Abstract
Prostatitis causes substantial morbidity to men, through associated urinary symptoms, sexual dysfunction, and pelvic pain; however, 90% to 95% of cases have an unknown etiology. Inflammation is associated with the development of carcinoma, and, therefore, it is imperative to identify and study the causes of prostatitis to improve our understanding of this disease and its role in prostate cancer. As estrogens cause prostatic inflammation, here we characterize the murine prostatic phenotype induced by elevated endogenous estrogens due to aromatase overexpression (AROM+). Early-life development of the AROM+ prostate was normal; however, progressive changes culminated in chronic inflammation and pre-malignancy. The AROM+ prostate was smaller at puberty compared with wild-type controls. Mast cell numbers were significantly increased at puberty and preceded chronic inflammation, which emerged by 40 weeks of age and was characterized by increased mast cell, macrophage, neutrophil, and T-lymphocyte numbers. The expression of key inflammatory mediators was also significantly altered, and premalignant prostatic intraepithelial neoplasia lesions emerged by 52 weeks of age. Taken together, these data link estrogens to prostatitis and premalignancy in the prostate, further implicating a role for estrogen in prostate cancer. These data also establish the AROM+ mouse as a novel, non-bacterial model for the study of prostatitis.
Prostatitis, an exceedingly common condition in the male population worldwide, has an incidence of ∼9% and a prevalence of ∼14%, and, unlike benign prostatic hyperplasia and prostate cancer (PCa), which are predominantly diseases of older men, prostatitis afflicts men of all ages. It is also the most common outpatient condition seen in men under 50 years of age and accounts for more clinical visits than either PCa and/or benign prostatic hyperplasia.1,2 At present our knowledge of prostatitis is lacking, with 90% to 95% of cases having an unknown etiology. This represents a significant problem, particularly as prostatitis has also been implicated in the development of PCa.3,4,5 Given the prevalence of prostatitis and this putative link to PCa, it is vitally important to identify the unknown causes of prostatitis.
Several causes of prostatitis have been postulated, including hormonal variation or exposure, infection due to sexually transmitted disease,6 dietary factors, and physical trauma from urine reflux.7 However, of particular interest is an apparent link between estrogen and prostatic inflammation,8 which has emerged mainly from the administration of pharmacological levels of exogenous estrogen to rodents.9 Additional data obtained from further rodent studies show that the prostate gland is particularly sensitive to estrogenic exposure during development in fetal and neonatal life; transient estrogen exposure before puberty results in inflammation later in life, well after the exposure has occurred.10,11 Several decades of research from various laboratories, including our own, has demonstrated that this action is mediated by the estrogen receptor (ER) α subtype and involves a cascade of events that permanently and irreversibly alter gene expression patterns in the gland.12 These data indicate that exposure to estrogen causes prostatic inflammation and directly links estrogen and prostatitis.
Studies have shown that chronic inflammation predisposes individuals to various types of cancer; indeed, underlying infection and inflammatory responses have been linked to 15% to 20% of all human cancers.5,13,14 This is also believed to be true for the prostate, and there is an emerging and growing body of evidence implicating inflammation in the etiology of PCa similar to other organs such as the liver and stomach.3,4,5 Epidemiological evidence also indicates that men with a history of prostatitis have an increased risk for PCa.15 Additionally, lesions characterized by proliferating epithelial cells and activated inflammatory cells (proliferative inflammatory atrophy) are frequently observed in juxtaposition to premalignant prostatic lesions (prostatic intraepithelial neoplasia; PIN) and PCa.3
To study the effects of estrogen on the prostate, previous studies have typically relied on the addition of exogenous estrogens at either low or pharmacological doses. This, however, introduces a number of complicating factors. Low dose effects can be difficult to discern, while pharmacological doses may not mimic normal physiological responses. In addition, this methodology also precludes the ability to examine the effects of estrogen and the progression of disease throughout development and adult life. Consequently, the development of new experimental animal models of prostatitis is essential to determine whether inflammation is linked to development of PCa. This need was highlighted and stressed in the report from the Bar Harbor Consensus meeting.16 Although two models of prostatitis have recently been developed, they are of limited utility: one is bacterial17 while the other is rat-based and requires the administration of exogenous hormones.18 As a result, both of these models preclude the ability to cross-breed with other types of genetically manipulated mice to delineate and study specific mechanisms. Consequently the imperative remains to develop new mouse models for the study of prostatitis.
The aromatase overexpressing (AROM+) transgenic mouse provides a novel model to examine the effect of altered aromatase activity, and therefore estrogen levels and action, in the prostate within physiological hormonal environment. Estrogen levels in these mice are significantly elevated and are associated with a concomitant decrease in androgens. It has been previously reported that the prostates of these mice are rudimentary due to the suppression of androgens.19
In this study, we show for the first time that the AROM+ mouse develops chronic prostatitis by 40 weeks of age. This inflammation is characterized by an elevation in mast cell numbers from puberty and ultimately leads to chronic inflammation and the development of PIN lesions. This demonstrates a continuum, with estrogen-inducing inflammation, which subsequently results in the onset of pre-malignancy.
Materials and Methods
Mice
AROM+ mice exhibit ubiquitous human aromatase expression, driven by the human ubiquitin C promoter.19 These mice are of FVB/N genetic background and were maintained under controlled conditions (lights on 0700 to 1900; temperature 20 °C to 24 °C), with access to mouse feed (Glen Forrest Stockfeeders, Glen Forrest, Australia) and water ad libitium.
Litters of AROM+ mice were generated by breeding transgenic females with wild-type FVB/N males to produce AROM+ and wild-type offspring. Anterior, ventral, dorsal and lateral prostate lobes (AP, VP, DL, and LP, respectively) and seminal vesicles (SV) were collected from AROM+ and wild-type animals for analysis at day 0 (d0; postnatal) and day 3 (d3; postnatal), then weekly from 3 to 52 weeks of age. For consistency and unless otherwise noted, all data presented were generated using VP tissues.
SCID mice were obtained from Central Animal Services, Monash University (Clayton, Australia) and housed as detailed for the AROM+ animals.
All animal studies were conducted in accordance with the National Health and Medical Research Council and Monash University animal ethics regulations and guidelines.
Quantitation of Branching Development
Analysis of branching morphogenesis in neonatal prostate tissues was conducted using combined confocal microscopy and computerized image analysis as previously described.20 Briefly, VP or AP were dissected from the urogenital tracts of neonatal day 0 and day 3 wild-type and AROM+ mice and prepared for wholemount immunostaining of epithelial cell cytokeratins. Six parameters of branching were recorded and analyzed; specifically, prostate ductal length, volume, number of main ducts, branches, branch points, and tips.
Immunohistochemistry
Immunohistochemistry was performed using a DAKO autostainer (DAKO, Carpentaria, CA) as previously described.21,22 Briefly, following fixation the prostate tissues or recombinants were processed and embedded in paraffin wax and serially sectioned at 5 μm for histological and/or stereological analysis. After dewaxing, the sections were rehydrated antigen retrieval (0.01 M/L citrate buffer at pH 6.0 or 0.05 M/L Tris/0.01% EDTA at pH 9.0) was performed. Peroxidase block (Dako) was used to inactivate endogenous peroxidase activity and nonspecific binding was blocked using CAS block (Zymed, San Francisco, CA) followed by incubation with primary antibody for 60 to 120 minutes at room temperature. Primary antibody binding was detected using a biotinylated secondary antibody (Zymed) followed by incubation with an avidin-biotin peroxidase kit (ABC Elite; Vector Laboratories, Burlingame, CA). Antibody localization was ultimately visualized using the chromogen diaminobenzidine tetrachloride (Dako). Finally, sections were counterstained with Mayer’s hematoxylin, gradually dehydrated with alcohol, cleared with Histolene, and covered with dansylated polymyxin B mounting solution and a coverslip.
The localization and expression of the androgen receptor (AR), ERα, ERβ, smooth muscle α-actin, proliferating cell nuclear antigen and high molecular weight cytokeratin (CKH) were determined using specific antibodies for AR (N-20, Santa Cruz Biotechnology, CA), ERα (Dako, a-actin, Sigma Aldrich, St. Louis, MO), proliferating cell nuclear antigen (Dako), CKH (Dako), and ERβ (Novocastra Laboratories Ltd., Newcastle, UK).
The presence and localization of specific leukocytes within the tissues was also examined using immunohistochemistry. The localization of neutrophils, T lymphocytes, B lymphocytes, and macrophages was determined using antibodies specific for Ly6g (BD Pharmingen, San Diego, CA), CD3 (Santa Cruz Biotechnology, CA), CD45R (BD Pharmingen), and F480 (Abcam, Cambridge, UK), respectively.
Mast Cell Analysis
Mast cells contain granules composed of heparin, sulfated glycosaminoglycan and histamine. Consequently, acidified toluidine blue will stain these cells metachromatically, making them readily distinguishable from the surrounding tissue; the mast cells will stain a red-purple (metachromatic staining) and the surrounding tissue will be blue (orthochromatic staining).
Briefly, 5-μm paraffin-embedded sections were deparaffinized and re-hydrated. The sections were then stained in a solution of 0.1% Toluidine Blue O (Sigma Aldrich), 7% alcohol, and 1% NaCl at pH 2.3 for 3 minutes. The sections were then washed in distilled water, rapidly dehydrated in 95% and 100% alcohol washes, mounted in dansylated polymyxin B, and coverslipped for analysis.
Stereology
All assessments were performed using a Bioprecision2 Microscope Stage (Ludl, NY), 99A400 Focus drive (Ludl), MAC5000 Controller (Ludl), and ND-281 Encoder (Heidenhain, IL) coupled to an Olympus BX-51 microscope (Olympus, Tokyo, Japan). The images were captured using a PixeLink PL-623C digital camera (PixeLink, Ottawa, ON, Canada) coupled to a computer. The newCAST component (version 2.14; Visiopharm, Hørsholm, Denmark) of the Visiopharm Integrator System (version 2.16.1.0; Visiopharm) was used to generate a set of counting frames and a point grid (grid properties were assessed individually for each marker).
Uniform systematic random sampling was then used to quantitate the number of positive cells on toluidine blue or immunohistochemical stained sections, as previously described.21,22,23 In brief, the sections were examined under ×40 magnification; they were mapped to define tissue boundaries and were sampled at predetermined intervals along the x- and y- axes using a single point grid-counting frame. Positive cells were then scored and totaled for each respective gland, the final number being expressed as the number of positive cells per unit area.
Serum Hormones
Serum levels of testosterone and estradiol were determined using the DSL-4000–coated tube radioimmunoassay and the DSL-4800 ultra-sensitive double antibody radioimmunoassay, respectively, as per the manufacturer’s instructions (Diagnostic System Laboratories Inc., Webster, TX). Values for the samples were derived via interpolation using standards provided with the kit.
Quantitative Real-Time PCR Array Analysis
The expression of 84 inflammatory cytokines and chemokines was examined using the Mouse Inflammatory Cytokines and Receptors RT2 Profiler PCR Array (SuperArray Bioscience Corporation, Frederick, MD). Quantitative real-time PCR array was performed on an Applied Biosystems 7900HT Fast Real-Time PCR System (Applied Biosystems, Forster City, CA).
VPs were dissected from AROM+ and wild-type mice at 40 weeks of age when chronic inflammation was evident. Total RNA was first extracted using TRIzol reagent, per the manufacturer’s instructions (Invitrogen, Carlsbad, CA), then further cleaned using the RT2 qPCR-Grade RNA isolation kit (SuperArray). RNA integrity was checked using Experion RNA StdSens Analysis kits and the Experion Automated Electrophoresis System (Biorad, Hercules, CA).
cDNA was synthesized from the purified RNA using the RT2 First Strand Kit (SuperArray) and samples were initially run on a RT2 RNA QC PCR Array (SuperArray) to check housekeeping gene expression levels, RT efficiency and for genomic DNA carry-over.
PCR array analysis was performed according to the manufacturer protocol with the RT2 Real-Time SYBR Green PCR Master Mix (SuperArray). mRNA expression for each gene was normalized using the expression of HPRT as a control housekeeping gene and compared with the data obtained with RNA from the control wild-type samples according the 2−ΔΔCT method.24 Results were considered significant when relative mRNA expression was at least twofold higher or lower than that of the wild-type control mice and the statistical P value was <0.05.
The results were also subsequently confirmed by quantitative real-time PCR on individual RNA samples using the QuantiTect SYBR Green PCR Kit (Qiagen Inc., Mississauga, Canada).
PCR Analysis and Relative Quantitation of Aromatase Expression
To examine aromatase expression in the wild-type mouse VP compared with the AROM+ VP, relative quantitative PCR was performed as previously described.25 Briefly, RNA was extracted from the VPs of wild-type and AROM+ mice using TRIzol (Invitrogen), DNase treated and cDNA generated. Real time PCR was subsequently performed using the Roche LightCycler per manufacturer’s instructions (Roche Diagnostics, Basel, Switzerland). cDNA was normalized to 18S expression and relative aromatase levels in the different tissues were subsequently determined. In total, samples from 5 AROM+ and four wild-type animals were examined, with all samples run in triplicate.
Tissue Recombination
Tissue recombinants were prepared as previously described.26,27,28,29 SVs were removed from day 0 wild-type or AROM+ mice and digested with 1% trypsin (Life Technologies, Gaithersburg, MD) in calcium- and magnesium-free Hanks’ balanced salt solution for 60 minutes at 4°C. Seminal vesicle mesenchyme was then separated from epithelium using a Graefe knife and fine forceps. APs were removed from adult male wild-type or AROM+ mice, and ductal tips of ∼1 mm in length were excised.
The tissue recombinants were constructed in vitro with wild-type or AROM+ epithelia (-E) and stroma (-S); specifically wild-type-E/wild-type-S or AROM+-E/AROM+-S. The assembled recombinant tissues were cultured in a humidified incubator at 37°C with 5% CO2 for 36 hours before being grafted under the renal capsules of intact male immune-deficient SCID mice. Grafts were harvested after 4 weeks of growth in vivo and fixed in Bouins for analysis. A minimum of five grafts were prepared for each group.
Statistics
Data were analyzed to determine normality and significant differences were determined by t-test with a significance threshold used at a level of 5% (P < 0.05). Analyses were conducted using Prism 5.00 software (GraphPad Software Inc., San Diego, CA). Data are expressed as mean ± SEM, unless otherwise noted.
Results
The AROM+ Mouse Develops Multiple Prostate and Genitourinary Pathologies, Including Chronic Inflammation, PIN, and Scrotal Herniation
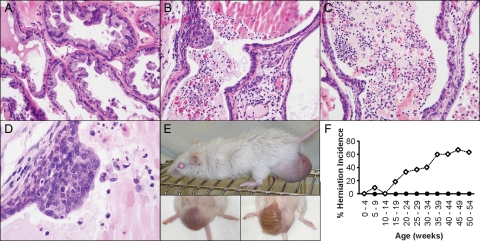
Analysis and characterization of the urological phenotype in the AROM+ mouse revealed that these animals develop a number of prostate and genitourinary pathologies. Particularly apparent was the development of chronic inflammation in the ventral prostates (VP’s) of 40-week-old AROM+ mice, which was characterized by a number of distinct lesions with varied cellular infiltrate. This included mononuclear lymphocyte-like cells and granulocytes throughout the stroma, as well as extensive infiltrate within the lumen, comprised of macrophages, plasma cells, neutrophils, and some cellular debris (Figure 1, B and C). No evidence of inflammation was apparent in the prostates of age-match wild-type littermate controls (Figure 1A). Following the emergence of the inflammation, the AROM+ mice were also found to develop PIN lesions that were reminiscent of PIN in the human (Figure 1D).
Figure 1.
The AROM+ prostate develops inflammation, PIN, and other genitourinary pathologies upon aging. On aging, the AROM+ mouse develops a number of prostate and genitourinary pathologies including the development of inflammation, PIN lesions, and scrotal hernias. Chronic inflammation developed by 40 weeks of age and was characterized by an abundance of mononuclear lymphocyte-like cells with a few granulocytes within the stroma (B; H&E stained section) along with a more mixed population of cells within the lumen, including macrophages, plasma cells, neutrophils and some cellular debris (B and C; H&E stained sections). No evidence of inflammation was seen in age-matched wild-type controls (A; H&E stained section). Further aging resulted in the development of ductal pre-malignant lesions identifiable as PIN (D). The AROM+ mice also developed scrotal hernias (E), with increasing incidence and severity with age (F; n ≥10 per genotype per age; open diamond = AROM+; closed circle = wild-type).
The AROM+ mice also developed scrotal hernias, leading to protrusions of abdominal fat and intestine into the scrotum (Figure 1E). This pathology increased in incidence and severity with age, affecting in excess of 60% of AROM+ males by 35 weeks of age (Figure 1F). In severe cases, the majority of the intestinal tract was found to have protruded through into the scrotum as a result. Although severe hernias such as these may ultimately cause inflammation due to drainage defects of blood vessels and possible bacterial infections, this is not the cause of the inflammation in the AROM+ mice: the chronic inflammation observed is seen in animals that develop hernias, as well as in those that do not.
Aromatase Overexpression Increases Serum Estradiol, Decreases Serum Testosterone and Reduces the Serum Testosterone/Estrogen Ratio
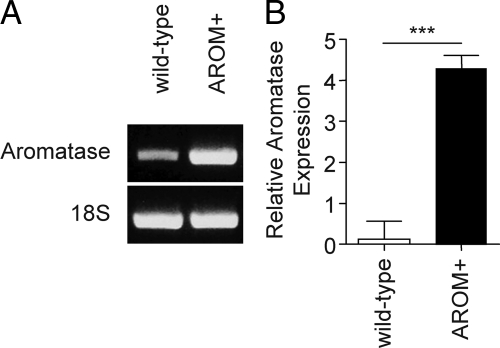
The AROM+ mouse has ubiquitous, constitutively activated expression of aromatase,19 including significant overexpression of aromatase within the prostate itself (Figure 2, A and B). Consequently, these animals should exhibit elevated levels of systemic estrogens with a concomitant decrease in androgen levels. Measurement of serum hormone concentrations in the AROM+ mice confirmed this, and, when compared with the wild-type littermate controls, showed that these mice have significantly decreased levels of serum testosterone at all ages, except in the 45- to 59-week age group (Table 1), and significantly increased levels of serum estrogens at all ages (Table 2).
Figure 2.
Relative aromatase expression in the mouse and AROM+ prostate. Expression of aromatase in the wild-type and AROM+ VP’s was determined and compared using relative quantitative PCR. Aromatase expression was detected in both the wild-type and AROM+ prostates (A; representative images), although the expression in the wild-type prostate was low, variable, and bordered on the threshold of detectability. In contrast, the expression of aromatase in the AROM+ prostate was readily detectable and significantly elevated compared with wild-type controls (B; n = 4 [wild-type] and n = 5 [AROM+]; ***P <0.001).
Table 1.
Reduced Serum Testosterone Levels in AROM+ Mice
| Age (weeks) | Testosterone (ng/ml)
|
Significance | |
|---|---|---|---|
| Wild-type | AROM+ | ||
| 5 | 8.54 ± 5.85 | 0.10 ± 0.06 | P = 0.0141 |
| 15 | 7.44 ± 9.48 | 0.66 ± 0.29 | P = 0.0216 |
| 26–36 | 7.50 ± 10.78 | 0.94 ± 0.69 | P = 0.0214 |
| 45–59 | 5.84 ± 8.18 | 3.07 ± 4.54 | NS |
Circulating serum testosterone levels in AROM+ mice were determined by RIA and were significantly reduced in AROM+ mice versus wild-type controls across all age groups, except at 45 to 59 weeks of age (n ≥10 for each age group).
Table 2.
Serum Estradiol Levels Are Increased in AROM+ Mice
| Age (weeks) | Estradiol (pg/ml)
|
Significance | |
|---|---|---|---|
| Wild-type | AROM+ | ||
| 5 | 15.61 ± 8.82 | 49.86 ± 18.5 | P < 0.0443 |
| 15 | 26.44 ± 12.26 | 143.9 ± 82.67 | P < 0.0003 |
| 26–36 | 42.61 ± 20.67 | 165.3 ± 92.68 | P < 0.0002 |
| 45–59 | 38.08 ± 10.91 | 302.6 ± 301.5 | P < 0.0089 |
Circulating serum estradiol levels in AROM+ mice were determined by RIA. Estradiol levels were found to be significantly increased in AROM+ mice when compared with wild-type controls across all age groups (n ≥10 for each age group).
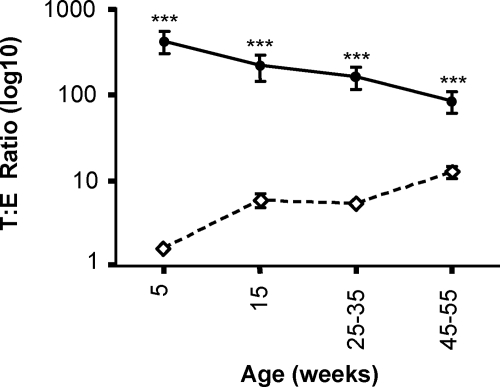
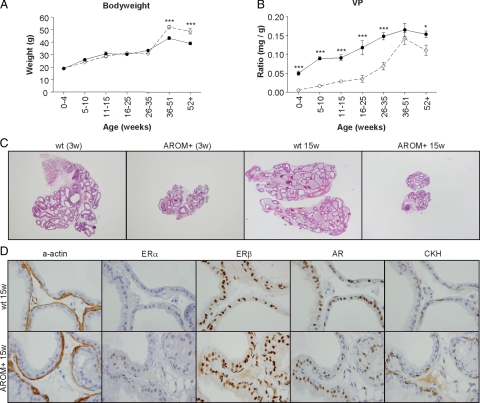
Closer analysis of the hormone levels in individual animals was made by expressing the absolute hormone levels as a ratio of serum testosterone to estradiol ratio. This analysis revealed a characteristic decline in the testosterone to estradiol ratio in wild-type mice that occurs with aging as testosterone levels fall. The ratio in the AROM+ animals did not decline, but, rather, increased slightly with aging. Despite this, the ratio in AROM+ animals was significantly reduced when compared with wild-type in all of the age groups examined (Figure 3).
Figure 3.
The serum testosterone to estradiol ratio is reduced in AROM+ mice. Temporal analysis of serum androgens and estrogens in the AROM+ mouse were determined by radioimmunoassay. When examined in detail, the serum testosterone/estradiol ratio was found to be significantly smaller in AROM+ animals versus wild-type at all ages. (open diamond = AROM+; close circle = wild-type; ***P <0.001; n ≥10 for each group).
Postnatal and Early Life Development of the AROM+ Prostate Is Normal
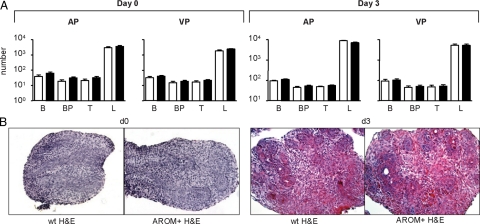
The development of the AROM+ prostate was examined to determine the etiology and causes of the prostate pathologies observed. The postnatal development of the AROM+ prostate was examined the computational analysis of branching morphogenesis, as previously described.20 This analysis revealed that the postnatal development of the AROM+ prostate was completely normal.
Specifically, there was no significant difference in any of the parameters examined (branches B, branch points BP, tips T, or ductal length L) between the wild-type and AROM+ prostates (both AP and VP) at day 0 and day 3 (Figure 4A). Similarly, the size and pathology of the AROM+ prostate at day 0 and day 3 were no different when compared with wild-type (Figure 4B).
Figure 4.
Postnatal development of the AROM+ prostate is normal. The postnatal and early-life development of the AROM+ prostate was normal. Computational analysis of branching morphogenesis in the AROM+ prostate demonstrated no significant difference between wild-type (open bars) and AROM+ (solid bars) in either AP or VP at day 0 or day 3 in any of the parameters examined (branches B, branch points BP, tips T, or ductal length L) (A; n = 5 per genotype per age). Similarly, the size and pathology of the AROM+ prostate at day 0 and day 3 were no different when compared with wild-type (B; H&E stained sections).
Divergence from Wild-Type and Emerging Pathology in the Pubertal and Adult AROM+ Prostate
Analysis of the pathology of the AROM+ prostate following puberty and into adulthood, however, revealed a divergence from wild-type and the development of distinct pathologies. When compared with wild-type, the bodyweights of the AROM+ mice were no different up until 35 to 51 weeks of age, when they became significantly increased due to an increase in the amount of abdominal fat deposition (Figure 5A). VP size, however, was significantly reduced in the AROM+ mice compared with the wild-type littermate controls, up until 35 to 51 weeks of age when the AROM+ VP size began to increase (Figure 5B). This was true of all lobes of the prostate (VP, AP, DL, and LP) as well as the seminal vesicles (Data not shown). Although the size of the AROM+ VP size was reduced, there was no still no evidence of any changes to the pathology of the tissues when compared with wild-type tissues (Figure 5C).
Figure 5.
Divergence of and emerging pathology in pubertal and adult AROM+ prostates. With puberty and further aging into adulthood the AROM+ prostate pathology begins to diverge from that of wild-type. Bodyweights of AROM+ mice were no different to wild-type until 40 weeks of age when they were significantly elevated due to increased body fat content (A; n ≥20 per group). AROM+ VP weights (as a ratio of bodyweight) were consistently smaller than their wild-type counterparts throughout life (B; open diamond = AROM+; closed circle = wild-type; n ≥20 per group; *P <0.05, ***P <0.001). Despite being significantly smaller than their age matched wild-type counterparts, the overall pathology of the AROM+ remained normal (C; H&E stained sections). Closer examination of the AROM+ tissues revealed differences to the wild-type tissue, in particular increased ERα expression, but no difference in AR, CKH, ERβ, and α-actin expression (D).
Consistent with the elevated levels of serum estrogens in the AROM+ animals, closer immunohistochemical analysis of AROM+ VPs revealed increased expression of ERα throughout the epithelial cells (Figure 5D), which was subsequently confirmed by stereology (Table 3). AR, ERβ, CKH and smooth muscle α-actin expression was unaltered, as demonstrated by both immunohistochemistry (Figure 5D) and stereology (data not shown).
Table 3.
Local Aromatase Overexpression Increases ERα Expression Independent of Systemic Hormones
| Tissue | Genotype | % Epithelial ERα−positive | Significance |
|---|---|---|---|
| VP | Wild-type | 0.8 ± 0.5 | |
| AROM+ | 14.1 ± 7.1 | P = 0.0463 | |
| Recombinants | wild-type-S/wild-type-E | 0.6 ± 0.3 | |
| AROM+-S/AROM+-E | 9.9 ± 5.4 | P = 0.0289 |
ERα expression in intact wild-type and AROM+ VP tissue, as well as in homotypic recombinant tissue, was quantitated using stereology. ERα expression was significantly increased in intact AROM+ tissues when compared with wild-type controls. Homotypic AROM ± S/AROM ± E tissue recombinants also demonstrated a significant increase in ERα expression when compared with wild-type-S/wild-type-E controls. Tissue recombinants were grown in the same host and, therefore, exposed to the same systemic hormonal milieu.
Importance of Local Intraprostatic Estrogen Metabolism
To assess whether the changes to the pathology of the AROM+ prostate resulted from local, intraprostatic increased estrogen metabolism or were a consequence of the altered systemic hormone levels, we used tissue recombination as previously described.26,30
Stromal and epithelial prostate tissues were derived from neonatal and adult tissues, respectively, and were taken from both wild-type and AROM+ mice. These tissues were recombined in vitro to form homotypic tissue recombinants (ie, wild-type-S/wild-type-E, AROM+-S/AROM+-E; Figure 6) and were then cultured in vivo under the kidney capsule of a host SCID mouse. As the recombinants are grown in the same host and in the same normal systemic hormone milieu any changes to pathology must be due to perturbation of local hormone levels and metabolism.
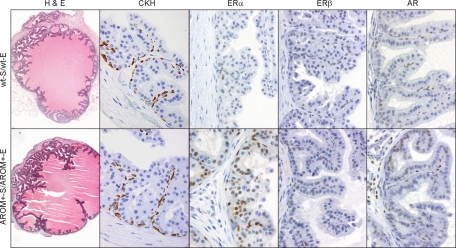
Figure 6.
Importance of local intra-prostatic estrogen metabolism. Tissue recombinants were prepared using seminal vesicle mesenchyme and epithelial tips from adult AROM+ AP. Following grafting into a normal male SCID mouse for 4 weeks, all grafts exhibited the same pathology as tissues from an adult intact AROM+ animal. ERα expression in AROM+-S/AROM+-E grafts was significantly elevated while no difference in AR, CKH, or ERβ expression was apparent (n ≥5 for each graft type).
Both wild-type and AROM+ recombinants gave rise to normal prostate tissue exhibiting a normal morphology (Figure 6). However, closer immunohistochemical analysis again revealed changes to, and increased expression of, ERα within the epithelial cells (Figure 6), which was subsequently confirmed by stereological quantitation (Table 3). These data are consistent with the effects of increased estrogen levels and mirror what is seen in the intact AROM+ mice (Figure 5). Although there was no inflammation observed in any of the grafts, this is due to the immune compromised nature of the host animals rather than being indicative of an essential systemic influence.
Ultimately, these data demonstrate that the difference in ERα expression that is observed must be due to increased local estrogen metabolism.
With Aging the AROM+ Mouse Develops Chronic Inflammation of the Prostate
Chronic inflammation was found to emerge in the VP’s of the AROM+ mice with aging and was characterized by a number of pathological patterns, including mononuclear lymphocyte-like cells and granulocytes throughout the stroma, as well as extensive infiltration and collection of macrophages, plasma cells, neutrophils, and some cellular debris within the lumen itself (Figure 1, B and C). This inflammation increased in incidence and severity with age (Table 4).
Table 4.
Incidence of Inflammation and PIN in AROM+ Mice
| Pathology | Genotype | 15w | 30w | 40w | 52w |
|---|---|---|---|---|---|
| Inflammation | wild-type | — | — | — | — |
| AROM+ | — | 21% | 67% | 88% | |
| PIN | wild-type | — | — | — | — |
| AROM+ | — | — | — | 33% |
The presence of inflammation and PIN lesions in the AROM+ and wild-type mice at different ages was scored following histological examination. Inflammatory lesions were first found to occur in AROM+ mice at 30 weeks of age, with increasing incidence concomitant with increasing age. PIN lesions were found to develop in AROM+ animals at 52 weeks of age and were observed in tissues that also demonstrated inflammation. No inflammation or PIN was noted in wild-type mice at any age (n ≥20 per genotype per age).
The specific nature of this inflammation and the infiltrating leukocytes was examined and quantitated using a combination of immunohistochemistry and stereology. Specifically, tissue sections were stained using specific stains for a mast cells (toluidine blue), neutrophils (ly6g/Gr1), T lymphocytes (CD3), B lymphocytes (B220/CD45R), or macrophages (F4-80) and then quantitated using systematic random sampling throughout the tissue (Figure 7, A, B, C, D, and E, respectively).
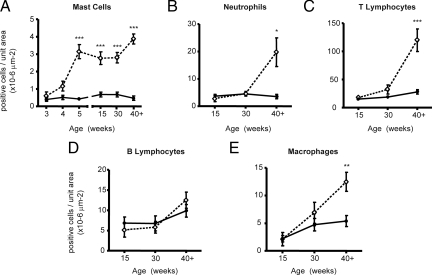
Figure 7.
Quantitation and characterization of the inflammatory infiltrate in the adult AROM+ prostate. Tissue sections were stained using specific stains for a mast cells (toluidine blue), neutrophils (ly6g/Gr1), T lymphocytes (CD3), B lymphocytes (B220/CD45R), or macrophages (F4–80) and then quantitated using systematic random sampling throughout the tissue and stereology. Significant increases in mast cell numbers were apparent immediately following puberty and persisted throughout life (A). Significant increases in neutrophils, T lymphocytes and macrophages from 40 weeks of age (B, C, and E) while B lymphocyte levels remained unaltered (D) (n ≥5 per genotype per age per marker; open diamond = AROM+; closed circle = wild-type; *P <0.05, **P <0.01, ***P <0.001).
This analysis revealed that the numbers of mast cells were significantly increased throughout the tissue immediately following puberty and that this persisted throughout life (Figure 7A). In contrast, the numbers of neutrophils, T-lymphocytes, and macrophages (Figure 7, B, C, and E, respectively), were all significantly increased by 40 weeks of age, while the number of B-lymphocytes were not significantly changed (Figure 7D).
Chronic Inflammation Leads to the Development of PIN-Like Lesions in the AROM+ Prostate
As described in The Consensus Report from the Bar Harbor Meeting of the Mouse Models of Human Cancer Consortium Prostate Pathology Committee,16 PIN is the focal neoplastic proliferation of atypical epithelial cells within pre-existing glands. This proliferation is most commonly recognized by the stratification of epithelial cells that demonstrate nuclear atypia, which can be in the form of nuclear enlargement, nuclear membrane irregularity, hyperchromasia, chromatin clumping, prominent nucleoli, or a combination of these features. These lesions begin focally and can also acquire a tufting, micropapillary, or cribriform growth pattern. Additionally, in the Bar Harbor classification mouse PIN can be further classified into two distinct subclasses: PIN with documented progression to invasive carcinoma, or PIN without documented progression.16
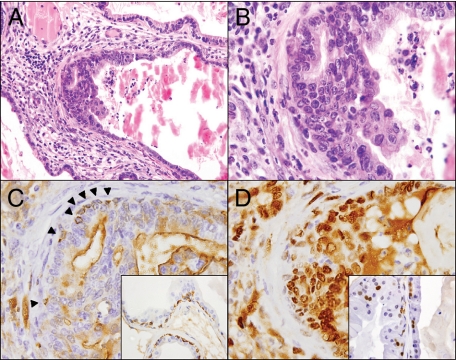
Using these parameters as a reference point, focal lesions classified as PIN (without progression to invasive carcinoma) were observed in the prostates of the AROM+ mice on further aging and following the emergence of the chronic inflammation (Figure 8A; Table 4). The diagnosis of these lesions as PIN was also independently confirmed by a urologic pathologist. These focal lesions were characterized by the stratification of atypical epithelial cells, micropapillary, and/or tufting growth patterns, along with enlarged atypical nuclei and very prominent nucleoli (Figure 1, D; Figure 8, A and B). CKH staining demonstrated the sparse presence of basal cells (Figure 8C, arrowheads indicate specific cells). These lesions also demonstrated significantly increased proliferation within the lesion as well as throughout the surrounding stroma (Figure 8D). This apparent loss of basal cells and increased proliferation was isolated to the PIN lesions and the surrounding tissue; tissue elsewhere within the same sections demonstrated typical proliferating cell nuclear antigen and CKH staining (C and D, insets). There were no differences in the expression of ERα, ERβ, E-Cadherin, or AR in the lesions or surrounding tissues (data not shown).
Figure 8.
Development and characterization of PIN-like lesions in the AROM+ prostate. Following the emergence of chronic inflammation, pre-malignant lesions identified as PIN developed in the AROM+ prostate (A). These lesions were characterized by stratified abnormal epithelial cells, some evidence of tufting, enlarged atypical nuclei, and very prominent nucleoli (B). Note also the inflammation within the lumen proximal to the lesion as well as the inflammation throughout the stroma (A and B). Staining for CKH demonstrated a relative lack of basal cells around the lesion itself (C; arrowheads indicate the few positive cells) while proliferating cell nuclear antigen staining revealed significantly increased proliferation within the lesion as well as the surrounding stroma (D). This loss of basal cells and increased proliferation is particularly evident when compared with the typical CKH and proliferating cell nuclear antigen staining that is seen elsewhere within the same sections (insets).
Chronic Inflammation as a Cause of Prostatic Premalignancy
The development of the PIN-like lesions in the AROM+ prostate occurred following the emergence of chronic inflammation. This is suggestive of the inflammation being a causative factor in the development of these lesions. Supporting this view was the observation of a large amount of inflammatory infiltrate within the lumen and stroma surrounding the PIN-like lesions, as well as within the lesions themselves.
To identify some of the potential mechanisms arising from the inflammation that may be mediating the development of premalignancy we examined the expression of a number of key inflammatory cytokines and chemokines in the AROM+ prostate. This analysis, performed using PCR type arrays and further investigated using qPCR, revealed significant changes to the expression of a number of cytokines, chemokines, and their receptors (Table 5). Of particular significance were significant increases in the expression of CCL20, CCL8, CCR6, CCR5, and CCR2, all of which have been implicated in the development of PCa.31,32,33
Table 5.
The Expression of Inflammatory Cytokines and Chemokines Is Altered in AROM+ Mice
| Gene | Name | Fold change | P value | Fold up- or Down-regulation |
|---|---|---|---|---|
| BCL6 | B-cell lymphoma 6 protein | 0.46 | 0.008094 | −2.18 |
| C3 | Complement component 3 | 4.03 | 0.020398 | 4.03 |
| Casp1 | Caspase 1 | 2.33 | 0.009186 | 2.33 |
| CCL12 | Chemokine (C-C motif) ligand 12 | 11.19 | 0.002889 | 11.19 |
| CCL17 | Chemokine (C-C motif) ligand 17 | 2.98 | 0.026750 | 2.98 |
| CCL19 | Chemokine (C-C motif) ligand 19 | 3.4 | 0.027870 | 3.4 |
| CCL20 | Chemokine (C-C motif) ligand 20 | 25.42 | 0.000015 | 25.42 |
| CCL8 | Chemokine (C-C motif) ligand 8 | 10.05 | 0.003893 | 10.05 |
| CCL9 | Chemokine (C-C motif) ligand 9 | 2.39 | 0.048203 | 2.39 |
| CCR2 | Chemokine (C-C motif) receptor 2 | 3.81 | 0.020781 | 3.81 |
| CCR3 | Chemokine (C-C motif) receptor 3 | 3.08 | 0.029387 | 3.08 |
| CCR4 | Chemokine (C-C motif) receptor 4 | 3.59 | 0.047536 | 3.59 |
| CCR5 | Chemokine (C-C motif) receptor 5 | 2.8 | 0.030673 | 2.8 |
| CCR6 | Chemokine (C-C motif) receptor 6 | 4.31 | 0.028034 | 4.31 |
| CD40LG | CD40 ligand | 3.58 | 0.026211 | 3.58 |
| CXCL1 | Chemokine (C-X-C motif) ligand 1 | 0.21 | 0.019112 | −4.74 |
| CXCL9 | Chemokine (C-X-C motif) ligand 9 | 2.37 | 0.046222 | 2.37 |
| CXCR5 | Chemokine (C-X-C motif) receptor 5 | 4.31 | 0.044996 | 4.31 |
| IL-10 | Interleukin 10 | 5.23 | 0.009659 | 5.23 |
| IL-10Ra | Interleukin 10 receptor, alpha subunit | 2 | 0.007194 | 2 |
| IL-1a | Interleukin 1, alpha | 0.5 | 0.019392 | −2 |
| IL-1R2 | Interleukin 1 receptor, type II | 3.9 | 0.007045 | 3.9 |
| IL-2Rg | Interleukin 2 receptor, gamma | 3.13 | 0.030638 | 3.13 |
| ITGB2 | Integrin beta 2 | 2.74 | 0.037346 | 2.74 |
| SPP1 | Secreted phosphoprotein 1 | 4.14 | 0.016364 | 4.14 |
| XCR1 | Chemokine (X-C motif) receptor 1 | 3.71 | 0.010119 | 3.71 |
Relative expression of inflammatory cytokines and chemokines in the inflamed AROM+ VP as compared with wild-type controls as determined by PCR-array. Only results with a relative mRNA expression at least two-fold higher or lower than that of the wt control mice and a statistical P value <0.05 were considered significant.
Discussion
In this study, we examine the prostate phenotype of the AROM+ transgenic mouse. Using this mouse model, we show a continuum with physiologically increased estrogens promoting the development of prostatitis, which, in turn, gives rise to the development of premalignant lesions within the prostate. These data also demonstrate, for the first time, a novel role for mast cells in this process and provide an indication of the potential mechanisms arising from the chronic inflammation that facilitate the development of PCa. Finally, these data also establish the AROM+ mouse as a new model of clinical prostatitis in the human.
Previously, a pivotal role for estrogen in the development of prostatic inflammation is evident mainly from the pharmacological administration of estrogen to rodents, as well as high-dose estrogen therapy given to transsexual males. Previous studies by us and others have shown prostatic epithelial dysplasia and inflammatory cell infiltration in the prostates of mice as they age, after they were transiently treated with estrogen during neonatal life.10,22,34,35 This estrogen-induced inflammatory response also appears to be specifically mediated by ERα: ERα knockout mice show no prostatic response to neonatal diethylstilbestrol, but ERβ knockout mice show a response similar to that observed in wild-type animals.12
The observation that estrogen is linked to the development of prostatic inflammation is particularly significant as estrogens, in addition to androgens, have also been implicated in the development of PCa. Androgens fall in men as they get older but the incidence of PCa rises, and animal studies have demonstrated that androgens—although necessary—cannot induce tumorigenesis alone.21 Indeed, the mechanism of hormonal induction of PCa requires both androgens and estrogens.36 Furthermore, increased levels of estrogens in men are linked to a higher incidence of PCa: serum levels of estrogens in African American men (who have the highest incidence of PCa in the United States) are significantly higher compared with Caucasian American men,37 while serum levels of estrogens are lower in Japanese men (who have a low risk of PCa) compared with Caucasian-Dutch men.38 Significantly, it is again ERα that mediates the development of carcinoma in response to estrogen.39
Despite convincing evidence that exogenous estrogen causes prostatitis and may be involved in the development of PCa, these data are frequently called into question. Studies investigating estrogen action in the rodent prostate typically rely on the addition of exogenous estrogens at either low or pharmacological doses, which introduces a number of complicating factors: low dose effects can be difficult to discern (particularly during fetal life when the fetus is exposed to higher maternal estrogens in the circulation); and pharmacological doses of estrogens may not mimic normal physiological responses. Therefore, given the potential role of estrogens in inflammation and PCa, it is imperative to examine the effects of estrogen on the prostate within the physiological setting.
The AROM+ mouse has a constitutively activated aromatase gene, and, therefore, provides an ideal means to examine the effects of elevated estrogens within a physiological setting. Despite having a significantly reduced serum testosterone/estrogen ratio when compared with wild-type controls, the early life development of the AROM+ VP was found to be completely normal. Indeed, no differences in the AROM+ and wild-type VP pathologies were apparent until puberty when the AROM+ VP remained significantly smaller that wild-type and demonstrated increased levels of ERα and CKH expression. Despite this, the overall pathology of the AROM+ VP was still comparable with wild-type.
The relevance of systemic hormone levels and their importance to the prostate is often unclear, particularly as the prostate expresses a number of enzymes involved in local hormone metabolism, including aromatase.25 To examine the influence and importance of local estrogen metabolism in the prostate, as opposed to systemic effects, we used tissue recombination. This is a powerful technique that has previously been used to elucidate the role of ERα in the development of squamous metaplasia, as well as the role of ERβ in the development of prostatic hypertrophy and hyperplasia.26,30 By grafting homotypic wild-type-S/wild-type-E and AROM+-S/AROM+-E recombinants into intact male SCID mice both graft types were exposed to the same, normal, systemic hormonal milieu. Consequently any differences in pathology that arise must be due to the altered local estrogen metabolism. These experiments demonstrated that local aromatase overexpression was sufficient to induce immunological changes, such as increased ERα expression, even in the presence of a normal systemic hormonal milieu.
There was extensive inflammation in the prostate of 40+ week-old AROM+ tissues. This inflammation was characterized by leukocyte accumulation within the stroma, the lymph vessels, and infiltration of the lumen. This response is reminiscent of the inflammation that has been reported to occur in neonatally estrogenized animals.10 We sought to extensively characterize the nature of this inflammation and found that it was associated with significantly increased numbers of mast cells, neutrophils, T-lymphocytes, and macrophages.
The elevation in mast cell numbers observed on the AROM+ mouse is a highly significant and novel finding. This increase in mast cells was found throughout the VP tissues, including within regions of chronic inflammation. Given the ubiquitous increase in mast cell numbers throughout the tissue, we could not discern any specific association between an increase in mast cells and the emergence of a specific inflammatory lesion. These data, however, are highly novel and do shed some insight into how estrogen may promote the development of chronic inflammation. Mast cell numbers rise immediately following puberty and this is likely a result of the profound changes to the hormone levels that occurs at this time. Mast cells are known to express ERα and are estrogen responsive,40 therefore the increase in estrogen levels that is evident in the AROM+ mice with puberty may be responsible for the increase in mast cell numbers. This putative role for estrogens increasing mast cells numbers is also supported by other previously reported data where mast cell numbers were increased in the inflamed testis of AROM+ mice.41
The fact that the increase in mast cell numbers precedes the emergence of the chronic inflammation also suggests these cells may be initiating and/or facilitating the recruitment of the other leukocytes; mast cells are well known to modulate leukocyte accumulation and facilitate the emergence inflammation in numerous tissues and organs.42,43,44,45 Of particular note is that mast cells have specifically been reported to mediate the recruitment of neutrophils and macrophages,46,47,48 as well as the activation of T-lymphocytes,49 all of which were significantly increased in the chronic inflammation observed in the AROM+ animals.
It is also significant that mast cells have previously been suggested to play a putative role in PCa. Specifically, it has been reported that mast cell numbers are increased within prostate tumors,50 and that low mast cell numbers in PCa may lead to a better prognosis.51 This evidence, however, is largely anecdotal and does not define the role of the mast cells in PCa. Despite this, and given the role of mast cells in inflammation and the emerging link between inflammation and the development of cancer, it may be that mast cells are playing an indirect role in PCa via the promotion of inflammation.
There is a significant and growing body of evidence implicating inflammation in the development of carcinoma in numerous organs and tissues, including the prostate. Our data support this, with the inflammation in the AROM+ mouse ultimately leading to the development of premalignant lesions reminiscent of PIN. These lesions were typified by focal stratification of atypical epithelial cells, tufting, and micropapillary growth patterns, as well as enlarged atypical nuclei and prominent nucleoli. They were also associated with high levels of proliferation throughout the surrounding stroma and within the lesion itself. The emergence of these lesions is indicative of a link between chronic inflammation and the development of carcinoma.
The activation and chemotaxis of inflammatory cells is regulated by specific cytokines, chemokines and their receptors. Although the primary role of these proteins is to control the trafficking of leukocytes during inflammatory responses, they also play pleiotropic roles in cancer development. In the AROM+ mouse we show that the expression of a number of these cytokines, chemokines and receptors are significantly altered. Of particular note are the significantly increased expression of CCL20, CCL8, CCR6, CCR5, and CCR2.
The increase in CCL20 and CCR6 is of particular note. CCL20 is strongly chemotactic for lymphocytes and also weakly attracts neutrophils and elicits its effects on target cells by binding and activating the chemokine receptor CCR6.52 The profound increase in CCL20 expression (∼25×) and its receptor CCR6 (∼5×) is of particular significance as increased CCR6 expression has been associated with advanced and aggressive PCa and CCL20/CCR6 have also been suggested as novel treatment target molecules for PCa.31 The increased expression of CCL8, CCR5, and CCR2 in the AROM+ tissues is also particularly significant (increased ∼10×, ∼3×, and ∼4×, respectively). Although the role of CCL8 in the prostate is unknown, this chemokine elicits its effects by binding to the receptors CCR5 and CCR2. CCR2 expression has been correlated with Gleason grade and pathological stages of PCa and has also been suggested to contribute to PCa development,32 while CCR5 has been shown to mediate PCa cell proliferation and invasion (albeit when activated by RANTES).33 Consequently, our data demonstrate that the induction of inflammation in the AROM+ mouse may facilitate the development of PCa via the altered expression of key cytokines and chemokines, specifically CCL20, CCL8, CCR6, CCR5, and CCR2.
The relevance of the AROM+ mouse as a model, not just of prostatitis per se, but of chronic pelvic pain syndrome, is supported by the presence of testicular abnormalities and inflammation,19,41 as well as infravesicle obstruction and bladder dysfunction,53 all of which are potential symptoms that are indicative of chronic pelvic pain syndrome.54,55 Indeed, chronic abacterial prostatitis with inflammation, such as is seen in the AROM+ mice, is termed Category IIIA prostatitis under the National Institutes of Health classification scheme and is specifically known as chronic pelvic pain syndrome.56 In light of this, the increase in IL-10 expression that was revealed by the array analyses is also particularly significant. IL-10 plays a number of roles in inflammation and can be both anti- and pro-inflammatory, and, consequently, the role that IL-10 is playing in the AROM+ mouse is uncertain. However, the increase in IL-10 expression is perhaps most intriguing when considering the AROM+ mouse as a model of chronic prostatitis and chronic pelvic pain syndrome. Specifically, IL-10 has been reported to be associated with these pain symptoms, with the development of chronic prostatitis and with chronic pelvic pain syndrome.56 These data, therefore, exemplify the AROM+ mouse as a model of human prostatitis.
Overall, this study demonstrates that physiologically elevated levels of estrogens are associated with the development of prostatitis, which subsequently leads to the development of premalignancy. This work also establishes the AROM+ mouse is a novel, nonbacterial mouse model of prostatitis with progression to premalignancy. This model is unique and particularly relevant to the study of prostatitis and its relationship to the development of PCa, sharing many of the features that occur in men with chronic prostatitis. Therefore, this is an ideal model to examine prostatitis, study its development throughout the various stages of life, as well as its role in the development of PCa.
Acknowledgments
We thank Ms. Shelley Hedwards and Dr. Stephen McPherson for their assistance with some of the work in this manuscript.
Footnotes
Address reprint requests to Dr. Stuart J. Ellem, 27-31 Wright Street, Clayton, Victoria, Australia 3168. E-mail: Stuart.Ellem@med.monash.edu.au.
Supported by a US Department of Defense Training Award to S.J.E. and an Australian NH & MRC Fellowship to G.P.R.
References
- Vykhovanets EV, Resnick MI, MacLennan GT, Gupta S. Experimental rodent models of prostatitis: limitations and potential. Prostate Cancer Prostatic Dis. 2007;10:15–29. doi: 10.1038/sj.pcan.4500930. [DOI] [PubMed] [Google Scholar]
- Collins MM, Stafford RS, O'Leary MP, Barry MJ. How common is prostatitis? A national survey of physician visits. J Urol. 1998;159:1224–1228. [PubMed] [Google Scholar]
- De Marzo AM, Marchi VL, Epstein JI, Nelson WG. Proliferative inflammatory atrophy of the prostate: implications for prostatic carcinogenesis. Am J Pathol. 1999;155:1985–1992. doi: 10.1016/S0002-9440(10)65517-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palapattu GS, Sutcliffe S, Bastian PJ, Platz EA, De Marzo AM, Isaacs WB, Nelson WG. Prostate carcinogenesis and inflammation: emerging insights. Carcinogenesis. 2005;26:1170–1181. doi: 10.1093/carcin/bgh317. [DOI] [PubMed] [Google Scholar]
- Balkwill F, Mantovani A. Inflammation and cancer: Back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- Strickler HD, Goedert JJ. Sexual behavior and evidence for an infectious cause of prostate cancer. Epidemiol Rev. 2001;23:144–151. doi: 10.1093/oxfordjournals.epirev.a000781. [DOI] [PubMed] [Google Scholar]
- Kirby RS, Lowe D, Bultitude MI, Shuttleworth KE. Intra-prostatic urinary reflux: an aetiological factor in abacterial prostatitis. Br J Urol. 1982;54:729–731. doi: 10.1111/j.1464-410x.1982.tb13635.x. [DOI] [PubMed] [Google Scholar]
- Naslund MJ, Strandberg JD, Coffey DS. The role of androgens and estrogens in the pathogenesis of experimental nonbacterial prostatitis. J Urol. 1988;140:1049–1053. doi: 10.1016/s0022-5347(17)41924-0. [DOI] [PubMed] [Google Scholar]
- Harris MT, Feldberg RS, Lau KM, Lazarus NH, Cochrane DE. Expression of proinflammatory genes during estrogen-induced inflammation of the rat prostate. Prostate. 2000;44:19–25. doi: 10.1002/1097-0045(20000615)44:1<19::aid-pros3>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Bianco JJ, McPherson SJ, Wang H, Prins GS, Risbridger GP. Transient neonatal estrogen exposure to estrogen-deficient mice (aromatase knockout) reduces prostate weight and induces inflammation in late life. Am J Pathol. 2006;168:1869–1878. doi: 10.2353/ajpath.2006.050623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins GS, Huang L, Birch L, Pu Y. The role of estrogens in normal and abnormal development of the prostate gland. Ann NY Acad Sci. 2006;1089:1–13. doi: 10.1196/annals.1386.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins GS, Birch L, Couse JF, Choi I, Katzenellenbogen B, Korach KS. Estrogen imprinting of the developing prostate gland is mediated through stromal estrogen receptor alpha: studies with alphaERKO and betaERKO mice. Cancer Res. 2001;61:6089–6097. [PubMed] [Google Scholar]
- Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuper H, Adami HO, Trichopoulos D. Infections as a major preventable cause of human cancer. J Intern Med. 2000;248:171–183. doi: 10.1046/j.1365-2796.2000.00742.x. [DOI] [PubMed] [Google Scholar]
- Dennis LK, Dawson DV. Meta-analysis of measures of sexual activity and prostate cancer. Epidemiology. 2002;13:72–79. doi: 10.1097/00001648-200201000-00012. [DOI] [PubMed] [Google Scholar]
- Shappell SB, Thomas GV, Roberts RL, Herbert R, Ittmann MM, Rubin MA, Humphrey PA, Sundberg JP, Rozengurt N, Barrios R, Ward JM, Cardiff RD. Prostate pathology of genetically engineered mice: definitions and classification. The consensus report from the Bar Harbor meeting of the Mouse Models of Human Cancer Consortium Prostate Pathology Committee. Cancer Res. 2004;64:2270–2305. doi: 10.1158/0008-5472.can-03-0946. [DOI] [PubMed] [Google Scholar]
- Elkahwaji JE, Zhong W, Hopkins WJ, Bushman W. Chronic bacterial infection and inflammation incite reactive hyperplasia in a mouse model of chronic prostatitis. Prostate. 2007;67:14–21. doi: 10.1002/pros.20445. [DOI] [PubMed] [Google Scholar]
- Bernoulli J, Yatkin E, Talvitie EM, Santti R, Streng T. Urodynamic changes in a noble rat model for nonbacterial prostatic inflammation. Prostate. 2007;67:888–899. doi: 10.1002/pros.20567. [DOI] [PubMed] [Google Scholar]
- Li X, Nokkala E, Yan W, Streng T, Saarinen N, Warri A, Huhtaniemi I, Santti R, Makela S, Poutanen M. Altered structure and function of reproductive organs in transgenic male mice overexpressing human aromatase. Endocrinology. 2001;142:2435–2442. doi: 10.1210/endo.142.6.8211. [DOI] [PubMed] [Google Scholar]
- Almahbobi G, Hedwards S, Fricout G, Jeulin D, Bertram JF, Risbridger GP. Computer-based detection of neonatal changes to branching morphogenesis reveals different mechanisms of and predicts prostate enlargement in mice haplo-insufficient for bone morphogenetic protein 4. J Pathol. 2005;206:52–61. doi: 10.1002/path.1753. [DOI] [PubMed] [Google Scholar]
- McPherson S, Wang H, Jones M, Pedersen J, Iismaa T, Wreford N, Simpson E, Risbridger G. Elevated androgens and prolactin in aromatase deficient (ArKO) mice cause enlargement but not malignancy of the prostate gland. Endocrinology. 2001;142:2458–2467. doi: 10.1210/endo.142.6.8079. [DOI] [PubMed] [Google Scholar]
- Bianco JJ, Handelsman DJ, Pedersen JS, Risbridger GP. Direct response of the murine prostate gland and seminal vesicles to estradiol. Endocrinology. 2002;143:4922–4933. doi: 10.1210/en.2002-220493. [DOI] [PubMed] [Google Scholar]
- Cancilla B, Jarred RA, Wang H, Mellor SL, Cunha GR, Risbridger GP. Regulation of prostate branching morphogenesis by activin a and follistatin. Dev Biol. 2001;237:145–158. doi: 10.1006/dbio.2001.0364. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C[T]) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Ellem SJ, Schmitt JF, Pedersen JS, Frydenberg M, Risbridger GP. Local aromatase expression in human prostate is altered in malignancy. J Clin Endocrinol Metab. 2004;89:2434–2441. doi: 10.1210/jc.2003-030933. [DOI] [PubMed] [Google Scholar]
- Risbridger G, Wang H, Young P, Kurita T, Wang YZ, Lubahn D, Gustafsson JA, Cunha G, Wong YZ. Evidence that epithelial and mesenchymal estrogen receptor-alpha mediates effects of estrogen on prostatic epithelium. Dev Biol. 2001;229:432–442. doi: 10.1006/dbio.2000.9994. [DOI] [PubMed] [Google Scholar]
- Cunha GR, Donjacour A. Stromal-epithelial interactions in normal and abnormal prostatic development. Prog Clin Biol Res. 1987;239:251–272. [PubMed] [Google Scholar]
- Cunha GR, Young P, Higgins SJ, Cooke PS. Neonatal seminal vesicle mesenchyme induces a new morphological and functional phenotype in the epithelia of adult ureter and ductus deferens. Development. 1991;111:145–158. doi: 10.1242/dev.111.1.145. [DOI] [PubMed] [Google Scholar]
- Day KC, McCabe MT, Zhao X, Wang Y, Davis JN, Phillips J, Von Geldern M, Ried T, KuKuruga MA, Cunha GR, Hayward SW, Day ML. Rescue of embryonic epithelium reveals that the homozygous deletion of the retinoblastoma gene confers growth factor independence and immortality but does not influence epithelial differentiation or tissue morphogenesis. J Biol Chem. 2002;277:44475–44484. doi: 10.1074/jbc.M205361200. [DOI] [PubMed] [Google Scholar]
- McPherson SJ, Ellem SJ, Simpson ER, Patchev V, Fritzemeier KH, Risbridger GP. Essential role for estrogen receptor beta in stromal-epithelial regulation of prostatic hyperplasia. Endocrinology. 2007;148:566–574. doi: 10.1210/en.2006-0906. [DOI] [PubMed] [Google Scholar]
- Ghadjar P, Loddenkemper C, Coupland SE, Stroux A, Noutsias M, Thiel E, Christoph F, Miller K, Scheibenbogen C, Keilholz U. Chemokine receptor CCR6 expression level and aggressiveness of prostate cancer. J Cancer Res Clin Oncol. 2008;134:1181–1189. doi: 10.1007/s00432-008-0403-5. [DOI] [PubMed] [Google Scholar]
- Lu Y, Cai Z, Xiao G, Liu Y, Keller ET, Yao Z, Zhang J. CCR2 expression correlates with prostate cancer progression. J Cell Biochem. 2007;101:676–685. doi: 10.1002/jcb.21220. [DOI] [PubMed] [Google Scholar]
- Vaday GG, Peehl DM, Kadam PA, Lawrence DM. Expression of CCL5 (RANTES) and CCR5 in prostate cancer. Prostate. 2006;66:124–134. doi: 10.1002/pros.20306. [DOI] [PubMed] [Google Scholar]
- Pylkkanen L, Santti R, Newbold R, McLachlan JA. Regional differences in the prostate of the neonatally estrogenized mouse. Prostate. 1991;18:117–129. doi: 10.1002/pros.2990180204. [DOI] [PubMed] [Google Scholar]
- Stoker TE, Robinette CL, Cooper RL. Perinatal exposure to estrogenic compounds and the subsequent effects on the prostate of the adult rat: evaluation of inflammation in the ventral and lateral lobes. Reprod Toxicol. 1999;13:463–472. doi: 10.1016/s0890-6238(99)00049-0. [DOI] [PubMed] [Google Scholar]
- Risbridger G, Frydenberg M. Groot LJD, editor. Philadelphia PA: W.B. Sanders,; Endocrinology of Prostate Cancer. 2004:p. 112–131. [Google Scholar]
- Hill P, Garbaczewski L, Walker AR. Age, environmental factors and prostatic cancer. Med Hypotheses. 1984;14:29–39. doi: 10.1016/0306-9877(84)90060-4. [DOI] [PubMed] [Google Scholar]
- de Jong FH, Oishi K, Hayes RB, Bogdanowicz JF, Raatgever JW, van der Maas PJ, Yoshida O, Schroeder FH. Peripheral hormone levels in controls and patients with prostatic cancer or benign prostatic hyperplasia: results from the Dutch-Japanese case-control study. Cancer Res. 1991;51:3445–3450. [PubMed] [Google Scholar]
- Ricke WA, McPherson SJ, Bianco JJ, Cunha GR, Wang Y, Risbridger GP. Prostatic hormonal carcinogenesis is mediated by in situ estrogen production and estrogen receptor alpha signaling. FASEB J. 2008;22:1512–1520. doi: 10.1096/fj.07-9526com. [DOI] [PubMed] [Google Scholar]
- Zaitsu M, Narita S, Lambert KC, Grady JJ, Estes DM, Curran EM, Brooks EG, Watson CS, Goldblum RM, Midoro-Horiuti T. Estradiol activates mast cells via a non-genomic estrogen receptor-alpha and calcium influx. Mol Immunol. 2007;44:1977–1985. doi: 10.1016/j.molimm.2006.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Strauss L, Kaatrasalo A, Mayerhofer A, Huhtaniemi I, Santti R, Makela S, Poutanen M. Transgenic mice expressing p450 aromatase as a model for male infertility associated with chronic inflammation in the testis. Endocrinology. 2006;147:1271–1277. doi: 10.1210/en.2005-0654. [DOI] [PubMed] [Google Scholar]
- Saperas E. The role of mast cells in gastrointestinal inflammation. Gastroenterology. 1996;110:1656–1658. doi: 10.1053/gast.1996.v110.agast961656. [DOI] [PubMed] [Google Scholar]
- Dumont N, Lepage K, Cote CH, Frenette J. Mast cells can modulate leukocyte accumulation and skeletal muscle function following hindlimb unloading. J Appl Physiol. 2007;103:97–104. doi: 10.1152/japplphysiol.01132.2006. [DOI] [PubMed] [Google Scholar]
- Cote CH, Tremblay MH, Duchesne E, Lapoite BM. Inflammation-induced leukocyte accumulation in injured skeletal muscle: role of mast cells. Muscle Nerve. 2008;37:754–763. doi: 10.1002/mus.20998. [DOI] [PubMed] [Google Scholar]
- Carlos D, de Souza DA, Junior, de Paula L, Jamur MC, Oliver C, Ramos SG, Silva CL, Faccioli LH. Mast cells modulate pulmonary acute inflammation and host defense in a murine model of tuberculosis. J Infect Dis. 2007;196:1361–1368. doi: 10.1086/521830. [DOI] [PubMed] [Google Scholar]
- Wershil BK, Furuta GT, Wang ZS, Galli SJ. Mast cell-dependent neutrophil and mononuclear cell recruitment in immunoglobulin E-induced gastric reactions in mice. Gastroenterology. 1996;110:1482–1490. doi: 10.1053/gast.1996.v110.pm8613053. [DOI] [PubMed] [Google Scholar]
- Wang Y, Thorlacius H. Mast cell-derived tumour necrosis factor-alpha mediates macrophage inflammatory protein-2-induced recruitment of neutrophils in mice. Br J Pharmacol. 2005;145:1062–1068. doi: 10.1038/sj.bjp.0706274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Stebut E, Metz M, Milon G, Knop J, Maurer M. Early macrophage influx to sites of cutaneous granuloma formation is dependent on MIP-1alpha /beta released from neutrophils recruited by mast cell-derived TNFalpha. Blood. 2003;101:210–215. doi: 10.1182/blood-2002-03-0921. [DOI] [PubMed] [Google Scholar]
- Nakae S, Suto H, Iikura M, Kakurai M, Sedgwick JD, Tsai M, Galli SJ. Mast cells enhance T cell activation: importance of mast cell costimulatory molecules and secreted TNF. J Immunol. 2006;176:2238–2248. doi: 10.4049/jimmunol.176.4.2238. [DOI] [PubMed] [Google Scholar]
- Aydin O, Dusmez D, Cinel L, Doruk E, Kanik A. Immunohistological analysis of mast cell numbers in the intratumoral and peritumoral regions of prostate carcinoma compared to benign prostatic hyperplasia. Pathol Res Pract. 2002;198:267–271. doi: 10.1078/0344-0338-00253. [DOI] [PubMed] [Google Scholar]
- Nonomura N, Takayama H, Nishimura K, Oka D, Nakai Y, Shiba M, Tsujimura A, Nakayama M, Aozasa K, Okuyama A. Decreased number of mast cells infiltrating into needle biopsy specimens leads to a better prognosis of prostate cancer. Br J Cancer. 2007;97:952–956. doi: 10.1038/sj.bjc.6603962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hieshima K, Imai T, Opdenakker G, Van Damme J, Kusuda J, Tei H, Sakaki Y, Takatsuki K, Miura R, Yoshie O, Nomiyama H. Molecular cloning of a novel human CC chemokine liver and activation-regulated chemokine (LARC) expressed in liver. Chemotactic activity for lymphocytes and gene localization on chromosome 2. J Biol Chem. 1997;272:5846–5853. doi: 10.1074/jbc.272.9.5846. [DOI] [PubMed] [Google Scholar]
- Streng T, Li X, Lehtoranta M, Makela S, Poutanen M, Talo A, Tekmal RR, Santti R. Infravesical obstruction in aromatase over expressing transgenic male mice with increased ratio of serum estrogen-to-androgen concentration. J Urol. 2002;168:298–302. [PubMed] [Google Scholar]
- Litwin MS, McNaughton-Collins M, Fowler FJ, Jr, Nickel JC, Calhoun EA, Pontari MA, Alexander RB, Farrar JT, O'Leary MP. The National Institutes of Health chronic prostatitis symptom index: development and validation of a new outcome measure. Chronic Prostatitis Collaborative Research Network. J Urol. 1999;162:369–375. doi: 10.1016/s0022-5347(05)68562-x. [DOI] [PubMed] [Google Scholar]
- McNaughton-Collins M, Joyce GF, Wise M, Pontari M. National Institutes of Health,; Urologic Diseases in America. 2007:p. 11–41. [Google Scholar]
- Miller LJ, Fischer KA, Goralnick SJ, Litt M, Burleson JA, Albertsen P, Kreutzer DL. Interleukin-10 levels in seminal plasma: implications for chronic prostatitis-chronic pelvic pain syndrome. J Urol. 2002;167:753–756. doi: 10.1016/S0022-5347(01)69139-0. [DOI] [PubMed] [Google Scholar]