Abstract
Permanent disability of patients suffering from central nervous system (CNS) inflammation such as multiple sclerosis, the most common chronic inflammatory disorder of the CNS, originates mainly from demyelination and axonal damage. Although many studies in the past focused on the role of CD4+ T cells, several recent findings postulate the relevance of autoaggressive, cytotoxic CD8+ T cells in the effector phase of multiple sclerosis. Yet, it remains unresolved whether axonal injury is the result of a CD8+ T cell-targeted hit against the axon itself or the consequence of an attack against the myelin structure. To address this issue of CD8-mediated tissue damage in CNS inflammation, we performed continuous confocal imaging of autoaggressive, cytotoxic CD8+ T cells in living organotypic cerebellar brain slices. We observed that loading brain slices with the cognate peptide antigen caused CD8-mediated damage of myelinated axons. To exclude the possibility that the cognate peptide loaded onto the brain slices was presented by axons directly, we restricted the cognate antigen expression exclusively to the cytosol of oligodendrocytes. Aside from vast myelin damage, extensive axonal bystander injury occurred. Using this model system of inflammatory CNS injury, we visualize that axonal loss can be the consequence from “collateral bystander damage” by autoaggressive, cytotoxic CD8+ T cells, targeting their cognate antigen processed and presented by oligodendrocytes.
Demyelination and axonal damage are key features of multiple sclerosis (MS).1 Mediators of both innate and adaptive immunity are thought to take part in the disease’s initiation and perpetuation.2 Recently, studies investigating the pathogenic role of autoaggressive, cytotoxic CD8+ T cells in MS pathogenesis were intensified.3 By virtue of histological investigations on MS brain tissue, it was shown that CD8+ T cells outnumber CD4+ T cells4 and that they closely interact with demyelinated axons.5 Furthermore, CD8+ T cells were found clonally expanded in the cerebrospinal fluid, in the blood, and in central nervous system (CNS) lesions of MS patients.4,6,7 Finally, CD8-mediated lysis of HLA-matched oligodendrocytes (ODCs)8 and transsection of dissociated single neurons9 provided functional evidence in vitro. In support, in vivo studies recently reported CNS injury after injecting antigen-specific CD8+ T cells either by using CD8-mediated experimental autoimmune encephalomyelitis,10,11 an animal model for MS, or by using novel approaches, where mice selectively express a neo-self antigen in ODCs.12,13
Despite the concept that CD8+ T cells hold an active role in MS pathogenesis, neither of the studies addressed the pathogenic role of myelin-reactive CD8+ T cells with regard to axonal damage. To clarify whether myelin-reactive, cytotoxic CD8+ T cells directly attack axons or whether axonal loss may be the result of a targeted hit against myelinated structures indicating “collateral bystander damage,” we established a novel experimental system of inflammatory CNS injury: autoaggressive, cytotoxic CD8+ T cells were traced by continuous confocal imaging in living cerebellar brain slices, which were derived from transgenic mice expressing green fluorescent protein (GFP) in myelin (PLP-GFP mouse14). In an inflammatory environment, loading of brain tissue with the cognate peptide antigen caused CD8+ T cells to damage myelinated axons. To ensure that the axonal damage we observed did not just occur due to the fact that the added cognate peptide was loaded on MHC class I molecules possibly present on axons, we crossed PLP-GFP mice with ODC-ovalbumin (OVA) mice,15 thus restricting the expression of the cognate (neo-self) antigen OVA exclusively to the cytosol of ODCs. Again in an inflammatory environment, we observed in real-time cytotoxic OVA-T cell receptor CD8+ T cells (OT-I T cells) directly attacking myelin structures. Subsequent morphological analysis demonstrated aside from vast myelin damage significant axonal injury. Thanks to this experimental setup in which the cognate antigen was sequestered to ODCs exclusively, we could show that these autoaggressive, cytotoxic CD8+ T cells target their cognate antigen processed and presented by ODCs. In conclusion, axonal loss can be the consequence of “collateral bystander damage,” resulting from of a CD8-mediated attack directed against the myelin.
Materials and Methods
Mice
Mice (all C57BL/6 background) were bred at the animal facilities of the University of Zurich under specific pathogen-free conditions, and all experimental procedures were approved by the Swiss Veterinary Office (109/2006). OT-I and OT-II mice carrying a transgenic T cell receptor specific for OVA amino acids 257–264 (SIINFEKL) in H-2Kb or for OVA323–339 in H-2IAb, respectively, were obtained from The Jackson Laboratory (Bar Harbor, ME). 2D2 transgenic mice carrying CD4+ T cells with a T cell receptor reactive for myelin ODC glycoprotein35–55 were provided by V. Kuchroo (Harvard Medical School, Boston, MA), PLP-GFP transgenic mice14 were a gift from B. Zalc (Institut National de la Santé et de la Recherche Médicale, Paris, France). ODC-OVA transgenic mice were previously generated and genotyped as described elsewhere.15
Organotypic Cerebellar Slice Culture
To study CNS tissue integrity, we obtained 500-μm-thick cerebellar slice cultures from 8- to 10-day-old mice in which the brain parenchyma is preserved in a proper network organization ex vivo.16 Slices were cultured on Millicell plate inserts (Millipore, Zug, Switzerland) for at least 1 week to allow tissue flattening as described elsewhere.16,17 Where indicated, slice culture medium was supplemented with 100 U/ml IFNγ (PeproTech (Rockhill, NJ)) 72 hours before coculture experiments.
Activation and Enrichment of T Cells
Splenocytes were obtained from OT-I, OT-II and 2D2 mice. OT-I cells were stimulated with their cognate antigen SIINFEKL peptide or Smcy738–746 (H-Y) control peptide (5 μg/ml; GenScript, Piscataway, NJ). OT-II and 2D2 splenocytes were incubated with their cognate antigen OVA323–339 peptide or myelin ODC glycoprotein35–55 peptide (both 5 μg/ml; GenScript), respectively. Splenocytes were kept for 24 hours in culture medium only for nonactivation or were incubated with anti-CD3 antibody (5 μg/ml; Bioexpress, West Lebanon, NJ) for unspecific stimulation. Supernatants were analyzed for IFNγ production, and T cells were enriched as described previously.18 Enriched T cell populations were labeled with the infrared DiD lipophilic tracer (Invitrogen, Basel, Switzerland) according to the manufacturer’s instructions. Twenty-four hours before time-lapse imaging, T cells were applied on cerebellar slices (1 × 106 cells/slice) and placed on ibiTreat chambers (ibidi, Munich, Germany) to allow T cell tissue invasion.
Confocal Time-Lapse Imaging
T cells and myelin in slices were visualized by an SP5 Leica motorized confocal laser scanning microscope (SP5; Leica, Heerbrug, Switzerland) using an argon and a helium neon laser with a ×20 objective (oil immersion, numerical aperture (NA) 0.7, Leica). Slices were maintained in an incubation chamber at 37°C using a heated stage, and 37°C warm slice culture medium was equilibrated with 95% O2 and 5% CO2. Three-dimensional data sets were acquired every 10 to 45 minutes over a period of up to 18 hours.
Immunostaining
Following confocal live imaging, slices were fixed and immunostained as described elsewhere.19 For primary antibodies monoclonal mouse anti-murine neurofilament 200 antibody (clone N52; Sigma-Aldrich, Buchs, Switzerland), monoclonal rat anti-murine MHC class I (clone ER-HR52; BMA Biomedicals, August, Switzerland), monoclonal rat anti-murine MHC class II (clone M5/114.15.2, BD Biosciences, Oxford, UK), and monoclonal rat anti-murine myelin basic protein (Serotec, Düsseldorf, Germany) were used as indicated and were detected with secondary goat anti-mouse Alexa Fluor 405-conjugated antibody or goat anti-rat Alexa Fluor 594-conjugated antibody (both by Invitrogen).
Laser Scanning Confocal Microscopy, Image Analysis, and Video Processing
Confocal microscopy from fixed slices was performed using SP2 or SP5 confocal laser microscopes (Leica) with ×10 (dry, NA 0.5), ×20 (oil immersion, NA 0.7), or ×63 (glycerol immersion, NA 1.3) objectives. Image series from fixed and stained slices and sequences from time-lapse imaging including z-stacks were collected, and images were analyzed using Imaris software (version 6.1.0; Bitplane, Zurich, Switzerland). Videos were processed using FinalCutPro (version 6.01; Apple).
Statistical Analysis
Data were obtained from at least three independent experiments performed in duplicate or triplicate. Results were expressed as means ± SEM. Statistical significance of mean differences was assessed by one-way analysis of variance followed by Bonferroni posttest using GraphPad Prism software (version 4.02; San Diego, CA).
Results
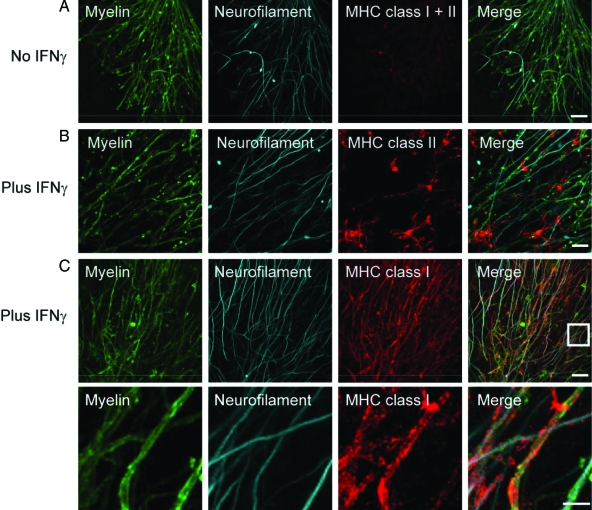
In this study, we established a model system of CNS inflammation to investigate the potential of autoaggressive, cytotoxic CD8+ T cells to cause axonal loss by “collateral bystander damage.” To obtain an inflammatory environment, living brain slices were preincubated with the proinflammatory cytokine IFNγ, described to enhance MHC expression. Subsequent confocal microscopic analysis of fixed slices, stained for neurofilament (NF) and either MHC class I or MHC class II molecules, demonstrated that both MHC class molecules were differentially inducible upon treatment with IFNγ (Figure 1A). As expected, MHC class II was neither observed on myelin nor axons but on microglia (Figure 1B), whereas MHC class I expression was most pronouncedly identified on myelin (Figure 1C and subset of C). As such, IFNγ-induced up-regulation of MHC class I molecules ensured the key prerequisite for antigen presentation to and recognition by CD8+ T cells.20
Figure 1.
Incubation with IFNγ induces MHC class I and II expression. Confocal images of costained MHC class I and II molecules (red) and NF (cyan) reveal only marginal MHC expression in untreated ODC-OVA × PLP-GFP brain slices (A). Yet, presence of IFNγ (100 U/ml, 72 hours) induces MHC expression (B and C). Whereas MHC class II was most likely observed on microglia (B), MHC class I was mainly detected on oligodendrocytes (C). The lower panels in C reflect the boxed area in the upper merged panel. Scale bars equal 20 μm in A, B, and upper panel in C and 7.5 μm for lower panel in C.
To assess the impact of cytotoxic CD8+ T cells on axonal injury, we pulsed IFNγ-pretreated brain slices from PLP-GFP mice14 with OVA peptide257–264 (SIINFEKL), the cognate antigen of CD8+ OT-I T cells. Addition of SIINFEKL-prestimulated CD8+ OT-I T cells (see supplemental Figure S1, B and D; see http://ajp.amjpathol.org) resulted in direct injury of myelinated fibers (supplemental Figure S2B; see http://ajp.amjpathol.org). In contrast, application of CD4+ OT-II T cells onto PLP-GFP brain slices loaded with the cognate MHC class II OVA peptide323–339 (supplemental Figure S1, A and C; see http://ajp.amjpathol.org) left myelin and axons intact in the previously described experimental setup (supplemental Figure S2A; see http://ajp.amjpathol.org).
Although we clearly observed CD8-mediated axonal damage in peptide-pulsed brain slices, in which we could not detect MHC class I expression by axons, we could not formally exclude the possibility that the peptides loaded onto the brain slices were presented by axons. To specifically address the question whether axonal loss is the result of a direct T cell-axon recognition or driven primarily by an attack against the myelin sheath, we restricted the cognate T cell antigen expression to ODCs. We therefore refined our system by crossing PLP-GFP mice with transgenic mice expressing OVA in the cytosol of ODCs only (ODC-OVA × PLP-GFP mice).
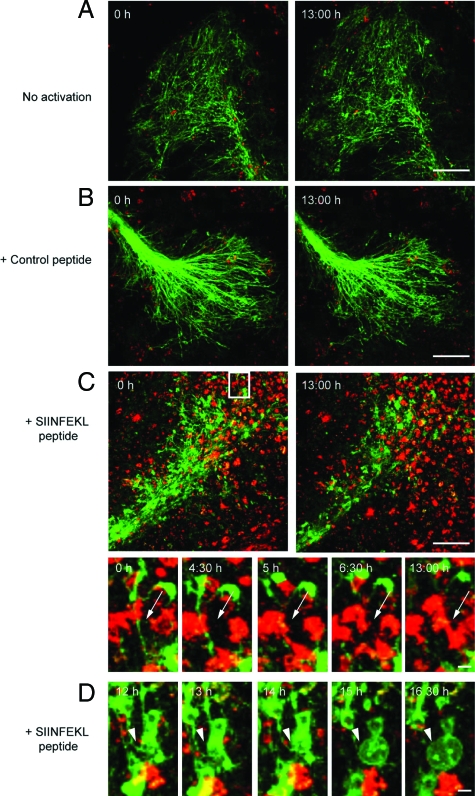
SIINFEKL or anti-CD3 antibody (data not shown) pre-stimulated CD8+ OT-I T cells directly attacked and transsected myelinated axons in ODC-OVA × PLP-GFP brain slices, causing extensive tissue injury (showing axonal transsection, Figure 2, A–D, and supplemental Video SV1; presenting the corresponding overview thereof, supplemental Video SV2; and depicting “blebbing” of an attacked ODC, supplemental Video SV3; see http://ajp.amjpathol.org; note that in the entire experimental setup confocal live imaging was performed after coculture of prestimulated T cells and slices for 24 hours). Yet, the observed pathology was neither detected when applying (i) nonactivated (Figure 2A and supplemental Video SV4, see http://ajp.amjpathol.org), nor (ii) control peptide prestimulated CD8+ OT-I T cells (Figure 2B and supplemental Video SV5, see http://ajp.amjpathol.org), nor when adding (iii) adequately prestimulated CD8+ OT-I cells to ODC-OVA negative slices (data not shown), or (iv) ODC-OVA-positive slices not pre-exposed to IFNγ (data not shown). Equally, loading of IFNγ pretreated PLP-GFP brain slices with whole OVA protein did not cause OT-I-mediated CNS injury (Figure 3, lower panel). In addition, both CD4+ OT-II and 2D2 T cells left myelin and axons intact in IFNγ-pretreated ODC-OVA × PLP-GFP brain slices (Figure 4, A and B).
Figure 2.
Direct transsection of myelinated axons by CD8+ OT-I T cells. Frames from confocal time-lapse sequences were captured and document only little invasion of nonactivated (A) or control peptide (B) prestimulated CD8+ OT-I T cells (red) into ODC-OVA × PLP-GFP brain slices. Myelin damage was not observed in either situation (A and B). Contrarily, SIINFEKL-prestimulated CD8+ OT-I T cells entered brain slices to a great extent (C) and directly attacked myelin (green) as pointed out by arrows (subregion of C). Additionally, pronounced blebbing of oligodendrocytes after T cell attack was observed as pointed out by arrowheads (D). In the upper left corner, time elapsed after start of sequence is documented, scale bars equal 30 μm in A, B, and C and 10 μm for subregion of C and in D.
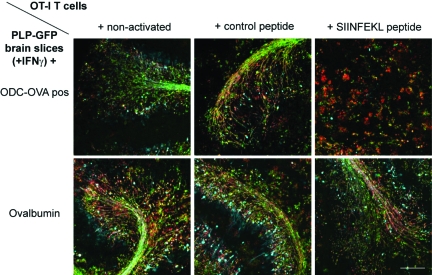
Figure 3.
SIINFEKL-prestimulated CD8+ OT-I T cells do not cause myelin damage when pulsing PLP-GFP brain slices with complete OVA protein. Immunostaining for neurofilament (cyan) and myelin basic protein (red) reveals that SIINFEKL-prestimulated CD8+ OT-I T cells leave myelin and axons intact in PLP-GFP brain slices pulsed with complete OVA protein (lower panel). In contrast, myelin and axons are extensively damaged by SIINFEKL-prestimulated CD8+ OT-I T cells in ODC-OVA × PLP-GFP brain slices (upper panel). Scale bars represents 150 μm.
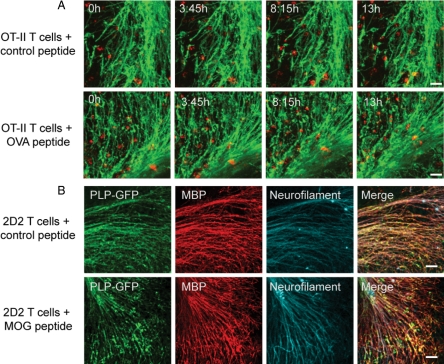
Figure 4.
CD4+ OT-II and 2D2 T cells leave myelin and axons intact in ODC-OVA × PLP-GFP brain slices. A: Frames from confocal time-lapse sequences were captured displaying myelin in green and differentially prestimulated OT-II T cells in red as indicated. No alteration of myelin morphology can be observed in either situation. In the upper left corner, time elapsed after start of the respective sequence is documented. B: Immunostaining for neurofilament (cyan) and myelin basic protein (red) from fixed ODC-OVA × PLP-GFP brain slices show no alteration in axonal and myelin morphology after incubation with differentially prestimulated 2D2 T cells as indicated. Scale bars represent 30 μm in A and 50 μm in B.
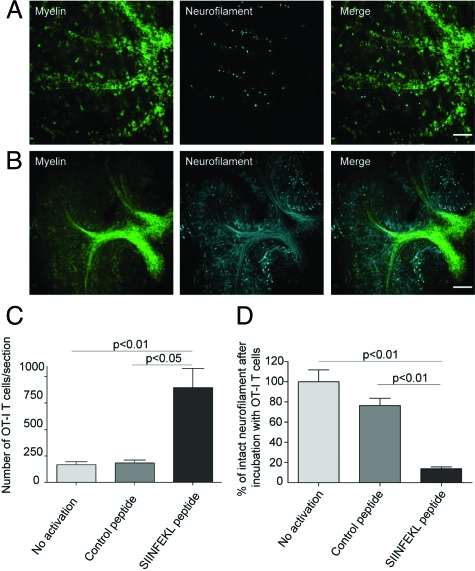
Injury of myelinated axons appeared to correlate directly with CD8+ T cell migration into and interaction with the brain parenchyma. Spot analysis from z-stacks of confocal time-lapse imaging underscored that SIINFEKL peptide-prestimulated OT-I T cells invaded IFNγ-pretreated ODC-OVA × PLP-GFP brain slices to a significantly greater extent than did nonactivated or control peptide prestimulated OT-I T cells (Figures 5C and 2). Additionally, longer lasting interactions of SIINFEKL-prestimulated OT-I T cells with CNS tissue were observed when compared with nonactivated and control peptide-prestimulated T cells. In line, trajectory analysis revealed a “scanning” behavior of SIINFEKL-prestimulated OT-I T cells, whereas nonactivated or control peptide-prestimulated T cells displayed a “straightforward” motion (supplemental Figure S3, see http://ajp.amjpathol.org), similarly to that behavior described for CD4+ T cells.21 As such, CD8-mediated CNS injury appeared to depend both on the T cell activation status and the cognate antigen being presented by MHC class I-expressing ODCs/myelin.
Figure 5.
SIINFEKL-prestimulated cytotoxic OT-I T cells invade brain tissue and cause damage of myelinated axons. Spot analysis from confocal time-lapse imaging revealed that SIINFEKL-prestimulated CD8+ OT-I T cells invaded ODC-OVA × PLP-GFP slices to a significantly greater extent than nonactivated or control peptide-prestimulated CD8+ OT-I T cells (C). Extensive disruption and irregularities of both myelin (green) and axons (cyan) in ODC-OVA × PLP-GFP brain slices were observed after incubation with SIINFEKL-prestimulated CD8+ OT-I T cells (A) in contrast to well-maintained structures in slices incubated with nonactivated CD8+ OT-I T cells (B). Quantification of axonal damage confirms that neurofilament was significantly injured in ODC-OVA × PLP-GFP brain slices incubated with SIINFEKL-prestimulated CD8+ OT-I T cells when compared with controls (D). Results are expressed as percentage of intact neurofilament compared with ODC-OVA × PLP-GFP brain slices incubated with nonactivated CD8+ OT-I T cells. Values are ± mean SEM, n = 3; for P value generation one-way analysis of variance followed by Bonferroni posttest was used to compare among groups (C and D). Scale bars equal 200 μm in A and B.
Subsequent morphological analysis of ODC-OVA × PLP-GFP brain slices exposed to SIINFEKL peptide-prestimulated OT-I T cells revealed widespread, patchy disintegration of axonal morphology (Figure 5, A and B, and supplemental Figure S4, see http://ajp.amjpathol.org). Quantification of axonal injury confirmed significantly more axonal loss in ODC-OVA × PLP-GFP brain slices exposed to SIINFEKL peptide-prestimulated OT-I T cells when compared with controls (Figure 5D).
In our experimental system, in which the cognate antigen was sequestered to ODCs exclusively, we could show that ODCs are capable of processing and presenting antigen to autoaggressive, cytotoxic CD8+ T cells. In turn, CD8+ T cells directly targeting the myelin sheath cause axonal loss due to “collateral bystander damage.”
Discussion
In this study, we revealed that cytotoxic CD8+ T cells specific for myelin components can evoke axonal loss by “collateral bystander damage.” Before causing tissue damage, CD8+ T cells required a sufficient stimulus to become cytotoxic. In line, the necessity of homeostatic T cell expansion and activation in a lymphopenic environment was recently reported12 in a mouse model, whereas the triggering event in MS remains to be resolved.
“Collateral bystander damage” and not direct axonal attack by CD8+ T cells was shown, as we restricted the expression of the cognate antigen to the cytosol of ODCs. In return, this demonstrated that ODCs can present peptides processed from endogenous protein efficiently to autoaggressive, cytotoxic CD8+ T cells. To challenge and to verify our findings, we incubated PLP-GFP brain slices with complete OVA protein. We based this investigation on a hypothetical scenario, in which myelin proteins would be released by myelin damage. Subsequent digestion could potentially lead to loading of myelin protein-derived peptides onto accessible MHC class I-positive cells, including axons, thus enabling CD8+ T cells to attack axons themselves. Yet, we could not detect any CD8-mediated axonal damage in PLP-GFP brain slices when mimicking myelin release by OVA addition. Apparently, extracellular processing of complete, 385-amino-acid-long OVA protein specifically to the cognate SIINFEKL peptide is a rather rare event. We therefore conclude that indeed processing of the intracellular OVA antigen to SIINFEKL peptide and consecutive presentation by ODCs/myelin is sufficient to cause “collateral bystander damage” of axons by myelin directed cytotoxic CD8+ T cells.
At least in our experimental system, the inflammatory environment evoked by IFNγ preincubation of brain slices was indispensable for a CD8-mediated attack. The significance of IFNγ in CD8-mediated CNS tissue damage was equally pointed out in the animal model,12 underscoring the differential effects of IFNγ in CD8- versus CD4-driven experimental autoimmune encephalomyelitis.22,23 In line with the necessity of IFNγ, CD8-driven tissue damage occurred in an MHC class I-restricted fashion. Antigen presentation by MHC class I molecules on ODCs was essential, permitting the formation of immune synapses even when expressed at low levels.24 As expected, we could induce expression of MHC class I and II molecules in brain slices by the addition of IFNγ to the culture medium. In contrast to MHC class II molecules, MHC class I expression was found most prominently on myelin (also reviewed in Ref. 25). With regard to these findings, it was expected that the integrity of myelinated axons was conserved when adding CD4+ OT-II or 2D2 T cells onto the slices, because CD4+ T cells recognize their cognate antigen in the context of MHC class II molecules. Remarkably, Nitsch et al26 report neuronal damage by PLP-specific CD4+ T cells independently of MHC restriction. For that finding, Nitsch et al26 used acute hippocampal brain slices from SJL/J mice, a strain known to be more susceptible to CD4-mediated experimental autoimmune encephalomyelitis than the C57BL/6 mice. SJL/J brain slices were incubated with PLP-specific CD4+ T cells, which had been generated by immunizing SJL/J mice with PLP 139-151 and had been restimulated with PLP 139-151 every 14 days in vitro. However, it is well established that long-term culture of CD4+ T cells selects for a T cell phenotype acquiring NK cell properties,27,28 which may explain the non-MHC-restricted target damage observed.26 Neurons in brain slices were visualized by the cell-permeable Ca2+ indicator Fluo-4, which is, however, not a neuron-specific marker. Changes in cellular calcium concentrations were then interpreted to represent CD4+ T cell-mediated neuronal damage. In our system, using CD4+ T cells as effectors, we could not detect any impact on the integrity of the cultured tissue. We would like to stress that the different experimental systems and readouts are likely to account for the diverging results observed regarding CD4-mediated CNS injury.
Finally, confocal live imaging allowed us to characterize CD8+ T cell migration through CNS structures, which has been poorly documented until now. Similarly to previous reports investigating the migration of CD4+ T cells through CNS tissue,21,26 we observed a significantly greater CNS tissue invasion and a “scanning” behavior of SIINFEKL-prestimulated OT-I CD8+ T cells contrary to nonactivated or control peptide-prestimulated CD8+ OT-I T cells. In line, local myelin damage along with heavily disrupted neurofilament morphology occurred most pronouncedly at sites of prolonged CD8+ T cell-myelin interactions.
In conclusion, we showed that an inflammatory CNS environment mimicked by IFNγ can enable ODCs to process and present endogenous antigen via MHC class I molecules. In turn, autoaggressive, cytotoxic CD8+ T cells directly targeting myelinated structures can cause axonal loss due to “collateral bystander damage.” Fully aware of its limitations, we chose the described ex vivo system to visualize the interaction of autoaggressive, cytotoxic CD8+ T cells with living CNS tissue. Although the outlined scenario is most likely not the only relevant mechanism of CNS tissue damage in inflammatory CNS diseases, we are confident that our findings critically contribute to the understanding of CD8-mediated neuropathology in CNS inflammation, strengthen a pathogenic role of CD8+ T cells in MS, and advocate for the development of future immunotherapies aiming at the CD8-myelin/ODC interface.
Supplementary Material
Acknowledgments
We thank Bernard Zalc for providing the PLP-GFP mice.
Footnotes
Address reprint requests to Norbert Goebels, M.D. Department of Neurology, Heinrich-Heine-University, Moorenstr. 5, D-40225 Duesseldorf, Germany. E-mail: norbert.goebels@uni-duesseldorf.de.
Supported by grants provided by the Deutsche Forschungsgemeinschaft through Sonderforschungs bereich 581 and Hertie Foundation (both to T.H.) and the National Center for Competence in Research “Neuronal Plasticity and Repair,” Neuroscience Center Zurich, Merck-Serono International S.A., Biogen-Idec, 3R Research Foundation Switzerland, Swiss Multiple Sclerosis Society, Swiss National Science Foundation, and the University of Zurich (to N.G.).
All authors report no financial or any other conflict of interest with the contents of the manuscript.
B.S. conducted the experimental studies, codesigned the study and wrote the manuscript. M.D.H. and U.Z. helped essentially to establish continuous confocal live imaging of brain slices. K.F. provided technical assistance. H.W. helped in designing the experimental approach and gave comments to the manuscript. T.H. provided ODC-OVA mice. B.B. helped with designing the study and writing the manuscript. N.G. designed and supervised the study and wrote the manuscript.
Supplemental material for this article can be found on http://ajp.amjpathol.org.
References
- Steinman L. Multiple sclerosis: a two-stage disease. Nat Immunol. 2001;2:762–764. doi: 10.1038/ni0901-762. [DOI] [PubMed] [Google Scholar]
- Frohman EM, Racke MK, Raine CS. Multiple sclerosis—the plaque and its pathogenesis. N Engl J Med. 2006;354:942–955. doi: 10.1056/NEJMra052130. [DOI] [PubMed] [Google Scholar]
- Friese MA, Fugger L. Autoreactive CD8+ T cells in multiple sclerosis: a new target for therapy? Brain. 2005;128:1747–1763. doi: 10.1093/brain/awh578. [DOI] [PubMed] [Google Scholar]
- Babbe H, Roers A, Waisman A, Lassmann H, Goebels N, Hohlfeld R, Friese M, Schroder R, Deckert M, Schmidt S, Ravid R, Rajewsky K. Clonal expansions of CD8+ T cells dominate the T cell infiltrate in active multiple sclerosis lesions as shown by micromanipulation and single cell polymerase chain reaction. J Exp Med. 2000;192:393–404. doi: 10.1084/jem.192.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann H, Medana IM, Bauer J, Lassmann H. Cytotoxic T lymphocytes in autoimmune and degenerative CNS diseases. Trends Neurosci. 2002;25:313–319. doi: 10.1016/s0166-2236(02)02154-9. [DOI] [PubMed] [Google Scholar]
- Crawford MP, Yan SX, Ortega SB, Mehta RS, Hewitt RE, Price DA, Stastny P, Douek DC, Koup RA, Racke MK, Karandikar NJ. High prevalence of autoreactive, neuroantigen-specific CD8+ T cells in multiple sclerosis revealed by novel flow cytometric assay. Blood. 2004;103:4222–4231. doi: 10.1182/blood-2003-11-4025. [DOI] [PubMed] [Google Scholar]
- Skulina C, Schmidt S, Dornmair K, Babbe H, Roers A, Rajewsky K, Wekerle H, Hohlfeld R, Goebels N. Multiple sclerosis: brain-infiltrating CD8+ T cells persist as clonal expansions in the cerebrospinal fluid and blood. Proc Natl Acad Sci USA. 2004;101:2428–2433. doi: 10.1073/pnas.0308689100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurewicz A, Biddison WE, Antel JP. MHC class I-restricted lysis of human oligodendrocytes by myelin basic protein peptide-specific CD8 T lymphocytes. J Immunol. 1998;160:3056–3059. [PubMed] [Google Scholar]
- Medana I, Martinic MA, Wekerle H, Neumann H. Transection of major histocompatibility complex class I-induced neurites by cytotoxic T lymphocytes. Am J Pathol. 2001;159:809–815. doi: 10.1016/S0002-9440(10)61755-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huseby ES, Liggitt D, Brabb T, Schnabel B, Ohlen C, Goverman J. A pathogenic role for myelin-specific CD8+ T cells in a model for multiple sclerosis. J Exp Med. 2001;194:669–676. doi: 10.1084/jem.194.5.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D, Whitaker JN, Huang Z, Liu D, Coleclough C, Wekerle H, Raine CS. Myelin antigen-specific CD8+ T cells are encephalitogenic and produce severe disease in C57BL/6 mice. J Immunol. 2001;166:7579–7587. doi: 10.4049/jimmunol.166.12.7579. [DOI] [PubMed] [Google Scholar]
- Na SY, Cao Y, Toben C, Nitschke L, Stadelmann C, Gold R, Schimpl A, Hunig T. Naive CD8 T cells initiate spontaneous autoimmunity to a sequestered model antigen of the central nervous system. Brain. 2008;131:2353–2365. doi: 10.1093/brain/awn148. [DOI] [PubMed] [Google Scholar]
- Saxena A, Bauer J, Scheikl T, Zappulla J, Audebert M, Desbois S, Waisman A, Lassmann H, Liblau RS, Mars LT. Cutting edge: multiple sclerosis-like lesions induced by effector CD8 T cells recognizing a sequestered antigen on oligodendrocytes. J Immunol. 2008;181:1617–1621. doi: 10.4049/jimmunol.181.3.1617. [DOI] [PubMed] [Google Scholar]
- Spassky N, Goujet-Zalc C, Parmantier E, Olivier C, Martinez S, Ivanova A, Ikenaka K, Macklin W, Cerruti I, Zalc B, Thomas JL. Multiple restricted origin of oligodendrocytes. J Neurosci. 1998;18:8331–8343. doi: 10.1523/JNEUROSCI.18-20-08331.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Toben C, Na SY, Stark K, Nitschke L, Peterson A, Gold R, Schimpl A, Hunig T. Induction of experimental autoimmune encephalomyelitis in transgenic mice expressing ovalbumin in oligodendrocytes. Eur J Immunol. 2006;36:207–215. doi: 10.1002/eji.200535211. [DOI] [PubMed] [Google Scholar]
- Gahwiler BH, Capogna M, Debanne D, McKinney RA, Thompson SM. Organotypic slice cultures: a technique has come of age. Trends Neurosci. 1997;20:471–477. doi: 10.1016/s0166-2236(97)01122-3. [DOI] [PubMed] [Google Scholar]
- Stoppini L, Buchs PA, Muller D. A simple method for organotypic cultures of nervous tissue. J Neurosci Methods. 1991;37:173–182. doi: 10.1016/0165-0270(91)90128-m. [DOI] [PubMed] [Google Scholar]
- Gutcher I, Urich E, Wolter K, Prinz M, Becher B. Interleukin 18-independent engagement of interleukin 18 receptor-α is required for autoimmune inflammation. Nat Immunol. 2006;7:946–953. doi: 10.1038/ni1377. [DOI] [PubMed] [Google Scholar]
- Harrer MD, von Budingen HC, Stoppini L, Alliod C, Pouly S, Fischer K, Goebels N. Live imaging of remyelination after antibody-mediated demyelination in an ex vivo model for immune mediated CNS damage. Exp Neurol. 2009;216:431–438. doi: 10.1016/j.expneurol.2008.12.027. [DOI] [PubMed] [Google Scholar]
- Garcia KC, Teyton L, Wilson IA. Structural basis of T cell recognition. Annu Rev Immunol. 1999;17:369–397. doi: 10.1146/annurev.immunol.17.1.369. [DOI] [PubMed] [Google Scholar]
- Kawakami N, Nagerl UV, Odoardi F, Bonhoeffer T, Wekerle H, Flugel A. Live imaging of effector cell trafficking and autoantigen recognition within the unfolding autoimmune encephalomyelitis lesion. J Exp Med. 2005;201:1805–1814. doi: 10.1084/jem.20050011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balabanov R, Strand K, Goswami R, McMahon E, Begolka W, Miller SD, Popko B. Interferon-γ-oligodendrocyte interactions in the regulation of experimental autoimmune encephalomyelitis. J Neurosci. 2007;27:2013–2024. doi: 10.1523/JNEUROSCI.4689-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renno T, Taupin V, Bourbonniere L, Verge G, Tran E, De Simone R, Krakowski M, Rodriguez M, Peterson A, Owens T. Interferon-γ in progression to chronic demyelination and neurological deficit following acute EAE. Mol Cell Neurosci. 1998;12:376–389. doi: 10.1006/mcne.1998.0725. [DOI] [PubMed] [Google Scholar]
- Faroudi M, Utzny C, Salio M, Cerundolo V, Guiraud M, Muller S, Valitutti S. Lytic versus stimulatory synapse in cytotoxic T lymphocyte/target cell interaction: manifestation of a dual activation threshold. Proc Natl Acad Sci USA. 2003;100:14145–14150. doi: 10.1073/pnas.2334336100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becher B, Bechmann I, Greter M. Antigen presentation in autoimmunity and CNS inflammation: how T lymphocytes recognize the brain. J Mol Med. 2006;84:532–543. doi: 10.1007/s00109-006-0065-1. [DOI] [PubMed] [Google Scholar]
- Nitsch R, Pohl EE, Smorodchenko A, Infante-Duarte C, Aktas O, Zipp F. Direct impact of T cells on neurons revealed by two-photon microscopy in living brain tissue. J Neurosci. 2004;24:2458–2464. doi: 10.1523/JNEUROSCI.4703-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergelli M, Le H, van Noort JM, Dhib-Jalbut S, McFarland H, Martin R. A novel population of CD4+CD56+ myelin-reactive T cells lyses target cells expressing CD56/neural cell adhesion molecule. J Immunol. 1996;157:679–688. [PubMed] [Google Scholar]
- Grimm EA, Mazumder A, Zhang HZ, Rosenberg SA. Lymphokine-activated killer cell phenomenon: lysis of natural killer-resistant fresh solid tumor cells by interleukin 2-activated autologous human peripheral blood lymphocytes. J Exp Med. 1982;155:1823–1841. doi: 10.1084/jem.155.6.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.