Abstract
Brk, a tyrosine kinase expressed in a majority of breast tumors, but not normal mammary tissue, promotes breast carcinoma cell proliferation. Normal epithelial cells are dependent on cell–cell or cell–matrix interactions for survival and undergo apoptosis after disruption of these interactions. Tumor cells are less sensitive to the induction of apoptosis and are predicted to have the potential to disseminate. We investigated whether Brk has further roles in breast tumor progression by relating its expression to tumor grade and demonstrating its role in the regulation of carcinoma cell survival under non-adherent conditions. Brk expression was determined by reverse transcription PCR on RNA extracted from surgical samples of human breast cancers. Breast carcinoma cell survival in suspension culture was examined when Brk protein levels were suppressed by RNA interference. Additionally, the effect of experimentally overexpressing Brk in otherwise Brk-negative breast carcinoma cells was assessed. Brk mRNA expression was notably higher in grade 3 breast tumors, as compared with lower tumor grades. In suspension culture, Brk suppression increased the rate of cell death, as compared with controls, and this cell death program exhibited characteristics of autophagy but not of apoptosis. Conversely, experimental expression of Brk in Brk-negative cells increased cell survival whereas kinase-inactive Brk did not. Therefore, Brk enhances breast carcinoma cell survival in suspension, suggesting a role for Brk in supporting breast cancer cell dissemination.
Breast tumor kinase (Brk)/protein tyrosine kinase 6 (PTK6) is a protein tyrosine kinase expressed in a high proportion of breast tumors, but not normal mammary tissue,1,2,3 and we and others have suggested that it may represent a novel therapeutic target.4 In support of this, we have previously shown that immortalized human mammary luminal epithelial cells, transfected to express Brk at pathologically relevant levels, show an increased sensitivity to the mitogenic effect of epidermal growth factor.5 In contrast, RNA interference-mediated down-regulation of Brk expression in T-47D breast carcinoma cells results in decreased proliferation.6 A role for Brk in promoting cell proliferation has also been found by other investigators, albeit in nontumorigenic human embryonic kidney cells (HEK 293) and rat astrocytes.7,8
In addition to proliferative deregulation, tumor cells are thought to acquire a variety of other characteristics that contribute to their survival and dissemination [eg,9], and we are interested in determining whether pathological Brk expression in breast carcinomas impacts on these properties. Notably, from the point of view of therapeutic target validation, we have sought to determine whether Brk might support carcinoma cell survival, and thus whether Brk-targeted therapies might be expected to kill tumor cells selectively under specific conditions operating during dissemination and metastasis.
Normal epithelial cells are dependent on either cell–cell or cell–matrix interactions for their survival, and can be induced to undergo apoptosis by disrupting their interactions with neighboring cells and the substratum/basement membrane.10 The process of apoptosis induced by loss of these interactions is termed “anoikis.”11 It has been suggested that loss of an adhesive function in transformed epithelial cells is a critical step in the promotion of a malignant phenotype and that even certain non-tumorigenic epithelial cells (Madin-Darby canine kidney) can acquire invasive properties when cellular adhesion is blocked.12
Reduced sensitivity to anoikis is an important step in the tumor metastatic process and cells with increased survival in the absence of attachment would be predicted to have an increased potential to disseminate successfully (eg, via the vascular or lymphatic circulation). Human mammary luminal epithelial cells modified to express Brk show an increased efficiency of colony formation and growth in soft agar, suggesting that Brk may play a role in anchorage-independent survival and/or proliferation in this cell type.5 We have therefore tested the hypothesis that Brk is involved in preventing the detachment-induced cell death of breast carcinoma cells.
Materials and Methods
Preparation of Tissue Samples
Samples were collected with approval of the Norwich District Ethics Committee, the Norwich Research and Development Committee, and the Partners in Cancer Research Tissue Bank Committee.
Human Breast Tumor Samples
Forty-six women ages 40 to 88 years (median age, 58 years) provided samples of 48 primary breast neoplasms (all of which were invasive carcinoma) after providing informed consent. Two patients who had bilateral disease gave two samples, one from each side. All samples were taken from fresh, unfixed carcinomas removed surgically. The specimens were sent immediately after removal to the laboratory, where, after inking the margins and slicing the specimen, a small part of the tumor was removed by a histopathologist for this study. Specimens were prepared and snap-frozen in the department’s (Norwich) human tissue bank as previously described.13 The rest of the surgical specimen was fixed in 10% formal saline and processed for routine histopathological diagnosis and establishment of prognostic factors.
Non-Neoplastic Human Mammary Tissue
Samples of non-neoplastic mammary tissue were obtained from seven patients, with informed consent, at reduction mammoplasty. All samples were snap-frozen and stored as described for the cancers.
RNA Extraction
RNA was extracted from 44 of the frozen human breast tumor surgical samples for which histological grade had been determined, and the seven non-neoplastic human mammary tissue samples. Total RNA was isolated using a modification of the SV Total RNA Isolation System (Promega, Madison, WI14;). Fifty to 100 mg of tumor tissue were homogenized in 1 ml of RNazol B reagent (Biogenesis Ltd., Poole, United Kingdom) using an UltraTurrax T8 homogenizer (IKA). The homogenate was stored at −80°C pending the following step of the protocol: the samples were allowed to thaw completely at room temperature, vortexed briefly, and collected into 200 μl of chloroform. They were shaken vigorously for 15 seconds, incubated for 3 minutes at room temperature, and centrifuged for 15 minutes at 14,000 rpm. The upper phase was collected into 200 μl of 95% ethanol, mixed, and transferred into a spin basket assembly. The Promega protocol was then followed from step 7 to the end according to the manufacturer’s instructions.
Reverse Transcription (for Real-Time PCR)
One μg of total RNA was primed with 0.5 μg of random hexameric primers (Promega) and reverse transcribed into cDNA in a 20 μl reaction volume containing 200 units of SuperScript II reverse transcriptase, 5 × first-strand buffer (final concentrations: 50 mmol/L Tris-HCl [pH8.3], 75 mmol/L KCl, and 3 mmol/L MgCl2), 10 mmol/L dithiothreitol, 0.5 mmol/L of each deoxynucleotide triphosphate (dATP, dCTP, dGTP, and dTTP; Life Technologies, Inc.), and 40 units of RNasin RNase inhibitor (Promega). Each priming reaction was performed at 70°C for 10 minutes and stopped by placing samples on ice; the reverse transcription was performed at 42°C for 1 hour followed by incubation at 70°C for 10 minutes.
TaqMan Real-Time PCR
Forward and reverse primers and fluorescence-labeled oligonucleotide probes (using 6-carboxy-fluorescein [FAM] as the reporter dye and 6-carboxytetramethylrhodamine [TAMRA] as the quencher dye) were designed for the human Brk/PTK6 gene using Primer Express 1.0 software (PE Applied Biosystems, Foster City, CA) and synthesized by PE Applied Biosystems (Warrington, United Kingdom). BLASTN searches were undertaken on our designed primers to confirm gene specificity. To ensure against genomic DNA amplification, the forward and reverse primers were placed in separate adjacent exons. Details of the primers are given in Table 1. A standard curve with concentrations ranging from 25 ng to 1 ng was produced using human placental cDNA dissected from fetal villi as the template. An XY scatter plot for each gene was produced using Microsoft Excel Chart Wizard software, and values for the equation y = mx + b (where m = the slope of the standard curve and b = the y intercept of that line) and R2 were obtained. The 18S rRNA gene was used as an endogenous control to normalize for differences in the amount of total RNA in each sample. PCR reactions for all samples were performed in duplicate in 96-well optical plates with 5 ng of cDNA (1 ng of cDNA for the 18S gene), 100 nmol/L probe, 200 nmol/L each primer, and 12.5 μl of TaqMan Universal 2 × PCR Master Mix (PE Applied Biosystems, Warrington, UK) in a 25 μl reaction volume. The amplification reaction was performed over 40 cycles with the following parameters: an initial holding stage of 2 minutes at 50°C and then 10 minutes at 95°C, followed by a two-step cycling program of 15 seconds at 95°C and 1 minute at 60°C.
Table 1.
PCR-Primer and siRNA Sequences
| Name | Sequence |
|---|---|
| Brk forward primer | 5′-ATGAAGAAGCTGCGGCACAA-3′ |
| Brk reverse primer | 5′-CCGAAACGGGCAGGACTT-3′ |
| Brk probe | 5′-FAM-ACATCATCACGGAGCTCATGGC CAAG-TAMRA-3′ |
| 18S primers and probe | Ribosomal RNA Control Reagent (PE Applied Biosystems) |
| Actin forward primer | 5′-AAGAGAGGCATCCTCACCCT-3′ |
| Actin reverse primer | 5′-TACATGGCTGGGGTGTTGAA-3′ |
| Beclin forward primer | 5′-GGCAAGATTGAAGACACAGG-3′ |
| Beclin reverse primer | 5′-ATGGCATGTTGTAGCTCTGG-3′ |
| Control siRNA | 5′-GGACACCAUCAAGUGUUCG-3′ |
| Brk siRNA A | 5′-GGUGAUUUCUCGAGACAAC-3′ |
| Brk siRNA B | 5′-GCUUGUGAACUACCACAGG-3′ |
Reverse Transcription
Seven hundred nanograms of total RNA was primed with 0.5 μg of random hexameric primers (Promega) and reverse transcribed into cDNA in a 20 μl reaction volume containing 200 units of SuperScript II reverse transcriptase, 5 × first-strand buffer (final concentrations: 50 mmol/L Tris-HCl [pH8.3], 75 mmol/L KCl, and 3 mmol/L MgCl2), 10 mmol/L dithiothreitol, 0.5 mmol/L of each deoxynucleotide triphosphate (dATP, dCTP, dGTP, and dTTP; Life Technologies, Inc.), and 40 units of RNasin RNase inhibitor (Promega). Each priming reaction was performed at 70°C for 10 minutes and stopped by placing samples on ice; the reverse transcription was performed at 42°C for 50 minutes followed by incubation at 70°C for 15 minutes.
PCR
PCR amplification was performed with the following parameters: denaturation at 94°C, followed by primer annealing at 55°C and an extension step of 72°C for 35 cycles (Beclin-1) and 25 Cycles (β-actin). Primer sequences are given in Table 1.
Cell Culture
T-47D and MDA-MB-157 breast carcinoma cells (ATCC HTB-133 and HTB-24) were cultured in RPMI-1640 supplemented with 10% fetal bovine serum, 2 mmol/L glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin at 37°C in a humidified atmosphere of 5%CO2 in air. GI101 cells were cultured under the same conditions but with additional medium supplementation of 10 μg/ml insulin. Cells were subcultured twice weekly.
RNA Interference
Small interfering (si)RNAs with dTdT overhangs (Dharmacon, Lafeyette, Co) (Table 1) were dissolved in annealing buffer (20 mmol/L KCl, 6 mmol/L HEPES-KOH pH 7.5, 0.2 mmol/L MgCl2) to a final concentration of 20 μmol/L. T-47D cells were seeded at 1.25 × 106 cells per 25 cm2 tissue culture flask and allowed to adhere overnight. Before transfection the medium was exchanged (with washing) for 4.8 ml serum free medium. Transfections of siRNA (sequences in Table 1) with 37.5 μl Oligofectamine (Invitrogen) were performed according to manufacturer’s instructions and as previously described6 to give a final siRNA concentration per flask of 120 nmol/L. Four hours after transfection fetal calf serum was added to a final concentration of 10%. A second transfection was performed 24 hours later. Duplicate independent Brk-targeting siRNAs were used in initial validation experiments and the BrkA sequence was prioritized as it gave more consistent reproducible suppression.
Stable Transfections
Duplicate stable Brk expressing MDA-MB-157 polyclonal cell populations were generated by transfecting MDA-MB-157 cells with pRcCMV encoding wild-type Brk (pRcCMV-WT-Brk) or pRcCMV alone (as a vector control). Transfections were performed with a 3:1 ratio of Fugene (Roche, West Sussex, United Kingdom):DNA following the manufacturer’s protocol. Forty eight hours after transfection, cells were subcultured into fresh medium containing 400 μg/ml G418 (Invitrogen, Paisley, United Kingdom). The medium was changed twice weekly and the cells were maintained in 400 μg/ml G418.
Cell Suspension Assay
Tissue culture plates were coated with a volume of poly(2-hydroxyethylmethacrylate) (polyHEMA, Sigma, Poole, United Kingdom) in 98% ethanol (20 mg/ml) to give a final polyHEMA concentration of 1.5 mg/cm2. Plates were air dried in a tissue culture cabinet. Cells were seeded into polyHEMA-coated wells at 0.75 × 104 cells/cm2. Cell viability was assessed by incubating a volume of cell suspension with an equal volume of 0.2% trypan blue solution for 1 minute, followed by counting on an improved Neubauer hemocytometer.
Annexin V Staining
The proportion of cells with exposed phosphatidyl serine on the cell surface was measured using the Annexin V-FITC fluorescein isothiocyanate (FITC) staining kit (BD Clontech) according to the manufacturer’s protocol and analyzing the stained cells by flow cytometry on a Beckman Coulter flow cytometer with Cell Quest software (Becton Dickinson, Oxford, United Kingdom).
Cell Death Enzyme-Linked Immunosorbent Assay
Levels of fragmented DNA were measured using the cell death detection sandwich ELISAPLUS assay (Roche Applied Science), which uses anti-DNA and histone antibodies to determine levels of mono- and oligonucleosomes in the cytoplasmic fraction of cell lysates, a marker of apoptosis-induced DNA cleavage. After 24 hours on polyHEMA 5 × 104 cells from duplicate wells were resuspended in 1 ml lysis buffer and the assay was performed according to manufacturer’s instructions. A reagent only background value was subtracted from the absorbance values for experimental samples.
Immunoblotting
Cells for analysis were lysed in 2 × SDS-polyacrylamide gel electrophoresis lysis buffer and proteins separated by SDS-polyacrylamide gel electrophoresis. After electro-transfer in Towbin buffer, membranes were blocked in 5% nonfat milk protein in TBS/0.1% Tween. Membranes were then incubated at 4°C overnight with primary antibody in 5% nonfat milk protein/TBS/0.1% Tween; glyceraldehyde-3-phosphate dehydrogenase and Beclin-1 (Abcam, Cambridge, United Kingdom), or 5% bovine serum albumin/TBS/0.1% Tween; Brk (ICR-100,5), caspase-3, caspase-8, poly (ADP-Ribose) polymerase (PARP), and Bcl-x (all Cell Signaling Technology, Hitchin, United Kingdom). Proteins were visualized with an appropriate horseradish peroxidase-conjugated secondary antibody (Dako, Cambridge, United Kingdom) and chemiluminescent substrate (Pierce, Warrington, United Kingdom).
Statistical Analysis
Statistical analysis was performed with Sigma STAT software. Data were assessed for normality by the Kolmogorov-Smirnov test and significance determined by either Mann Whitney U or Student’s t-test. P < 0.05 was considered significant.
Results
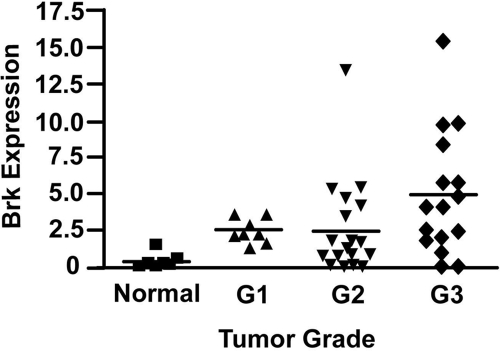
To assess whether Brk expression is associated with breast tumor grade, we analyzed Brk mRNA expression levels using quantitative reverse-transcription PCR (Q-RT-PCR) in 44 surgical samples of breast carcinoma of known grade, and in seven non-neoplastic (normal) mammary tissue samples obtained from reduction mammoplasty. We have previously shown by Western blotting that over 60% of breast tumor samples express Brk,1 and others have used Q-RT-PCR to corroborate this finding.2,3 The data presented here suggest that approximately 85% of breast tumor samples tested were positive for Brk mRNA expression. As Q-RT-PCR is a more sensitive technique than Western blotting, this difference might be predicted; however, it remains a possibility that a subset of tumors express Brk mRNA but not protein. Expression of mRNA confirms the previous Western blotting data1 showing that Brk is frequently over expressed in breast tumor samples compared with normal mammary tissue (P = 0.001). By plotting mRNA expression levels against histopathological grade, a correlation between Brk expression and tumor grade was also noted (Figure 1). Brk mRNA levels were significantly higher in grade 3 breast tumors compared with those classed as grades 1 and 2 (P = 0.024).
Figure 1.
Brk expression increases with tumor grade. Relative levels of Brk mRNA expression are plotted against histological grade. The horizontal bars represent the mean normalized expression for each grade. Thirty-nine out of 44 samples were positive for Brk expression (determined by subtracting the median of the normal breast samples from the tumor samples).
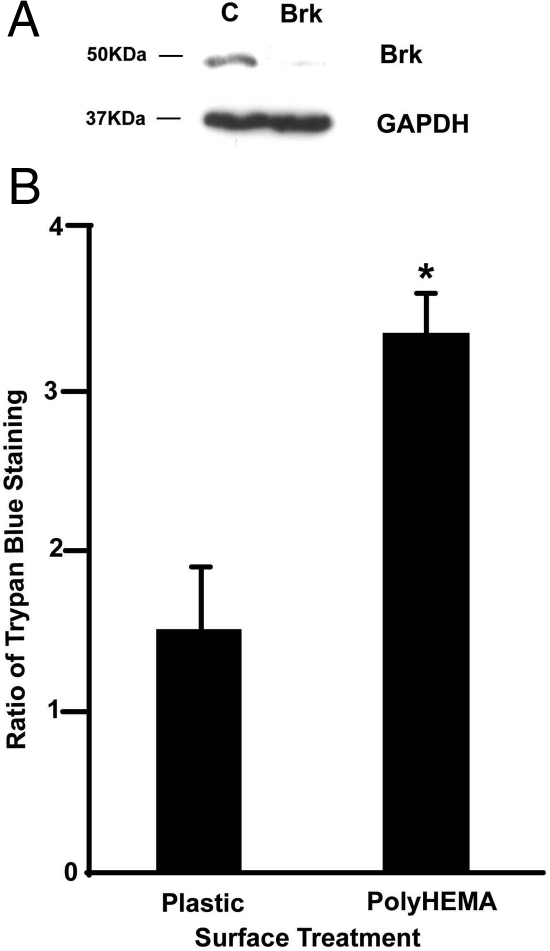
Following from these findings on clinical samples, we sought to determine whether Brk expression has an influence on breast carcinoma cell biology beyond its pro-proliferative role. As histological grade reflects patterns of metastatic spread and high grade tumors are associated with poor prognosis [eg,15], we hypothesized that Brk may affect some of the processes involved in dissemination such as anchorage independent survival. To determine whether Brk has a role in protecting breast cancer cells from anoikis, T-47D cells were transfected with siRNAs to suppress Brk expression (Figure 2A), and plated into tissue culture wells that had either been coated with polyHEMA to prevent attachment or left untreated. Initially we assessed the effects of Brk suppression on cell survival using the trypan blue exclusion assay, and the proportion of trypan blue permeable (and therefore dead) cells was compared 24 hours after plating. When adhering to plastic, T-47D cells transfected with Brk targeting siRNA showed only a small, statistically insignificant increase in the proportion of trypan blue-permeable cells compared with control transfectants. In contrast, after 24 hours on polyHEMA, which completely inhibited attachment, at least threefold more Brk-targeted cells had incorporated trypan blue than cells transfected with the control siRNA (Figure 2B). Additional transfections with a second Brk-targeting siRNA sequence6 gave comparable results (data not shown). This suggests that Brk targeting decreases the ability of T-47D breast carcinoma cells to survive when detached from their normal substratum.
Figure 2.
Brk suppression increases cell death in suspension culture. T-47D cells were transfected twice with either control or Brk-targeting siRNAs and, 24 hours after the second transfection, cells were seeded into untreated or polyHEMA-coated wells. A: Parallel wells of adherent cells were lysed in SDS-polyacrylamide gel electrophoresis buffer 24 hours after the second transfection, and Brk expression analyzed by immunoblotting (upper panel), with glyceraldehyde-3-phosphate dehydrogenase as a loading control (lower panel). B: Cell viability of cells plated onto plastic or polyHEMA was assessed by trypan blue exclusion. As the percentage of trypan-positive cells varied with the efficacy of the knock-down, the proportion of trypan blue-positive cells in Brk-targeted wells compared with controls for each experiment was determined. The mean of three independent experiments is plotted; *P = 0.002.
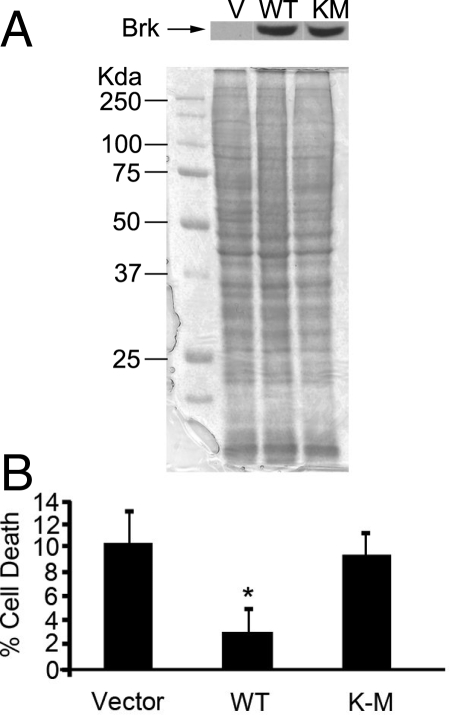
If the expression of Brk protects T-47D cells from suspension-induced cell death, we reasoned that transfecting a Brk-negative breast carcinoma cell line with wild-type Brk should reduce the levels of cell death in suspension. We transfected the breast cancer cell line MDA-MB-157 with plasmid alone or plasmid expressing wild-type Brk (Brk WT) or kinase-inactive Brk (Brk K219M) and selected stable polyclonal populations. After incubation on polyHEMA for 24 hours the levels of cell death observed using the trypan blue exclusion assay decreased by almost half in the Brk WT transfected cells compared with MDA-MB-157 cells transfected with vector only (Figure 3, A and B), showing that a gain in Brk expression increases the survival of this breast cancer cell line in suspension. Interestingly the levels of cell death detected in cells transfected with kinase inactive Brk were similar to those observed in the MDA-MB-157 cells transfected with vector only. Similar results were also seen with MDA-MB-468 cells (data not shown).
Figure 3.
Brk promotes survival of MDA-MD-157 cells in suspension. MDA-MB-157 cells were transfected with pRcCMV vector alone (V), pRcCMV-Brk WT) or pRcCMV-Brk K219M (KM). A: Expression of Brk was confirmed by Western blotting with anti-Brk antibodies and Coomassie staining was performed to ensure equal loading. B: Cells were seeded into polyHEMA-coated wells, and cell viability was assessed by trypan blue exclusion. The ratio of trypan blue permeable cells in the Brk expressing cells was expressed as a percentage of the vector controls. The mean of four wells is plotted. *P = 0.029.
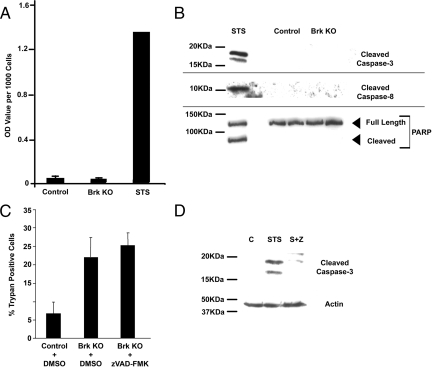
We next wished to determine whether the Brk targeting-induced increase in the proportion of trypan blue incorporating cells in suspension reflected death by apoptosis, using traditional markers such as cell-surface exposure of phosphatidylserine, the appearance of cytosolic histone-bound DNA, activation of caspases 3 and 8, and cleavage of PARP.
We assayed for loss of cell membrane polarity with Annexin V-FITC using fluorescence-activated cell sorting analysis to detect the cell surface exposure of phosphatidylserine molecules, one of the early markers of apoptosis [reviewed in16]. Only low levels of staining were detected and no difference was observed between control and Brk-targeted cells after 24 hours on polyHEMA (Figure 4A). We also observed very little difference in Annexin-v-FITC staining when T-47D cells were treated with the broad spectrum kinase inhibitor staurosporine, as a positive control for the induction of apoptosis, although HeLa cells treated in parallel showed a shift in Annexin-V-FITC fluorescence, confirming that the assay was functioning (data not shown) and indicating that phosphatidylserine exposure is not a classical feature of cell death in T-47D cells.
Figure 4.
Brk-regulated cell death is not ‘classical’ apoptosis. T-47D cells were transfected with either control (Control) or Brk–targeting (Brk KO) siRNAs and seeded into polyHEMA-coated plates. Additional adherent T-47D cells were treated with 1.5 μmol/L staurosporine (STS) as positive controls where necessary. After 24 hours cells were harvested and assayed for markers of apoptosis by (A) cell death enzyme-linked immunosorbent assay measuring cytosolic histone-bound DNA; or (B) immunoblotting for the presence of cleaved caspases 3 and 8, as well as PARP cleavage. C: T-47D cells were transfected with siRNAs and seeded into polyHEMA-coated plates in the presence of carrier (dimethyl sulfoxide) or 20 μmol/L zVAD-FMK. The proportion of trypan blue incorporating cells was assessed as a percentage. D: Adherent T-47D cells were treated with carrier (dimethyl sulfoxide), 1.5 μmol/L staurosporine (STS), or 1.5 μmol/L staurosporine plus 20 μmol/L zVAD-FMK (S+Z) and lysed in SDS-polyacrylamide gel electrophoresis sample buffer. Lysates were analyzed by Western blotting for the presence of cleaved caspase-3 to confirm efficacy of the zVAD-FMK.
By assessing histone-bound DNA in the cytoplasm resulting from cell death–associated DNA cleavage, we observed no difference in DNA fragmentation between control and Brk-targeted cells after 24 hours on polyHEMA (Figure 4A). Activation-associated cleavage of caspases 3 and 8 were not detected in response to Brk suppression in non-adherent T-47D cells; nor was cleavage of PARP, a caspase target in apoptotic cells (Figure 4B). T-47D cells treated with 1.5 μmol/L staurosporine were included in these experiments as a positive control to confirm that DNA breaks, caspase activation, and PARP cleavage could be detected in T-47D cells undergoing apoptosis (Figure 4, A and B).
Caspases are widely believed to be important executioners of apoptosis, and their activation is documented in the induction of apoptosis by staurosporine in T-47D cells.17 Untransformed epithelial cells undergoing detachment-induced cell death show an increase in the activation of both caspases 3 and 8.18 Although caspase-8 is believed to be the effector caspase that initiates the cellular events after detachment that result in apoptosis, it is also activated at a later stage, possibly through a positive feedback mechanism in some cell types.19 To determine whether other caspases might be involved, we repeated the siRNA transfections and seeded cells into polyHEMA-coated wells, with the addition of the broad spectrum caspase inhibitor zVAD-FMK in replicate wells transfected with Brk siRNA (Figure 4C). There was no change in trypan blue inclusion observed in the Brk-suppressed cells treated with zVAD-FMK, when compared with the dimethyl sulfoxide carrier-treated and Brk-suppressed cells (P > 0.24). To confirm the efficacy of the caspase inhibitor, adherent T-47D cells were also treated with 1.5 μmol/L staurosporine and zVAD-FMK, and caspase-3 cleavage assessed by Western blotting. Caspase-3 cleavage was detected in cells treated with staurosporine; however, cleavage was notably reduced on inclusion of the caspase inhibitor, confirming that 20 μmol/L zVAD-FMK is sufficient to inhibit caspase activation-associated cleavage in this cell line (Figure 4D).
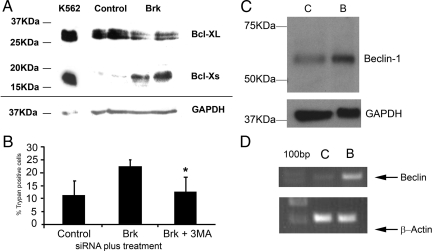
We now know that there are caspase-independent cell death pathways, such as autophagy [reviewed in20], and the promotion of cell death by some agents in cells, including T-47D, has already been shown to be independent of any known caspases.21,22 Western blots of lysates from Brk-suppressed cells in suspension culture showed a decrease in the levels of Bcl-xL relative to control transfectants. An increase in Bcl-xS was also observed (Figure 5A). As Bcl-xL has been shown to play a role in regulating autophagy,23 the hypothesis that Brk-targeting and suspension culture induced autophagic, not apoptotic, cell death was tested. Cells were transfected with siRNA and analyzed for the presence of membrane bound vesicles by electron microscopy and for changes in levels of Beclin-1. Beclin-1 is a critical protein in the induction of autophagy.24 It interacts with Vps34 and Vps15 in the Beclin-1 interactome to induce cell death25,26 and is inhibited by binding to Bcl-2 and Bcl-xL.27,28,29 Cells that were transfected with either control or Brk-targeting siRNA were found to contain membrane bound vesicles, characteristic of autophagic cells, although little difference in absolute number was seen at the time points measured (data not shown). Inclusion of the autophagy inhibitor 3-methyladenine in the suspension cultures resulted in a significant decrease in cell death in Brk-targeted cells compared with treatment with Brk siRNA alone. The levels of cell death were decreased to a comparable level to that shown by the control transfectants (Figure 5B). Beclin-1 mRNA and protein were found to be increased in Brk-suppressed suspension cultures compared with controls (Figure 5, C and D).
Figure 5.
Brk-regulated cell death has hallmarks of autophagy. T-47D cells were transfected with either control (Control/C) or Brk–targeting (Brk/B) siRNAs and seeded into polyHEMA coated plates. After 24 hours cells were harvested and assayed for (A) Bcl-x expression by Western blotting. B: Cells were seeded with or without the autophagy inhibitor 3-methyladenine and % cell death determined by trypan blue inclusion. *P = 0.005. After 24 hours on polyHEMA, protein and RNA were harvested and assayed by (C) Western blotting and (D) reverse transcription-PCR for Beclin-1 expression.
Thus detachment-induced cell death due to Brk suppression does not manifest many of the common molecular features of apoptosis, but is more characteristic of autophagy.
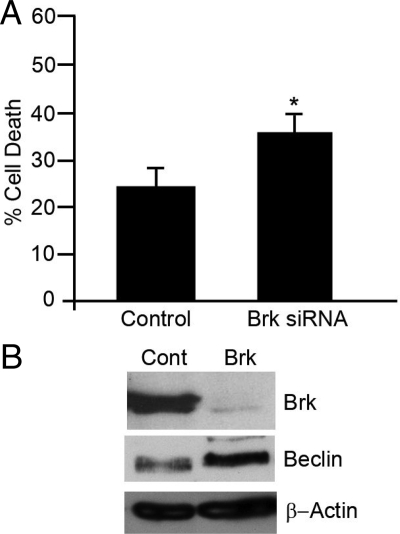
To confirm that the protection from autophagic cell death was not cell line specific, GI101 cells were transfected with Brk targeting or control siRNA, and cells were subsequently suspended in polyHEMA-coated wells for 20 hours. The levels of cell death were significantly increased in the Brk-suppressed cells (Figure 6A), but to a slightly lesser extent than previously seen in T-47D cells, possibly because the background level of cell death in the control transfectants was higher. In addition, the increase in cell death in GI101 cells was accompanied by an increase in Beclin expression (Figure 6B).
Figure 6.
Autophagic phenotype is not cell line-specific. GI101 cells were transfected with either control (Control/Cont) or Brk–targeting (Brk) siRNAs and seeded into polyHEMA-coated plates. After 20 hours, cells were harvested and (A) % cell death determined by trypan blue inclusion; *P = 0.007. B: Beclin-1 levels were determined by Western blotting.
Discussion
We have previously shown that Brk suppression results in decreased cell proliferation in the breast carcinoma cell line T-47D,6 although we have not previously established a link between Brk expression and breast cancer progression. We show here that Brk expression levels are significantly higher in grade 3 breast cancers, as compared with those samples graded as either 1 or 2. This study corroborates the findings of other groups,3,30 showing that Brk is expressed in a higher proportion of tumors than previously reported and that there is a relationship between expression levels and breast cancer grade,31 with Brk expression being elevated in tumors whose grade is typically associated with poor prognosis [eg,15]. Our results show a marked similarity to those published as part of a larger study by Lange and colleagues,30 suggesting that, at least in the Western hemisphere, Brk expression is not a prognostic indicator that is confined to geographical location. Other investigators have identified positive associations between Brk mRNA levels in breast tumors and positive estrogen receptor/postmenopausal status2 and HER2 expression.3 Positive association of Brk expression with estrogen receptor expression may also explain why it has been reported previously that Brk expression is correlated with increased disease-free survival, despite expression levels being higher in tumors of higher grade. The positive association of Brk with HER2 is further strengthened by the demonstration of co-amplification of Brk and HER2, as well as co-expression, in breast cancers. Brk overexpression promoted cell cycle progression induced by HER2 through activation of the MAPK pathway and the Cyclin E/CDK2 complex in the non-transformed mammary cell line MCF-10A.32 More recent studies have indicated that the previously identified Brk substrate BKS/STAP-233 may be required for Brk’s modulation of downstream signaling elements such as STAT3.34 Future studies will reveal whether Brk’s role in promoting HER2-induced proliferation also requires BKS/STAP-2.
As Brk expression levels correlate with tumor grade, we hypothesized that Brk plays a role in disease progression in Brk positive tumors. We used RNA interference to reduce Brk protein levels to assess its role in protecting breast cancer cells from suspension-induced cell death as anchorage independent survival is a key process in metastasis.12,35 We found that Brk-targeted cells showed an increase in the percentage of dead cells when suspended on polyHEMA for 24 hours, relative to control transfectants, but did not exhibit the recognized hallmarks of apoptosis. The cells rather demonstrated some of the features of autophagy, suggesting that decreased Brk expression in conjunction with suspension culture can initiate autophagic cell death in breast carcinoma cells. Conversely, experimental Brk expression protected breast tumor cells from suspension-induced death and this protection appeared to be dependent on the kinase activity of Brk. These data support the hypothesis that Brk plays a role in protecting breast cancer cells from detachment-induced cell death. Therefore Brk expression in carcinomas may confer a survival advantage on metastasizing tumor cells. This is in contrast to the data presented recently, where nontransformed rat fibroblasts transfected with Brk were sensitized to inducers of apoptosis rather than gaining a survival advantage, suggesting that Brk may not be oncogenic in such cells.36 Indeed, Brk’s ability to inhibit Akt activity (in COS1 cells) might underlie such a pro-apoptotic role for Brk.37 However, in support of Brk having pro-tumorigenic activity, parallels can be drawn with data on c-myc, as myc has long been known to have oncogenic properties but is also capable of sensitizing fibroblasts to induction of apoptosis by serum deprivation [38 and refs therein,39,40]. Constitutively active Brk has been shown to increase proliferation, anchorage-independent colony formation and cell survival in HEK293 cells,7 further underlining the fact that that the functions of Brk are likely to be dependent on cellular context.31
So far, physiological Brk expression has not been detected in nonepithelial cells, its normal expression pattern being restricted to differentiating or differentiated epithelial cells in the skin, gut, prostate, and oral mucosa.41,42,43,44 Our data identify a role for Brk in protecting cells from a form of cell death that is not classically apoptotic but has features of autophagy. Altering the ratio of Bcl-xL:Bcl-xS by targeting Brk could reduce the Bcl-xL-mediated inhibition of Beclin-1. Binding of Beclin to Bcl-xL or Bcl-227,28,29 inhibits Beclin, as it is then not available to bind to Vps34 and Vps15 in the Beclin interactome.25,26 Dissociation of Beclin-1 from Bcl- xL is required for the induction of autophagy and this process can be mediated by phosphorylation of Beclin on Thr119.45,46 In this study, Bcl-xL levels were reduced on Brk targeting, suggesting that there could be reduced inhibition of Beclin. We also demonstrated that Brk suppression resulted in increased Beclin expression, which contributed to the increased cell death observed. Recently epidermal growth factor has been shown to protect bovine mammary cells from autophagic cell death47 providing further evidence that Brk’s role in augmenting the effects of epidermal growth factor signaling in mammary cells5,32 may not be limited solely to proliferation and may include protection from cell death. In addition, given that allelic loss of Beclin-1 is common in breast cancers,24,48,49 our expression data suggest that abrogation of Brk function may provide a mechanism for restoring the autophagy pathway in cells with decreased Beclin-1 levels. It remains to be seen however whether this function is manifest in other Brk-positive tumor cells from tissues other than the breast, such as the colon and prostate.
Together with previous studies showing that experimental Brk expression increases colony formation by nontransformed breast epithelial cells on detachment5 and more recent data proposing a role for Brk in invasion,50 our data show a potential role for Brk in one of the processes that control metastasis of breast cancer cells: promoting anchorage-independent survival. When considered alongside the fact that Brk expression is highest in grade 3 tumors, targeting Brk, possibly by kinase inhibition may provide the basis for antimetastatic therapy.
Supplementary Material
Acknowledgments
We thank Dr. Fiona Foster for help with the fluorescence-activated cell sorting analysis.
Footnotes
Address reprint requests to Amanda Harvey, the Brunel Institute for Cancer Genetics and Pharmacogenomics, Biosciences, School of Health Sciences and Social Care, Brunel University, Kingston Lane, Uxbridge, Middlesex, UB8 3PH. United Kingdom. E-mail: amanda.harvey@brunel.ac.uk.
Supported by project grants from the Breast Cancer Campaign (awarded to M.R.C./A.J.H./S.A.E. and D.E.) and the EU Framework Programme 6 Cancer Degradome project (LSHC-CT-2003-503297). We acknowledge NHS funding to the NIHR Biomedical Research Centre.
This work was initiated at Royal Holloway and completed at Brunel University.
References
- Barker KT, Jackson LE, Crompton MR. Brk tyrosine kinase expression in a high proportion of human breast carcinomas. Oncogene. 1997;15:799–805. doi: 10.1038/sj.onc.1201241. [DOI] [PubMed] [Google Scholar]
- Zhao C, Yasui K, Lee CJ, Kurioka H, Hosokawa Y, Oka T, Inazawa J. Elevated levels of NCOA3. TOP1, TFAP2C in breast tumors as predictors of poor prognosis. Cancer. 2003;98:18–23. doi: 10.1002/cncr.11482. [DOI] [PubMed] [Google Scholar]
- Born M, Quintanilla-Fend L, Braselmann H, Reich U, Richter M, Hutzler P, Aubele M. Simultaneous over-expression of the Her2/neu and PTK6 tyrosine kinases in archival invasive ductal breast carcinomas. J Pathol. 2005;205:592–596. doi: 10.1002/path.1720. [DOI] [PubMed] [Google Scholar]
- Harvey AJ, Crompton MR. The Brk tyrosine kinase as a novel therapeutic target in breast cancer: opportunities and challenges. Anticancer Drugs. 2004;15:107–111. doi: 10.1097/00001813-200402000-00002. [DOI] [PubMed] [Google Scholar]
- Kamalati T, Jolin HE, Mitchell PJ, Barker KT, Jackson KE, Dean CJ, Page MJ, Gusterson BA, Crompton MR. Brk, a breast tumor derived non-receptor tyrosine kinase, sensitizes mammary epithelial cells to epidermal growth factor. J Biol Chem. 1996;271:30956–30963. doi: 10.1074/jbc.271.48.30956. [DOI] [PubMed] [Google Scholar]
- Harvey AJ, Crompton MR. Use of RNA interference to validate Brk as a novel therapeutic target in breast cancer: brk promotes breast carcinoma cell proliferation. Oncogene. 2003;22:5006–5010. doi: 10.1038/sj.onc.1206577. [DOI] [PubMed] [Google Scholar]
- Kim HI, Lee S-T. An intramolecular interaction between SH2-kinase linker and kinase domain is essential for the catalytic activity of protein-tyrosine kinase-6. J Biol Chem. 2005;280:28973–28980. doi: 10.1074/jbc.M504568200. [DOI] [PubMed] [Google Scholar]
- Lukong KE, Larocque D, Tyner AL, Richard S. Tyrosine phosphorylation of sam68 by breast tumor kinase regulates intranuclear localization and cell cycle progression. J Biol Chem. 2005;280:38639–38647. doi: 10.1074/jbc.M505802200. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Frisch SM, Francis H. Disruption of epithelial cell-matrix interactions induces apoptosis. J Cell Biol. 1994;124:619–626. doi: 10.1083/jcb.124.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruoslahti E, Reed JC. Anchorage dependence, integrins and apoptosis. Cell. 1994;77:477–478. doi: 10.1016/0092-8674(94)90209-7. [DOI] [PubMed] [Google Scholar]
- Behrens J, Mareel MM, Van Roy FM, Birchmeier W. Dissecting tumor cell invasion: epithelial cells acquire invasive properties after the loss of uvomorulin-mediated cell-cell adhesion. J Cell Biol. 1989;108:2435–2447. doi: 10.1083/jcb.108.6.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddick ACP, Barker C, Sheriffs I, Bass R, Ellis V, Sethis KK, Edwards DR, Ball RY. Banking of fresh-frozen prostate tissue: methods, validation and use. BJU Int. 2003;91:315–323. doi: 10.1046/j.1464-410x.2003.03041.x. [DOI] [PubMed] [Google Scholar]
- Wall SJ, Edwards DR. Quantitative reverse transcription-polymerase chain reaction (RT-PCR): a comparison of primer-dropping, competitive, and real-time RT-PCRs. Anal Biochem. 2002;300:269–273. doi: 10.1006/abio.2001.5458. [DOI] [PubMed] [Google Scholar]
- Porter GJR, Evans AJ, Pinder SE, James JJ, Cornford EC, Burrell HC, Chan SY, Cheung KL, Robertson JFR. Patterns of metastatic breast cancer: influence of tumor histological grade. Clinical Radiology. 2004;59:1094–1098. doi: 10.1016/j.crad.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Van Engeland M, Nieland LJ, Ramaekers FC, Schutte B, Reutelingsperger CP. Annexin V-affinity assay: a review on an apoptosis detection system based on phosphatidylserine exposure. Cytometry. 1998;31:1–9. doi: 10.1002/(sici)1097-0320(19980101)31:1<1::aid-cyto1>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Mooney LK, Al-Sakkaf KA, Brown BL, Dobson PR. Apoptotic mechanisms in T47D and MCF-7 human breast cancer cells. Br J Cancer. 2002;87:909–917. doi: 10.1038/sj.bjc.6600541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rytomaa M, Martins LM, Downward J. Involvement of FADD and caspase-8 signalling in detachment induced apoptosis. Curr Biol. 1999;9:1043–1046. doi: 10.1016/s0960-9822(99)80454-0. [DOI] [PubMed] [Google Scholar]
- Grossmann J, Walther K, Artinger M, Kiessling S, Scholmerich J. Apoptotic signalling during initiation of detachment-induced apoptosis (“anoikis”) of primary human intestinal epithelial cells. Cell Growth Diff. 2001;12:147–155. [PubMed] [Google Scholar]
- Broker LE, Kruyt FAE, Giaccone G. Cell death independent of caspases: a review. Clin Cancer Res. 2005;11:3155–3162. doi: 10.1158/1078-0432.CCR-04-2223. [DOI] [PubMed] [Google Scholar]
- Cheng EH, Wei MC, Weiler S, Flavell RA, Mak TW, Lindsten T, Korsmeyer SJ. Bcl-2. Bcl-xl sequester BH3 domain-only molecules preventing Bax- and Bak- mediated mitochondrial apoptosis. Mol Cell. 2001;8:705–711. doi: 10.1016/s1097-2765(01)00320-3. [DOI] [PubMed] [Google Scholar]
- Mathiasen IS, Lademann U, Jaattela M. Apoptosis induced by vitamin D compounds in breast cancer cells is inhibited by Bcl-2 but does not involve known caspases or p53. Cancer Res. 1999;59:4848–4856. [PubMed] [Google Scholar]
- Yanagisawa H, Miyashita T, Nakano Y, Yamamoto D. HSpin1, a transmembrane protein interacting with Bcl-2/Bcl-xL, induces a caspase-independent autophagic cell death. Cell Death Differ. 2003;10:798–807. doi: 10.1038/sj.cdd.4401246. [DOI] [PubMed] [Google Scholar]
- Liang XH, Jackson S, Seaman M, Brown K, Kempkes B, Hibshoosh H, Levine B. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature. 1999;402:672–676. doi: 10.1038/45257. [DOI] [PubMed] [Google Scholar]
- Kametaka S, Okano T, Ohsumi M, Ohsumi Y. Apg14p and Apg6/Vps30p form a protein complex essential for autophagy in the yeast, Saccharomyces cerevisiae. J Biol Chem. 1998;273:22284–22291. doi: 10.1074/jbc.273.35.22284. [DOI] [PubMed] [Google Scholar]
- Kihara A, Kabeya Y, Ohsumi Y, Yoshimori T. Beclin-phosphatidylinositol 3-kinase complex functions at the trans-Golgi network. EMBO Rep. 2001;2:330–335. doi: 10.1093/embo-reports/kve061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiuri MC, Le Toumelin G, Criollo A, Rain JC, Gautier F, Juin P, Tasdemir E, Pierron G, Troulinaki K, Tavernarakis N, Hickman JA, Geneste O, Kroemer G. Functional and physical interaction between Bcl-X(L) and a BH3-like domain in Beclin-1. EMBO J. 2007;26:2527–2539. doi: 10.1038/sj.emboj.7601689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, Mizushima N, Packer M, Schneider MD, Levine B. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005;122:927–939. doi: 10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Levine B, Sinha S, Kroemer G. Bcl-2 family members: dual regulators of apoptosis and autophagy. Autophagy. 2008;4:600–606. doi: 10.4161/auto.6260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrander JH, Daniel AR, Lofgren K, Kleer CG, Lange CJ. Breast tumor kinase (protein tyrosine kinase 6) regulates heregulin-induced activation of ERK5 and p38 MAP kinases in breast cancer cells. Cancer Res. 2007;67:4199–209. doi: 10.1158/0008-5472.CAN-06-3409. [DOI] [PubMed] [Google Scholar]
- Aubele M, Auer G, Walch AK, Munro A, Atkinson MJ, Braselmann H, Fornander T, Bartlett JM. PTK (protein tyrosine kinase)-6 and HER2 and 4, but not HER1 and 3 predict long-term survival in breast carcinomas. Br J Cancer. 2007;96:801–807. doi: 10.1038/sj.bjc.6603613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang B, Chatti K, Qiu H, Lakshmi B, Krasnitz A, Hicks J, Yu M, Miller WT, Muthuswamy SK. Brk is coamplified with ErbB2 to promote proliferation in breast cancer. Proc Natl Acad Sci USA. 2008;105:12463–12468. doi: 10.1073/pnas.0805009105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell PJ, Sara EA, Crompton MR. A novel adaptor-like protein that is a substrate for the non-receptor tyrosine kinase. BRK Oncogene. 2000;19:4273–4282. doi: 10.1038/sj.onc.1203775. [DOI] [PubMed] [Google Scholar]
- Ikeda O, Miyasaka Y, Sekine Y, Mizushima A, Muromoto R, Nanbo A, Yoshimura A, Matsuda T. STAP-2 is phosphorylated at tyrosine-250 by Brk and modulates Brk-mediated STAT3 activation. Biochem Biophys Res Commun. 2009;384:71–75. doi: 10.1016/j.bbrc.2009.04.076. [DOI] [PubMed] [Google Scholar]
- Simpson CD, Anyiwe K, Schimmer AD. Anoikis resistance and tumor metastasis. Cancer Lett. 2008;272:177–185. doi: 10.1016/j.canlet.2008.05.029. [DOI] [PubMed] [Google Scholar]
- Haegebarth A, Nunez R, Tyner AL. The intracellular tyosine kinase Brk sensitizes non-transformed cells to inducers of apoptosis. Cell Cycle. 2005;4:1239–1246. doi: 10.4161/cc.4.9.1965. [DOI] [PubMed] [Google Scholar]
- Zhang P, Ostrander JH, Faivre EJ, Olsen A, Fitzimmons D, Lange CA. Regulated association of protein kinase B/Akt with breast tumor kinase. J Biol Chem. 2005;280:1982–1991. doi: 10.1074/jbc.M412038200. [DOI] [PubMed] [Google Scholar]
- Ramsay M, Evan GI, Bishop JM. The protein encoded by the human proto-oncogene c-myc. Proc Nat Acad Sci USA. 1984;81:7742–7746. doi: 10.1073/pnas.81.24.7742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evan GI, Wyllie AH, Gilbert CS, Littlewood TD, Land H, Brooks M, Waters CM, Penn LZ, Hancock DC. Induction of apoptosis in fibroblasts by c-myc protein. Cell. 1992;69:119–128. doi: 10.1016/0092-8674(92)90123-t. [DOI] [PubMed] [Google Scholar]
- Harrington EA, Bennett MR, Fanidi A, Evan GI. c-Myc-induced apoptosis in fibroblasts is inhibited by specific cytokines. EMBO J. 1994;139:3286–3295. doi: 10.1002/j.1460-2075.1994.tb06630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petro BJ, Tan RC, Tyner AL, Lingen MW, Watanbabe K. Differential expression of the non-receptor tyrosine kinase BRK in oral squamous cell carcinoma and normal oral epithelium. Oral Oncol. 2004;40:1040–1047. doi: 10.1016/j.oraloncology.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Llor X, Serfas MS, Bie W, Vasioukhin Y, Polonskaia M, Derry J, Abbott CM, Tyner AL. BRK/Sik expression in the gastrointestinal tract and in colon tumors. Clin Cancer Res. 1999;5:1767–1777. [PubMed] [Google Scholar]
- Vasioukhin V, Serfas MS, Siyanova EY, Polonskaia M, Costigan VJ, Liu B, Thomason A, Tyner AL. A novel intracellular epithelial cell tyrosine kinase is expressed in the skin and gastrointestinal tract. Onogene. 1995;10:349–357. [PubMed] [Google Scholar]
- Wang TC, Jee SH, Tsai TF, Huang YL, Tsai WL, Chen RH. Role of breast tumor kinase in the in vitro differentiation of HaCaT cells. Br J Dermatol. 2005;153:282–289. doi: 10.1111/j.1365-2133.2005.06604.x. [DOI] [PubMed] [Google Scholar]
- Zalckvar E, Berissi H, Mizrachy L, Idelchuk Y, Koren I, Eisenstein M, Sabanay H, Pinkas-Kramarski R, Kimchi A. DAP-kinase-mediated phosphorylation on the BH3 domain of beclin 1 promotes dissociation of beclin 1 from Bcl-XL and induction of autophagy. EMBO Reports. 2009;10:285–92. doi: 10.1038/embor.2008.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalckvar E, Berissi H, Eisenstein M, Kimchi A. Phosphorylation of Beclin 1 by DAP-kinase promotes autophagy by weakening its interactions with Bcl-2 and Bcl-X(L). Autophagy. 2009;5:720–722. doi: 10.4161/auto.5.5.8625. [DOI] [PubMed] [Google Scholar]
- Sobolewska A, Gajewska M, Zarzynska J, Gajkowska B, Motyl T. IGF-I. EGF, and sex steroids regulate autophagy in bovine mammary epithelial cells via the mTOR pathway. Eur J Cell Biol. 2009;88:117–130. doi: 10.1016/j.ejcb.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Aita VM, Liang XH, Murty VV, Pincus DL, Yu W, Cayanis E, Kalachikov S, Gilliam TC, Levine B. Cloning and genomic organization of beclin 1, a candidate tumor suppressor gene on chromosome 17q21. Genomics. 1999;59:59–65. doi: 10.1006/geno.1999.5851. [DOI] [PubMed] [Google Scholar]
- Karantza-Wadsworth V, White E. Role of autophagy in breast cancer. Autophagy. 2007;3:610–613. doi: 10.4161/auto.4867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HY, Shen CH, Tsai YT, Lin FC, Huang YP, Chen RH. Brk activates rac1 and promotes cell migration and invasion by phosphorylating paxillin. Mol Cell Biol. 2004;24:10558–10572. doi: 10.1128/MCB.24.24.10558-10572.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.