Abstract
AIM: To investigate the effects of prednisolone on cell membrane bleb formation, calpain μ activation and talin degradation during hepatic ischemia-reperfusion injury in rats.
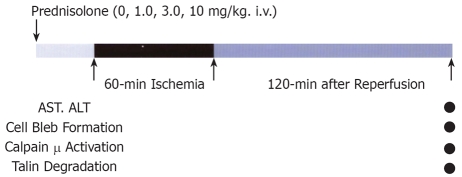
METHODS: The hilar area of the left lateral and median lobes of rat liver (68%) was clamped for 60 min and followed by 120 min reperfusion. Prednisolone was administered at 1.0, 3.0, or 10 mg/kg at 30 min before ischemia. In addition to biochemical and microscopic analyses, activation of calpain μ was determined using specific antibodies against the intermediate (activated) form of calpain μ. Degradation of talin was also studied by Western blotting.
RESULTS: In the control and prednisolone (1.0 mg/kg) groups, serum aspartate transaminase (AST) and alanine transaminase (ALT) level were elevated, and cell membrane bleb formation was observed after 120 min of reperfusion. Moreover, calpain μ activation and talin degradation were detected. Infusion of prednisolone at 3.0 or 10 mg/kg significantly suppressed serum AST and ALT, and prevented cell membrane bleb formation. At 10 mg/kg, prednisolone markedly suppressed calpain μ activation and talin degradation.
CONCLUSION: Prednisolone can suppress ischemia-reperfusion injury of the rat liver. Its cytoprotective effect is closely associated with the suppression of calpain μ activation and talin degradation.
Keywords: Ischemia-reperfusion, Prednisolone, Cell membrane bleb, Calpain μ, Talin
INTRODUCTION
Hepatic ischemia-reperfusion injury is a serious complication but unavoidable problem in liver surgery including liver transplantation and hepatic resection[1]. The most important consequence of this pathological process is multiple organ failure with a high mortality rate. Therefore, there is a considerable interest in the prevention of hepatic ischemia-reperfusion injury. Steroid therapy suppresses liver injury by a variety of mechanisms, including increased tissue blood flow and suppression of oxygen free radicals, arachidonic acid derivatives, lysosomal proteases (cathepsins) and cytokine production[2–5]. However, the exact intracellular mechanisms of steroid action on hepatic ischemia-reperfusion injury remains unknown.
Exposure of hepatocytes to hypoxia or oxidative stress is thought to result in a rise in intracellular Ca2+ concentrations ([Ca2+]i), initiating cell membrane bleb formation, an early event leading to cell death[6,7]. Although the molecular mechanisms of bleb formation are unknown at present, Ca2+-dependent disruption of the cytoskeleton is considered to play an important role in the blebbing of plasma membrane[8,9]. Calpain μ, a Ca2+-sensitive form of Ca2+-activated neutral protease (EC 3, 4, 22, 17), has been shown to degrade various cytoskeletal proteins such as talin, α-actinin and filamin[10–14].
Steroids are the most potent anti-inflammatory and immunosuppressive agents[15]. They inhibit the synthesis of almost all known cytokines and cell surface molecules required for immune function, and suppress the activity of nuclear factor kappa B (NF-κB)[16]. This inhibition is mediated by the induction of IκBα inhibitory protein, which traps activated NF-κB in inactive cytoplasmic complexes. On the other hand, inflammatory cytokines, such as interleukin-1β and tumor necrosis factor-α, are involved in the pathophysiology of hepatic ischemia-reperfusion injury[17]. The liver also plays a central role in the metabolism of these acute reactant cytokines. In the liver, these mediators are produced in large amounts by Kupffer cells or endothelial cells[18], and are released rapidly under various insults like hypoxia[17].
In the present study, we investigated the cytoprotective mechanisms of prednisolone using an experimental model of ischemia-reperfusion injury of rat liver, with special emphasis on the activation of calpain μ and degradation of talin.
MATERIALS AND METHODS
Animals
Thirty-seven adult male Wistar (Aburabi Laboratory, Shiga, Japan) rats weighing 220-260 g were used in the study. All procedures were carried out in accordance with the guidelines set forth in the Animal Welfare Act and the Guide for the Care and Use of Laboratory Animals by the National Institutes of Health. Rats were anesthetized by an interperitoneal injection of pentobarbital sodium (50 mg/kg body weight), and underwent laparotomy. Before vascular clamping, heparin sodium (50 units) was intravenously injected to prevent blood coagulation.
Prednisolone
A highly potent antagonist of prednisolone was obtained from Shionogi Pharmaceutical Co., Japan. Its molecular formula is C25H31NaO8 and molecular weight is 482.51 (Figure 1). Animals were divided into two groups: rats treated with 0.9% normal saline solution were assigned as the control group (n = 10), and those treated with intravenous injection of prednisolone (1.0, 3.0, or 10 mg/kg) as the prednisolone group (n = 27) (1.0, 3.0 and 10 mg/kg) (Figure 2).
Figure 1.
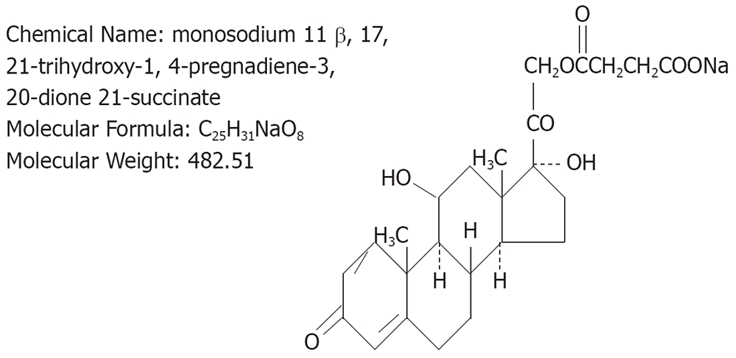
Structure of prednisolone.
Figure 2.
Experimental model.
Partial liver ischemia
An intravenous catheter was placed in the tail vein, through which prednisolonewas infused at 30 min before vascular clamping. Partial (68%) hepatic ischemia was induced by clamping the branches of the portal vein, and hepatic artery feeding the left lateral and median lobes, the branches of the right lateral (24%) and caudate (8%) lobes were not clamped[13,14]. In this condition, intestinal congestion or other complications did not occur throughout the experiment (Figure 3).
Figure 3.
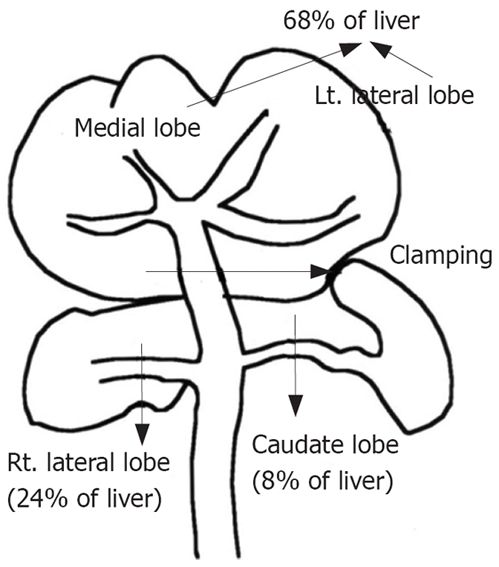
Partial liver ischemia by clamping hilar area of left lateral and medial lobes without causing intestinal congestion. Lt.: Left; Rt.: Right.
Measurement of serum aspartate transaminase (AST) and alanine transaminase (ALT)
Blood samples were collected at 120 min after declamping, and serum samples were stored at -80°C until biochemical analysis. Serum AST and ALT concentrations were determined by a Spot Chem kit (Spotchem Co., Kyoto, Japan).
Cell membrane bleb formation
In a separate set of experiments similar to those described above, tissue samples were obtained for histological examination after in situ perfusion and fixation with 0.1% glutaraldehyde and 4% paraformaldehyde, which were infused via the portal vein. Biopsy specimens were also processed for routine histopathologic examination[13,14].
Antibodies against intermediate (activated) form of calpain μ
The preparation and characteristics of an antibody that specifically recognizes the N-terminal peptide of intermediate (activated) (NH2-AQVQKQC-COOH) form of calpain μ (78 kDa) have been described previously[13,14].
Western blot analysis
Liver tissues were immediately frozen by liquid nitrogen and stored at -80°C for 3 d. For Western blot analysis, samples were homogenized in an ice-water bath using a radioimmune protein assay buffer [1.0% Nonidet P-40 (Iwai Kagaku Co Ltd, Tokyo, Japan); 0.1% deoxycholic acid; 150 mmol/L sodium chloride; 50 mmol/L Tris hydrochloride; 1.0 mmol/L phenylmethylsul-fonyl fluoride, pH 7.5] containing 5 mmol/L ethylene glycol-bis (b-aminoethyl ether)-N, N, N’ N’-tetra acetic acid (EGTA) and 5 μmol/L leupeptin. After centrifugation at 3000 r/min for 20 min at 4°C, the supernatant (25 μg of protein) was subjected to Western blot analysis using an antibody against the intermediate form of calpain μ or an anti-talin antibody (Sigma Chemical Co., St. Louis, MO). The amount of these polypeptides was determined by a densitometric analysis.
Statistical analysis
Data were expressed as means ± SD. Differences in transaminase levels, calpain μ activation and talin degradation among various groups were tested for statistical significance using the Student’s t test and Dunnett’s multiple comparison test. A P value of less than 0.05 denoted the presence of a statistically significant difference.
RESULTS
Serum AST and ALT
Serum AST and ALT concentrations were increased at 120 min after declamping in the control group. Prednisolone reduced the concentration of both transaminases in a dose-dependent manner. Differences between control and prednisolone levels were significant at prednisolone dose of 3.0 and 10 mg/kg (Figure 4).
Figure 4.
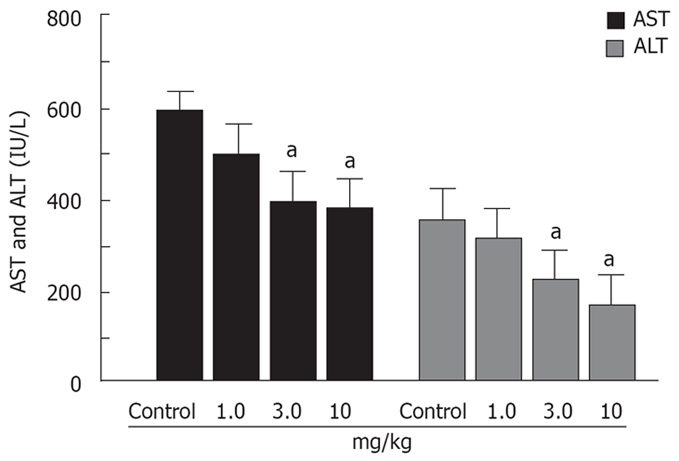
Effects of prednisolone on the AST and ALT levels in animals with 60 min of partial hepatic ischemia. aP < 0.05.
Histological findings
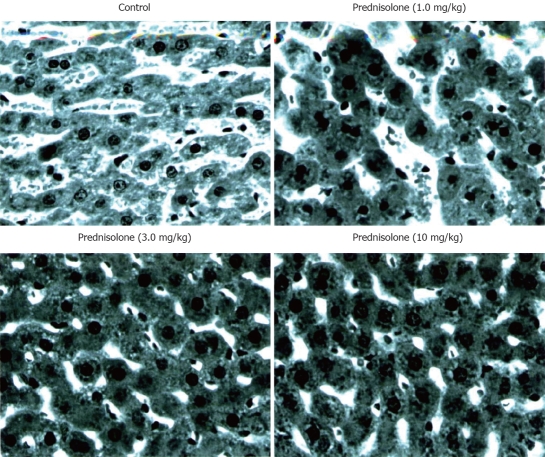
To further investigate the cytoprotective effect of prednisolone, liver tissues were examined histologically (Figure 5). In the control group, at 120 min after declamping, the cell structure of hepatocytes was not clear due to membrane bleb formation. Furthermore, numerous membrane microparticles were present in the sinusoidal space. In contrast, membrane blebbing was rarely seen and the cell structure was well preserved in prednisolone-treated (3.0 and 10 mg/kg) rats.
Figure 5.
Effects of prednisolone on histopathological findings in animals with 60 min partial hepatic ischemia. T (HE, × 400).
Activation of calpain μ
Using a specific antibody to the intermediate (78 kDa) form of calpain μ, Western blotting was performed to examine the relationship between calpain μ activation and cell injury of the liver (Figure 6). The activated form of calpain μ appeared in all groups at 120 min after vascular declamping. However, prednisolone inhibited calpain μ activation in a dose-dependent manner. The difference in calpain μ activation was significant between control and high dose prednisolone (10 mg/kg) rats.
Figure 6.
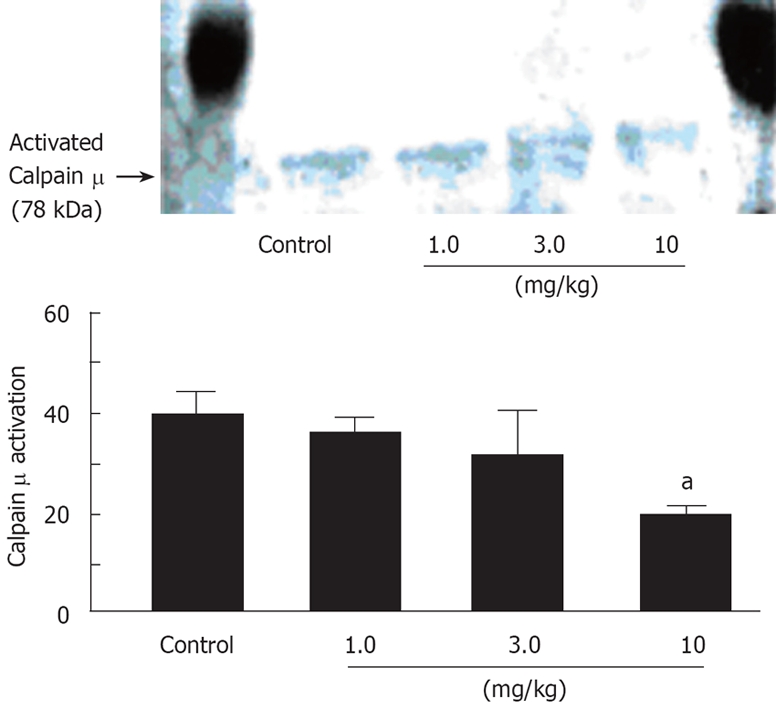
Effects of prednisolone on calpain μ activation in animals with 60 min of partial hepatic ischemia. aP < 0.05.
Degradation of talin
Proteolysis of talin, a favorable intracellular substrate of calpain μ was also investigated by Western blotting (Figure 7). Talin was markedly degraded in control and low-dose prednisolone (1.0 mg/kg) rats 120 min after vascular declamping. However, at 3.0 and 10 mg/kg, prednisolone significantly suppressed talin degradation, compared to the control group.
Figure 7.
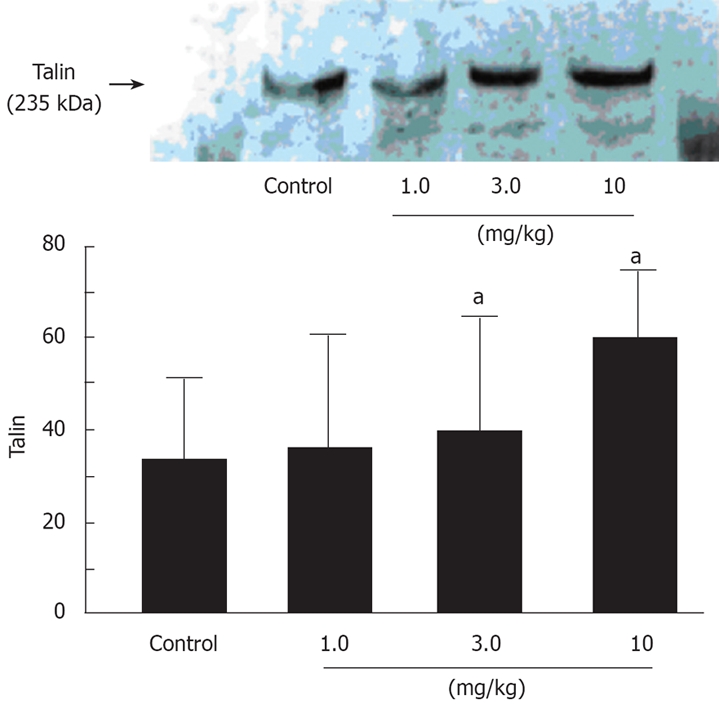
Effects of prednisolone on the degradation of talin in animals with 60 min of partial hepatic ischemia. aP < 0.05.
DISCUSSION
The major findings of our study were that prednisolone inhibited calpain μ activation in ischemia-reperfusion injury of the rat liver and that the degree of calpain μ activation closely correlated to the morphological changes in hepatocytes, i.e., cell membrane bleb formation. Furthermore, we also showed that prednisolone reduced the level of talin degradation in ischemic liver tissues. These changes were associated with improved overall liver function as reflected by lowering of serum AST and ALT concentrations, relative to the control.
Calpain μ is a major Ca2+-dependent cytosolic protease so far described[13,14] and is activated following increased [Ca2+]i in hepatocyte injury. Since cell membrane bleb formation is an irreversible phenomenon leading to cell necrosis, the role of calpain activation in cell membrane blebbing through the proteolysis of cytoskeletal proteins[15] is considered to be particularly important. Furthermore, we have previously reported that talin and α-actinin were degraded simultaneously with calpain μ activation in oxidative stress-induced hepatocyte injury, and that all these events were suppressed by a specific calpain inhibitor, calpeptin[10–14]. Thus, the beneficial effects of prednisolone on hepatic ischemia-reperfusion injury may be at least due to inhibition of calpain μ activation.
Recent studies have demonstrated that hepatocyte injury is initiated by a rise in [Ca2+]i, which results from Ca2+ release from the internal storage sites and Ca2+ influx[7,11,12], and is closely correlated with the magnitude of ischemic insult. On the other hand, the pathogenesis of hepatic ischemia-reperfusion injury is complex and multifactorial. Various factors such as the reactive oxygen species[19], platelet-activating factor[20], thromboxane A2[21], leukotriene B4[22] and endothelin-1[23] have been identified. In addition, inflammatory cytokines like interleukin-1β and tumor necrosis factor-α also play an important role in hepatic ischemia-reperfusion injury[17]. All these substances induce a rise in [Ca2+]i. These findings indicate that in addition to prostaglandin E1 and prostacyclin[13] which directly suppress the rise in [Ca2+]i, other agents like corticosteroids may inhibit hepatic ischemia-reperfusion injury by suppressing the production of these extracellular mediators. In fact, corticosteroids have been shown to inhibit the generation of oxygen free radicals[24], and reduce hepatic ischemia-reperfusion injury[25]. In the present study, we demonstrated that at a dose of 10 mg/kg, prednisolone inhibited degradation of talin, as well as calpain μ activation in the ischemic liver. Since the increase in [Ca2+]i induces calpain μ activation, inhibition of calpain μ activation by prednisolone suggests a possible suppression of increased [Ca2+]i in hepatocytes. Therefore, it is possible that prednisolone inhibits hepatic ischemia-reperfusion injury by suppressing the generation of extracellular mediators with subsequent abrogation of the rise in [Ca2+]i.
Consistent with the beneficial effects on extra- and intracellular mediators, these findings suggest that preoperative administration of glucocorticoids may prevent hepatic ischemia-reperfusion injury and subsequent systemic reactions. However, the inhibitory effect of prednisolone on the calpain μ activation was not completely similar to the results of the serum transaminase levels, and membrane bleb formation. From the clinical point of view, a complete inhibition of hepatic ischemia-reperfusion injury may be an ultimate goal in liver surgery. To accomplish this, treatment with a combination of several agents that inhibit various steps of hepatic ischemia-reperfusion injury is probably necessary since all agents reported so far produce only a partial inhibitory effect. The multiplicity approach for hepatic ischemia-reperfusion injury (e.g. prostaglandin E1 and prednisolone) is now under investigation.
In conclusion, we demonstrated in the present study that prednisolone suppressed ischemia-reperfusion injury of the rat liver. Its cytoprotective effect was partial, but was closely associated with inhibition of calpain μ activation and suppression of talin degradation. The effects of a combination of two or more agents of different inhibitory mechanisms should be examined in the future to reduce the complications encountered during hepatic surgery.
COMMENTS
Background
Hepatic ischemia-reperfusion injury is a serious complication but unavoidable problem in liver surgery including liver transplantation and hepatic resection. The most important consequence of this pathological process is multiple organ failure with a high mortality rate. Steroid therapy suppresses liver injury by a variety of mechanisms, including increased tissue blood flow and suppression of oxygen free radicals, arachidonic acid derivatives, lysosomal proteases (cathepsins) and cytokine production. In this study, authors investigated the cytoprotective mechanisms of prednisolone using an experimental model of ischemia-reperfusion injury of rat liver, with special emphasis on the activation of calpain μ and degradation of talin.
Research frontiers
Steroids are the most potent anti-inflammatory and immunosuppressive agents. They inhibit the synthesis of almost all known cytokines and cell surface molecules required for immune function, and suppress the activity of nuclear factor kappa B (NF-κB). This inhibition is mediated by the induction of IκBα inhibitory protein, which traps activated NF-κB in inactive cytoplasmic complexes. On the other hand, inflammatory cytokines, such as interleukin-1β and tumor necrosis factor-α, are involved in the pathophysiology of hepatic ischemia-reperfusion injury. The liver also plays a central role in the metabolism of these acute reactant cytokines. In the liver, these mediators are produced in large amounts by Kupffer cells or endothelial cells, and are released rapidly under various insults like hypoxia.
Innovations and breakthroughs
Recent studies have demonstrated that hepatocyte injury is initiated by a rise in [Ca2+]i, which results from Ca2+ release from the internal storage sites and Ca2+ influx, and is closely correlated with the magnitude of ischemic insult. On the other hand, the pathogenesis of hepatic ischemia-reperfusion injury is complex and multifactorial. All these reactive oxygen species induce a rise in [Ca2+]i. This studyfound that at a dose of 10 mg/kg, prednisolone inhibited degradation of talin, as well as calpain μ activation in the ischemic liver.
Applications
In this research, authors found calpain μ is an important enzyme related to hepatic ischemia-reperfusion injury. The mechanism of calpain μ is first reported in living organism in this research.
Peer review
The study deals with protective effect of prednisolone antagonist on ischemia-induced liver injury. The authors have shown that the compound used offered protection for the liver. This is a well written and interesting study.
Peer reviewers: Kunissery A Balasubramanian, Professor, Christian Medical College, Gastrointestinal Sciences, Ida Scudder Road, Vellore 632004, India; Wendy M Mars, PhD, Department of Pathology, University of Pittsburgh, S-411B South Biomedical Science Tower Pittsburgh PA 15261, United States
S- Editor Li DL L- Editor Ma JY E- Editor Zhang WB
References
- 1.Pachter HL, Spencer FC, Hofstetter SR, Coppa GF. Experience with finger fracture technique to achieve intrahepatic hemostasis in 75 patients with severe injuries of liver. Ann Surg. 1983;197:771–778. doi: 10.1097/00000658-198306000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hess ML, Manson NH. The paradox of steroid therapy: inhibition of oxygen free radicals. Circ Shock. 1983;10:1–5. [PubMed] [Google Scholar]
- 3.Liu P, Vonderfecht SL, Fisher MA, McGuire GM, Jaeschke H. Priming of phagocytes for reactive oxygen production during hepatic ischemia-reperfusion potentiates the susceptibility for endotoxin-induced liver injury. Circ Shock. 1994;43:9–17. [PubMed] [Google Scholar]
- 4.Fornander J, Hellman A, Hasselgren PO. Effects of methylprednisolone on protein synthesis and blood flow in the postischemic liver. Circ Shock. 1984;12:287–295. [PubMed] [Google Scholar]
- 5.Glenn TM, Lefer AM. Role of lysosomes in the pathogenesis of splanchnic ischemia shock in cats. Circ Res. 1970;27:783–797. doi: 10.1161/01.res.27.5.783. [DOI] [PubMed] [Google Scholar]
- 6.Nicotera P, Hartzell P, Davis G, Orrenius S. The formation of plasma membrane blebs in hepatocytes exposed to agents that increase cytosolic Ca2+ is mediated by the activation of a non-lysosomal proteolytic system. FEBS Lett. 1986;209:139–144. doi: 10.1016/0014-5793(86)81099-7. [DOI] [PubMed] [Google Scholar]
- 7.Sakaida I, Thomas AP, Farber JL. Increases in cytosolic calcium ion concentration can be dissociated from the killing of cultured hepatocytes by tert-butyl hydroperoxide. J Biol Chem. 1991;266:717–722. [PubMed] [Google Scholar]
- 8.Rosser BG, Gores GJ. Liver cell necrosis: cellular mechanisms and clinical implications. Gastroenterology. 1995;108:252–275. doi: 10.1016/0016-5085(95)90032-2. [DOI] [PubMed] [Google Scholar]
- 9.Gores GJ, Herman B, Lemasters JJ. Plasma membrane bleb formation and rupture: a common feature of hepatocellular injury. Hepatology. 1990;11:690–698. doi: 10.1002/hep.1840110425. [DOI] [PubMed] [Google Scholar]
- 10.Miyoshi H, Umeshita K, Sakon M, Imajoh-Ohmi S, Fujitani K, Gotoh M, Oiki E, Kambayashi J, Monden M. Calpain activation in plasma membrane bleb formation during tert-butyl hydroperoxide-induced rat hepatocyte injury. Gastroenterology. 1996;110:1897–1904. doi: 10.1053/gast.1996.v110.pm8964416. [DOI] [PubMed] [Google Scholar]
- 11.Murata M, Monden M, Umeshita K, Nakano H, Kanai T, Gotoh M, Mori T. Role of intracellular calcium in superoxide-induced hepatocyte injury. Hepatology. 1994;19:1223–1228. [PubMed] [Google Scholar]
- 12.Schanne FA, Kane AB, Young EE, Farber JL. Calcium dependence of toxic cell death: a final common pathway. Science. 1979;206:700–702. doi: 10.1126/science.386513. [DOI] [PubMed] [Google Scholar]
- 13.Wang M, Sakon M, Miyoshi H, Umeshita K, Kishimoto S, Taniguchi K, Gotoh M, Imajoh-Ohmi S, Monden M. Prostacyclin analog-suppressed ischemia-reperfusion injury of the rat liver: evaluation by calpain mu activation. J Surg Res. 1997;73:101–106. doi: 10.1006/jsre.1997.5200. [DOI] [PubMed] [Google Scholar]
- 14.Wang M, Sakon M, Umeshita K, Miyoshi H, Taniguchi K, Kishimoto S, Imajoh-Ohmi S, Monden M. Determination of a safe vascular clamping method for liver surgery: evaluation by measuring activation of calpain mu. Arch Surg. 1998;133:983–987. doi: 10.1001/archsurg.133.9.983. [DOI] [PubMed] [Google Scholar]
- 15.Cohen JJ. In anti-inflammatory steroid action, basic and clinical. Academic Press San Diego. 1989:111–131. [Google Scholar]
- 16.Auphan N, DiDonato JA, Rosette C, Helmberg A, Karin M. Immunosuppression by glucocorticoids: inhibition of NF-kappa B activity through induction of I kappa B synthesis. Science. 1995;270:286–290. doi: 10.1126/science.270.5234.286. [DOI] [PubMed] [Google Scholar]
- 17.Colletti LM, Remick DG, Burtch GD, Kunkel SL, Strieter RM, Campbell DA Jr. Role of tumor necrosis factor-alpha in the pathophysiologic alterations after hepatic ischemia/reperfusion injury in the rat. J Clin Invest. 1990;85:1936–1943. doi: 10.1172/JCI114656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Decker K. Biologically active products of stimulated liver macrophages (Kupffer cells) Eur J Biochem. 1990;192:245–261. doi: 10.1111/j.1432-1033.1990.tb19222.x. [DOI] [PubMed] [Google Scholar]
- 19.Jaeschke H. Reactive oxygen and ischemia/reperfusion injury of the liver. Chem Biol Interact. 1991;79:115–136. doi: 10.1016/0009-2797(91)90077-k. [DOI] [PubMed] [Google Scholar]
- 20.Wang KS, Monden M, Kanai T, Gotoh M, Umeshita K, Ukei T, Mori T. Protective effect of platelet-activating factor antagonist on ischemia-induced liver injury in rats. Surgery. 1993;113:76–83. [PubMed] [Google Scholar]
- 21.Hanazaki K, Kuroda T, Haba Y, Shiohara E, Kajikawa S, Iida F. Protective effect of the thromboxane A2 receptor antagonist ONO 3708 on ischemia-reperfusion injury in the dog liver. Surgery. 1994;116:896–903. [PubMed] [Google Scholar]
- 22.Hughes H, Farhood A, Jaeschke H. Role of leukotriene B4 in the pathogenesis of hepatic ischemia-reperfusion injury in the rat. Prostaglandins Leukot Essent Fatty Acids. 1992;45:113–119. doi: 10.1016/0952-3278(92)90226-9. [DOI] [PubMed] [Google Scholar]
- 23.Kawamura E, Yamanaka N, Okamoto E, Tomoda F, Furukawa K. Response of plasma and tissue endothelin-1 to liver ischemia and its implication in ischemia-reperfusion injury. Hepatology. 1995;21:1138–1143. [PubMed] [Google Scholar]
- 24.Casey JP, Short BL, Rink RD. The effect of methylpre-dnisolone on hepatic oxygen supply and plasma lactate and glucose in endotoxemia. Circ Shock. 1979;6:245–253. [PubMed] [Google Scholar]
- 25.Yamaguchi Y, Akizuki E, Ichiguchi O, Matsumura F, Goto M, Miyanari N, Mori K, Yamada S, Ogawa M. Neutrophil elastase inhibitor reduces neutrophil chemoattractant production after ischemia-reperfusion in rat liver. Gastroenterology. 1997;112:551–560. doi: 10.1053/gast.1997.v112.pm9024309. [DOI] [PubMed] [Google Scholar]