nervous system damage, monocytes and macrophages, and neurological disorders in AIDS. Annu Rev Neurosci. 2002;25:537–562. doi: 10.1146/annurev.neuro.25.112701.142822. [DOI] [PubMed] [Google Scholar]
- Anthony IC, Bell JE. The neuropathology of HIV/AIDS. Int Rev Psychiatry. 2008;20:15–24. doi: 10.1080/09540260701862037. [DOI] [PubMed] [Google Scholar]
- Dore GJ, Correll PK, Li Y, Kaldor JM, Cooper DA, Brew BJ. Changes to AIDS dementia complex in the era of highly active antiretroviral therapy. Aids. 1999;13:1249–1253. doi: 10.1097/00002030-199907090-00015. [DOI] [PubMed] [Google Scholar]
- Thomas SA. Anti-HIV drug distribution to the central nervous system. Curr Pharm Des. 2004;10:1313–1324. doi: 10.2174/1381612043384835. [DOI] [PubMed] [Google Scholar]
- Peluso R, Haase A, Stowring L, Edwards M, Ventura P. A Trojan Horse mechanism for the spread of visna virus in monocytes. Virology. 1985;147:231–236. doi: 10.1016/0042-6822(85)90246-6. [DOI] [PubMed] [Google Scholar]
- Buckner C, Luers A, Calderon T, Eugenin E, Berman J. Neuroimmunity and the blood–brain barrier: molecular regulation of leukocyte transmigration and viral entry into the nervous system with a focus on NeuroAIDS. J NeuroImmune Pharmacol. 2006;1:160–181. doi: 10.1007/s11481-006-9017-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eugenin EA, Osiecki K, Lopez L, Goldstein H, Calderon TM, Berman JW. CCL2/monocyte chemoattractant protein-1 mediates enhanced transmigration of human immunodeficiency virus (HIV)-infected leukocytes across the blood-brain barrier: a potential mechanism of HIV-CNS invasion and neuroAIDS. J Neurosci. 2006;26:1098–1106. doi: 10.1523/JNEUROSCI.3863-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu DT, Woodman SE, Weiss JM, McManus CM, D'Aversa TG, Hesselgesser J, Major EO, Nath A, Berman JW. Mechanisms of leukocyte trafficking into the CNS. J Neurovirol. 2000;6(Suppl 1):S82–S85. [PubMed] [Google Scholar]
- Williams KC, Corey S, Westmoreland SV, Pauley D, Knight H, deBakker C, Alvarez X, Lackner AA. Perivascular macrophages are the primary cell type productively infected by simian immunodeficiency virus in the brains of macaques: implications for the neuropathogenesis of AIDS. J Exp Med. 2001;193:905–915. doi: 10.1084/jem.193.8.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazeux R. AIDS encephalopathy and tropism of HIV for brain monocytes/macrophages and microglial cells. Pathobiology. 1991;59:214–218. doi: 10.1159/000163648. [DOI] [PubMed] [Google Scholar]
- Anderson E, Zink W, Xiong H, Gendelman HE. HIV-1-associated dementia: a metabolic encephalopathy perpetrated by virus-infected and immune-competent mononuclear phagocytes. J Acquir Immune Defic Syndr. 2002;31(Suppl 2):S43–S54. doi: 10.1097/00126334-200210012-00004. [DOI] [PubMed] [Google Scholar]
- Kramer-Hammerle S, Rothenaigner I, Wolff H, Bell JE, Brack-Werner R. Cells of the central nervous system as targets and reservoirs of the human immunodeficiency virus. Virus Res. 2005;111:194–213. doi: 10.1016/j.virusres.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Aquaro S, Ronga L, Pollicita M, Antinori A, Ranazzi A, Perno CF. Human immunodeficiency virus infection and acquired immunodeficiency syndrome dementia complex: role of cells of monocyte-macrophage lineage. J Neurovirol. 2005;11(Suppl 3):58–66. doi: 10.1080/13550280500513416. [DOI] [PubMed] [Google Scholar]
- Davies J, Everall IP, Weich S, McLaughlin J, Scaravilli F, Lantos PL. HIV-associated brain pathology in the United Kingdom: an epidemiological study. AIDS. 1997;11:1145–1150. doi: 10.1097/00002030-199709000-00010. [DOI] [PubMed] [Google Scholar]
- Nath A, Maragos WF, Avison MJ, Schmitt FA, Berger JR. Acceleration of HIV dementia with methamphetamine and cocaine. J Neurovirol. 2001;7:66–71. doi: 10.1080/135502801300069737. [DOI] [PubMed] [Google Scholar]
- Ferris MJ, Mactutus CF, Booze RM. Neurotoxic profiles of HIV, psychostimulant drugs of abuse, and their concerted effect on the brain: current status of dopamine system vulnerability in NeuroAIDS. Neurosci Biobehav Rev. 2008;32:883–909. doi: 10.1016/j.neubiorev.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdo TH, Katner SN, Taffe MA, Fox HS. Neuroimmunity, drugs of abuse, and neuroAIDS. J Neuroimmune Pharmacol. 2006;1:41–49. doi: 10.1007/s11481-005-9001-3. [DOI] [PubMed] [Google Scholar]
- Nath A, Hauser KF, Wojna V, Booze RM, Maragos W, Prendergast M, Cass W, Turchan JT. Molecular basis for interactions of HIV and drugs of abuse. J Acquir Immune Defic Syndr. 2002;31(Suppl 2):S62–S69. doi: 10.1097/00126334-200210012-00006. [DOI] [PubMed] [Google Scholar]
- Roth MD, Tashkin DP, Choi R, Jamieson BD, Zack JA, Baldwin GC. Cocaine enhances human immunodeficiency virus replication in a model of severe combined immunodeficient mice implanted with human peripheral blood leukocytes. J Infect Dis. 2002;185:701–705. doi: 10.1086/339012. [DOI] [PubMed] [Google Scholar]
- Ellis RJ, Childers ME, Cherner M, Lazzaretto D, Letendre S, Grant I. Increased human immunodeficiency virus loads in active methamphetamine users are explained by reduced effectiveness of antiretroviral therapy. J Infect Dis. 2003;188:1820–1826. doi: 10.1086/379894. [DOI] [PubMed] [Google Scholar]
- Dhillon NK, Williams R, Peng F, Tsai YJ, Dhillon S, Nicolay B, Gadgil M, Kumar A, Buch SJ. Cocaine-mediated enhancement of virus replication in macrophages: implications for human immunodeficiency virus-associated dementia. J Neurovirol. 2007;13:483–495. doi: 10.1080/13550280701528684. [DOI] [PubMed] [Google Scholar]
- Liang H, Wang X, Chen H, Song L, Ye L, Wang SH, Wang YJ, Zhou L, Ho WZ. Methamphetamine enhances HIV infection of macrophages. Am J Pathol. 2008;172:1617–1624. doi: 10.2353/ajpath.2008.070971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: from structure to function. Physiol Rev. 1998;78:189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- Bradberry CW. Acute and chronic dopamine dynamics in a nonhuman primate model of recreational cocaine use. J Neurosci. 2000;20:7109–7115. doi: 10.1523/JNEUROSCI.20-18-07109.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher C, Ungless MA. The mechanistic classification of addictive drugs. PLoS Med. 2006;3:e437. doi: 10.1371/journal.pmed.0030437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce RC, Kumaresan V. The mesolimbic dopamine system: the final common pathway for the reinforcing effect of drugs of abuse? Neurosci Biobehav Rev. 2006;30:215–238. doi: 10.1016/j.neubiorev.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Kreek MJ. Cocaine, dopamine and the endogenous opioid system. J Addict Dis. 1996;15:73–96. doi: 10.1300/J069v15n04_05. [DOI] [PubMed] [Google Scholar]
- Koob GF, Bloom FE. Cellular and molecular mechanisms of drug dependence. Science. 1988;242:715–723. doi: 10.1126/science.2903550. [DOI] [PubMed] [Google Scholar]
- Talloczy Z, Martinez J, Joset D, Ray Y, Gacser A, Toussi S, Mizushima N, Nosanchuk J, Goldstein H, Loike J, Sulzer D, Santambrogio L. Methamphetamine inhibits antigen processing, presentation, and phagocytosis. PLoS Pathog. 2008;4:e28. doi: 10.1371/journal.ppat.0040028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz Y, Lee CJ, Schmauss C, Gonon F, Sulzer D. Amphetamine distorts stimulation-dependent dopamine overflow: effects on D2 autoreceptors, transporters, and synaptic vesicle stores. J Neurosci. 2001;21:5916–5924. doi: 10.1523/JNEUROSCI.21-16-05916.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SR, Garris PA, Wightman RM. Different effects of cocaine and nomifensine on dopamine uptake in the caudate-putamen and nucleus accumbens. J Pharmacol Exp Ther. 1995;274:396–403. [PubMed] [Google Scholar]
- Moghaddam B, Bunney BS. Differential effect of cocaine on extracellular dopamine levels in rat medial prefrontal cortex and nucleus accumbens: comparison to amphetamine. Synapse. 1989;4:156–161. doi: 10.1002/syn.890040209. [DOI] [PubMed] [Google Scholar]
- Pontieri FE, Tanda G, Di Chiara G. Intravenous cocaine, morphine, and amphetamine preferentially increase extracellular dopamine in the “shell” as compared with the “core” of the rat nucleus accumbens. Proc Natl Acad Sci USA. 1995;92:12304–12308. doi: 10.1073/pnas.92.26.12304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna F, McLaughlin PJ, Lewis BJ, Sibbring GC, Cummerson JA, Bowen-Jones D, Moots RJ. Dopamine receptor expression on human T- and B-lymphocytes, monocytes, neutrophils, eosinophils and NK cells: a flow cytometric study. J Neuroimmunol. 2002;132:34–40. doi: 10.1016/s0165-5728(02)00280-1. [DOI] [PubMed] [Google Scholar]
- Kirillova GP, Hrutkay RJ, Shurin MR, Shurin GV, Tourkova IL, Vanyukov MM. Dopamine receptors in human lymphocytes: radioligand binding and quantitative RT-PCR assays. J Neurosci Methods. 2008;174:272–280. doi: 10.1016/j.jneumeth.2008.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santambrogio L, Lipartiti M, Bruni A, Dal Toso R. Dopamine receptors on human T- and B-lymphocytes. J Neuroimmunol. 1993;45:113–119. doi: 10.1016/0165-5728(93)90170-4. [DOI] [PubMed] [Google Scholar]
- Ghosh MC, Mondal AC, Basu S, Banerjee S, Majumder J, Bhattacharya D, Dasgupta PS. Dopamine inhibits cytokine release and expression of tyrosine kinases. Lck and Fyn in activated T cells. Int Immunopharmacol. 2003;3:1019–1026. doi: 10.1016/S1567-5769(03)00100-0. [DOI] [PubMed] [Google Scholar]
- Levite M, Chowers Y, Ganor Y, Besser M, Hershkovits R, Cahalon L. Dopamine interacts directly with its D3 and D2 receptors on normal human T cells, and activates beta1 integrin function. Eur J Immunol. 2001;31:3504–3512. doi: 10.1002/1521-4141(200112)31:12<3504::aid-immu3504>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Czub S, Czub M, Koutsilieri E, Sopper S, Villinger F, Muller JG, Stahl-Hennig C, Riederer P, Ter Meulen V, Gosztonyi G. Modulation of simian immunodeficiency virus neuropathology by dopaminergic drugs. Acta Neuropathol (Berl) 2004;107:216–226. doi: 10.1007/s00401-003-0801-3. [DOI] [PubMed] [Google Scholar]
- Czub S, Koutsilieri E, Sopper S, Czub M, Stahl-Hennig C, Muller JG, Pedersen V, Gsell W, Heeney JL, Gerlach M, Gosztonyi G, Riederer P, ter Meulen V. Enhancement of central nervous system pathology in early simian immunodeficiency virus infection by dopaminergic drugs. Acta Neuropathol (Berl) 2001;101:85–91. doi: 10.1007/s004010000313. [DOI] [PubMed] [Google Scholar]
- Bolan EA, Kivell B, Jaligam V, Oz M, Jayanthi LD, Han Y, Sen N, Urizar E, Gomes I, Devi LA, Ramamoorthy S, Javitch JA, Zapata A, Shippenberg TS. D2 receptors regulate dopamine transporter function via an extracellular signal-regulated kinases 1 and 2-dependent and phosphoinositide 3 kinase-independent mechanism. Mol Pharmacol. 2007;71:1222–1232. doi: 10.1124/mol.106.027763. [DOI] [PubMed] [Google Scholar]
- Martin-Negrier ML, Charron G, Bloch B. Receptor recycling mediates plasma membrane recovery of dopamine D1 receptors in dendrites and axons after agonist-induced endocytosis in primary cultures of striatal neurons. Synapse. 2006;60:194–204. doi: 10.1002/syn.20296. [DOI] [PubMed] [Google Scholar]
- Pedrosa R, Soares-da-Silva P. Oxidative and non-oxidative mechanisms of neuronal cell death and apoptosis by L-3,4-dihydroxyphenylalanine (L-DOPA) and dopamine. Br J Pharmacol. 2002;137:1305–1313. doi: 10.1038/sj.bjp.0704982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Shi L, Javitch JA. The fourth transmembrane segment forms the interface of the dopamine D2 receptor homodimer. J Biol Chem. 2003;278:4385–4388. doi: 10.1074/jbc.C200679200. [DOI] [PubMed] [Google Scholar]
- Sacerdote P, Martucci C, Vaccani A, Bariselli F, Panerai AE, Colombo A, Parolaro D, Massi P. The nonpsychoactive component of marijuana cannabidiol modulates chemotaxis and IL-10 and IL-12 production of murine macrophages both in vivo and in vitro. J Neuroimmunol. 2005;159:97–105. doi: 10.1016/j.jneuroim.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Gomez F, Ruiz P, Briceno F, Rivera C, Lopez R. Macrophage Fcgamma receptors expression is altered by treatment with dopaminergic drugs. Clin Immunol. 1999;90:375–387. doi: 10.1006/clim.1998.4665. [DOI] [PubMed] [Google Scholar]
- Coutinho-Silva R, Ojcius DM, Gorecki DC, Persechini PM, Bisaggio RC, Mendes AN, Marks J, Burnstock G, Dunn PM. Multiple P2X and P2Y receptor subtypes in mouse J774, spleen and peritoneal macrophages. Biochem Pharmacol. 2005;69:641–655. doi: 10.1016/j.bcp.2004.11.012. [DOI] [PubMed] [Google Scholar]
- Carvalho-Freitas MI, Rodrigues-Costa EC, Nasello AG, Palermo-Neto J, Felicio LF. In vitro macrophage activity: biphasic effect of prolactin and indirect evidence of dopaminergic modulation. Neuroimmunomodulation. 2008;15:131–139. doi: 10.1159/000148196. [DOI] [PubMed] [Google Scholar]
- Sarkar C, Das S, Chakroborty D, Chowdhury UR, Basu B, Dasgupta PS, Basu S. Cutting Edge: stimulation of dopamine D4 receptors induce T cell quiescence by up-regulating Kruppel-like factor-2 expression through inhibition of ERK1/ERK2 phosphorylation. J Immunol. 2006;177:7525–7529. doi: 10.4049/jimmunol.177.11.7525. [DOI] [PubMed] [Google Scholar]
- Gosztonyi G, Schmidt V, Nickel R, Rothschild MA, Camacho S, Siegel G, Zill E, Pauli G, Schneider V. Neuropathologic analysis of postmortal brain samples of HIV-seropositive and -seronegative i.v. drug addicts. Forensic Sci Int. 1993;62:101–105. doi: 10.1016/0379-0738(93)90052-c. [DOI] [PubMed] [Google Scholar]
- Goodkin K, Shapshak P, Metsch LR, McCoy CB, Crandall KA, Kumar M, Fujimura RK, McCoy V, Zhang BT, Reyblat S, Xin KQ, Kumar AM. Cocaine abuse and HIV-1 infection: epidemiology and neuropathogenesis. J Neuroimmunol. 1998;83:88–101. doi: 10.1016/s0165-5728(97)00225-7. [DOI] [PubMed] [Google Scholar]
- Bowyer JF, Ali S. High doses of methamphetamine that cause disruption of the blood-brain barrier in limbic regions produce extensive neuronal degeneration in mouse hippocampus. Synapse. 2006;60:521–532. doi: 10.1002/syn.20324. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Is there a common molecular pathway for addiction? Nat Neurosci. 2005;8:1445–1449. doi: 10.1038/nn1578. [DOI] [PubMed] [Google Scholar]
- Miyazaki I, Asanuma M, Diaz-Corrales FJ, Miyoshi K, Ogawa N. Direct evidence for expression of dopamine receptors in astrocytes from basal ganglia. Brain Res. 2004;1029:120–123. doi: 10.1016/j.brainres.2004.09.014. [DOI] [PubMed] [Google Scholar]
- Reyes MG, Faraldi F, Senseng CS, Flowers C, Fariello R. Nigral degeneration in acquired immune deficiency syndrome (AIDS). Acta Neuropathol. 1991;82:39–44. doi: 10.1007/BF00310921. [DOI] [PubMed] [Google Scholar]
- Aylward EH, Henderer JD, McArthur JC, Brettschneider PD, Harris GJ, Barta PE, Pearlson GD. Reduced basal ganglia volume in HIV-1-associated dementia: results from quantitative neuroimaging. Neurology. 1993;43:2099–2104. doi: 10.1212/wnl.43.10.2099. [DOI] [PubMed] [Google Scholar]
- Nath A, Anderson C, Jones M, Maragos W, Booze R, Mactutus C, Bell J, Hauser KF, Mattson M. Neurotoxicity and dysfunction of dopaminergic systems associated with AIDS dementia. J Psychopharmacol. 2000;14:222–227. doi: 10.1177/026988110001400305. [DOI] [PubMed] [Google Scholar]
- Fujimura RK, Goodkin K, Petito CK, Douyon R, Feaster DJ, Concha M, Shapshak P. HIV-1 proviral DNA load across neuroanatomic regions of individuals with evidence for HIV-1-associated dementia. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;16:146–152. doi: 10.1097/00042560-199711010-00002. [DOI] [PubMed] [Google Scholar]
- Navia BA, Cho ES, Petito CK, Price RW. The AIDS dementia complex: II. Neuropathology. Ann Neurol. 1986;19:525–535. doi: 10.1002/ana.410190603. [DOI] [PubMed] [Google Scholar]
- King JE, Eugenin EA, Buckner CM, Berman JW. HIV tat and neurotoxicity. Microbes Infect. 2006;8:1347–1357. doi: 10.1016/j.micinf.2005.11.014. [DOI] [PubMed] [Google Scholar]
- McManus CM, Weidenheim K, Woodman SE, Nunez J, Hesselgesser J, Nath A, Berman JW. Chemokine and chemokine-receptor expression in human glial elements: induction by the HIV protein. Tat, and chemokine autoregulation. Am J Pathol. 2000;156:1441–1453. doi: 10.1016/S0002-9440(10)65013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Aversa TG, Eugenin EA, Berman JW. NeuroAIDS: contributions of the human immunodeficiency virus-1 proteins Tat and gp120 as well as CD40 to microglial activation. J Neurosci Res. 2005;81:436–446. doi: 10.1002/jnr.20486. [DOI] [PubMed] [Google Scholar]
- Theodore S, Cass WA, Nath A, Maragos WF. Progress in understanding basal ganglia dysfunction as a common target for methamphetamine abuse and HIV-1 neurodegeneration. Curr HIV Res. 2007;5:301–313. doi: 10.2174/157016207780636515. [DOI] [PubMed] [Google Scholar]
- Flora G, Lee YW, Nath A, Hennig B, Maragos W, Toborek M. Methamphetamine potentiates HIV-1 Tat protein-mediated activation of redox-sensitive pathways in discrete regions of the brain. Exp Neurol. 2003;179:60–70. doi: 10.1006/exnr.2002.8048. [DOI] [PubMed] [Google Scholar]
- Theodore S, Stolberg S, Cass WA, Maragos WF. Human immunodeficiency virus-1 protein tat and methamphetamine interactions. Ann NY Acad Sci. 2006;1074:178–190. doi: 10.1196/annals.1369.018. [DOI] [PubMed] [Google Scholar]
- Rohr O, Sawaya BE, Lecestre D, Aunis D, Schaeffer E. Dopamine stimulates expression of the human immunodeficiency virus type 1 via NF-kappaB in cells of the immune system. Nucleic Acids Res. 1999;27:3291–3299. doi: 10.1093/nar/27.16.3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheller C, Sopper S, Jassoy C, ter Meulen V, Riederer P, Koutsilieri E. Dopamine activates HIV in chronically infected T lymphoblasts. J Neural Transm. 2000;107:1483–1489. doi: 10.1007/s007020070012. [DOI] [PubMed] [Google Scholar]
- Kearn CS, Blake-Palmer K, Daniel E, Mackie K, Glass M. Concurrent stimulation of cannabinoid CB1 and dopamine D2 receptors enhances heterodimer formation: a mechanism for receptor cross-talk? Mol Pharmacol. 2005;67:1697–1704. doi: 10.1124/mol.104.006882. [DOI] [PubMed] [Google Scholar]
- Seeman P, Van Tol HH. Dopamine receptor pharmacology. Trends Pharmacol Sci. 1994;15:264–270. doi: 10.1016/0165-6147(94)90323-9. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Le Moine C. Dopamine D1/5 receptor stimulation induces c-fos expression in the subthalamic nucleus: possible involvement of local D5 receptors. Eur J Neurosci. 2002;15:133–142. doi: 10.1046/j.0953-816x.2001.01840.x. [DOI] [PubMed] [Google Scholar]
- Marcellino D, Carriba P, Filip M, Borgkvist A, Frankowska M, Bellido I, Tanganelli S, Muller CE, Fisone G, Lluis C, Agnati LF, Franco R, Fuxe K. Antagonistic cannabinoid CB1/dopamine D2 receptor interactions in striatal CB1/D2 heteromers: a combined neurochemical and behavioral analysis. Neuropharmacology. 2008;54:815–823. doi: 10.1016/j.neuropharm.2007.12.011. [DOI] [PubMed] [Google Scholar]
- So CH, Verma V, O'Dowd BF, George SR. Desensitization of the dopamine D1 and D2 receptor hetero-oligomer mediated calcium signal by agonist occupancy of either receptor. Mol Pharmacol. 2007;72:450–462. doi: 10.1124/mol.107.034884. [DOI] [PubMed] [Google Scholar]