Abstract
WNK1 [with-no-lysine (K)−1] is a ubiquitous serine/threonine kinase with a unique placement of the catalytic lysine residue. Increased WNK1 expression levels in humans causes a hypertension-hyperkalemia syndrome by altering renal Na+ and K+ transport. The function of WNK1 outside of the kidney remains elusive. In this study, we report that Wnk1 ablation causes cardiovascular developmental defects. The developing heart of null mutant embryos has smaller chambers and reduced myocardial trabeculation at E10.5. Yolk sac vessels in the E10.5 null mutant fail to remodel into a network of large and small vessels, and embryonic vessels show defective angiogenesis that involves both arteries and veins. The arterial marker neuropilin-1 and venous marker EphB4 are ectopically expressed in mutant veins and arteries, respectively. However, the orphan nuclear receptor COUP-TFII as well as the Notch signaling pathway, which are known to be critical for angiogenesis and artery-vein specification, are not significantly altered in Wnk1−/− mutants. Conditional deletion of Wnk1 in endothelial cells phenotypically copies defects caused by global Wnk1 ablation. Moreover, endothelial-specific expression of a Wnk1 transgene rescues cardiovascular developmental defects in Wnk1−/− mice. These findings identify a novel function of WNK1 in endothelial cells that is critical for angiogenesis and heart development, raising the possibility for a role of endothelial WNK1 in the control of blood pressure and postnatal angiogenesis and cardiac growth.
The cardiovascular system is the first organ system to develop during embryogenesis. In developing mouse embryos, blood circulation begins at about day 8.5 of gestation.1,2 The development of the vascular system occurs via two separate processes known as vasculogenesis and angiogenesis. Vasculogenesis involves in situ differentiation of endothelial cells from precursor angioblasts and assembly of these differentiating endothelial cells into tube-shaped blood vessels. This process results in the formation of the primordial heart tube and major axial vessels as well as a homogenous primary capillary plexus in the embryo proper and extraembryonic structures such as the yolk sac. Angiogenesis involves further growth, sprouting, branching from the primary vessels and remodeling of the vascular plexus to form a tree-like system of large and small vessels, eventually resulting in a mature vasculature.1,2 Angiogenesis is responsible for the formation of blood vessels in organs, such as the brain and kidney. Integral to the development of vasculature is the recruitment of mural cells (smooth muscle and/or pericytes) to form the external walls of mature vessels. The arterial and venous sides of the vascular system are anatomically, functionally and molecularly distinct. Specification of arteries and veins is an essential process of vascular development that requires reciprocal interactions between arterial and venous fated endothelial cells. Failure in artery/vein specification results in angiogenesis defects and almost always in embryonic lethality.3,4
The molecular mechanisms controlling vascular development have been under intensive investigation, but much remains unknown. Dozens of genes have been identified that regulate the processes of vasculogenesis, angiogenesis, and artery-vein specification.5 Vascular endothelial growth factor (VEGF) and its receptors VEGF-R1 (Flt1) and VEGF-R2 (Flk1), and transforming growth factor-β are essential regulators of the initial differentiation and assembly of endothelial cell into primary vessel plexus in vasculogenesis. Additional factors, including angiopoietins/Tie receptors, VEGFs/VEGF receptors, fibroblast growth factors (FGFs), Notch signaling pathway, transforming growth factor-β/Endoglin/Smad5, matrix metalloproteinases (MMPs), ephrins and receptors, neuropilins, are also involved in modulation of angiogenesis. Early hypotheses proposed that activation of the VEGF-Notch signaling pathway confers arterial fate and a lack of activation results in the formation of veins by default.6 A more recent study, however, reports that the orphan nuclear receptor COUP-TFII is specifically expressed in venous-fated endothelial cells and actively confers venous fate by suppressing the VEGF-Notch signaling.7 Mice homozygous for deletion of COUP-TFII, however, do not display vascular defects in yolk sac,8 suggesting that yet additional factors are involved in the molecular determination of vein identity.
WNK1 [with-no-lysine (K)−1] is a member of unique serine/threonine protein kinase family characterized by an atypical location of the catalytic lysine.9 Despite the atypical kinase domain structure, WNK1 exhibits kinase activity and catalyzes phosphorylation of endogenous substrates.9 Three additional members, WNK2, WNK3, and WNK4, are present in mammals.9,10,11 Each member of the WNK family is encoded by a separate gene. WNK1 protein is over 2100 amino acids long, whereas WNK2, WNK3, and WNK4 range between 1200 and 1600 amino acids in length. The four WNK kinases share a conserved kinase domain, an autoinhibitory domain, 1–2 coiled-coil domains, and multiple proline-rich motifs for potential protein-protein interactions.9,10,11 Few recognizable motifs exist beyond the aforementioned conserved domains/ motifs.
The human as well as mouse WNK1 gene each consists of 28 exons and undergoes tissue-specific alternative splicing.12,13 A full-length WNK1 encoded by all 28 exons is ubiquitously expressed.9,12,13 A shorter splice variant of WNK1 that lacks the region encoded by the first four exons is predominantly expressed in the kidney.12 WNK2 is predominantly expressed in heart, brain and colon.11 WNK3 and WNK4 are expressed in kidney, heart, and brain.11,14,15 In addition, WNK4 is expressed in many epithelial tissues.15
WNK1 regulates sodium channel ENaC and the thiazide-sensitive sodium-chloride cotransporter by altering their cell surface abundance.16,17 WNK1 has also been reported to interact with intersectin, a multimodular endocytic scaffold protein, to regulate internalization of a renal potassium channel ROMK1 from the cell surface.18 Large intronic deletions of WNK1 that lead to its overexpression cause pseudohypoaldosteronism type II (PHA II), an autosomal dominant disorder featuring hypertension and hyperkalemia (high blood potassium level).10 Alterations in WNK1 regulation of sodium and potassium transport in kidney contribute, at least partly, to the pathogenesis of hypertension and hyperkalemia in patients with WNK1 mutations.
Mice heterozygous for Wnk1 mutation generated by a gene-trapping insertion within intron-1 have hypotension, supporting an important role of WNK1 in the control of blood pressure.19 Wnk1 heterozygous mice, however, do not display apparent defects in renal Na+ transport, raising the possibility for additional role of WNK1 in other tissues in the control of blood pressure. Mice homozygous for Wnk1 mutation die before embryonic day 13 (E13), indicating an essential role of WNK1 in embryogenesis. However, the precise role of WNK1 in the embryonic development is not known. Here, we show that Wnk1-ablated mice display cardiac developmental and angiogenesis defects. The angiogenesis defect is evident in both yolk sac and embryos and is associated with an aberrant expression of arterial and venous markers. The defects in Wnk1-null embryos, however, are distinct from arterial-venous specification abnormalities observed in embryos with alterations of COUP-TFII and Notch signaling pathway. Conditional deletion of Wnk1 in endothelial cells phenocopies defects caused by global Wnk1 ablation. Endothelial-specific expression of a Wnk1 transgene rescues cardiovascular developmental defects in Wnk1−/− mice. These findings suggest that function of WNK1 in endothelial cells defines a novel mechanism for regulating angiogenesis and establishing or maintaining artery/vein identity and raise the possibility for a role of endothelial WNK1 in the control of blood pressure.
Materials and Methods
Mouse Strains and Animal Procedure
Wnk1+/− mice produced by a gene-trap insertion in intron-1 have been described.19 Tie2-Cre transgenic mice were from the Jackson Laboratories (Bar Harbor, Maine). Flk1-Cre/+ knockin mice were the gift of Dr. T. Sato.20 Sox2-Cre transgenic mice were the gift of Dr. T. Carroll and Dr. A. McMahon.21 All animal maintenance and experiments were conducted in accordance with the Guide for the Use and Care of Laboratory Animals and approved by the Institutional Animal Care and Use Committee. For timed breeding, the appearance of vaginal plug was checked twice a day at 9 a.m. and 6 p.m. At 1 a.m. or 1 p.m. of the day in which a plug was found was designated as embryonic day 0. The embryonic stage was further confirmed by the number of somites at the time of embryo dissection. Mouse tail tips, ear punches, toe clips, or portions of yolk sacs of embryos were digested in Viagen DirectPCR reagents (Viagen Biotech, Los Angeles, CA) with 0.2 mg/ml proteinase K at 55°C overnight, heat-inactivated at 85°C for 45 minutes, and analyzed by polymerase chain reaction (PCR). All genotyping PCR reactions were performed with 35 cycles of 95°C, 20 seconds; 60°C, 30 seconds; and 72°C, 1 minute. The primers for genotyping of Wnk1 knockout were as described.19 Sequences of other primers for genotyping are in Table 1.
Table 1.
List of Primers Used for Genotyping and Real-Time PCR
| Primer | Sequence |
|---|---|
| cF1 | 5′-TCTGTCTCTGCCTCCCAAGT-3′ |
| cF2 | 5′-TGACATCTGGAACACTTAAGACG-3′ |
| cR1 | 5′-CTGAGTTCAGCTTTCAGCATCCATGTC– TAG-3′ |
| cR2 | 5′-AGGGGATCGGCAATAAAAAG-3′ |
| F1 | 5′-GAGTTCTCTGCTGCCTCCTG-3′ |
| F2 | 5′-CCCCCTGAACCTGAAACATA-3′ |
| R1 | 5′-CTCCCACTCAGCACTGACAA-3′ |
| VEGF | 5′-GGCTTTACTGCTGTACCTCCA-3′, |
| 5′-ACAGGACGGCTTGAAGATGTA-3′ | |
| Ang1 | 5′-TCCTGACTCAGCACCATGAC-3′, |
| 5′-GATGGCCTTGATGTTGCTCT-3′ | |
| Ang2 | 5′-CGACTACGACGACTCAGTGC-3′, |
| 5′-TCTGGTTCTGCACCACATTC-3′ | |
| Hey1 | 5′-GGTACCCAGTGCCTTTGAGA-3′, |
| 5′-ACCCCAAACTCCGATAGTCC-3′ | |
| Hey2 | 5′-GGTCCAATTCACCGACAACT-3′, |
| 5′-TGGCAGATCCTTGTTTTTCA-3′ | |
| Neuropilin1 | 5′-CGTCACACTCATGCACTGG-3′, |
| 5′-CCCTGAGAGAGCCACACACA-3′ | |
| Neuropilin2 | 5′-TGTTCTGTCATTGGGGTTAGC-3′, |
| 5′-AACTGCAACTTTGATTTTCCG-3′ | |
| Ephrin-B2 | 5′-GCGGGATCCAGGAGATCCCCAC– TTGGACT-3′, |
| 5′-GTGCGCAACCTTCTCCTAAG-3′ | |
| EphB4 | 5′-GCGGGATTCCAGCGCTCTGGACAA– GATGAT-3′, |
| 5′-CATCTCAAAGGAGCCGAATC-3′ | |
| COUP-TFII | 5′-AAGCACTACGGCCAGTTCAC-3′, |
| 5′-AGGCATCCTGCCTCTCTGTA-3′ | |
| Flt-1 | 5′-GCTGCTTGGAGATCTCACTG-3′, |
| 5′-TCAGCAGCTCAAGTGTCACC-3′ | |
| Flt-4 | 5′-TGCATGCTGGGTGGACTATCA-3′, |
| 5′-GCAGGAGGAGGAAGAGGAGC-3′ | |
| Tie2 | 5′-GGACAGTGCTCCAACCAAATG-3′, |
| 5′-GACGGAAATGTTGAAAGGC-3′ | |
| VCAM1 | 5′-GCTCAAATCGGTGACTCCAT-3′, |
| 5′-AACTGACAGGCTCCATGGTC-3′ | |
| SM22 | 5′-ACGATGGAAACTACCGTGGA-3′, |
| 5′-GCTGGCCTTCCCTTTCTAAC-3′ | |
| Sox18 | 5′-CTCCCTGTCACCAACGTCTC-3′, |
| 5′-GTGCAGATCCGGATTTTGTT-3′ |
ROSA-Wnk1 Targeting and Rescue
A full-length rat WNK1 cDNA fragment was released from a pBluescript-WNK1 plasmid9 by digestion with XbaI and SalI. The XbaI-SalI fragment was inserted between compatible NheI and XhoI sites in the multiple cloning sites of pBigT plasmid22 downstream of the loxP-PGKneo-tpA-loxP cassette. The resulting pBigT-WNK1 construct was then digested with PacI and AscI into a ∼6-kb PacI-AscI fragment and a ∼5-kb PacI-PacI fragment. These two fragments were subsequently inserted into PacI-AscI sites of ROSA26PAS vector,22 between the short arm and long arm of ROSA26 genomic sequence, to create the ROSA26-WNK1 targeting construct. Targeting construct was linearized by XhoI before electroporation.
Culture and electroporation of ES cells and injection of ES cells into blastocysts were performed by the Transgenic Core Facility of University of Texas Southwestern Medical Center. For screening of ES cell clones positive for targeted ROSA-Wnk1 (RW) allele, electroporated G418-resistant ES cells were digested with Viagen DirectPCR reagent and 0.2 mg/ml proteinase K at 55°C for 4 hours, heat inactivation at 85°C for 40 minutes, and analyzed by PCR. PCR primers consisted of forward primer located upstream of the ROSA26 short arm (5′-AGGTAGGGGATCGGGACTCT-3′) and reverse primer in the 5′ loxP site of targeting vector (5′-GCAGGTCGAGGGACCTAATA-3′). Another reverse primer located in the ROSA26 short arm (5′-TAAGCCTGCCCAGAAGACTC-3′) was included in the reaction for internal PCR control. PCR was performed according to the manufacturer’s instructions (HotStarTaq, Qiagen, Valencia, CA) with 35 cycles of 95°C, 30 seconds; 60°C, 30 seconds; and 72°C, 2 minutes. PCR product for wild-type ROSA26 allele was about 1.2 kb; product for targeted ROSA26-Wnk1 allele was about 1.4 kb (data not shown). ES cell clones positive for the targeted RW allele were expanded and injected into C57BL/6 blastocysts to generate chimera founders. More than 30 male chimeras with 80% to 100% agouti coat color were backcrossed with C57BL/6 females. Germ-line transmission was determined by presence of agouti offspring and confirmed by genotyping PCR using primers specific for the targeting vector or rat WNK1 cDNA.
Generation of Wnk1flox/flox Mice
The exon 2 of Wnk1 and two ∼3-kb genomic fragments flanking exon 2 (left and right arms) were amplified from mouse genomic DNA and subcloned into OsDupDel targeting vector (gift of Dr. L. James and Dr. O. Smithies)23, so that the exon 2 is in front of a reverse-oriented Neo cassette. The exon2-neo fragment was flanked at each end by single loxP site. This targeting construct was used to generate Wnk1flox mice as described above.
Histology and Immunohistochemistry
For routine histological analysis, embryos were fixed with 4% paraformaldehyde at 4°C overnight. Thereafter, they were either embedded in OCT and frozen by dry ice/ethanol for cryosectioning or dehydrated (serial submersion in 50%, 70%, 95%, and 100% ethanol solution) and embedded in paraffin wax for sectioning. Hematoxylin and eosin staining was performed on paraffin-embedded sections. Immunostaining using polyclonal antibodies to Neuropilin-1 (R&D Systems, Minneapolis, MN; 1:200) and EphB4 (R&D Systems, Minneapolis, MN; 1:100) was performed on cryosections as described.7 Fluorescence-labeled second antibodies from the Jackson ImmunoResearch Laboratories (West Grove, PA) were used as instructed. Whole-mount immunostaining by anti-PECAM1 antibodies (at 1:300 dilution) was performed as described with modifications.7 Signal was detected using a biotinylated second antibody (at 1:500) and Vectastain Elite ABC kit (Vector Labs, Burlingame, CA).
In Situ Hybridization of Wnk1
Exons 6-9 of Wnk1 was PCR-subcloned into pBluescript using the primers previously described.12 Digoxigenin-labeled riboprobe was made from this fragment using digoxigenin-UTP (Roche, Indianapolis, IN) and MaxiScript in vitro transcription kit (Ambion, Austin, TX). For in situ hybridization on sections, the glass slides were washed three times for 3 minutes in phosphate-buffered saline, treated with 15 μg/ml proteinase K for 10 minutes, then rinsed in phosphate-buffered saline and fixed in 4% PFA for 5 minutes, and acetylated for 10 minutes (acetylation solution: 2.66 ml of triethanolamine, 350 μg of HCl, 750 μg of acetic anhydride, and 200 ml of water). Then the slides were transferred to a slide mailer containing hybridization buffer (50% formamide (Fisher, Pittsburgh, PA), 5X standard saline citrate (SSC), pH 4.5, 50 μg/ml ribonucleic acid from torula yeast, type VI (Sigma, St. Louis, MO), 1% sodium dodecyl sulfate, 50 μg/ml heparin (Sigma) for prehybridization at room temperature for 1 hour. Probe hybridization was performed with 100 μl of probe/slide (1 μg/ml Digoxigenin-labeled probe in hybridization solution, covered with coverslip) in a humidified chamber (humidified with 50% formamide/5X SSC) at 68°C overnight. After hybridization, the slides were immersed in 2X SSC at 72°C until coverslips separated, rinsed in 0.2X SSC at 72°C and room temperature for 1 minute each, rinsed briefly in MBST buffer (100 mmol/L maleic acid, 150 mmol/L NaCl, pH 7.5, 0.1% Tween 20), and then incubated in blocking solution (2% blocking reagent from Roche, 5% heat-inactivated sheep serum in MBST) for 1 hour at room temperature. Then the slides were applied with 250 μl of anti-Digoxigenin antibody (Roche), covered with parafilm, and incubated in a chamber humidified with MBST at 4°C overnight. The slides were then washed in MBST three times 30 minutes, and treated in NTMT (100 mmol/L NaCl, 100 mmol/L Tris, pH 9.5, 50 mmol/L MgCl2, 0.1% Tween 20) three times for 5 minutes. Color reaction was done in BM purple solution (Roche), and the slides were sealed using Permount (Fisher SP15-500).
Quantitative Real-Time PCR
Total RNAs were extracted from tissues (embryo, yolk sac, etc) using TRIzol (Invitrogen, Carlsbad, CA). DNase-treated RNAs (1–2 μg) were reverse-transcribed using a TaqMan reverse-transcription kit (Applied Biosystems, Foster City, CA). The resulting cDNA samples (5 ng at 1–2 ng/μl dilution) were analyzed in real-time PCR reactions using SYBR PCR reagents (Bio-Rad, Hercules, CA) and the real-time PCR system from Applied Biosystems. Sequences of primers for genes analyzed by real time are in Table 1.
Results
Wnk1−/− Embryos Die between E10.5 and E12.5 with Circulation Problems
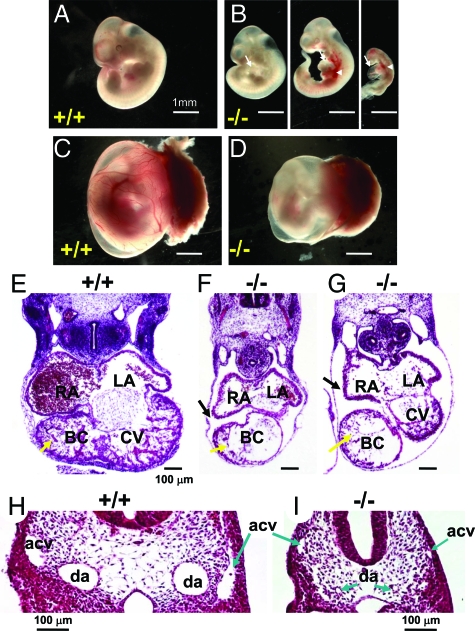
Ablation of Wnk1 in mice causes death before embryonic day 13 (E13).19 The cause of death in Wnk1-ablated mice, however, is not known. To examine the role of WNK1 in embryogenesis, we crossed heterozygous mice and collected embryos at various embryonic stages. Viable Wnk1 homozygous null embryos were obtained with expected Mendelian ratios at E8.5-E9.5 (Table 2). At E9.5, most of the mutant embryos (10 of 15, Table 2) are normal and phenotypically indistinguishable from their wild-type and heterozygous littermates. A small portion of the E9.5 mutant embryos, however, show signs of growth retardation (five of 15, Table 2; see also Supplementary Figure S1 at http://ajp.amjpathol.org). At E10.5, homozygous null embryos, although recovered at expected Mendelian ratios, are all abnormal (Table 2). Compared with wild-type embryos (Figure 1A), Wnk1−/− embryos at E10.5 exhibit growth retardation of varying severity or death (Figure 1B). Pericardial edema (arrow) and hemorrhage (arrowhead) are evident in various regions of null mutant embryos, including around the heart, common cardinal veins, and head (Figure 1B), indicating that integrity of the vasculature is defective. Despite smaller overall size, body axis and somite number of living Wnk1−/− mutant embryos at E10.5 are similar to those of wild-type (Figure 1, A and B). Wnk1+/− embryos are phenotypically indistinguishable from wild-type embryos (not shown).
Table 2.
Genotyping of Progeny from Wnk1 Heterozygous Crosses
| Age | Total | Wnk1+/+ | Wnk1+/− | Wnk1−/− |
|---|---|---|---|---|
| E8.5 | 31 | 7 (23%) | 16 (51%) | 8 (26%) |
| E9.5 | 66 | 18 (27%) | 33 (50%) | 15 (23%)/5* |
| E10.5 | 92 | 21 (23%) | 48 (52%) | 23* (25%)/9† |
| E11.5 | 41 | 11 (27%) | 21 (51%) | 9* (22%)/7† |
| E12.5 | 38 | 12 (32%) | 22 (58%) | 4† (11%) |
| E13.5 | 56 | 16 (29%) | 35 (63%) | 5† (9%) |
| Pups | 254 | 82 (32%) | 172 (68%) | 0 (0%) |
Abnormal: defective (including dead) embryos.
Dead: dead embryos.
Figure 1.
Developmental defects of Wnk1−/− mutant. A–D: Yolk sac, placenta, and embryo of E10.5 wild-type (A, C) and Wnk1−/− mutants (B, D). White arrows and arrowheads in D indicate pericardial edema and hemorrhage in some mutant embryos, respectively. E–I: H&E staining of transverse sections of E10.5 wild-type (E, H) and mutant embryos (F, G, I). RA, right atrium; LA, left atrium; BC, bulbus cordis; CV, common ventricle; da, dorsal aorta; acv, anterior cardinal vein. Yellow and black arrows in E–G indicate ventricular trabeculation and dilatation of pericardial sac in mutants, respectively. Arrows in H and I indicate dorsal aorta (da) or anterior cardinal vein (acv).
Wild-type yolk sacs at E10.5 display a network of large and small vessels filled with circulating blood (Figure 1C). In contrast, yolk sacs of E10.5 mutants are noticeably thinner, paler, and lack larger branching vitelline vessels and obvious blood circulation (Figure 1D). At E11.5, only a small percentage of homozygous mutant embryos are alive (two of nine, Table 2), and they display severe abnormalities, including growth retardation and hemorrhage (not shown). All homozygous null embryos are dead by E12.5 and E13.5. No Wnk1−/− embryos were recovered beyond E13.5.
The death of embryos at midgestation, as well as the lack of detectable blood circulation and large vessels in the mutant yolk sac, hemorrhage and pericardial edema, are all indicative of cardiovascular developmental defects. To analyze cardiovascular defects, we sectioned the embryos and examined the histology of heart and blood vessels. Transverse sections through the heart of E10.5 wild-type and Wnk1−/− embryos showed that all four cardiac chambers initiate formation normally (Figure 1, E–G). However, both atrial and ventricular chambers of mutant embryos are hypoplastic compared with wild-type embryos. The myocardial trabeculations of the ventricles and bulbus cordis are extensive in wild-type, but significantly reduced in mutants (Figure 1, E–G). The outer myocardial wall is also significantly thinner in mutant ventricles (Figure 1, F and G). Pericardial edema is evident from the dilatation of the pericardial sac (Figure 1, F and G). Thus, Wnk1-ablated embryos exhibit defective heart development, including reduced heart chamber size and reduced myocardial trabeculation. In addition, transverse sections of wild-type and mutant embryos, anterior and posterior to the heart, reveal smaller or collapsed dorsal aortae and cardinal veins in the mutants when compared with wild-type (Figure 1, H and I). Formation of these axial vessels, however, appears relatively normal in E8.5-9.5 mutant embryos (see Supplementary Figure S1 at http://ajp.amjpathol.org). Ink injection, via the posterior cardinal vein, in live E10 mutant embryos revealed normal blood flow from heart to dorsal aorta (data not shown), indicating that no hemodynamically significant blockage exists in the outflow tract. Thus, the decrease in the size of dorsal aortae and cardinal veins in E10.5 mutants is probably secondary to systemic circulatory insufficiency, and not a result of reduced cardiac contractility or anatomical blockage in the outflow tract.
PECAM1 Immunostaining of Vascular Endothelium
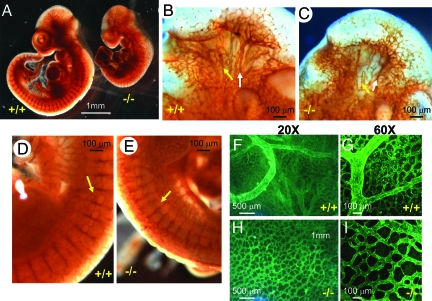
We further examined the developing vasculature using an antibody against a pan-endothelial cell marker, platelet endothelial cell adhesion molecule-1 (PECAM1). Whole-mount immunostaining using anti-PECAM1 antibody revealed that the formation of trunk vessels (indicated by arrow) and the initial stage of sprouting angiogenesis forming intersomitic vessels occur relatively normally in E8.5-E9.5 mutants (see Supplementary Figure S1 at http://ajp.amjpathol.org). In E10.5 mutant embryos, the density of blood vessels in the head region, however, is markedly reduced compared with wild-type (Figure 2A). The intersomitic vessels and their branches are less organized in E10.5 mutant than in wild-type embryos (Figure 2A). Close examination of enlarged images reveals that branches of the internal carotid artery (white arrows) and primary head veins (yellow arrows) of the mutants were atretic (Figure 2, B and C). The primary branches of intersomitic vessels are present in both wild-type and mutant embryos (Figure 2, D and E). However, the secondary and tertiary branching (yellow arrow) from intersomitic vessels are significantly reduced in the mutants (Figure 2, D and E). In wild-type yolk sacs at E10.5, the initial primitive vascular plexus develops into a highly organized hierarchical vascular network, with large and small branched vitelline arteries and veins (Figure 2, F and G). In contrast, the blood vessels of mutant yolk sacs fail to remodel by E10.5, retaining the primitive plexus pattern (Figure 2, H and I). Furthermore, the density of small capillary vessels in mutant yolk sacs is significantly lower than in wild type, leaving increased avascular space between plexus vessels (Figure 2, H and I). Thus, Wnk1 deletion in mice causes defects in sprouting angiogenesis, remodeling angiogenesis, as well as cardiac developmental defects. The presence of primary capillary plexus in E10.5 mutant yolk sacs, together with the normal formation of trunk vessels and the initial sprouting of intersomitic vessels in E8.5-9.5 mutant embryos, indicate that vasculogenesis is not affected by Wnk1 deletion.
Figure 2.
Immunostaining with anti-PECAM1 antibody shows vascular endothelial defects. A–E: Whole-mount staining of E10.5 wild-type and mutant embryos. White and yellow arrows (B, C) indicate internal carotid artery and head vein, respectively. Yellow arrows in D and E indicate position and density of small lateral branches sprouting between intersomitic vessels. F–I: PECAM1 staining of wild-type (F, G) and mutant yolk sacs (H, I) shown at ×20 (F, H) or ×60 magnification (G, I).
Placental Defects in Wnk1−/− Mice
Placental defects are frequently observed in mice with disruption of genes involved in the development of extraembryonic vasculature.24,25 As shown in Figure 1D, Wnk1−/− mice display defects in the development of extraembryonic vasculature in yolk sac. Given that WNK1 is expressed in the placenta,26 we examined placental development in Wnk1 null embryos. Wild-type and mutant placentas were analyzed. Normally, the fetal embryonic vessels intermingle with maternal circulation in the labyrinthine layer of placenta (labeled La in Figure 3,A and B). The hematoxylin and eosin-stained sections of E10.5 wild-type placenta revealed an intricate labyrinthine vascular network evident with deep penetration of embryonic vessels and juxtaposition of maternal sinuses (Figure 3B). Arrows in Figure 3B indicate embryonic erythrocytes with immature larger nuclei within the lumen of embryonic vessels; arrowheads indicate maternal erythrocytes in maternal sinuses. In contrast to wild-type placenta, E10.5 Wnk1−/− placenta showed a thinner and less vascularized labyrinthine layer. Moreover, the penetration of embryonic vessels (identified by arrows indicating embryonic erythrocytes) in mutant placenta is relatively superficial and there is much less contact between embryonic and maternal vessels.
Figure 3.
Placental defects of Wnk1−/− mutant. A and B: H&E-stained sections of E10.5 placenta (A, ×25; B, ×100 magnification) show that the labyrinthine layer (La, where embryonic vessels are in contact with maternal circulation) is thinner in mutant placenta. In the wild-type placenta, an intricate labyrinthine vascular network is evident with deep penetration of embryonic vessels and juxtaposition of maternal sinuses. In mutant placenta, the penetration of embryonic vessels is relatively superficial and there is much less contact between embryonic and maternal vessels. Arrows in B indicate embryonic erythrocytes (immature, larger nuclei) in embryonic vessels. Arrowheads indicate maternal erythrocytes in maternal sinuses. Al, allantois; La, labyrinth; Sp, spongiotrophoblast; Gc, giant cells; De, maternal decidua.
Ectopic Expression of Arterial and Venous Markers in Blood Vessels of Wnk1−/− Embryos
Specification of arteries and veins in the developing vasculature is a critical process associated with angiogenesis.5,6 Failure in artery-vein specification results in angiogenesis defects.3,4 To determine whether Wnk1 deletion affects artery-vein specification, we analyzed the expression pattern of the arterial marker neuropilin-1 (NP1)4 and the venous marker EphB43 in Wnk1−/− embryos by immunostaining. In E9.5 wild-type embryos, expression of NP1 and EphB4 are restricted to the dorsal aorta and anterior cardinal vein, respectively (Figure 4, A and B). In contrast, NP1 and EphB4 are ectopically expressed in the presumptive cardinal vein and dorsal aorta of mutant embryos, respectively (Figure 4, C–E). Co-staining with PECAM1 verified that ectopic expression of NP1 in the mutant cardinal vein is localized to the endothelium (Figure 4, F–I).
Figure 4.
Aberrant expression of arterial and venous markers in mutant arteries and veins. A–D: The arterial marker neuropilin-1 (NP1, green) and venous marker ephrin receptor B4 (EphB4, yellow) are expressed in the dorsal aorta (A) and cardinal vein (V) of wild-type embryos, respectively (A and B). In an E9.5 mutant (C), NPI, however, is expressed in veins (V) as well as aortae (A). Similarly, EphB4 is expressed in both aorta (A) and vein (V) of mutant embryo (D). E: H&E-stained transverse sections at the level similar to that in A–D. nt, neural tube; fg, foregut; da, dorsal aorta; acv, anterior cardinal vein. F–H: Double staining of NP1 (F, red) and PECAM1 (G, green) in mutant vessels. H is an overlaid image of F and G. I: A H&E-stained transverse section at the level similar to that in F–H.
Quantitative Real-Time PCR Analysis of Expression of Genes Involved in Angiogenesis
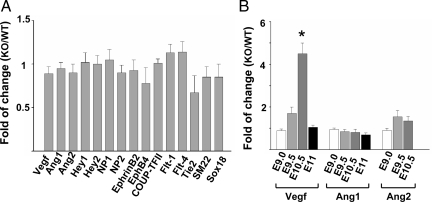
To identify downstream genes that may be regulated by WNK1, we compared the expression of multiple candidate genes involved in angiogenesis5,27 in wild-type and Wnk1−/− yolk sacs and embryos, from E9.0 to E10.5. Genes analyzed include VEGFs and VEGF receptors, angiopoietins and Tie receptors, neuropilins, ephrins, Eph receptors, Notch target genes Hey1 and Hey2, and COUP-TFII. We found few differences in expression levels between wild-type and Wnk1−/− mutant at E9.0 (Figure 5A and B). However, the expression of VEGF was up-regulated in E10.5 Wnk1−/− embryos (Figure 5B). The timing and direction of changes suggest that the dysregulation of VEGF is not the primary cause of vascular developmental defects but rather it is secondary to circulatory failure and tissue hypoxia.
Figure 5.
Expression of angiogenesis-related genes in Wnk1−/− embryos relative to wild-type at embryonic days 9–11. A: Expression of gene in Wnk1−/− embryos (KO) relative to wild-type (WT) at embryonic day 9 (E9.0). B: Relative expression of a subset of genes at embryonic days 9, 9.5, 10.5, and 11. Gene expression was analyzed by quantitative real-time PCR. Each experiment includes four to six samples for KO and WT, respectively. Results are mean ± SEM from three separate experiments. Except for VEGF at E10.5 (*P < 0.01 by unpaired two-tailed t-test), none of the genes analyzed are statistically significantly different between KO and WT.
Wnk1 Expression in Developing Embryos Analyzed by in Situ Hybridization
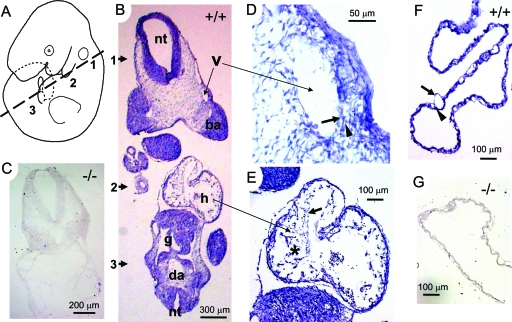
A recent study showed that Wnk1 is expressed ubiquitously in the developing mouse embryos as early as E7.5.26 Using a bacterial artificial chromosome (BAC) bearing a mouse Wnk1 transgene and an integrated lacZ reporter, this group was able to follow the expression of Wnk1 and observed that the expression becomes enriched in the cardiovascular system during development. We examined the expression of endogenous Wnk1 in E10 mouse embryos using in situ hybridization. We used an RNA probe specific for exons 6 to 9 of mouse Wnk1 gene, which would recognize the full length transcript as well as all alternatively spliced transcripts known to exist.12,13 Examination of multiple sections along the entire anteroposterior body axis reveals generalized expression of Wnk1 in E10 wild-type embryos. Figure 6B is a representative image from an oblique section illustrated by Figure 6A. The approximate position of tissues along the dorsal-ventral plane is marked by numbers, 1 through 3. We first examined expression in Wnk1−/− mice, which were produced by insertional mutation within intron-1 of Wnk1. In contrast to the ubiquitous expression of Wnk1 observed in wild-type embryos (Figure 6B), no signal was detected in null embryos (Figure 6C), confirming the specificity of the probe. Close examination of enlarged images shows Wnk1 expression in vascular endothelium (arrow) and surrounding cells (arrowhead) (Figure 6D). Wnk1 is also expressed in all layers of developing heart tissue (Figure 6E), including the endocardium (arrow) and myocardial trabeculation (asterisk). Moreover, Wnk1 is expressed in the vascular endothelium (arrow) and supporting cells (arrowhead) of wild-type yolk sac (Figure 6F) but not of mutant yolk sac (Figure 6G).
Figure 6.
Expression of Wnk1 in E10 embryos and yolk sacs. In situ hybridization using RNA probe against exon 6-9 of Wnk1 was performed in E10 embryos sectioned as diagrammed (A). Wnk1 is ubiquitously expressed in wild-type embryos (B). nt, neural tube; v, a branch of vein; ba, brachial arch; h, heart; g, gut; da, dorsal aorta. In the developing vessels of embryo proper (D) and yolk sac (F), strong Wnk1 signal is detected in both endothelial cells (arrow) and pericytes (arrowhead). In the developing heart (E), both endothelial lining (arrow) and trabeculated myocardium (asterisk) are positive for Wnk1. As negative controls, no signal is detected in Wnk1−/− embryo (C) and yolk sac (G).
Endothelial-Specific Conditional Deletion of Wnk1 Phenocopies Developmental Defects Caused by Global Wnk1 Ablation
Ubiquitous expression of Wnk1 raised the question as to which tissue failed in the Wnk1-ablated mutant embryos. Nonendothelial cell types surrounding developing vasculature play essential roles in the angiogenesis.2,5,28 These perivascular cells secrete a large array of proangiogenic or antiangiogenic factors to regulate the angiogenesis remodeling and they actively participate in the formation of the external wall of mature vessels.5,29 Disruptions of the function of these perivascular cells cause angiogenesis defects.
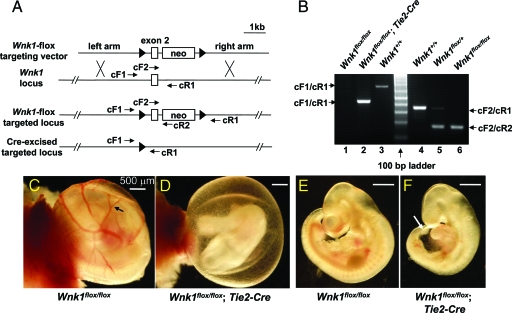
Wnk1 is expressed in endothelial and all vessel support cells. To define the cell lineages in which WNK1 function is essential for cardiovascular development, we generated mice to be used for conditional ablation of Wnk1 in endothelial cells. A Wnk1-floxed allele was produced by inserting loxP sites in the 5′ and 3′ region of exon 2 of mouse Wnk1 (Figure 7A). Mice bearing targeted Wnk1-floxed allele(s) were crossed with a Tie2-Cre transgenic mouse line that expresses Cre recombinase specifically in endothelial cell lineage.30,31 Offspring embryos were genotyped by PCR using specific primers that allow distinction between wild-type Wnk1, floxed Wnk1, and floxed Wnk1 allele excised by Cre recombinase (Figure 7, A and B). Among 58 embryos collected between E10.5 and 12.5, from multiple breeding pairs of parents bearing one or two floxed Wnk1 alleles and/or Tie2-Cre allele, we found that all five embryos bearing Wnk1flox/flox;Tie2-Cre genotype are abnormal (Table 3). Furthermore, no Wnk1flox/flox;Tie2-Cre embryos or pups were recovered at or beyond E13.5. The defects observed and the timing of lethality were identical to those displayed by the global Wnk1 deletion.
Figure 7.
Endothelial-specific conditional knockout of Wnk1. A: Diagram for Wnk1 exon 2-floxed targeting construct and location of primers used for genotyping of targeted locus and Cre-mediated excised targeted locus. The exon 2 of Wnk1 and two ∼3-kb genomic fragments flanking exon 2 (left and right arm) were PCR-amplified and subcloned into a targeting vector such that exon 2 of Wnk1 was followed by a neo cassette, and exon 2-neo was flanked by two loxP sites. This targeting construct was used to generate Wnk1flox mice, which were crossed with Tie2-Cre mice to produce an endothelial-specific conditional Wnk1 knockout. Forward primers cF1 and cF2 are located in the 3′ end of left arm and exon 2, respectively. Reverse primers cR1 and cR2 are located in the right arm and at the beginning of neo cassette, respectively. Sequences of primers are in Table 1. B: Genotyping of conditional knockout mice. The cF2 and cR2 primer pair identifies the targeted Wnk1flox allele with a ∼180-bp product; while cF2 and cR1 primer pair amplifies the wild-type allele into a ∼350-bp band. Thus, PCR reaction using mixtures of cF2 forward primer and cR1 and cR2 reverse primers distinguishes among Wnk1+/+, Wnk1flox/+, and Wnk1flox/flox (lanes 3, 4, and 5, respectively). The cF1 and cR1 primer pair amplifies the Cre-excised Wnk1flox/flox locus into a ∼420-bp product (lane 2), which can be distinguished from the ∼680-bp product of wild-type locus (lane 3). The much larger product of unexcised Wnk1flox/flox locus was not amplified by PCR under the condition used (lane 1). C and D: Yolk sac of E10.5 endothelial-specific conditional Wnk1 mutant (D) and Wnk1flox/flox but Cre-negative control (C). Arrow in C indicates the network of large and small vessels evident in the control yolk sac, which is notably absent in conditional Wnk1 mutant (D). E and F: Embryo of E10.5 endothelial-specific conditional Wnk1 mutant (F) and Wnk1flox/flox but Cre-negative control (E). White arrow in F indicates the rim of the dilated pericardial sac in conditional Wnk1 mutants (ie, pericardial edema).
Table 3.
Developmental Cardiovascular Defects in Endothelial-Specific Wnk1-Deficient Mice Result in Embryonic Lethality by E13.5
| Breeding Parents | Age | Total | Wnk1flox/flox | Wnk1flox/flox;Tie2-Cre |
|---|---|---|---|---|
| Wnk1flox/+;Tie2-Cre | ||||
| X | E10.5-E12.5 | 58 | 16 | 5* |
| (Wnk1flox/+ | ||||
| Or | E13.5-E18.5 | 19 | 4 | 0 |
| Wnk1flox/+;Tie2-Cre | ||||
| Or | Pups | 47 | 11 | 0 |
| Wnk1flox/flox) |
Dead or dying with cardiovascular defects.
We examined phenotypic features of E10.5 endothelial-specific Wnk1-deleted embryos and yolk sacs. We found that yolk sacs of E10.5 Wnk1flox/flox mice are morphologically identical to those of wild-type mice (Figure 7C versus Figure 1C). However, yolk sacs of E10.5 Wnk1flox/flox;Tie2-Cre mutants lack visible large vessels (Figure 7D), as in the global Wnk1−/− mutants (Figure 1D). Similarly, Wnk1flox/flox embryos are phenotypically indistinguishable from wild-type embryos (Figure 7E versus Figure 1A). Wnk1flox/flox;Tie2-Cre embryos are smaller and display pericardial edema compared with Wnk1flox/flox embryos (Figure 7, E and F). Hematoxylin and eosin-stained transverse sections anterior to the heart show collapsed aortae and cardinal veins in E10.5 Wnk1flox/flox;Tie2-Cre embryos compared with Wnk1flox/flox embryos (Figure 8, A and B). Sections across the heart show hypoplastic atrial and ventricular chambers, thinning of myocardium, and reduced myocardial trabeculation in Wnk1flox/flox;Tie2-Cre embryos (Figure 8, C and D). The vasculature of Wnk1flox/flox;Tie2-Cre yolk sac retains the pattern of primitive plexus, lacking angiogenesis remodeling (Figure 8, E and F). Whole-mount PECAM staining reveals reduced branching angiogenesis and vascular density in the head region (Figure 8H versus Figure 8G) and collapsed aortae and cardinal veins and smaller heart chambers (Figure 8, J and L versus Figure 8, I and K) of E10.5 Wnk1flox/flox;Tie2-Cre embryos (versus Wnk1flox/flox embryos). These results suggest that the primary defect in the Wnk1 global null embryos is a direct result of endothelial requirement for WNK1 function.
Figure 8.
Detailed phenotypic features of E10.5 embryos and yolk sacs in mice of endothelial-specific deletion of Wnk1. A–D: H&E-stained transverse sections of E10.5 Wnk1flox/flox (A, C) and Wnk1flox/flox;Tie2-Cre (B, D) embryos. nt, neural tube; da, dorsal aorta; acv, anterior cardinal vein; RA, right atrium; LA, left atrium; BC, bulbus cordis; CV, common ventricle. The conditional knockout embryos have smaller or collapsed vessels (B), less trabeculation (yellow arrow in B, D), and dilated pericardial cavity (black arrow in D). E–J: PECAM-stained E10.5 yolk sacs (E, F) and embryos (G–J). Compared with Wnk1flox/flox, Wnk1flox/flox,Tie2-Cre yolk sacs have defective remodeling (E versus F). Compared with Wnk1flox/flox embryos, Wnk1flox/flox,Tie2-Cre embryos have atretic and disorganized branches of internal carotid artery and head veins (white and yellow arrow, respectively, in G and H), and collapsed aorta and cardinal veins (yellow arrow and yellow arrowhead, respectively, in I and J). K and L: Tracing of heart and major vessels in I and J, respectively. a, atria; aa, aortic arches; ao, aorta; cv, cardinal vein; ccv, common cardinal vein; sv, sinus venosus, v, ventricle.
Rescue of Cardiovascular Developmental Defects in Wnk1−/− Embryos by Endothelial-Specific Expression of a Wnk1 Transgene
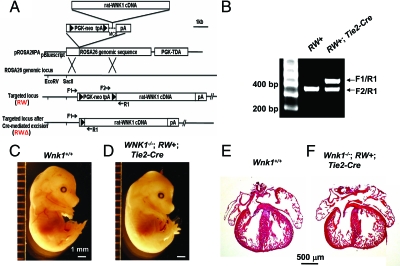
Cardiac developmental defects can be caused by disruption of endothelial signaling.3,7,32 However, Wnk1 is also abundantly expressed in the myocardium of developing mouse heart (Figure 6E). We thus asked whether cardiac developmental defects in the global Wnk1 knockout mice is due to deletion of Wnk1 in myocardium or deletion of Wnk1 in endothelium and/or endocardium. To answer this question, we generated a mouse line that allows conditional tissue-specific expression of WNK1. This line was produced by targeting a full-length rat Wnk1 cDNA downstream of a floxed transcriptional terminator cassette to the ROSA26 locus (“RW” allele, Figure 9, A and B). Conditional expression of the Wnk1 transgene can be achieved by crossing mice bearing RW allele(s) with mouse lines that express Cre recombinase in tissues of interest.
Figure 9.
Endothelial-specific expression of Wnk1 transgene rescues cardiovascular developmental defects and the embryonic lethality that results from global Wnk1 ablation. A: Construct for targeting full-length rat WNK1 cDNA downstream of a floxed transcriptional stopper cassette (PGK-neo tpA) to the ROSA26 locus and diagram for location of primers used for genotyping of targeted ROSA-Wnk1 (RW) allele and Cre-excised targeted allele (RWΔ). Reverse primer of RW targeted allele (R1) is located at the 5′ end of the rat WNK1 cDNA. Forward primers located upstream of the PGK-neo-tpA cassette (F1) and within the cassette (F2) distinguish the excised (active) ROSA26-Wnk1 targeted allele (RWΔ) from the unexcised (inactive) targeted allele (RW). B: Tail DNA was genotyped by PCR using mixtures of F1 and F2 forward primers and R2 reverse primer. The ∼450-bp band amplified by F1/R1 primer pair indicates presence of the excised targeted allele (RWΔ). The ∼370-bp band amplified by F2/R1 primer pair indicates presence of unexcised targeted allele (RW). PCR primers for transgenic Cre lines were as described in the Jackson Laboratories genotyping protocol. C and D: E15.5 wild-type (C) and rescued (D) embryos. E and F: Longitudinal sections of the heart of E15.5 wild-type (E) and rescued (F) embryos.
Our results demonstrated that it is the blood vessel endothelium that requires WNK1 function. Crossing of Wnk1+/−;RW+/− mice with Wnk1+/−;Tie2-Cre mice produced 37 Wnk1−/− embryos from a total of 261 embryos collected between E12.5 and E17.5 (Table 4). Among Wnk1−/− embryos, 15 were positive for both RW and Tie2-Cre. Strikingly, seven of the 15 double-positives were phenocopies of wild-type (Figure 9, C and D), suggesting that transgenic expression of WNK1 in endothelial cells was sufficient to rescue cardiovascular failure and early embryonic lethality. The developing hearts of E15.5 rescued embryos (Wnk1−/−;RW+;Tie2-Cre) were normal in size and structure compared with wild-type hearts (Figure 9, E and F). Thus, expression of WNK1 in the myocardium is not essential for embryonic cardiac development. Function of WNK1 in endothelial cells and/or endocardium is critical for myocardial development, as well as angiogenesis.
Table 4.
Rescue of Wnk1−/− Phenotype By Conditional Wnk1 Transgenic Expression
| Breeding parents | Age | Total | Wnk1−/− | Wnk1−/−;RW+;Cre | Rescued |
|---|---|---|---|---|---|
| (RW+ or RW−) | |||||
| Wnk1+/−;Tie2-Cre | E12.5-E17.5 | 261 | 37 | 15 | 7 |
| X | |||||
| Wnk1+/−;RW+ | Pups | 436 | 6 | 6 | 6/5* |
| Wnk1+/−;Flk1-Cre/+ | E10.5-E17.5 | 81 | 9 | 5 | 3 |
| X | |||||
| Wnk1+/−;RW+ | Pups | 91 | 0 | 0 | 0 |
| Wnk1+/−;Sox2-Cre | E10.5-E17.5 | 145 | 29 | 6 | 0 |
| X | |||||
| WNK1+/−;RW+ | Pups | 113 | 0 | 0 | 0 |
Died at birth.
RW: ROSA26-WNK1; Tie2-Cre, Flk1-Cre: endothelial specific; Sox2-Cre: epiblast-derived somatic cells (extraembryonic negative). Tie2-Cre and Sox2-Cre are transgenic lines. Flk1-Cre/+ is a knockin line.
Other organs including lung, liver, kidney and gastrointestinal tract were grossly similar between wild-type and rescued embryos (see Supplementary Figure S2 at http://ajp.amjpathol.org). Some of rescued mice were delivered at term but all except one died within the first day of birth (Table 4). The only surviving mouse was generally smaller compared with Wnk1+/− littermates of the same gender (see Supplementary Figure S2 at http://ajp.amjpathol.org). Loss of function of WNK1 in organs, such as kidney, may be responsible for the observed perinatal death and growth retardation of endothelial-WNK1 “rescued” mutants. Transgenic expression of WNK1 using another endothelial-specific Cre recombinase (Flk1-Cre)20,30 also rescued cardiovascular defects of Wnk1−/− embryos (Table 4). Importantly, expression of WNK1 in somatic embryonic cells, activated by Sox2-Cre, did not rescue Wnk1−/− embryos (Table 4). Sox2 is expressed in all epiblast-derived somatic cells but not in some extraembryonic tissues or endoderm of yolk sac.21 Thus, WNK1 expression specifically in the vasculature of extraembryonic tissues (yolk sac and/or placenta) is critically required for normal embryogenesis.
We observed that some of the Wnk1−/−;RW(+); Cre(+) embryos collected between E12.5 and E17.5 were defective or dead (Table 4). The percentage of defective or dead Wnk1−/−;RW(+); Cre(+) embryos is higher than that observed (occasionally) for wild-type embryos (not shown). The precise reason(s) for the incomplete rescue is unknown. Heterogeneous expression of Cre recombinase (an inherent potential problem of transgenic promoters) in endothelial cells is probably not the main reason because Flk1-Cre is generated by knockin, thus using the endogenous promoter for the expression of Cre recombinase. Differential temporal expression of Flk1 and Tie2 in the developing mouse embryos, yolk sac, and placenta may be a contributory cause. In the embryos, the expression of Flk1 and Tie2 begins in E7.0 and 8.5, respectively, and continues thereafter throughout embryogenesis.30 Thus, transgenic expression of Wnk1 driven by Flk1- and Tie2-Cre can prevent growth retardation and death of Wnk1-null embryos that begins at ∼E9.5.
Expression of Flk1 and Tie2 in the yolk sac, even though it also begins around E7.5–8.5, decreases over time to nearly undetectable beyond E12.5.30 As above, transgenic expression of WNK1 mediated by Sox2-Cre fails to rescue Wnk1−/− embryos, indicating an essential role of yolk sac and/or placenta for normal embryogenesis. Thus, the decline in expression in the yolk sac may conceivably contribute to the demise and incomplete rescue of Wnk1−/− embryos by Flk1- or Tie2-Cre. In contrast to that in the embryo and yolk sac, the expression of Flk1 and Tie2 in placenta begins much later at E12.5.30 The fact that Flk1- and Tie2-Cre mediated expression of Wnk1 can rescue Wnk1−/− embryos indicates that placental requirement for WNK1 is not the primary cause of early embryonic lethality of Wnk1−/− embryos between E10.5 and 12.5. Function of WNK1 in placenta, however, may be important for normal embryogenesis beyond E12.5.
Discussion
The development of blood vessels during embryogenesis occurs via an initial vasculogenesis followed by angiogenesis.1,2 Vasculogenesis forms the major axial vessels in the embryo proper and a homogenous primary capillary plexus in both the embryo proper and in extraembryonic structures such as the yolk sac. Angiogenesis, through sprouting and branching from the primary vessels and remodeling of the vascular plexus into a hierarchical tree-like system, gives rise to intersomitic vessels and blood vessels in organs, such as the brain and kidney. WNK1 is a member of the newly discovered serine-threonine protein kinase family. To date, its function is relatively unknown in tissues and organs outside the kidney; however, recent work has described its expression in vascular endothelium, suggesting a possible function in cardiovascular development.26 In the present study, we report that indeed WNK1 is essential for embryonic development of blood vessels and heart. WNK1 appears to regulate the later stages of angiogenesis; however, it is dispensable for vasculogenesis and the initial stages of angiogenesis. WNK1 is expressed in perivascular mesenchyme besides endothelial cells. Using endothelial-specific conditional deletion of Wnk1, we further report that function of WNK1 in endothelial cells is essential for the embryonic angiogenesis.
We also observed defects in proper artery/vein differentiation in the Wnk1−/− mutants. Specification of arteries versus veins is a critical step during vascular development. Differentiation of arteries and veins during vascular development requires reciprocal interaction between ephrin-B2 expressed in arteries and its cognate receptor EphB4 expressed in veins.3,27 Specific expression of ephrin-B2 and EphB4 in arteries and veins is believed to be genetically programmed and mediated by expression/activation of NP1-VEGF-Notch signaling pathway or of COUP-TFII, respectively. Stimulation of the heterodimeric NP1/Flk1-VEGF2 receptors4 in endothelial cells activates Notch target genes, Hey1 and Hey2, and confers arterial fate by inducing the expression of ephrin-B2 and suppressing the expression of EphB4.27,33 In contrast, COUP-TFII is expressed in veins and confers venous fate by suppressing NP1 and down-regulating ephrin-B2, thus promoting the expression of EphB4.7 In support of this model, transgenic expression of COUP-TFII or disruption of Notch signaling in endothelium both result in a loss of expression of arterial markers and aberrant expression of venous markers in arteries.7,34 Conversely, knockout of COUP-TFII or ectopic activation of Notch signaling produces the reverse phenotype.7,33
Unlike embryos with alteration of COUP-TFII or Notch signaling pathway, Wnk1−/− embryos exhibit concurrent ectopic expression of arterial marker in veins and of venous marker in arteries. These findings are unprecedented. Moreover, the expression of Notch downstream genes Hey1/2 is not altered in Wnk1 mutants. Thus, WNK1 is likely not directly upstream of the Notch pathway with respect to arterial-venous cell fate specification in embryonic vessels. However, as a protein kinase that plays an important role in regulating membrane trafficking of proteins,18,35 WNK1 may conceivably regulate the activity and/or trafficking of proteins of the VEGF-Notch pathway critical for the genetic determination of arterial-venous cell fate. This mechanism of regulation, if occurs, would be at the level downstream to the Notch and Hey1/2 expression.
Alternatively, WNK1 may not be involved in the genetic determination of arterial-venous fate, but rather it may be important for the maintenance of established vascular fate. Cell-lineage tracing studies in zebrafish have shown that genetic predetermination of arterial and venous fates occurs early in the progenitor cells.36 In the chick, arterial- and venous-fated progenitors are also present in the early extraembryonic blood islands before formation of blood vessels.37 The genetically predetermined arterial-venous identity, however, is plastic and can be influenced later in development by external cues. For example, experimental manipulation of flow to the uterine artery alters the expression of arterial markers and cell fate.38 If WNK1 in endothelial cells is important for maintaining arterial-venous identity, loss of function of WNK1 may lead to de-repression of arterial and venous markers in veins and arteries, respectively. This effect would be identified by the coexpression of arterial and venous markers in both arteries and veins, as we observed in Wnk1-null embryos.
We found that blood flow from heart to dorsal aorta is relatively normal in the live Wnk1−/− embryos at E10 (data not shown), a stage in which angiogenesis defects and ectopic expression of arterial and venous markers are already quite apparent (Figure 4). Thus, the angiogenesis and arterial-venous specification defect in Wnk1-deleted mice is not primarily due to a decrease in blood flow from depressed cardiac contractility. Nonetheless, changes in blood flow from systemic circulatory insufficiency (and with or without mild cardiac insufficiency) may contribute to angiogenesis and remodeling defects.
We found that global deletion of Wnk1 also causes cardiac defects, including reductions in the thickness of myocardium and in myocardial trabeculation. WNK1 is expressed in the developing myocardium as well endothelium, raising the question in which tissues WNK1 is important. We find that endothelial expression of Wnk1 transgene rescues cardiac defects, indicating a requirement of endothelial WNK1 for cardiac development. Loss of WNK1 in endothelial cells could cause the observed myocardial defects by two mechanisms. First, failure of proper angiogenesis and vascular development impede the normal function of cardiovascular circulation, which provides developing tissues with proper gas and nutritional exchange. Indeed, vascular defects in placenta lead to impaired cardiac development.39,40 Additionally, a number of reports have described the interdependence of endothelial/endocardial and myocardial development and have identified a number of reciprocal signals exchanged between these tissues.32,41,42,43,44 Thus, failure of the embryonic vascular endothelium in the Wnk1−/− mutants may result in defects in normal endothelial-myocyte interactions, such as paracrine signals from endocardium to myocardium, and ultimately failure in proper myocyte growth. We note, however, that myocyte development at least initiates normally, as most heart markers examined appeared relatively normal in mutant embryos (eg, Nkx2.5, ANF, desmin; data not shown). It remains to be seen what secondary signals from the endocardium might be lost in the Wnk1−/− mutants.
In conclusion, our results provide strong evidence suggesting that function of WNK1 in endothelial cells defines a novel pathway or mechanism in the determination and/or maintenance of arterial-venous identity and is essential for embryonic cardiac development. Wnk1 heterozygous mice have lower blood pressure without apparent defects in renal Na+ transport,19 raising the possibility that function of WNK1 outside the kidney is also important in the control of blood pressure. Whether endothelial WNK1 is involved in the extrarenal control of blood pressure remains to be determined. Finally, angiogenesis and endothelial cell mass have also been shown to be a critical determinant of the adult heart size and function independent of external stimuli.45 It would be interesting to investigate in the future the role of endothelial WNK1 in postnatal angiogenesis and cardiac growth in physiological and/or pathophysiological states.
Supplementary Material
Acknowledgments
We thank Lexicon Genetics (Woodlands, TX) for providing Wnk1 mutant mice, and Dr. Thomas Carroll, Dr. Leighton James, and Dr. Peter Igarashi for discussions and comments.
Footnotes
Address reprint requests to Chou-Long Huang, M.D., Ph.D., UT Southwestern Medical Center, Department of Medicine, 5323 Harry Hines Blvd., Dallas, TX 75390-8856. E-mail: chou-long.huang@utsouthwestern.edu.
Supported by National Institutes of Health grants DK-59530 and DK-079328, American Heart Association grants 0440019N and 0755054Y, and March of Dimes Basil O'Connor award 5-FY05-1213.
Supplemental material for this article can be found on http://ajp.amjpathol.org.
References
- Risau W, Flamme I. Vasculogenesis. Annu Rev Cell Dev Biol. 1995;11:73–91. doi: 10.1146/annurev.cb.11.110195.000445. [DOI] [PubMed] [Google Scholar]
- Folkman J, D'Amore PA. Blood vessel formation: what is its molecular basis? Cell. 1996;87:1153–1155. doi: 10.1016/s0092-8674(00)81810-3. [DOI] [PubMed] [Google Scholar]
- Wang HU, Chen ZF, Anderson DJ. Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell. 1998;93:741–753. doi: 10.1016/s0092-8674(00)81436-1. [DOI] [PubMed] [Google Scholar]
- Gu C, Rodriguez ER, Reimert DV, Shu T, Fritzsch B, Richards LJ, Kolodkin AL, Ginty DD. Neuropilin-1 conveys Semaphorin and VEGF signaling during neural and cardiovascular development. Dev Cell. 2003;5:45–57. doi: 10.1016/s1534-5807(03)00169-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coultas L, Chawengsaksophak K, Rossant J. Endothelial cells and VEGF in vascular development. Nature. 2005;438:937–945. doi: 10.1038/nature04479. [DOI] [PubMed] [Google Scholar]
- Adams RH. Molecular control of arterial-venous blood vessel identity. J Anat. 2003;202:105–112. doi: 10.1046/j.1469-7580.2003.00137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You LR, Lin FJ, Lee CT, DeMayo FJ, Tsai MJ, Tsai SY. Suppression of notch signaling by the Coup-TFII transcription factor regulates vein identity. Nature. 2005;435:98–104. doi: 10.1038/nature03511. [DOI] [PubMed] [Google Scholar]
- Pereira FA, Qiu Y, Zhou G, Tsai MJ, Tsai SY. The orphan nuclear receptor COUP-TFII is required for angiogenesis and heart development. Genes Dev. 1995;13:1037–1049. doi: 10.1101/gad.13.8.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B, English JM, Wilsbacher JL, Stippec S, Goldsmith EJ, Cobb MH. WNK1, a novel mammalian serine/threonine protein kinase lacking the catalytic lysine in subdomain II. J Biol Chem. 2000;275:16795–16801. doi: 10.1074/jbc.275.22.16795. [DOI] [PubMed] [Google Scholar]
- Wilson FH, Disse-Nicodeme S, Choate KA, Ishikawa K, Nelson-Williams C, Desitter I, Gunel M, Milford DV, Lipkin GW, Achard JM, Feely MP, Dussol B, Berland Y, Unwin RJ, Mayan H, Simon DB, Farfel Z, Jeunemaitre X, Lifton RP. Human hypertension caused by mutations in WNK kinases. Science. 2001;293:1107–1112. doi: 10.1126/science.1062844. [DOI] [PubMed] [Google Scholar]
- Verissimo F, Jordan P. WNK kinases, a novel protein kinase subfamily in multi-cellular organisms. Oncogene. 2001;20:5562–5569. doi: 10.1038/sj.onc.1204726. [DOI] [PubMed] [Google Scholar]
- Delaloy C, Lu J, Houot AM, Disse-Nicodeme S, Gasc JM, Corvol P, Jeunemaitre X. Multiple promoters in the WNK1 gene: one controls expression of a kidney-specific kinase-defective isoform. Mol Cell Biol. 2003;23:9208–9221. doi: 10.1128/MCB.23.24.9208-9221.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Reilly M, Marshall E, Speirs HJ, Brown RW. WNK1, a gene within a novel blood pressure control pathway, tissue-specifically generates radically different isoforms with and without a kinase domain. J Am Soc Nephrol. 2003;14:2447–2456. doi: 10.1097/01.asn.0000089830.97681.3b. [DOI] [PubMed] [Google Scholar]
- Holden S, Cox J, Raymond FL. Cloning, genomic organization, alternative splicing and expression analysis of the human gene WNK3 (PRKWNK3). Gene. 2004;335:109–119. doi: 10.1016/j.gene.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Kahle KT, Gimenez I, Hassan H, Wilson FH, Wong RD, Forbush B, Aronson PS, Lifton RP. WNK4 regulates apical and basolateral Cl− flux in extrarenal epithelia. Proc Natl Acad Sci USA. 2004;101:2064–2069. doi: 10.1073/pnas.0308434100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CL, Angell J, Mitchell R, Ellison DH. WNK kinases regulate thiazide-sensitive Na-Cl cotransport. J Clin Invest. 2003;111:1039–1045. doi: 10.1172/JCI17443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B, Stippec S, Chu PY, Li XJ, Lazrak A, Ortega B, Lee BH, English JM, Huang CL, Cobb MH. WNK1 activates SGK1 to regulate the epithelial sodium channel. Proc Natl Acad Sci USA. 2005;102:10315–10320. doi: 10.1073/pnas.0504422102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He G, Wang HR, Huang SK, Huang CL. Intersectin links WNK kinases to endocytosis of ROMK. J Clin Invest. 2007;117:1078–1087. doi: 10.1172/JCI30087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambrowicz BP, Abuin A, Ramirez-Solis R, Richter LJ, Piggott J, BeltrandelRio H, Buxton EC, Edwards J, Finch RA, Friddle CJ, Gupta A, Hansen G, Hu Y, Huang Wenhu, Jaing C, Key BW, Jr, Kipp P, Kohlhauff B, Ma Z-Q, Markesich D, Payne R, Potter DG, Quan N, Shaw J, Schrick J, Shi Z-Z, Sparks MJ, Van Slightenhorst I, Vogel P, Walke W, Xu N, Zhu Q, Person C, Sands AT. Wnk1 kinase deficiency lowers blood pressure in mice: A gene-trap screen to identify potential targets for therapeutic intervention. Proc Natl Acad Sci USA. 2003;100:14109–14114. doi: 10.1073/pnas.2336103100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motoike T, Markham DW, Rossant J, Sato TN. Evidence for novel fate of Flk1+ progenitor: contribution to muscle lineage. Genesis. 2003;35:153–159. doi: 10.1002/gene.10175. [DOI] [PubMed] [Google Scholar]
- Hayashi S, Lewis P, Pevny L, McMahon AP. Efficient gene modulation in mouse epiblast using a Sox2Cre transgenic mouse strain. Mech Dev. 2002;119(Suppl 1):S97–S101. doi: 10.1016/s0925-4773(03)00099-6. [DOI] [PubMed] [Google Scholar]
- Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM, Costantini F. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smithies O, Kim HS. Targeted gene duplication and disruption for analyzing quantitative genetic traits in mice. Proc Natl Acad Sci USA. 1994;91:3612–3615. doi: 10.1073/pnas.91.9.3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs LT, Xue Y, Norton CR, Shutter JR, Maguire M, Sundberg JP, Gallahan D, Closson V, Kitajewski J, Callahan R, Smith GH, Stark KL, Gridley T. Notch signaling is essential for vascular morphogenesis in mice. Genes Dev. 2000;14:1343–1352. [PMC free article] [PubMed] [Google Scholar]
- Fischer A, Schumacher N, Maier M, Sendtner M, Gessler M. The Notch target genes Hey1 and Hey2 are required for embryonic vascular development. Genes Dev. 2004;18:901–911. doi: 10.1101/gad.291004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaloy C, Hadchouel J, Imbert-Teboul M, Clemessy M, Houot AM, Jeunemaitre X. Cardiovascular expression of the mouse WNK1 gene during development and adulthood revealed by a BAC reporter assay. Am J Pathol. 2006;169:105–118. doi: 10.2353/ajpath.2006.051290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin FJ, Tsai MJ, Tsai SY. Artery and vein formation: a tug of war between different forces. EMBO Rep. 2007;8:920–924. doi: 10.1038/sj.embor.7401076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain RK. Molecular regulation of vessel maturation. Nat Med. 2003;9:685–693. doi: 10.1038/nm0603-685. [DOI] [PubMed] [Google Scholar]
- Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9:653–660. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- Dumont DJ, Fong GH, Puri MC, Gradwohl G, Alitalo K, Breitman ML. Vascularization of the mouse embryo: a study of flk-1, tek, tie, and vascular endothelial growth factor expression during development. Dev Dyn. 1995;203:80–92. doi: 10.1002/aja.1002030109. [DOI] [PubMed] [Google Scholar]
- Kisanuki YY, Hammer RE, Miyazaki J, Williams SC, Richardson JA, Yanagisawa M. Tie2-Cre transgenic mice: a new model for endothelial cell-lineage analysis in vivo. Dev Biol. 2001;230:230–242. doi: 10.1006/dbio.2000.0106. [DOI] [PubMed] [Google Scholar]
- Chang S, Young BD, Li S, Qi X, Richardson JA, Olson EN. Histone deacetylase 7 maintains vascular integrity by repressing matrix metalloproteinase 10. Cell. 2006;26:321–334. doi: 10.1016/j.cell.2006.05.040. [DOI] [PubMed] [Google Scholar]
- Lawson ND, Scheer N, Pham VN, Kim CH, Chitnis AB, Campos-Ortega JA, Weinstein BM. Notch signaling is required for arterial-venous differentiation during embryonic vascular development. Development. 2001;128:3675–3683. doi: 10.1242/dev.128.19.3675. [DOI] [PubMed] [Google Scholar]
- Lawson ND, Vogel AM, Weinstein BM. Sonic hedgehog and vascular endothelial growth factor act upstream of the Notch pathway during arterial endothelial differentiation. Dev Cell. 2002;3:127–136. doi: 10.1016/s1534-5807(02)00198-3. [DOI] [PubMed] [Google Scholar]
- Anselmo AN, Earnest S, Chen W, Juang YC, Kim SC, Zhao Y, Cobb MH. WNK1 and OSR1 regulate the Na+, K+, 2Cl- cotransporter in HeLa cells. Proc Natl Acad Sci USA. 2006;103:10883–10888. doi: 10.1073/pnas.0604607103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog Y, Guttmann-Raviv N, Neufeld G. Segregation of arterial and venous markers in subpopulations of blood islands before vessel formation. Dev Dyn. 2005;232:1047–1055. doi: 10.1002/dvdy.20257. [DOI] [PubMed] [Google Scholar]
- Zhong TP, Childs S, Leu JP, Fishman MC. Gridlock signaling pathway fashions the first embryonic artery. Nature. 2001;414:216–220. doi: 10.1038/35102599. [DOI] [PubMed] [Google Scholar]
- le Noble F, Moyon D, Pardanaud L, Yuan L, Djonov V, Matthijsen R, Breant C, Fleury V, Eichmann A. Flow regulates arterial0venous differentiation in the chick embryo yolk sac. Development. 2004;131:361–375. doi: 10.1242/dev.00929. [DOI] [PubMed] [Google Scholar]
- Antonson P, Schuster GU, Wang L, Rozell B, Holter E, Flodby P, Treuter E, Holmgren L, Gustafsson JA. Inactivation of the nuclear receptor coactivator RAP250 in mice results in placental vascular dysfunction. Mol Cell Biol. 2003;23:1260–1268. doi: 10.1128/MCB.23.4.1260-1268.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh V, McEwen A, Barbour V, Takahashi Y, Rehg JE, Jane SM, Cunningham JM. Defective extraembryonic angiogenesis in mice lacking LBP-1a, a member of the grainyhead family of transcription factors. Mol Cell Biol. 2004;24:7113–7129. doi: 10.1128/MCB.24.16.7113-7129.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CB, Boyer AS, Runyan RB, Barnett JV. Requirement of type III TGF-β receptor for endocardial cell transformation in the heart. Science. 1999;283:2080–2082. doi: 10.1126/science.283.5410.2080. [DOI] [PubMed] [Google Scholar]
- Chang S, Young BD, Li S, Qi X, Richardson JA, Olson EN. Histone deacetylase 7 maintains vascular integrity by repressing matrix metalloproteinase 10. Cell. 2006;126:321–334. doi: 10.1016/j.cell.2006.05.040. [DOI] [PubMed] [Google Scholar]
- Stankunas K, Hang CT, Tsun ZY, Chen H, Lee NV, Wu JI, Shang C, Bayle JH, Shou W, Iruela-Arispe ML, Chang CP. Endocardial Brg1 represses ADAMTS1 to maintain the microenvironment for myocardial morphogenesis. Dev Cell. 2008;14:298–311. doi: 10.1016/j.devcel.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleaver O, Melton DA. Endothelial signaling during development. Nat Med. 2003;9:661–668. doi: 10.1038/nm0603-661. [DOI] [PubMed] [Google Scholar]
- Tirziu D, Chorianopoulos E, Moodie KL, Palac RT, Zhuang ZW, Tjwa M, Roncal C, Eriksson U, Fu Q, Elfenbein A, Hall AE, Carmeliet P, Moons L, Simons M. Myocardial hypertrophy in the absence of external stimuli is induced by angiogenesis in mice. J Clin Invest. 2007;117:3188–3197. doi: 10.1172/JCI32024. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.