Abstract
Mounting evidence points to a role for the sympathetic nervous system in suppressing inflammation. This role might be of specific relevance for immune privilege in the eye, where, sporadically, patients with denervated sympathetic fibers develop chronic inflammation. The present study used mice to investigate whether the robust innervation of intraocular structures by the sympathetic system plays a role in maintaining ocular immune privilege. We first performed surgical removal of the superior cervical ganglion, which supplies sympathetic fibers to the eye, and studied the immune response generated against soluble antigens or allogeneic tumor cells injected into the ocular anterior chamber under these conditions. Our results show that in the absence of functional sympathetic fibers, the eye loses its ability to prevent either the immune rejection of intraocular allogeneic tumor cells or the suppression of delayed type hypersensitivity responses against soluble antigens injected in the anterior chamber. This loss of immune privilege is accompanied by a decrease in the concentration of transforming growth factor-β in the aqueous humor. These results suggest that immune privilege is lost in the absence of a functional sympathetic innervation of the eye, allowing intraocular immune responses to become exaggerated. We conclude that ocular sympathetic nerves are critical for the generation and maintenance of immune privilege in the eye through the facilitation of local transforming growth factor-β production.
Several decades of research are beginning to unveil a close structural and functional interconnection between the sympathetic and the immune systems (reviewed in Kin and Sanders1). Not only are the primary and secondary lymphoid organs extensively innervated by sympathetic nerve termini,2 but every potential inflammatory site, inside virtually every organ, is also influenced by these nerves due to their ubiquitous association with the vascular system. This close anatomical relationship is further complemented at the cellular and molecular levels by the presence of adrenergic and neuropeptide receptors on immune cells, which in turn are coupled with the necessary secondary messenger systems that allow them to respond adequately to sympathetic signals (reviewed by Kin and Sanders1).
Although the effects of the sympathetic system on immune function are far from being completely understood, much work indicates that, overall, sympathetic signals exert a down-regulatory effect on cell-mediated inflammation. For example, norepinephrine, which is the main neurotransmitter released by sympathetic nerve terminals, suppresses the production of cytokines required for the propagation of Th-1 mediated inflammatory responses, namely interleukin-2 and interferon gamma.3,4 It also blocks the production of pro-inflammatory cytokines such as tumor necrosis factor-α from stimulated macrophages and cytotoxic CD8+ T cells.5,6 More importantly, at target tissues, adrenergic signals promote the expression of transforming growth factor-β (TGF-β),7,8,9,10,11 a potent immunosuppressive cytokine.
These immuno-regulatory properties might be of major relevance in the function of immune privileged sites like the eye, whose function could potentially be destroyed by even a single episode of inflammation.12 This view is supported by clinico-pathological observations that patients with aberrant or absent sympathetic innervation of eye structures (eg, Horner syndrome) can develop a chronic inflammatory condition of the anterior chamber (AC) known as Fuch’s iridocyclitis.13,14,15,16,17 Although it has been hypothesized that this condition is caused by a propensity for certain intraocular viral infections,18,19,20 another potential explanation for this condition is that local mechanisms of immune privilege are compromised by the absence of sympathetic signals to ocular structures. Traditionally ocular immune privilege is thought to be mediated by classical immunosuppressive molecules such as TGF-β, Fas ligand, thrombospondin-1, and neuropeptides such as α-melanocortin stimulating hormone (α-MSH), vasoactive intestinal peptide (VSP), calcitonin gene-related peptide (CGRP).21 However, intraocular nerves also appear to play an important role, as corneal grafts fail to survive if the corneal nerves are transected before transplantation.22 Furthermore, chemical sympathectomy with 6-hydroxydopamine (6-OHDA) abolishes the normal systemic immunosuppressive response against intraocular antigens.23
Given the robust innervation of intraocular structures by the sympathetic system, we investigated whether the sympathetic innervation of the eye contributes to the generation and maintenance of ocular immune privilege. Our experiments, which used superior cervical ganglionectomy (SCGX) as a method of intraocular sympathectomy, demonstrate that this is indeed the case.
Methods and Materials
Mice
BALB/C (H-2Kd) mice (6 to 8 weeks old) were purchased from Jackson Laboratories (Bar Harbor, ME). All animal experiments were conducted as approved by institutional guidelines (protocol number S-005-0703) and all animals were treated in accordance with the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research.
Culture Media and Reagents
Serum free medium consisted of RPMI 1640 supplemented with 10 mmol/L HEPES, 0.1 mmol/L nonessential amino acids, 1 mmol/L sodium pyruvate, 100 U/ml penicillin, 100 μg/ml streptomycin, (Lonza, Basel, Switzerland), and 10−5 M 2-ME (Sigma-Aldrich, St. Louis, MO), 0.1% bovine serum albumin (Sigma-Aldrich), 0.2% insulin, transferrin, and selenium, culture supplement (Sigma-Aldrich). The medium used for the TGF-β quantification bioassay consisted of Eagle’s MEM (Lonza, Basel, Switzerland), supplemented with 2 mmol/L l-glutamine, 10 mmol/L HEPES, 0.1 mmol/L nonessential amino acids, 1 mmol/L sodium pyruvate, 100 U/ml penicillin, 100 μg/ml streptomycin, and 0.5% fetal bovine serum.
Surgical Removal of the Superior Cervical Ganglion
After being anesthetized, mice were placed in a supine position and a ventral midline incision was made in the neck. The submaxillary gland was separated, and the superior cervical ganglion (SCG) was identified at the levels of the carotid bifurcation. Using fine forceps, the ganglion was picked up and its inputs transected with microscissors 2 mm proximal and distal to the ganglion. The SCG was then removed and the surgical area was closed in layers. All animals were allowed to recover for 7 days before AC inoculations. This recovery period is based on prior studies in rats in which sympathetic terminals underwent complete structural degeneration 7 days after SCGX.24 However, successful SCGX surgery was easily confirmed on clinical examination just a few hours after the surgery, as the sympathectomized side developed ptosis and its pupil lost the ability to dilate in the dark.
Sham operation (SHAM) involved identical surgical exposure of the SCG on the side of interest with exception that the fibers entering and leaving the SCG were not surgically transected.
Suppression of the Delayed Type Hypersensitivity Response against Eye-Derived Antigens
BALB/c mice (n = 6/group) underwent SCGX or SHAM surgery on the right side. Seven days later the mice received AC-injections of ovalbumin (OVA) (50 μg in 2 μl of HBSS), and 7 days after that they were immunized in the nape of the neck with 100 μg of OVA emulsified in a 1:1 volume with complete Freund’s adjuvant so that a total volume of 100 μl was delivered. Seven days later the mice received an ear-challenge with 200 μg OVA in 10 μl of HBSS (20 mg/ml) and ear swelling was measured 24 and 48 hours later for comparison with a baseline measurements of ear thickness obtained immediately before the ear challenge. Measurements were performed with a digital micrometer (Mitutoyo, Aurora, IL) placed at the location of the ear injection. Three individual measurements were taken per ear at each time point and their averages were used for final tabulation. Each group of mice (SCGX, SHAM, or intact) was tested against its own internal positive control that did not receive AC injections of antigen (positive control) and its own internal negative control, which only received antigen challenge in the ear.
AC Inoculations and Subcutaneous Immunizations
The procedures for AC inoculation have been described in detail elsewhere.25 In brief, the cornea is punctured with a sterile 30 gauge needle (BD and Co., Franklin Lakes, NJ) and approximately 2 μl of air is then dispensed into the AC by using a Hamilton microinjector (Hamilton Co. Inc., Whittier, CA). Cells (10,000 P815 tumor cells of DBA2 background) or OVA (50 μg suspended in 2 μl in sterile HBSS) was then delivered into the AC by using a heat-pulled 10 μl glass micropipette (Drummond Scientific Co., Broomall, PA) fitted into a sterile infant feeding tube (No. 5 French; Cutter Laboratories, Inc., Berkeley, CA). The latter was attached to a 1 ml insulin syringe, which was used to deliver the antigen load into the AC.
Mixed Lymphocyte Reaction
Three groups of mice (SCGX 2, SHAM, and intact) received AC injections of DBA2-P815 (10,000 cells). The SCGX and SHAM groups received their injections 7 days after surgery. Twenty-one days later the submandibular lymph nodes were harvested and used to make cell suspensions to be plated in 24 well plates (1 × 106 cells/ml/well). The cervical and submandibular lymph nodes were chosen based on three previous reports that these are the lymph nodes where tumor cells injected in the AC preferentially drain.26 Irradiated DBA-2 splenocytes, which share the same major histocompatibility complex antigens with P815 tumor cells were then added (500,000 splenocytes per 1 million lymph node cells [LNCs]). The cultures were then allowed to proceed for 72 hours in serum free medium at which time lymphocytic proliferation was tested via a standard tritiated thymidine assay.
Aqueous Humor Collection
Aqueous humor (AH) was collected under general anesthesia. To collect the AH, the eye of interest was gently picked up with small blunt curved forceps from its base at the orbit. A puncture was carefully made in the center of the cornea by using a sterile needle (30 g). Concurrently, a disposable glass micropipette was placed against the center of the cornea immediately adjacent to the puncture site. AH then moves passively into the micropipette by capillary action. The collected AH is immediately transferred to a siliconized microcentrifuge tube for experimental use. AH from multiple eyes was pooled, diluted to different concentrations, and stored at −80°C until it was used for TGF-β quantification.
TGF-β Quantification
The Mink Lung epithelial (Mv1Lu) cell assay was used for TGF-β quantification. Mv1Lu cells were recovered from subconfluent cultures by using trypsin (1.0 mg/ml). This was followed by washing in HBSS supplemented with 2% fetal calf serum. Fifty thousand Mv1Lu cells were diluted in 100 μl of serum free medium and incubated in 96-well flat bottom microtiter plates with AH (1:13 dilution) for 16 hours at 37°C in 5% CO2. AH samples used to assess total levels of TGF-β were acidified for 1 hour at 4°C with HCl (5 μl HCl/100 μl AH) and then neutralized with 1 N NaOH and 1 M HEPES. At the end of this culture period individual wells were pulsed with 1 μCi [3H]thymidine. Four hours later, cells were trypsinized and harvested by an automated cell harvester. The incorporated radioactivity was then measured by a liquid scintillation counter. TGF-β concentrations were calculated from the suppression of Mv1Lu cell proliferation as compared with the suppression of proliferation by known amounts of TGF-β1 (R&D Systems, Minneapolis, MN). The bioassay had a detection limit of 3 pg/ml TGF-β.
Histology
Histology was performed on intact and experimental eyes at different time points after SCGX or SHAM surgery and/or tumor cell inoculations. After sacrifice, animals were perfused with 10 ml of normal saline to remove blood from the blood vessels. Then, the eyes were enucleated by pulling the eye gently with blunt curved forceps until all of the extraocular muscles were exposed and cut, along with the optic nerve. The eyes were then fixed overnight in 4% paraformaldehyde and processed for regular H&E staining.
Statistics
Data are presented as the SEM, and statistical analyses were performed by using Prism software 4.0. A one-way analysis of variance with a posthoc Tukey test were routinely used unless otherwise specified. A P value of <0.05 was considered statistically significant.
Results
SCGX Results in the Loss of Ocular Immune Privilege
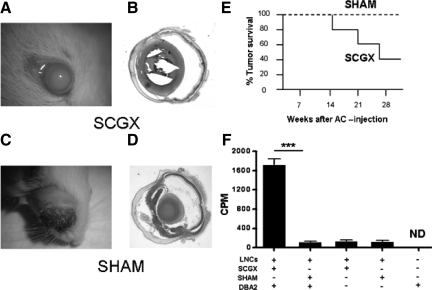
The most clear experimental demonstration of ocular immune privilege consists of the indefinite growth of histoincompatible allogeneic tumor cells injected into the AC of a normal eye.27 To test if intraocular sympathetic fibers are necessary for the maintenance of ocular immune privilege we set out to determine whether sympathetic denervation accomplished by SCGX results in the rejection of histoincompatible allogeneic tumor cells. To this end, SCGX or SHAM surgery was performed on BALB/c mice, and 7 days later P815 mastocytoma (DBA/2 origin) cells were injected in the AC, as described in Methods. Mice were followed clinically and their eyes were collected at the end of the experiment for histological analysis (Figure 1A–D). Four weeks after AC tumor cell injection, 60% (6 of 10) of SCGX animals fully rejected the injected cells, while none of the SHAM animals did (Figure 1E). It is worth noting that, although the absence of sympathetic fibers is likely to cause changes in the P815 tumor cells such as the release of chemokines28 and cytokines,29 the nature of these responses and their relationship with the ensuing immune response is beyond the scope of this investigation.
Figure 1.
Sympathectomized eyes allow for the immunological rejection of AC-injected allogeneic tumor cells. Mice underwent either SCGX or SHAM on day 0. On day 7, 10,000 P815 tumor cells were delivered into the AC (n = 10/group). Sixty percent of SGCX animals rejected their tumor, whereas none of the SHAM animals did (E). Gross pictures of the injected eyes and their cross sections are shown in A–B (SCGX) and C–D (SHAM), respectively. Bar graph (F): In a separate experiment, submandibular LNCs from SCGX or SHAM mice previously injected in the AC with P815 cells (DBA-2 background) were tested in culture for their ability to recognize irradiated DBA-2 cells as foreign (see Materials and Methods). Only SCGX LNCs mounted a proliferative response against DBA2 cells. A representative experiment of three is shown. ***P < 0.001.
In separate experiments we assessed whether the rejection of tumor cells coincided with the priming of lymphocytes in the draining lymph nodes. To this end, the submandibular lymph nodes26 of tumor-bearing SCGX and SHAM-operated mice were harvested 21 days after AC inoculation with P815 tumor cells. The LNCs were then co-cultured with irradiated DBA/2 tumor cells and their proliferation was tested via conventional tritiated thymidine incorporation. Only the LNCs from SCGX mice proliferated in the presence of DBA/2 cells (Figure 1F) indicating the presence of effectors primed against DBA/2 alloantigens. This response coincided with a significant decrease in interleukin-10 in the supernatant of LNCs derived from SCGX mice co-cultured with P815 cells as compared with the levels detected in the control supernatants from similarly cultured LNCs derived from SHAM-operated mice (data not shown). Together these results suggest that local sympathetic fibers prevent the sensitization of immune cells against eye derived antigens.
In the Absence of a Trigger, SCGX Does Not Appear to Cause Inflammation
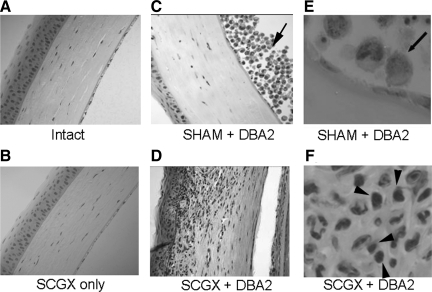
We then investigated whether sympathetic denervation, independently of AC injections, might induce a low level of inflammation that could set the stage for the rejection of AC tumor cells in SCGX mice. To this end, histology was performed in the eyes of mice at 7, 14, and 28 days after SCGX surgery, for comparison with intact eyes. To the extent that can be observed with H&E staining, sympathetic denervation of the eye did not lead to the recruitment of mononuclear cells anywhere along the ocular axis. Only the corneas of representative eyes are shown for illustration (Figure 2, A and B). Mononuclear cell infiltration that typically represents inflammatory response was detected only in SCGX mice injected with P815 tumor cells (Figure 2, D and F) and not in the SHAM-operated mice in which thriving tumor cells were easily detectable in the AC (Figure 2, C and E). These results strongly suggest that disruption of the sympathetic innervation of the eye, by itself, does not induce an inflammatory response. Instead, SCGX appears to remove an important mechanism of local immune suppression, thus allowing for the expansion of an inflammatory response against tumor cells injected in the AC.
Figure 2.
The absence of sympathetic innervation by itself does not induce intraocular inflammation. Mice underwent unilateral ocular sympathectomy (SCGX) or SHAM and their eyes were assessed for inflammation 7 (n = 3), 14 (n = 3), and 28 days (n = 3) after surgery. To the extent that can be assessed with H&E staining, neither group exhibited mononuclear infiltrates in the eye as a result of surgery alone. Only representative corneas harvested on day 14 are shown for illustration, but identical results were found at all times tested. For comparison, the corneas of representative SHAM and SCGX mice that received injections of P815 cells are shown (C and E, and D and F, respectively). Note the striking absence of mononuclear infiltrates in the AC of a SHAM-operated mouse (C and E) despite the growing tumor cells adjacent to it (arrow). By contrast, SCGX corneas (D and F) display a significant amount of inflammation. Original magnification: ×200 (C and D); ×600 (E and F of representative areas from C and D, respectively). Arrowheads in F show mononuclear cells.
Suppression of the Delayed Type Hypersensitivity Response against Eye-Derived Antigens Requires an Intact Sympathetic Innervation of the Eye
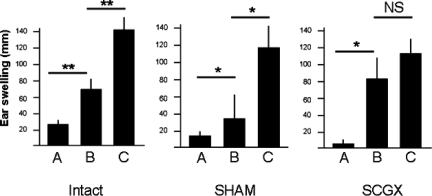
Antigens injected in the periphery induce a Th1-mediated immune response, which can be re-elicited experimentally when the antigen is re-injected subcutaneously along with complete Freund’s adjuvant. However, whenever an antigen is first injected into the AC, local antigen presenting cells (APCs) are influenced by local factors to propagate a suppressive or regulatory immune response, instead of an inflammatory one. This causes a suppression of the delayed type hypersensitivity (DTH) response against that antigen, and is considered a key mechanism of ocular immune privilege. In this experiment, we set out to determine whether the loss of immune privilege in SCGX mice is also associated with the loss of this regulatory immune response. To this end, a soluble antigen was injected into the AC (see Methods) of intact, SCGX, and SHAM-operated animals, and their subsequent DTH responses to a peripheral re-challenge with the same antigen were compared. As shown in Figure 3 (left and middle panels), intact and SHAM-operated mice, respectively (Bars A and B), displayed significantly suppressed DTH responses, by comparison with those of their positive controls (Bar C). It should be noted that the SCGX and SHAM operated mice exhibited a reduction in the normal DTH response by comparison with intact mice (Figure 3; bar C in all three groups). This, we believe, is the result of an expected postoperative effect on immunity, which is not seen in the intact mice. However, the reduction was not statistically significant as determined by a one-way analysis of variance test (n = 3 experiments; Mean ± SEM: Intact = 124.6 ± 11.97; SHAM = 98.26 ± 10.86; SCGX = 86.19 ± 12.51; P > 0.1). Furthermore, DTH responses in SCGX mice injected with intraocular OVA were almost identical to those of SCGX mice injected with intraocular saline (Figure 3, right panel, n = 3 independent experiments; Mean ± SEM: 82.22 ± 12.51 versus 86.19 ± 10.13, respectively), suggesting that denervation of the sympathetic nerves to the eye abolishes the normal suppression of the immune response against intraocular antigens. This lack of DTH suppression in SCGX mice could be observed even if AC injections were given 31 days after surgery (data not shown).
Figure 3.
Sympathetic innervation of the eye by the SCG is required for suppression of the DTH response against eye-derived antigens. Seven days after anesthesia alone (intact), SHAM, or SCGX mice were injected into the AC with OVA (B) or saline (C). Ten days later all mice were injected with subcutaneous OVA in the ear as described in Materials and Methods. Ear swelling was measured 24 hours later, also as described in Materials and Methods. *P < 0.05; **P < 0.01. A representative experiment of three is shown.
Ocular Sympathetic Fibers Regulate Local TGF-β Production
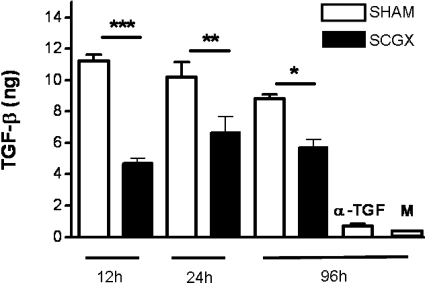
TGF-β is one of the most potent immunosuppressive cytokines found in the ocular microenvironment. Because several lines of evidence suggest that TGF-β production can be regulated by sympathetic fibers,7,10,11,30,31,32 we then wondered whether TGF-β production could be affected by the sympathetic deafferentation of the eye. To this end, we performed unilateral surgical resection of the SCG on one side (typically the right side). The contralateral side was left untouched. We then compared the levels of TGF-β in the AH from the two sides at several time points after the surgery by using the MVLU bioassay for the detection of TGF-β. We detected a significant decline in the TGF-β content of the AH derived from eyes on the side of SCGX as compared with that from eyes on the contralateral side (Figure 4). It should be noted that TGF-β levels in the AH of unoperated eyes were not significantly different from those seen in the eyes of intact mice (data not shown). TGF-β levels of samples treated with pan anti-TGF-β (negative control) were nearly undetectable (Figure 4). The decreased TGF-β levels persisted until 7 days post-SCGX, the last time point tested (day 7; data not shown). Similar results were obtained when AH was pooled from groups of mice, which had undergone bilateral SCGX (data not shown). This decline in AH TGF-β content in SCGX eyes strongly supports the hypothesis that sympathetic nerves regulate TGF-β synthesis in the eye. Furthermore, these results correlate with both the loss of the regulatory immune response to AC-injected antigen and the loss of ocular immune privilege.
Figure 4.
Removal of the SCG results in reduced TGF-β levels in the AC. AH was collected at different times after SCGX or SHAM, and the Mv1Lu assay was used to quantify its TGF-β levels as described in Materials and Methods. ***P < 0.001; **P < 0.01; *P < 0.05. α-TGF = negative control treated with a pan-anti-TGF-β antibody; M = medium alone. A representative experiment of five is shown.
Discussion
The present study establishes that the local sympathetic innervation of the eye is a key factor in the generation and maintenance of ocular immune privilege. Specifically, our studies show that transection of sympathetic fibers to the eye results in a loss of immune privilege, as evidenced by the rapid immunological rejection of allogeneic tumor cells, the loss of suppression of the DTH response against eye-derived antigens, and the reduction of TGF-β levels in the AC. These results are in agreement with mounting evidence that adrenergic signals from the sympathetic system down-regulate both innate and adaptive immune responses. For example, in vitro studies have shown that β−2 adrenergic stimulation of activated human peripheral blood mononuclear cells causes them to produce decreased levels of the pro-inflammatory cytokines interferon gamma,33 and interleukin-234 and increased levels of the anti-inflammatory cytokine interleukin-10.34 Furthermore, in some animal models of head trauma and spinal cord injury, immune suppression due to systemic adrenergic catecholamines (ie, sympathetic storm) can be reversed with adrenergic receptor blockers.35,36,37 These findings help to explain why head trauma and stroke patients secrete massive amounts of systemic interleukin-10, become immunosupressed, and experience higher infection and mortality rates.38,39,40,41
Aside from supplying ocular structures, the sympathetic nerves that originate in the SCG also supply lymph nodes thought to process intraocular antigens including the cervical and submandibular lymph nodes.42,43 In agreement with our results, prior studies have identified alterations in immune responsiveness within these lymph nodes as a result of SCGX. They include enhanced contact hypersensitivity, DTH, and graft versus host reactions.44 Furthermore, norepinephrine dramatically suppresses the function of activated F4/80+ macrophages, and diminishes the expansion of tumor necrosis factor-α- expressing CD8+ antitumor cytotoxic lymphocytes.5 Placed in the context of the present investigation these data suggest that norepinephrine released from sympathetic nerves inside the eye and lymph nodes might suppress both the function of resident F4/80+ macrophages involved in the processing and presentation of ocular antigens (in this case tumor cells), and in the subsequent generation of inflammatory effector lymph node cells involved in mounting a response against them.
The decreased level of TGF-β in the AH after sympathectomy supports the view that tonic sympathetic influences on eye structures facilitate the constant production of TGF-β, a molecule that is released in large quantities by multiple cell types within the eye.45,46 In support of this view are data showing that norepinephrine up-regulates TGF-β in several tissue and cell-types7,8,9,10,11 and that adrenergic receptor blockers decrease pathological processes induced by high levels of TGF-β such as allergic asthma and chronic renal fibrosis.20,35 Importantly, norepinephrine’s maximal effect appears to be on TGFβ2, as evident from findings that an intravenous bolus of norepinephrine induces a 16-fold increase in this isoform in the rat myocardium.7 The latter study is of particular relevance to the present investigation as TGF-β2 accounts for 80% to 90% of the total TGF-β in the AH, and is thought to be the principal isoform mediating ocular immune privilege.47 Furthermore, a correlation has been made between low levels of TGF-β2 in the aqueous and vitreous humors, and the presence of uveitis.48 Taken together these data support the view that TGF-β expression is modulated by the sympathetic system, and leads us to favor the hypothesis that the breakdown in ocular immune privilege after sympathetic denervation is caused, at least partially, by a decrease in the local production of TGF-β.
Using systemic injections of 6-OHDA, other investigators have found a role for the sympathetic system in suppressing the DTH response against AC-injected antigens.23 In that study, eye-derived APCs from 6-OHDA-injected animals transferred the suppression of DTH responses to secondary recipients, suggesting that systemic sympathectomy does not prevent the generation of tolerogenic APCs inside the eye. Nonetheless, it is likely that those results are largely due to extraocular effects of 6-OHDA injections, because this chemical does not cross the blood-brain barrier49 and, to our knowledge, it has not been shown to cross the blood-ocular barrier. Consequently, the combination of our results with those of those of Li et al23 suggest that both intraocular and extraocular sympathetic fibers play important roles in enforcing ocular immune privilege.
Finally, it should be noted that the present investigation provides a plausible explanation for multiple case reports in the medical literature about patients with pathological sympathetic denervation of the eye (eg, in the context of Horner syndrome) who later in their clinical course unexpectedly develop chronic inflammation of the AC (Fuch’s iridocyclitis).13,14,15,16,17 In these patients the stimulus for inflammation might be an otherwise innocuous viral eye infection, which in the absence of ocular immune privilege unleashes an inflammatory response that ultimately becomes chronic and progressive. And in fact, many reports suggest that Fuch’s iridocyclitis is tightly linked to various intraocular infections.18,19,20
In conclusion, the present study establishes a previously unknown requirement for the local sympathetic innervation of the eye in the generation and maintenance of ocular immune privilege. While much research is needed to understand the exact mechanisms underlying these observations, our experiments support a role mediated by local sympathetic fibers in the facilitation of TGF-β production inside the eye.
Footnotes
Address reprint requests to Sharmila Masli, Schepens Eye Research Institute, 20 Staniford St., Boston, MA 02114. E-mail: sharmila.masli@schepens.harvard.edu.
This work was supported in part by a grant from the National Institute of Allergy And Infectious Diseases, NIAID 1F32AI066677-01 to J.L.V. and a National Institute of Health grant EY015472 to S.M.
Current address of J.L.V.: Department of Neurology, Columbia University Medical Center, Columbia University College of Physicians and Surgeons, New York, NY; and H.K.: Department of Ophthalmology, Kyorin University School of Medicine, Tokyo, Japan.
References
- Kin NW, Sanders VM. It takes nerve to tell T and B cells what to do. J Leukoc Biol. 2006;79:1093–1094. doi: 10.1189/jlb.1105625. [DOI] [PubMed] [Google Scholar]
- Straub RH. Complexity of the bi-directional neuroimmune junction in the spleen. Trends Pharmacol Sci. 2004;25:640–646. doi: 10.1016/j.tips.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Swanson MA, Lee WT, Sanders VM. IFN-gamma production by Th1 cells generated from naive CD4+ T cells exposed to norepinephrine. J Immunol. 2001;166:232–240. doi: 10.4049/jimmunol.166.1.232. [DOI] [PubMed] [Google Scholar]
- Ramer-Quinn DS, Swanson MA, Lee WT, Sanders VM. Cytokine production by naive and primary effector CD4+ T cells exposed to norepinephrine. Brain Behav Immun. 2000;14:239–255. doi: 10.1006/brbi.2000.0603. [DOI] [PubMed] [Google Scholar]
- Kalinichenko VV, Mokyr MB, Graf LH, Jr, Cohen RL, Chambers DA. Norepinephrine-mediated inhibition of antitumor cytotoxic T lymphocyte generation involves a beta-adrenergic receptor mechanism and decreased TNF-alpha gene expression. J Immunol. 1999;163:2492–2499. [PubMed] [Google Scholar]
- Ignatowski TA, Spengler RN. Regulation of macrophage-derived tumor necrosis factor production by modification of adrenergic receptor sensitivity. J Neuroimmunol. 1995;61:61–70. doi: 10.1016/0165-5728(95)00074-c. [DOI] [PubMed] [Google Scholar]
- Briest W, Homagk L, Rassler B, Ziegelhöffer-Mihalovicová B, Meier H, Tannapfel A, Leiblein S, Saalbach A, Deten A, Zimmer H-G. Norepinephrine-induced changes in cardiac transforming growth factor-beta isoform expression pattern of female and male rats. Hypertension. 2004;44:410–418. doi: 10.1161/01.HYP.0000141414.87026.4d. [DOI] [PubMed] [Google Scholar]
- Dixon IM, Drobic V. Gender dependency in the pathogenesis of cardiac hypertrophy: effect of norepinephrine on transforming growth factor-beta release in female heart. Hypertension. 2004;44:392–393. doi: 10.1161/01.HYP.0000141484.53649.6f. [DOI] [PubMed] [Google Scholar]
- Oben JA, Roskams T, Yang S, Lin H, Sinelli N, Torbenson M, Smedh U, Moran TH, Li Z, Huang J, Thomas SA, Diehl AM. Hepatic fibrogenesis requires sympathetic neurotransmitters. Gut. 2004;53:438–445. doi: 10.1136/gut.2003.026658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai SY, Schluns KS, Le PT, McNulty JA. TGF-beta1 and IL-6 expression in rat pineal gland is regulated by norepinephrine and interleukin-1beta. Histol Histopathol. 2001;16:1135–1141. doi: 10.14670/HH-16.1135. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Culmsee C, Roth-Eichhorn S, Krieglstein J. Beta(2)-adrenoceptor stimulation enhances latent transforming growth factor-beta-binding protein-1 and transforming growth factor-beta1 expression in rat hippocampus after transient forebrain ischemia. Neuroscience. 2001;107:593–602. doi: 10.1016/s0306-4522(01)00357-8. [DOI] [PubMed] [Google Scholar]
- Streilein JW. Ocular immune privilege: the eye takes a dim but practical view of immunity and inflammation. J Leukoc Biol. 2003;74:179–85. doi: 10.1189/jlb.1102574. [DOI] [PubMed] [Google Scholar]
- Aggarwal RK, Luck J, Coster DJ. Horner’s syndrome and Fuchs’ heterochromic uveitis. Br J Ophthalmol. 1994;78:949. doi: 10.1136/bjo.78.12.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calmettes, Deodati, Amalric A case of Fuch’s heterochromia associated with Claude-Bernard-Horner syndrome. Arch Ophtalmol Rev Gen Ophtalmol. 1953;13:512–513. [PubMed] [Google Scholar]
- Makley TA, Abbott K. Neurogenic heterochromia: report of an interesting case. Am J Ophthalmol. 1965;59:927–928. [PubMed] [Google Scholar]
- Regenbogen LS, Naveh-Floman N. Glaucoma in Fuchs’ heterochromic cyclitis associated with congenital Horner’s syndrome. Br J Ophthalmol. 1987;71:844–849. doi: 10.1136/bjo.71.11.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcher C, Humbert P, Bron A, Chirpaz L, Royer J. Optic neuropathy and Parry-Romberg syndrome: apropos of a case. J Fr Ophtalmol. 1990;13:557–561. [PubMed] [Google Scholar]
- de Visser L, Braakenburg A, Rothova A, de Boer JH. Rubella virus-associated uveitis: clinical manifestations and visual prognosis. Am J Ophthalmol. 2008;146:292–297. doi: 10.1016/j.ajo.2008.04.011. [DOI] [PubMed] [Google Scholar]
- Kim EC, Margolis TP. Hypertensive iridocyclitis. Br J Ophthalmol. 2006;90:812–813. doi: 10.1136/bjo.2006.091876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barequet IS, Li Q, Wang Y, O'Brien TP, Hooks JJ, Stark WJ. Herpes simplex virus DNA identification from aqueous fluid in Fuchs heterochromic iridocyclitis. Am J Ophthalmol. 2000;129:672–673. doi: 10.1016/s0002-9394(00)00409-8. [DOI] [PubMed] [Google Scholar]
- Streilein JW, Masli S, Takeuchi M, Kezuka T. The eye’s view of antigen presentation. Human Immunol. 2002;63:435–443. doi: 10.1016/s0198-8859(02)00393-2. [DOI] [PubMed] [Google Scholar]
- Streilein JW, Bradley D, Sano Y, Sonoda Y. Immunosuppressive properties of tissues obtained from eyes with experimentally manipulated corneas. Invest Ophthalmol Vis Sci. 1996;37:413–424. [PubMed] [Google Scholar]
- Li X, Taylor S, Zegarelli B, Shen S, O'Rourke J, Cone RE. The induction of splenic suppressor T cells through an immune-privileged site requires an intact sympathetic nervous system. J Neuroimmunol. 2004;153:40–49. doi: 10.1016/j.jneuroim.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Huhtala A. Origin of myelinated nerves in the rat iris. Exp Eye Res. 1976;22:259–265. doi: 10.1016/0014-4835(76)90053-1. [DOI] [PubMed] [Google Scholar]
- Saban DR, Elder IA, Nguyen CQ, Smith WC, Timmers AM, Grant MB, Peck AB. Characterization of intraocular immunopathology following intracameral inoculation with alloantigen. Mol Vis. 2008;14:615–624. [PMC free article] [PubMed] [Google Scholar]
- Boonman ZF, van Mierlo GJ, Fransen MF, Franken KL, Offringa R, Melief CJ, Jager MJ, Toes RE. Intraocular tumor antigen drains specifically to submandibular lymph nodes, resulting in an abortive cytotoxic T cell reaction. J Immunol. 2004;172:1567–1574. doi: 10.4049/jimmunol.172.3.1567. [DOI] [PubMed] [Google Scholar]
- Streilein JW, Niederkorn JY, Shadduck JA. Systemic immune unresponsiveness induced in adult mice by anterior chamber presentation of minor histocompatibility antigens. J Exp Med. 1980;152:1121–1125. doi: 10.1084/jem.152.4.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsugami K, Tamada K, Abe K, Harada M, Nomoto K. Local injections of OK432 can help the infiltration of adoptively transferred CD8+ T cells into the tumor sites and synergistically induce the local production of Th1-type cytokines and CXC3 chemokines. Cancer Immunol Immunother. 2000;49:361–368. doi: 10.1007/s002620000131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Zhang H, Yang H, Zhang L, Chen X, Zheng X, He S. Induction of T-helper type 2 cytokine release and up-regulated expression of protease-activated receptors on mast cells by recombinant American cockroach allergen Per a 7. Clin Exp Allergy. 2008;38:1160–1167. doi: 10.1111/j.1365-2222.2008.02991.x. [DOI] [PubMed] [Google Scholar]
- Cobelens PM, Kavelaars A, Vroon A, Ringeling M, van der Zee R, van Eden W, Heijnen CJ. The beta 2-adrenergic agonist salbutamol potentiates oral induction of tolerance, suppressing adjuvant arthritis and antigen-specific immunity. J Immunol. 2002;169:5028–5035. doi: 10.4049/jimmunol.169.9.5028. [DOI] [PubMed] [Google Scholar]
- Fisher SA, Absher M. Norepinephrine and ANG II stimulate secretion of TGF-beta by neonatal rat cardiac fibroblasts in vitro. Am J Physiol. 1995;268:C910–C917. doi: 10.1152/ajpcell.1995.268.4.C910. [DOI] [PubMed] [Google Scholar]
- Wong VY, Laping NJ, Nelson AH, Contino LC, Olson BA, Gygielko E, Campbell WG, Jr, Barone F, Brooks DP. Renoprotective effects of carvedilol in hypertensive-stroke prone rats may involve inhibition of TGF beta expression. Br J Pharmacol. 2001;134:977–984. doi: 10.1038/sj.bjp.0704329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal SK, Marshall GD., Jr Beta-adrenergic modulation of human type-1/type-2 cytokine balance. J Allergy Clin Immunol. 2000;105:91–98. doi: 10.1016/s0091-6749(00)90183-0. [DOI] [PubMed] [Google Scholar]
- Loop T, Bross T, Humar M, Hoetzel A, Schmidt R, Pahl HL, Geiger KK, Pannen BH. Dobutamine inhibits phorbol-myristate-acetate-induced activation of nuclear factor-kappaB in human T lymphocytes in vitro. Anesth Analg. 2004;99:1508–1515. doi: 10.1213/01.ANE.0000132976.19021.1B. [DOI] [PubMed] [Google Scholar]
- Vega JL, Ganea D, Jonakait GM. Acute down-regulation of antibody production following spinal cord injury: role of systemic catecholamines. J Neuropathol Exp Neurol. 2003;62:848–854. doi: 10.1093/jnen/62.8.848. [DOI] [PubMed] [Google Scholar]
- Woiciechowsky C, Asadullah K, Nestler D, Eberhardt B, Platzer C, Schöning B, Glöckner F, Lanksch WR, Volk H-D, Döcke W-D. Sympathetic activation triggers systemic interleukin-10 release in immunodepression induced by brain injury. Nat Med. 1998;4:808–813. doi: 10.1038/nm0798-808. [DOI] [PubMed] [Google Scholar]
- Lucin KM, Sanders VM, Jones TB, Malarkey WB, Popovich PG. Impaired antibody synthesis after spinal cord injury is level dependent and is due to sympathetic nervous system dysregulation. Exp Neurol. 2007;207:75–84. doi: 10.1016/j.expneurol.2007.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klehmet J, Harms H, Richter M, Prass K, Volk HD, Dirnagl U, Meisel A, Meisel C. Stroke-induced immunodepression and post-stroke infections: lessons from the Preventive Antibacterial Therapy in Stroke trial. Neuroscience. 2009;158:1184–1193. doi: 10.1016/j.neuroscience.2008.07.044. [DOI] [PubMed] [Google Scholar]
- Quattrocchi KB, Frank EH, Miller CH, Amin A, Issel BW, Wagner FC., Jr Impairment of helper T-cell function and lymphokine-activated killer cytotoxicity following severe head injury. J Neurosurg. 1991;75:766–773. doi: 10.3171/jns.1991.75.5.0766. [DOI] [PubMed] [Google Scholar]
- Quattrocchi KB, Miller CH, Wagner FC, Jr, DeNardo SJ, DeNardo GL, Ovodov K, Frank EH. Cell-mediated immunity in severely head-injured patients: the role of suppressor lymphocytes and serum factors. J Neurosurg. 1992;77:694–699. doi: 10.3171/jns.1992.77.5.0694. [DOI] [PubMed] [Google Scholar]
- Chamorro A, Amaro S, Vargas M, Obach V, Cervera A, Gomez-Choco M, Torres F, Planas AM. Catecholamines, infection, and death in acute ischemic stroke. J Neurol Sci. 2007;252:329–335. doi: 10.1016/j.jns.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Ksander BR, Streilein JW. Analysis of cytotoxic T cell responses to intracameral allogeneic tumors. Invest Ophthalmol Vis Sci. 1989;30:323–329. [PubMed] [Google Scholar]
- Giron LT, Jr, Crutcher KA, Davis JN. Lymph nodes–a possible site for sympathetic neuronal regulation of immune responses. Ann Neurol. 1980;8:520–525. doi: 10.1002/ana.410080509. [DOI] [PubMed] [Google Scholar]
- Alito AE, Romeo HE, Baler R, Chuluyan HE, Braun M, Cardinali DP. Autonomic nervous system regulation of murine immune responses as assessed by local surgical sympathetic and parasympathetic denervation. Acta Physiol Pharmacol Latinoam. 1987;37:305–319. [PubMed] [Google Scholar]
- Wilbanks GA, Mammolenti M, Streilein JW. Studies on the induction of anterior chamber-associated immune deviation (ACAID). III. Induction of ACAID depends upon intraocular transforming growth factor-beta. Eur J Immunol. 1992;22:165–173. doi: 10.1002/eji.1830220125. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Takeuchi M, Streilein JW. Participation of pigment epithelium of iris and ciliary body in ocular immune privilege. 1. Inhibition of T-cell activation in vitro by direct cell-to-cell contact. Invest Ophthalmol Vis Sci. 2000;41:811–821. [PubMed] [Google Scholar]
- Cousins SW, McCabe MM, Danielpour D, Streilein JW. Identification of transforming growth factor-beta as an immunosuppressive factor in aqueous humor. Invest Ophthalmol Vis Sci. 1991;32:2201–2211. [PubMed] [Google Scholar]
- de Boer JH, Limpens J, Orengo-Nania S, de Jong PT, La Heij E, Kijlstra A. Low mature TGF-beta 2 levels in aqueous humor during uveitis. Invest Ophthalmol Vis Sci. 1994;35:3702–3710. [PubMed] [Google Scholar]
- Kostrzewa RM, Jacobowitz DM. Pharmacological actions of 6-hydroxydopamine. Pharmacol Rev. 1974;26:199–288. [PubMed] [Google Scholar]