Abstract
AIM:To investigate the efficacy of 6-mercaptopurine (6-MP) in cases of azathioprine (AZA) hypersensitivity in patients with inflammatory bowel disease.
METHODS: Twenty nine previously confirmed Crohn’s disease (CD) (n = 14) and ulcerative colitis (UC) (n = 15) patients with a known previous (AZA) hypersensitivity reaction were studied prospectively. The 6-MP doses were gradually increased from 0.5 up to 1.0-1.5 mg/kg per day. Clinical activity indices (CDAI/CAI), laboratory variables and daily doses of oral 5-ASA, corticosteroids, and 6-MP were assessed before and in the first, sixth and twelfth months of treatment.
RESULTS: In 9 patients, 6-MP was withdrawn in the first 2 wk due to an early hypersensitivity reaction. Medication was ineffective within 6 mo in 6 CD patients, and myelotoxic reaction was observed in two. Data were evaluated at the end of the sixth month in 12 (8 UC, 4 CD) patients, and after the first year in 9 (6 UC, 3 CD) patients. CDAI decreased transiently at the end of the sixth month, but no significant changes were observed in the CDAI or the CAI values at the end of the year. Leukocyte counts (P = 0.01), CRP (P = 0.02), and serum iron (P = 0.05) values indicated decreased inflammatory reactions, especially in the UC patients at the end of the year, making the possibility to taper oral steroid doses.
CONCLUSION: About one-third of the previously AZA-intolerant patients showed adverse effects on taking 6MP. In our series, 20 patients tolerated 6MP, but it was ineffective in 8 CD cases, and valuable mainly in ulcerative colitis patients.
Keywords: Inflammatory bowel disease, Azathioprine, 6-mercaptopurine, Side effects, Efficacy
INTRODUCTION
5-aminosalicylate is usually ineffective in the main-tenance treatment of steroid induced remission in idiopathic inflammatory bowel (IBD) diseases, i.e. ulcerative colitis (UC) and Crohn’s disease (CD)[1]. In the vast majority of cases immunosuppressive treatment is necessary to maintain the remission. Azathioprine (AZA) has been advised in the treatment of UC and CD since the middle of 1960’s[2]. It is worth starting if the patient is corticosteroid resistant (the effective dose does not lead to remission) or dependent (discontinuation of the corticosteroid causes relapse)[3]. Azathioprine and its first metabolite 6-mercaptopurine are effective immunomodulators, but contrary to the corticosteroids, purine analogues have a late onset of action[4]. Their maximum effect can only be expected after 3-6 mo[5]. Azathioprine is offered as first choice, but it can cause early hypersensitivity reaction (fever), or gastrointestinal side effects (nausea, vomiting, and diarrhoea) in the first two weeks in 5%-10% of patients. In these cases, 6-mercaptopurine (6-MP) may be effective without side effects[6]. However, there are few data about the clinical efficacy of changing the AZA to 6-MP therapy in cases having hypersensitivity reactions after the first AZA medication.
MATERIALS AND METHODS
Between 2002 and 2005, 29 IBD patients (15 women and 14 men) were treated with 6-mercaptopurine due to azathioprine hypersensitivity. The drug Purinethol, 50 mg (Laboratoire GlaxoSmithKline) was approved by the National Institute of Pharmacy. The mean age of the patient’s was 40.1 years (range 19-66 years), and mean time to 6-MP treatment from the IBD diagnosis was 5.4 years (range 0.1-16.4 years). Fifteen patients had UC and 14 patients had CD. Table 1 contains the number of IBD patients in the 3 groups, according to extents, fistulas and surgeries. 6-MP test dose (50 mg/d for 7-10 d) was administered first. The therapy had to be discontinued in the first two weeks because of the same or similar hypersensitivity reactions as taking azathioprine during the initial doses in 9/29 patients (7/9 UC, 2/9 CD). Among the 9 patients, 4 had hypersensitivity reactions, including fever, and 5 were intolerant due to GI side-effects. During the very short interval (7-10 d) between the AZA start and the appearance of adverse events, we did not observe side-effects, such as leucopenia, abnormal LFTs or pancreatitis. Medication had to be suspended in 8/20 patients during the first 6 mo because it was ineffective. Decreasing the CDAI score not more than 70, and at least 3 score values in the CAI, was considered to be a treatment failure. All of them were CD patients; their treatment was continued by methotrexate and/or infliximab, if required.
Table 1.
The number of IBD patients in the three groups, according to extents, fistulas and surgeries
| Disease | No. of patients | A | B | C | D | E | F | |
| Group1 | UC | 7 | 1 | 2 | 4 | - | - | - |
| Group1 | CD | 2 | - | 2 | - | - | - | - |
| Group2 | CD | 8 | 3 | 2 | 2 | 1 | 8 | 16 |
| Group3 | UC | 8 | 1 | - | 7 | 1 | ||
| Group3 | CD | 4 | - | 3 | 1 | - | 4 | 4 |
| 29 | 4 | 9 | 14 | 1 | 12 | 21 |
UC-A: Distal; UC-B: Left sided; UC-C: Pancolitis; CD-A: Small bowel only; CD-B: Colon only; CD-C: Small bowel + colon, CD-D: Upper GI + small bowel + colon; CD-E: Number of fistulas; CD-F: Number of surgeries.
Twelve patients tolerated 6-mercaptopurine without side-effects for more than six months with clinical efficiency. Four of 12 had Crohn’s disease, 8/12 had UC. During the study 9 patients (6 UC, 3 CD) were treated for more than a year. The initial dose of 6-MP was 50 mg/d and it was increased, if possible, up to 1.5 mg/kg per day. The clinical activity, 6-MP and corticosteroid (prednisolone, methylprednisolone, separately) as well as the laboratory variables of acute inflammatory process were recorded. The medication was initiated at the first visit, and follow-up visits after the first, third, and sixth, twelfth months after the initial therapy. Crohn’s Disease Activity Index (CDAI) and Clinical Activity Index of Ulcerative Colitis (CAI) were scored and calculated at each visit. According to the calculated values, the actual activity was grouped as inactive (1), mild (2), moderate (3) or severe (4). The following blood chemistry variables were determined, erythrocyte sedimentation rate, hematocrit, white cell and platelet counts, blood iron level, CRP, and fibrinogen.
STATA V8 program was used for statistical analysis. The repeated measurements of ANOVA and correlation analysis were used. Associations of variables between activity groups were analyzed by one way method of ANOVA.
RESULTS
The baseline means ± SD of CDAI/CAI, CRP, ESR, WBC, platelets, and the changes over time demonstrated decreased inflammatory reactions (Table 2). Relationships between time (at six months and one year), activity, and laboratory variables are presented in Table 3. Results of the analysis corresponded to 12 patients at 6 mo (8 UC, 4 CD), and 9 mo (6 UC, 3 CD) at the end of the first year (repeated measurement of ANOVA). CDAI decreased significantly only at the end of the sixth month in 4 of the 12 patients in whom 6-MP treatment was successful, but a similar decrease could not be demonstrated in the CAI of UC. At the end of the year, such an alteration could not be detected in any of the activity indices. CRP, referring to the severity of the inflammation, decreased and the iron level increased significantly at the end of the year (Table 3). Correlation between the calculated activity index numeric values (CDAI, CAI) and laboratory variables (correlation analysis) is presented in Table 4. The laboratory variables associated with the numeric values of the activity revealed a decrease in the inflammatory reaction in UC, but not in CD, due to the small number of CD cases. Besides the amelioration of the inflammatory reaction, the direct suppressive effect on the bone marrow may also be associated with reductions in the numbers of leukocytes and platelets. Treatment had to be discontinued temporarily in 1 case because of leukopenia at the end of the year. In another case, a significant bone marrow depression developed, necessitating surgical intervention (ileal-pouch anal anastomosis - IPAA) after drug cessation. Hepatotoxic side-effects and pancreatitis were not observed during the year. Table 5 shows the correlation between the activity groups - inactive (1), mild (2), moderate (3), severe (4) - and the values of laboratory variables in UC and CD patients (one way ANOVA). The laboratory variables correlated substantially better with the group classification than with the activity index numeric values.
Table 2.
Changes of CDAI/CAI, CRP, ESR, WBC, PLT variables in the third group (mean ± SD)
| Disease | Months | CDAI | CAI | CRP | ESR | WBC | PLT |
| UC | 0 | - | 7.8 ± 6.3 | 16 ± 10 | 30.4 ± 16.2 | 9.3 ± 3.3 | 384 ± 175 |
| UC | 3 | - | 6.9 ± 6 | 8.3 ± 3.5 | 28.3 ± 16.5 | 10.3 ± 4.5 | 336 ± 119 |
| UC | 6 | - | 6.1 ± 6.7 | 11 ± 19 | 31.9 ± 32.7 | 6.8 ± 2 | 348 ± 197 |
| UC | 12 | - | 1.1 ± 1 | 4.6 ± 3.5 | 27 ± 17.2 | 6.1 ± 2.6 | 283 ± 87 |
| CD | 0 | 146 ± 128 | - | 39.8 ± 26.7 | 41.3 ± 11.6 | 9.9 ± 2.7 | 431 ± 136 |
| CD | 3 | 145 ± 124 | - | 23.3 ± 10.7 | 29.2 ± 14.1 | 7 ± 3 | 332 ± 66 |
| CD | 6 | 167 ± 68 | - | 21 ± 15 | 17.6 ± 28.6 | 9.9 ± 4.2 | 317 ± 89 |
| CD | 12 | 46 ± 56 | - | 6 ± 4 | 18 ± 14.7 | 6.2 ± 1.3 | 247 ± 80 |
Table 3.
Significant changes after six and twelve months of 6-MP treatment
| Variable | 6 mo | 12 mo |
| CDAI | P = 0. 0144 (n = 4) | NS n = 3 |
| CAI | NS n = 8 | NS n = 6 |
| Leukocyte | NS | P = 0. 0057 |
| CRP | NS | P = 0. 0206 |
| Serum iron | NS | P = 0. 0459 |
NS: Not significant.
Table 4.
Correlation of activity index numbers with the laboratory variables
| Variable | CAI/UC | CDAI/CD |
| Thrombocyte | P < 0.001 | NS |
| Serum iron | P = 0.006 | NS |
| CRP | P = 0.008 | NS |
| Haematocrit | P = 0.022 | NS |
| Leukocyte | P = 0.071 (BS) | NS |
| ESR 1st h | P = 0.077 (BS) | NS |
NS: Not significant; BS: Borderline significance.
Table 5.
Correlation between activity groups and laboratory variables
| Variable | 1-4 UC (P) | 1-4 CD (P) |
| Fibrinogen | 0.0046 | NS |
| Thrombocyte | 0.0060 | 0.0350 |
| CRP | 0.0061 | NS |
| Leukocyte | 0.0144 | 0.071 (BS) |
| Haematocrit | 0.0270 | NS |
| Serum iron | 0.0808 (BS) | NS |
BS: Borderline significance.
The average doses of 6-MP and corticosteroids administered at the time of the visits is presented as mg/kg (Figure 1). Prednisolone treatment could be omitted and the dose of methylprednisolone could be tapered to one third at the end of the year. At the end of the first year 5/9 patients (3 UC, and 2 CD) became steroid free.
Figure 1.
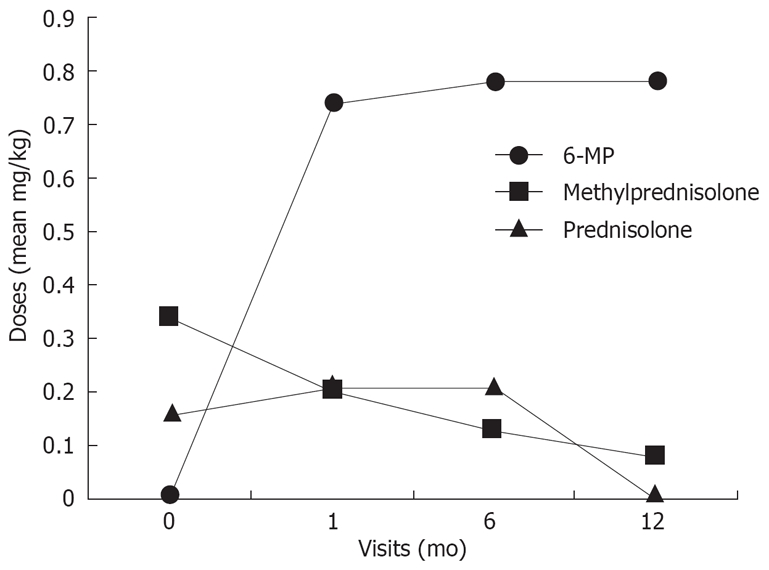
Changes of the 6-MP and oral steroid mean doses.
DISCUSSION
Systemic corticosteroid treatment is often needed in relapses of idiopathic inflammatory bowel diseases (UC, CD)[7]. The dose depends on the activity of the disease with severe cases treated intravenously[8]. If the patient responds to the intravenous regime, treatment should be switched to oral administration, and oral doses should be tapered gradually and finally terminated. If the patient proves to be steroid resistant, or dependent, immunosuppressive treatment is suggested[9]. Azathioprine is recommended first, where it is the most frequently used immunosuppressive drug for IBD[10]. An oral dose of 1.5-2.5 mg/kg per day is usually effective, but requires monitoring[11]. Therapeutic effects and side-effects of AZA show great variability among the patients due to the various concentrations of the therapeutic and toxic metabolites[12]. The application is hampered in 9%-25% of the patients due to its toxic effects. Hypersensitivity reactions (fever), or gastrointestinal side effects (nausea, diarrhea), can occur during the first weeks of the treatment[13]. According to McGovern et al, imidazole that is cleaved of the AZA molecule can be responsible for the development of this process[14]. AZA is a pro-drug that is converted to 6-MP through a non-enzymatic step. Further metabolism of 6-MP depends on three competing enzyme pathways. Hepatotoxic 6-methylmercaptopurine (6-MMP) is produced by thiopurine methyltransferase (TPMT), a key enzyme of toxic and therapeutic metabolites. Measurement of its activity helps determine individual doses[14,15]. Catabolism, via xanthine oxidase (XO), forms inactive 6-thiouric acid which is eliminated through the urine. Metabolism, via hypoxantin-guanin phosphoribosyl transferase (HPRT), leads to the formation of cytoactive 6-thioguanine nucleotide (6-TGN) that binds to the DNA and RNA, and is the active molecule responsible for the late side-effects in a dose-dependent manner. The proportion of these three enzymes determines the effective 6-TGN level. In case of low TPMT activity (due to enzyme polymorphisms) metabolism shifts to the production of 6-TGN, where high concentrations are associated with efficacy, but above a certain level (> 450 pmol/108 erythrocyte), 6-TGN has a myelotoxic effects[16]. Bone marrow depression that is a late toxic reaction usually occurs in the first three months[17]. Other mechanisms participate in the development of this process as well since this side-effect is noticed with normal and high TPMT activity. During the occurrence of immunosuppressor activity in IBD, a decrease in the number of lamina propria plasma cells and altered function of the lymphocytes and killer cells is detected. According to previous studies that need further confirmation, the therapeutic effect of AZA is explained by its apoptosis inducing property that is independent from 6-TGN. Tiede et al showed that azathioprine and its 6-thioGTP metabolite alters apoptosis of T cells[18]. The activity of certain genes in the cells is repressed by the metabolite which induces apoptosis in a mitochondrial manner.
In the view of the clinical efficacy, results achieved by 6-MP treatment can be compared with azathioprine. Considering bioequivalence, 6-MP shows the same efficacy as half (0.75-1.5 mg/kg per day) of the daily dose of AZA (1.5-2.5 mg/kg per day)[19]. There are few data about the clinical use of 6-MP[20] and only some investigated treatment results of 6-MP in AZA-intolerant patients suffering from IBD. Boulton-Jones et al reported a cohort of 19 patients who had failed AZA therapy. Ten had CD and 9 UC[21]. The AZA dose prior to discontinuation ranged from 50 to 150 mg. Eleven of 19 patients were able to tolerate 6-MP in a dose ranging from 50 to 100 mg (median 100 mg). Two of 8 patients developed fever; other adverse events were vomiting, leucopenia, skin rash, headache, and abdominal pain. The treatment could be continued in 6 patients from the 11 in the study performed by Bowen et al and were successful only in 4 cases[22]. McGovern et al achieved steroid independent remission in 15 of 29 patients[23]. Similar results were achieved by Doménech et al[24].
In our CD patients, favourable effects were attained only in 4/14 cases, at the end of the first 6 mo with 6-MP (average: 0.75 mg/kg per day). Eight CD patients were withdrawn due to ineffective therapy within the same time. Later, at the end of the first year, the benefit of the therapy was lost in all patients. In these cases, higher doses (more than 1.5 mg/kg per day) might increase the response and perhaps the number of adverse events[25]. Decreased inflammatory reactions were more significant in UC. The efficacy of the drug was indicated by the improvement of biological parameters associated with inflammation, which permitted a gradual reduction in the steroid. It is worth mentioning that clinical activity indexes (CDAI and CAI) were less sensitive than the laboratory variables at presenting decreased inflammation[26]. The correlation between activity groups and the laboratory variables showed substantially better statistical association. Considering the efficacy in case of our patients without 6-MP intolerance, it should be mentioned that more significant improvement could be achieved by using higher doses (mainly in CD). Measurement of the 6-TGN and 6-MMP metabolites and TPMT activity is recommended for the safe dosage of thiopurine analogues, and to avoid the development of late toxic effects, although none of these parameters ensure total safety[27]. In patients with early AZA intolerance treated with 6-MP thereafter, our results showed wide individual variation of 6-MP doses and responses. Patients on effective doses may develop adverse reactions at any time; therefore, blood counts and liver function tests have to be checked in every second week at the beginning, then every month, and later on in every third month. Because of the potential for life-threatening side-effects, the drug is recommended in cooperating patients only[28].
AZA and 6-MP have been accepted as maintenance therapies in IBD, but controversy exists regarding optimal dosing and benefits of therapeutic drug monitoring of metabolites[29]. The AZA baseline dose (1.7 mg/kg per day) proved to be effective in maintaining remission in a French cohort of CD patients, which was lower than the dose used in clinical trials (2.5 mg/kg per day). Nielsen et al in their review in the year 2001, suggested a 0.25 ± 0.5 mg/kg daily initial (AZA equivalent) dose for the 6-MP, increasing to 1.0 ± 1.5 mg/kg daily[30]. Su and Lichtenstein, three years later, advocated “the optimal dose for the treatment of active CD is generally considered 2.5 mg/kg per day for azathioprine and 1.5 mg/kg per day for 6-MP”[17]. In our AZA intolerant patients, the initial dose was 50 mg/d with a stepwise increase after the third and sixth month with 25 or 50 mg/d up to 1.5 mg/kg per day according to the tolerability of the patients. The low dose and the step up policy may play a role in the poor therapeutic response, especially in very active Crohn disease patients in the second group.
It is worth starting with 6-MP therapy in patients with hypersensitivity reactions to AZA. One third of the patients will be intolerant, and another third will not gain any benefit, especially those who had serious active CD. However, patients with mild or moderate UC/CD might have the advantage of being able to tolerate the daily dose of more than 1 mg/kg.
Supported by Astellas-Pharma
S- Editor Liu Y L- Editor Rippe RA E- Editor Lin YP
References
- 1.Nielsen OH, Munck LK. Drug insight: aminosalicylates for the treatment of IBD. Nat Clin Pract Gastroenterol Hepatol. 2007;4:160–170. doi: 10.1038/ncpgasthep0696. [DOI] [PubMed] [Google Scholar]
- 2.Goldstein F. Immunosuppressant therapy of inflammatory bowel disease. Pharmacologic and clinical aspects. J Clin Gastroenterol. 1987;9:654–658. doi: 10.1097/00004836-198712000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Katz JA. Advances in the medical therapy of inflammatory bowel disease. Curr Opin Gastroenterol. 2002;18:435–440. doi: 10.1097/00001574-200207000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Gilissen LP, Derijks LJ, Bos LP, Bus PJ, Hooymans PM, Engels LG. Therapeutic drug monitoring in patients with inflammatory bowel disease and established azathioprine therapy. Clin Drug Investig. 2004;24:479–486. doi: 10.2165/00044011-200424080-00006. [DOI] [PubMed] [Google Scholar]
- 5.Robinson M. Optimizing therapy for inflammatory bowel disease. Am J Gastroenterol. 1997;92:12S–17S. [PubMed] [Google Scholar]
- 6.Lees CW, Maan AK, Hansoti B, Satsangi J, Arnott ID. Tolerability and safety of mercaptopurine in azathioprine-intolerant patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2008;27:220–227. doi: 10.1111/j.1365-2036.2007.03570.x. [DOI] [PubMed] [Google Scholar]
- 7.Stein RB, Hanauer SB. Medical therapy for inflammatory bowel disease. Gastroenterol Clin North Am. 1999;28:297–321. doi: 10.1016/s0889-8553(05)70058-3. [DOI] [PubMed] [Google Scholar]
- 8.Tremaine WJ. Refractory IBD: medical management. Neth J Med. 1997;50:S12–S14. doi: 10.1016/s0300-2977(96)00065-4. [DOI] [PubMed] [Google Scholar]
- 9.Mate-Jimenez J, Hermida C, Cantero-Perona J, Moreno-Otero R. 6-mercaptopurine or methotrexate added to prednisone induces and maintains remission in steroid-dependent inflammatory bowel disease. Eur J Gastroenterol Hepatol. 2000;12:1227–1233. doi: 10.1097/00042737-200012110-00010. [DOI] [PubMed] [Google Scholar]
- 10.Sandborn WJ. Azathioprine: state of the art in inflammatory bowel disease. Scand J Gastroenterol Suppl. 1998;225:92–99. doi: 10.1080/003655298750027290. [DOI] [PubMed] [Google Scholar]
- 11.Carter MJ, Lobo AJ, Travis SP. Guidelines for the management of inflammatory bowel disease in adults. Gut. 2004;53 Suppl 5:V1–V16. doi: 10.1136/gut.2004.043372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coulthard S, Hogarth L. The thiopurines: an update. Invest New Drugs. 2005;23:523–532. doi: 10.1007/s10637-005-4020-8. [DOI] [PubMed] [Google Scholar]
- 13.Makins R, Ballinger A. Gastrointestinal side effects of drugs. Expert Opin Drug Saf. 2003;2:421–429. doi: 10.1517/14740338.2.4.421. [DOI] [PubMed] [Google Scholar]
- 14.Egan LJ, Derijks LJ, Hommes DW. Pharmacogenomics in inflammatory bowel disease. Clin Gastroenterol Hepatol. 2006;4:21–28. doi: 10.1016/j.cgh.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 15.Gearry RB, Barclay ML. Azathioprine and 6-mercaptopurine pharmacogenetics and metabolite monitoring in inflammatory bowel disease. J Gastroenterol Hepatol. 2005;20:1149–1157. doi: 10.1111/j.1440-1746.2005.03832.x. [DOI] [PubMed] [Google Scholar]
- 16.Winter JW, Gaffney D, Shapiro D, Spooner RJ, Marinaki AM, Sanderson JD, Mills PR. Assessment of thiopurine methyltransferase enzyme activity is superior to genotype in predicting myelosuppression following azathioprine therapy in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2007;25:1069–1077. doi: 10.1111/j.1365-2036.2007.03301.x. [DOI] [PubMed] [Google Scholar]
- 17.Su C, Lichtenstein GR. Treatment of inflammatory bowel disease with azathioprine and 6-mercaptopurine. Gastroenterol Clin North Am. 2004;33:209–234, viii. doi: 10.1016/j.gtc.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 18.Tiede I, Fritz G, Strand S, Poppe D, Dvorsky R, Strand D, Lehr HA, Wirtz S, Becker C, Atreya R, et al. CD28-dependent Rac1 activation is the molecular target of azathioprine in primary human CD4+ T lymphocytes. J Clin Invest. 2003;111:1133–1145. doi: 10.1172/JCI16432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pierik M, Rutgeerts P, Vlietinck R, Vermeire S. Pharma-cogenetics in inflammatory bowel disease. World J Gastroenterol. 2006;12:3657–3667. doi: 10.3748/wjg.v12.i23.3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dubinsky MC. Azathioprine, 6-mercaptopurine in inflammatory bowel disease: pharmacology, efficacy, and safety. Clin Gastroenterol Hepatol. 2004;2:731–743. doi: 10.1016/s1542-3565(04)00344-1. [DOI] [PubMed] [Google Scholar]
- 21.Boulton-Jones JR, Pritchard K, Mahmoud AA. The use of 6-mercaptopurine in patients with inflammatory bowel disease after failure of azathioprine therapy. Aliment Pharmacol Ther. 2000;14:1561–1565. doi: 10.1046/j.1365-2036.2000.00872.x. [DOI] [PubMed] [Google Scholar]
- 22.Bowen DG, Selby WS. Use of 6-mercaptopurine in patients with inflammatory bowel disease previously intolerant of azathioprine. Dig Dis Sci. 2000;45:1810–1813. doi: 10.1023/a:1005569808947. [DOI] [PubMed] [Google Scholar]
- 23.McGovern DP, Travis SP, Duley J, Shobowale-Bakre el M, Dalton HR. Azathioprine intolerance in patients with IBD may be imidazole-related and is independent of TPMT activity. Gastroenterology. 2002;122:838–839. doi: 10.1053/gast.2002.32124. [DOI] [PubMed] [Google Scholar]
- 24.Domenech E, Nos P, Papo M, Lopez-San Roman A, Garcia-Planella E, Gassull MA. 6-mercaptopurine in patients with inflammatory bowel disease and previous digestive intolerance of azathioprine. Scand J Gastroenterol. 2005;40:52–55. doi: 10.1080/00365520410009492. [DOI] [PubMed] [Google Scholar]
- 25.Lichtenstein GR, Abreu MT, Cohen R, Tremaine W. American Gastroenterological Association Institute medical position statement on corticosteroids, immunomodulators, and infliximab in inflammatory bowel disease. Gastroenterology. 2006;130:935–939. doi: 10.1053/j.gastro.2006.01.047. [DOI] [PubMed] [Google Scholar]
- 26.Jorgensen LG, Fredholm L, Hyltoft Petersen P, Hey H, Munkholm P, Brandslund I. How accurate are clinical activity indices for scoring of disease activity in inflammatory bowel disease (IBD)? Clin Chem Lab Med. 2005;43:403–411. doi: 10.1515/CCLM.2005.073. [DOI] [PubMed] [Google Scholar]
- 27.Scholmerich J. Which immunosuppressors do you use to treat Crohn's disease and ulcerative colitis? In which order of priority and how worried are you about toxicity? Inflamm Bowel Dis. 1998;4:248–252; discussion 253-254. doi: 10.1097/00054725-199808000-00009. [DOI] [PubMed] [Google Scholar]
- 28.Ananthakrishnan AN, Attila T, Otterson MF, Lipchik RJ, Massey BT, Komorowski RA, Binion DG. Severe pulmonary toxicity after azathioprine/6-mercaptopurine initiation for the treatment of inflammatory bowel disease. J Clin Gastroenterol. 2007;41:682–688. doi: 10.1097/01.mcg.0000225577.81008.ee. [DOI] [PubMed] [Google Scholar]
- 29.Hanauer SB, Thisted RA. Treatment of Crohn's disease: the "long" of it. Gastroenterology. 2005;128:2164–2166. doi: 10.1053/j.gastro.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 30.Nielsen OH, Vainer B, Rask-Madsen J. Review article: the treatment of inflammatory bowel disease with 6-mercaptopurine or azathioprine. Aliment Pharmacol Ther. 2001;15:1699–1708. doi: 10.1046/j.1365-2036.2001.01102.x. [DOI] [PubMed] [Google Scholar]


