Abstract
AIM: To investigate the prognostic impact of the metastatic lymph node ratio (MLR) in advanced gastric cancer from the cardia and fundus.
METHODS: Two hundred and thirty-six patients with gastric cancer from the cardia and fundus who underwent D2 curative resection were analyzed retrospectively. The correlations between MLR and the total lymph nodes, positive nodes and the total lymph nodes were analyzed respectively. The influence of MLR on the survival time of patients was determined with univariate Kaplan-Meier survival analysis and multivariate Cox proportional hazard model analysis. And the multiple linear regression was used to identify the relation between MLR and the 5-year survival rate of the patients.
RESULTS: The MLR did not correlate with the total lymph nodes resected (r = -0.093, P = 0.057). The 5-year overall survival rate of the whole cohort was 37.5%. Kaplan-Meier survival analysis identified that the following eight factors influenced the survival time of the patients postoperatively: gender (χ2 = 4.26, P = 0.0389), tumor size (χ2 = 18.48, P < 0.001), Borrmann type (χ2 = 7.41, P = 0.0065), histological grade (χ2 = 5.07, P = 0.0243), pT category (χ2 = 49.42, P < 0.001), pN category (χ2 = 87.7, P < 0.001), total number of retrieved lymph nodes (χ2 = 8.22, P = 0.0042) and MLR (χ2 = 34.3, P < 0.001). Cox proportional hazard model showed that tumor size (χ2 = 7.985, P = 0.018), pT category (χ2 = 30.82, P < 0.001) and MLR (χ2 = 69.39, P < 0.001) independently influenced the prognosis. A linear correlation between MLR and the 5-year survival was statistically significant based on the multiple linear regression (β = -0.63, P < 0.001). Hypothetically, the 5-year survival would surpass 50% when MLR was lower than 10%.
CONCLUSION: The MLR is an independent prognostic factor for patients with advanced gastric cancer from the cardia and fundus. The decrease of MLR due to adequate number of total resected lymph nodes can improve the survival.
Keywords: Stomach neoplasms, Lymph node metastasis, Metastatic lymph node ratio, Lymphadenectomy, Prognosis
INTRODUCTION
At present, patients with advanced gastric cancer from the cardia and fundus still have a poor prognosis despite some therapeutic modalities. Lymph node metastasis is considered one of the most important prognostic factors[1–3]. And lymphadenectomy is fundamentally critical in radical surgery. Its standardization highly depends on the accuracy of prognosis evaluation according to the classification of lymph node metastasis. The current American Joint Committee on Cancer/International Union Against Cancer (AJCC/UICC) TNM system (1997) has established the classification system based on the number of metastatic lymph nodes. D2 curative resection, which includes gastrectomy and D2 lymphadenectomy, required dissection of all the Group 1 and Group 2 nodes classified by anatomical location. However, with the development of D2 lymphadenectomy, larger lymph nodes dissected may enable to fine larger metastatic lymph nodes, which induces a migration in the staging system. The ratio of the number of metastatic lymph nodes over the total number of resected lymph nodes is introduced to prognosis evaluation. It was reported that metastatic lymph node ratio (MLR) can minimize the stage migration effect caused by increasing total dissected lymph nodes, also can help refine the current TNM stage system[4,5]. Though many studies on the prognostic significance of MLR in gastric cancer have been carried out, relevant researches on advanced gastric cancer from the cardia and fundus are still rare. Therefore, the aim of this retrospective study was to discuss the clinical impact of MLR in patients with gastric cancer from the cardia and fundus, and provide further evidence for rational lymphadenectomy.
MATERIALS AND METHODS
Materials
Two hundred and thirty-six cases, diagnosed as primary gastric cancer from the cardia and fundus were treated with curative resection at the Department of Oncology, Affiliated Union Hospital of Fujian Medical University, Fuzhou China, between January 1996 and June 2002. The surgical procedure was defined as curative when no grossly visible tumor tissue (metastasis or lymph node involvement) remained after the resection and the resection margins were histologically normal. There were 197 male (83.5%) and 39 female (16.5%) patients aged from 30 to 79 years with a mean of 58.8 ± 9.8 years. All patients received a D2 or more extended dissection of all the Group 1 and Group 2 lymph nodes or more according to the Japanese Classification of Gastric Carcinoma (JCGC)[6]. Among the 236 patients, total gastrectomy (TG) was performed in 190 patients, and proximal subtotal gastrectomy (PSG) in 46. Lymph nodes were meticulously dissected from the en bloc specimens, and the classification of the dissected lymph nodes was determined by specialized surgeons who reviewed the excised specimens after surgery based on the JCGC. Clinical and histopathologic data of each patient were collected and recorded in a specifically designed data collection form. The histopathologic spectrum included papillary adenocarcinomas (47/236, 20%), tubular adenocarcinomas (101/236, 43%), mucinous adenocarcinomas (29/236, 12%), poorly differentiated adenocarcinomas (36/236, 15%), undifferentiated carcinomas (8/236, 3%) and others (15/236, 6%) according to the World Health Organization classification system. Based on the 5th Edition of UICC TNM system[7], T category is defined as follows: T2: tumor invades muscularis propria or submucosa; T3: tumor penetrates serosa without invasion of adjacent structure; T4: tumor invades adjacent structures. N category is defined as follows: N0: no regional lymph node metastasis; N1: metastasis in 1 to 6 regional lymph nodes; N2: metastasis in 7 to 15 regional lymph nodes; N3: metastasis in more than 15 regional lymph nodes. Among our patients, there were 25 at stage pT2, 118 at pT3 and 93 at pT4, respectively, while there were 42 pN0, 97 pN1, 68 pN2 and 29 pN3 respectively. Finally, 48 cases (20%) were categorized as stage II, 128 (54%) as stage III and 60 (25%) as stage IV. All the patients received postoperative chemotherapy, using 5-FU as the dominant agent. No patientreceived postoperative radiotherapy. The follow-up was carried out by specialized investigators, who were trained about the follow-up system for clinical observation. The median follow-up for the entire cohort was 44 mo (range: 1-136 mo). A total of 222 cases were followed up with a rate of 94.0%.
Methods
All calculations were performed using the SPSS 11.5 statistical package. Correlation analysis was made to find the relationship between MLR and the total lymph nodes, positive nodes and the total lymph nodes. Cumulative survival was determined via the Kaplan-Meier method, with univariate comparisons between groups through the log-rank test. Covariates that remained significant through the univariate analysis were selected for multivariate analysis. Cox regression was used for multivariate analysis, with a forward stepwise elimination model. A multiple linear regression model to correlate MLR with 5-year survival was obtained based on Kaplan-Meier 5-year survival estimates for each MLR interval, using midpoint of MLR interval as the independent variate. Significance of differences was assumed at P values of less than 0.05.
RESULTS
MLR of different pT/pN subcategories
From the 236 cases, a total of 5615 lymph nodes were picked up and histologically examined, with 1610 positive and 4005 negative. The median total LN number was 23 (range 7-74, mean 23.8 ± 8.8 per patient), the median number of positive LNs was 5 (range 0-44, mean 6.8 ± 6.8 per patient), and that of negative LNs was 16 (range 0-48, mean 16.9 ± 9.3 per patient). The MLRs were 12.0%, 26.3% and 36.2% in cases with pT2, pT3and pT4, (χ2 = 138.9, P < 0.001), and 16.8%, 42.7% and 68.9% in cases with pN1, pN2, and pN3, respectively (χ2 = 820.7, P < 0.001). Figure 1 shows a trend of MLR according to different pT/pN subcategories. The MLR ascended as the invasion deepened, or the number of metastatic nodes increased.
Figure 1.
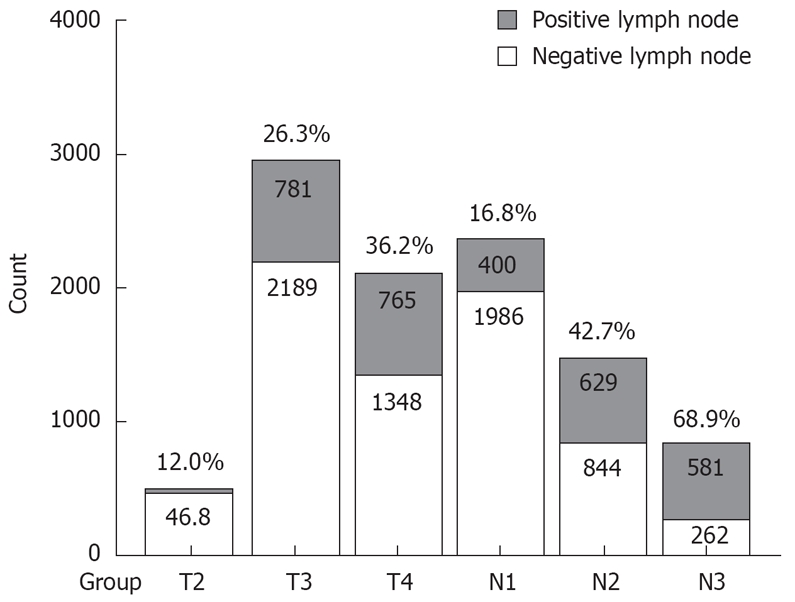
MLR of different pT/pN subcategories.
Correlation analysis between MLR and total lymph nodes, positive lymph nodes and total lymph nodes
The MLR did not correlate with the total lymph nodes dissected (r = -0.093, P = 0.057), whereas positive lymph nodes did (r = 0.173, P = 0.008). Figures 2 and 3 show the scatters of these two groups. The results revealed that, in the same extent of lymphadenectomy, MLR would not increase with the number of total lymph nodes, but the number of positive lymph nodes would.
Figure 2.
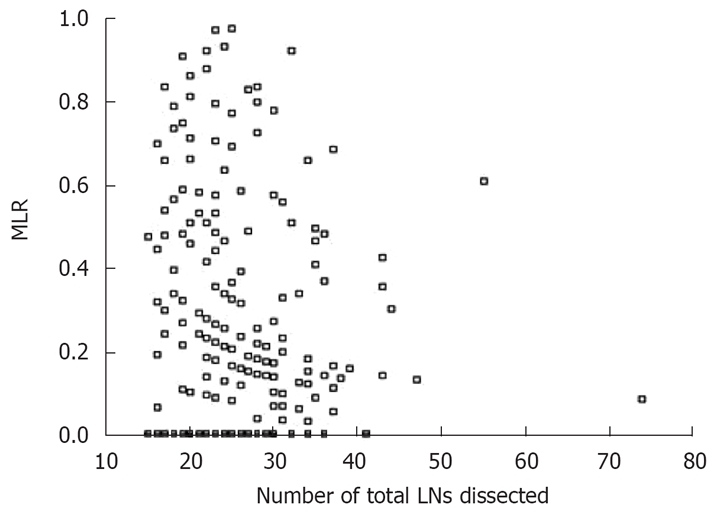
Scatter of MLR and total LNs dissected.
Figure 3.
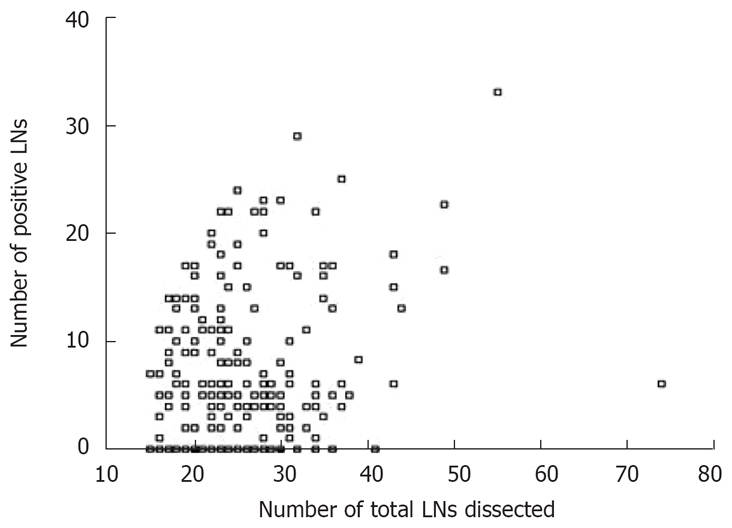
Scatter of positive LNs and total LNs dissected.
Univariate survival analysis
The 5-year overall survival rate of the entire cohort was 37.5%. The clinicopathological variables tested in the univariate analysis are shown in Table 1. Factors influencing the 5-year survival rate were patient gender (P = 0.0389), tumor size (P < 0.001), Borrmann type (P = 0.0065), histological grade (P = 0.0243), pT category (P < 0.001), pN category (P < 0.001), total number of dissected lymph nodes (P = 0.0042) and MLR (P < 0.001). Patient age (P = 0.2777) and type of gastrectomy (P = 0.0844) had no significant influence on the survival.
Table 1.
Univariate survival analysis of MLR and clinicopathological characteristics in 236 patients undergoing curative surgery
| Characteristics | n | Median survival time (mo) | 5-yr survival (%) | χ2 | P |
| Gender | 4.26 | 0.0389 | |||
| Male | 197 | 45 | 36.3 | ||
| Female | 39 | 51 | 43.6 | ||
| Age (yr) | 1.18 | 0.2777 | |||
| < 60 | 103 | 45 | 39.0 | ||
| ≥ 60 | 133 | 46 | 36.6 | ||
| Tumor size (cm) | 18.48 | 0.0000 | |||
| < 3 | 88 | 60 | 45.5 | ||
| 3-6 | 97 | 47 | 39.7 | ||
| > 6 | 51 | 32 | 18.1 | ||
| Borrmann’s type1 | 7.41 | 0.0065 | |||
| BorrmannI, II | 172 | 50 | 41.7 | ||
| Borrmann III, IV | 64 | 41 | 26.5 | ||
| Histological type | 5.07 | 0.0243 | |||
| Differentiated | 213 | 47 | 38.9 | ||
| Undifferentiated | 23 | 35 | 19.9 | ||
| pT category | 49.42 | 0.0000 | |||
| pT2 | 25 | 72 | 84.0 | ||
| pT3 | 118 | 54 | 40.2 | ||
| pT4 | 93 | 29 | 20.8 | ||
| pN category | 87.7 | 0.0000 | |||
| pN0 | 42 | 65 | 73.2 | ||
| pN1 | 97 | 55 | 42.4 | ||
| pN2 | 68 | 27 | 19.9 | ||
| pN3 | 29 | 17 | 0.0 | ||
| Num of dissected LNs | 8.22 | 0.0042 | |||
| < 15 | 36 | 27 | 22.4 | ||
| ≥ 15 | 200 | 49 | 39.1 | ||
| MLR | 34.3 | 0.0000 | |||
| < 10% | 59 | 63 | 60.5 | ||
| ~ 20% | 58 | 46 | 34.1 | ||
| ~ 30% | 33 | 44 | 33.5 | ||
| > 30% | 86 | 28 | 21.0 | ||
| Type of gastrectomy | 2.98 | 0.0844 | |||
| TG | 190 | 46 | 39.3 | ||
| PSG | 46 | 45 | 31.3 |
Borrmann’s type: Macroscopic appearances of primary tumor, classified as TypeI: polypoid tumors; Type II: ulcerated carcinomas with demarcated and raised margins; Type III: ulcerated carcinomas without definite limits, infiltrating into the surrounding wall; Type IV: diffusely infiltrating carcinomas.
Multivariate survival analysis
Multiple survival analysis was calculated by the Cox’s proportional hazard regression model. In order to confirm the influence of MLR, the prognostic factors considered at univariate analysis were analyzed first by stepwise regression, including gender, tumor size, Borrmann type, histological grade, pT category, pN category and total number of dissected lymph nodes except MLR. As a result, there were four independent, statistically significant prognostic parameters: tumor size (P = 0.039), pT category (P = 0.002), pN category (P < 0.001) and total number of dissected lymph nodes (P = 0.001). When MLR was included in the Cox’s regression, the overall fit of the Cox model increased (likelihood ratio test with and without MLR: 397 and 305, respectively). Tumor size (P = 0.018), pT category (P < 0.001) and MLR (P < 0.001) were remained to be independent prognostic factors, with MLR being the most significantly independent factor. The risk ratios and their 95% confident interval are listed in Table 2.
Table 2.
Multiple stepwise regression analysis with Cox proportional hazards model
| Characteristics | β | Wald | P | RR |
95% CI for RR |
|
| Low | High | |||||
| MLR excluded | ||||||
| Tumor size | 6.665 | 0.039 | ||||
| 3-6 cm vs < 3 cm | 0.222 | 1.938 | 0.164 | 1.249 | 0.913 | 1.707 |
| > 6 cm vs < 3 cm | 0.501 | 6.636 | 0.010 | 1.650 | 1.127 | 2.414 |
| pT category | 27.747 | 0.002 | ||||
| pT3 vs pT2 | 0.761 | 9.184 | 0.000 | 2.140 | 1.308 | 3.499 |
| pT4 vs pT2 | 1.286 | 24.369 | 0.000 | 3.617 | 2.171 | 6.026 |
| pN category | 65.066 | 0.000 | ||||
| pN1 vs pN0 | 0.618 | 9.792 | 0.002 | 1.855 | 1.260 | 2.733 |
| pN2 vs pN0 | 1.224 | 30.727 | 0.000 | 3.402 | 2.206 | 5.244 |
| pN3 vs pN0 | 2.156 | 57.470 | 0.000 | 8.639 | 4.947 | 15.090 |
| Num of total LNs | -0.682 | 10.684 | 0.001 | 0.506 | 0.336 | 0.761 |
| MLR included | ||||||
| Tumor size | 7.985 | 0.018 | ||||
| 3-6 cm vs < 3 cm | 0.307 | 3.863 | 0.049 | 1.359 | 1.001 | 1.845 |
| > 6 cm vs < 3 cm | 0.512 | 7.353 | 0.007 | 1.668 | 1.152 | 2.415 |
| pT category | 30.821 | 0.000 | ||||
| pT3 vs pT2 | 0.772 | 9.940 | 0.002 | 2.165 | 1.339 | 3.500 |
| pT4 vs pT2 | 1.343 | 26.759 | 0.000 | 3.832 | 2.303 | 6.37 |
| MLR | 2.569 | 69.390 | 0.000 | 13.06 | 7.134 | 23.900 |
MLR impact on overall survival
Linear trend test found that tumor size and MLR both had a linear correlation to the 5-year overall survival rate, and the correlation coefficients (r) were -0.157 (P = 0.019) and -0.655 (P < 0.0001), respectively. Obviously, MLR showed a much stronger correlation than tumor size. Despite tumor size and overall survival related, only MLR can reach statistically significant differences according to the multiple linear regressions (P < 0.0001). As shown in Table 3, the regression equation of the assumed statistically linearity was y = -0.63x + 0.56. Figure 4 presents a regression line of this calculated MLR effect on the 5-year survival. The hypothetical baseline 5-year survival (based on the y-intercept, i.e. MLR 0%) was 56%. For every 10% added to MLR, the calculated 5-year survival rate decreased by 6.3%. Hypothetically, the 5-year survival would surpass 50% when MLR was lower than 10%.
Table 3.
Estimation based on multiple linear regression model (stepwise method)
| Parameters | β | t | P | 95% CI for β |
| Tumor size | -0.11 | -1.97 | 0.051 | |
| MLR | -0.63 | -10.4 | 0.000 | -0.71 to -0.51 |
| Intercept (constant) | 0.56 | 19.0 | 0.000 | 0.48 to 0.59 |
Figure 4.
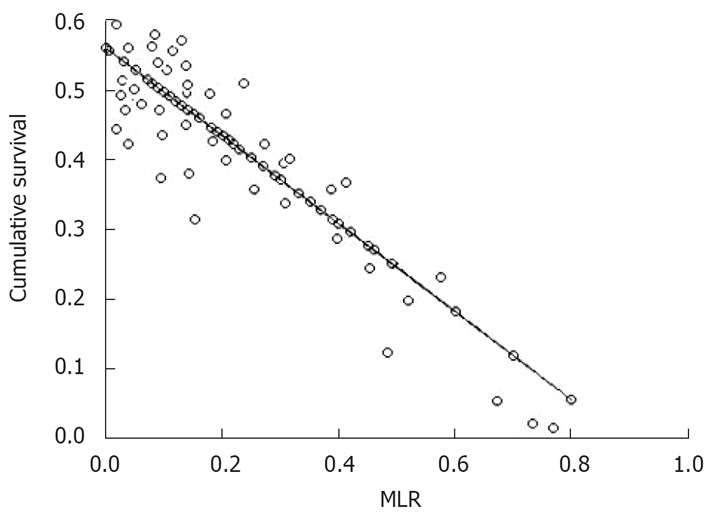
Regression line of MLR impact on the 5-year survival rate.
DISCUSSION
Over the last few decades, the rising trends in incidence rates for upper third gastric cancer have been reported by many investigators around the world[8,9]. Cancer of the gastric cardia and fundus is commonly found in late or the advanced stage at the initial diagnosis[10,11]. It was warranted to link the poor prognosis to lymph node metastases for cancer of this anatomical location reported with a higher frequency of perigastric lymph nodes and higher proportion of overall lymph node metastasis[12,13]. A prospective study conducted by de Manzoni et al[14] showed that 56.9% of patients with types II and III cardia cancer had nodal spread. Di et al[15] set up a research on lymph node involvement in gastric cancer for different sites, showing an involvement in 80.4% of cases for upper third cancers. In the present study, 82.2% of cases were found to have lymph node metastasis. And the overall MLR was 28.7%. These data confirmed the cited reports.
Lymph node metastasis is one of the most important prognostic factors for gastric cancer after curative resection. Methods of metastatic lymph nodes evaluation are still under investigation and being continuously improved, including some immunohistochemical methods, in order to predict the prognosis and guide the therapy. But few special proteins expressed in gastric cardia cancer, compared with non-cardia cancer. For example, with regard to the mucin phenotype, MUC1 and MUC5AC expression was less frequent in cardia carcinomas than in non-cardia carcinomas[16]. Therefore, lymph node status mainly depends on routine pathological examinations, previously based on the anatomical station of metastatic lymph nodes, and is classified by the number of metastatic regional lymph nodes[17–19]. The problem of lymph node classification based on number of metastatic ones is the stage migration, induced by larger lymph nodes dissected. Researches on prognostic impact of MLR have been done in patients with colon cancer, pancreatic cancer, breast cancer and other carcinomas[20–23] to find its advantage in predicting survival outcome after curative resection. MLR was introduced as a more reliable prognostic factor for gastric cancer[24,25]. In our study, a greater MLR was associated with poorer survival by univariate analysis. Multivariate analysis further identified that the MLR was a most important independent prognostic factor among the other factors evaluated, including pN category. This phenomenon was in agreement with those reported by some other investigators. It may be a superior indicator for lymph node classification system. Relevant data were reported about the grouping of patients with gastric cancer with lymph node metastasis ratios. By imitating the pN category of UICC/AJCC, most researchers selected three or four different groups. Different N ratio cutoffs have been proposed[26–29], such as 0%, 1% to 9%, 10% to 25%, > 25%; 0%, < 25%, < 50%, > 50% and so on. Many authors did not describe a specific method for the selection of the reported cutoffs. Hyung et al[30] discovered that the cutoff values were 10% for T3N1M0 and 25% for T3N2M0 by analysis of the prognosis according to MLR. In the present study, we found the 5-year survival would surpass 50% when MLR was lower than 0.10.
Our study also showed that MLR can reflect the number of LNs examined and the quality of LN dissection. Curative resection was the determining factor to improve the 5-year survival in this type of tumor[31]. Although D2 radical resection for patients with cancer of gastric cardia and fundus is widely accepted[32], how many lymph nodes should be removed to accurately predict clinical outcome has not been determined. Barbour et al[33] suggested patients with Siewert types II and III adenocarcinoma of the GEJ should undergo adequate lymphadenectomy to permit examination of ≥ 15 lymph nodes allowing the accurate identification of prognostic variables. Removal of ≥ 15 lymph nodes is associated with more accurate survival estimates for patients with advanced disease. Gee et al[34] stated that preferably 20-25 lymph nodes were necessary for determining prognosis and treatment for tumors of the gastroesophageal junction. In the present study, MLR did not correlate with the total lymph nodes while the number of metastatic LNs did. This finding indicated that the extent of lymphadenectomy was adequate when MLR value did not fluctuate with the resected number of lymph nodes. Obviously, it requires a certain amount of lymph nodes to be dissected. McKee et al[35] pointed out, MLR may be confounded by the small number of nodes examined from each patient, it should not be used for prognostic information in patients with fewer than 15 nodes examined. The median total LN number was 23 (mean 23.8 ± 8.8 per patient) in this study, so we suggested D2 lymphadenectomy in order to ensure adequate dissection.
In conclusion, MLR has advantages in providing a more precise prognostic evaluation. We should pay attention to the clinical impact of MLR on prognosis of gastric cancer located in cardia and fundus when performing a D2 radical resection. It is warranted to make efforts to reduce MLR, preferably lower than 10%, in order to achieve better therapeutic efficiency.
COMMENTS
Background
The incidence rates of gastric cancer located in cardia and fundus have increased in recent years. Though the staging system of gastric cancer refines step by step, staging techniques never stop updating. So far, few studies have investigated the relative contribution of metastatic lymph node (LN) ratio to the prognosis evaluation with advanced gastric cancer from cardia and fundus.
Research frontiers
Some researches have shown that metastatic lymph node ratio (MLR) is an excellent predictor for survival outcome in patients with colon cancer, pancreatic cancer, breast cancer and other carcinomas. Some related studies on gastric cancer also found the potential of the MLR on prognostic evaluation, but without a consensus on stratification cutoffs, especially lack of data for advanced gastric cancer located in cardia and fundus.
Innovations and breakthroughs
The authors retrospectively reviewed 236 patients with gastric cancer from cardia and fundus who were treated with D2 radical resection in a hospital in Fujian between 1996 and 2002, to investigate the validity of metastatic LN ratio as a prognostic factor. The study not only divided MLR into some different grades for survival analysis, but also set up a regression to discover the relation between MLR and survival.
Applications
The authors suggest that metastatic LN ratio can provide more dependable and accurate information on the extent of LN metastasis and lymphadenectomy for advanced gastric cancer located in cardia and fundus. Moreover, it is an important prognostic factor and can provide further evidence for rational lymphadenectomy.
Peer review
This is an interesting and well-done study investigating the prognostic role of MLR in cancer of gastric cardia and fundus. This is a well-written and significant paper.
Peer reviewers: Da-Jun Deng, Professor, Department of Cancer Etiology, Peking University School of Oncology, 1 Da- Hong-Luo-Chang Street, Western District, Beijing 100034, China; Yaron Niv, Professor, Department of Gastroenterology, Rabin Medical Center, Beilinson Campus, Tel Aviv University, 2 Hadekel St., Pardesia 42815, Israel; Giovanni Maconi, MD, Department of Gastroenterlogy, ‘L.Sacco’ University Hospital, Via G.B. Grassi, 74, Milan 20157, Italy
S- Editor Li DL L- Editor Kerr C E- Editor Zheng XM
References
- 1.Yokota T, Ishiyama S, Saito T, Teshima S, Narushima Y, Murata K, Iwamoto K, Yashima R, Yamauchi H, Kikuchi S. Lymph node metastasis as a significant prognostic factor in gastric cancer: a multiple logistic regression analysis. Scand J Gastroenterol. 2004;39:380–384. doi: 10.1080/00365520310008629. [DOI] [PubMed] [Google Scholar]
- 2.Wu ZY, Li JH, Zhan WH, He YL, Wan J. Effect of lymph node micrometastases on prognosis of gastric carcinoma. World J Gastroenterol. 2007;13:4122–4125. doi: 10.3748/wjg.v13.i30.4122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Higashi H, Natsugoe S, Ishigami S, Uenosono Y, Matsumoto M, Nakajo A, Miyazono F, Hokita S, Takao S, Aikou T. Distribution of lymph node metastasis including micrometastasis in gastric cancer with submucosal invasion. World J Surg. 2003;27:455–459. doi: 10.1007/s00268-002-6601-4. [DOI] [PubMed] [Google Scholar]
- 4.Saito H, Fukumoto Y, Osaki T, Yamada Y, Fukuda K, Tatebe S, Tsujitani S, Ikeguchi M. Prognostic significance of the ratio between metastatic and dissected lymph nodes (n ratio) in patients with advanced gastric cancer. J Surg Oncol. 2008;97:132–135. doi: 10.1002/jso.20929. [DOI] [PubMed] [Google Scholar]
- 5.Celen O, Yildirim E, Berberoglu U. Prognostic impact of positive lymph node ratio in gastric carcinoma. J Surg Oncol. 2007;96:95–101. doi: 10.1002/jso.20797. [DOI] [PubMed] [Google Scholar]
- 6.Japanese Gastric Cancer Association. Japanese Classification of Gastric Carcinoma-2nd English Edition. Gastric Cancer. 1998;1:10–24. doi: 10.1007/s101209800016. [DOI] [PubMed] [Google Scholar]
- 7.Sobin LH, Fleming ID. TNM Classification of Malignant Tumors, fifth edition (1997). Union Internationale Contre le Cancer and the American Joint Committee on Cancer. Cancer. 1997;80:1803–1804. doi: 10.1002/(sici)1097-0142(19971101)80:9<1803::aid-cncr16>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 8.Devesa SS, Fraumeni JF Jr. The rising incidence of gastric cardia cancer. J Natl Cancer Inst. 1999;91:747–749. doi: 10.1093/jnci/91.9.747. [DOI] [PubMed] [Google Scholar]
- 9.Kubo A, Corley DA. Marked regional variation in adenocarcinomas of the esophagus and the gastric cardia in the United States. Cancer. 2002;95:2096–2102. doi: 10.1002/cncr.10940. [DOI] [PubMed] [Google Scholar]
- 10.Pinto-De-Sousa J, David L, Seixas M, Pimenta A. Clinicopathologic profiles and prognosis of gastric carcinomas from the cardia, fundus/body and antrum. Dig Surg. 2001;18:102–110. doi: 10.1159/000050109. [DOI] [PubMed] [Google Scholar]
- 11.Griffiths EA, Pritchard SA, Mapstone NP, Welch IM. Emerging aspects of oesophageal and gastro-oesophageal junction cancer histopathology-an update for the surgical oncologist. World J Surg Oncol. 2006;4:82. doi: 10.1186/1477-7819-4-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dresner SM, Lamb PJ, Bennett MK, Hayes N, Griffin SM. The pattern of metastatic lymph node dissemination from adenocarcinoma of the esophagogastric junction. Surgery. 2001;129:103–109. doi: 10.1067/msy.2001.110024. [DOI] [PubMed] [Google Scholar]
- 13.Kim DY, Seo KW, Joo JK, Park YK, Ryu SY, Kim HR, Kim YJ, Kim SK. Prognostic factors in patients with node-negative gastric carcinoma: a comparison with node-positive gastric carcinoma. World J Gastroenterol. 2006;12:1182–1186. doi: 10.3748/wjg.v12.i8.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Manzoni G, Morgagni P, Roviello F, Di Leo A, Saragoni L, Marrelli D, Guglielmi A, Carli A, Folli S, Cordiano C. Nodal abdominal spread in adenocarcinoma of the cardia. Results of a multicenter prospective study. Gastric Cancer. 1998;1:146–151. doi: 10.1007/s101200050009. [DOI] [PubMed] [Google Scholar]
- 15.Di Leo A, Marrelli D, Roviello F, Bernini M, Minicozzi A, Giacopuzzi S, Pedrazzani C, Baiocchi LG, de Manzoni G. Lymph node involvement in gastric cancer for different tumor sites and T stage: Italian Research Group for Gastric Cancer (IRGGC) experience. J Gastrointest Surg. 2007;11:1146–1153. doi: 10.1007/s11605-006-0062-2. [DOI] [PubMed] [Google Scholar]
- 16.Kim MA, Lee HS, Yang HK, Kim WH. Clinicopathologic and protein expression differences between cardia carcinoma and noncardia carcinoma of the stomach. Cancer. 2005;103:1439–1446. doi: 10.1002/cncr.20966. [DOI] [PubMed] [Google Scholar]
- 17.Ichikura T, Tomimatsu S, Uefuji K, Kimura M, Uchida T, Morita D, Mochizuki H. Evaluation of the New American Joint Committee on Cancer/International Union against cancer classification of lymph node metastasis from gastric carcinoma in comparison with the Japanese classification. Cancer. 1999;86:553–558. doi: 10.1002/(sici)1097-0142(19990815)86:4<553::aid-cncr2>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 18.Lee HK, Yang HK, Kim WH, Lee KU, Choe KJ, Kim JP. Influence of the number of lymph nodes examined on staging of gastric cancer. Br J Surg. 2001;88:1408–1412. doi: 10.1046/j.0007-1323.2001.01875.x. [DOI] [PubMed] [Google Scholar]
- 19.Roder JD, Bottcher K, Busch R, Wittekind C, Hermanek P, Siewert JR. Classification of regional lymph node metastasis from gastric carcinoma. German Gastric Cancer Study Group. Cancer. 1998;82:621–631. [PubMed] [Google Scholar]
- 20.Berger AC, Sigurdson ER, LeVoyer T, Hanlon A, Mayer RJ, Macdonald JS, Catalano PJ, Haller DG. Colon cancer survival is associated with decreasing ratio of metastatic to examined lymph nodes. J Clin Oncol. 2005;23:8706–8712. doi: 10.1200/JCO.2005.02.8852. [DOI] [PubMed] [Google Scholar]
- 21.Lee HY, Choi HJ, Park KJ, Shin JS, Kwon HC, Roh MS, Kim C. Prognostic significance of metastatic lymph node ratio in node-positive colon carcinoma. Ann Surg Oncol. 2007;14:1712–1717. doi: 10.1245/s10434-006-9322-3. [DOI] [PubMed] [Google Scholar]
- 22.Pawlik TM, Gleisner AL, Cameron JL, Winter JM, Assumpcao L, Lillemoe KD, Wolfgang C, Hruban RH, Schulick RD, Yeo CJ, et al. Prognostic relevance of lymph node ratio following pancreaticoduodenectomy for pancreatic cancer. Surgery. 2007;141:610–618. doi: 10.1016/j.surg.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 23.van der Wal BC, Butzelaar RM, van der Meij S, Boermeester MA. Axillary lymph node ratio and total number of removed lymph nodes: predictors of survival in stage I and II breast cancer. Eur J Surg Oncol. 2002;28:481–489. doi: 10.1053/ejso.2002.1239. [DOI] [PubMed] [Google Scholar]
- 24.Bando E, Yonemura Y, Taniguchi K, Fushida S, Fujimura T, Miwa K. Outcome of ratio of lymph node metastasis in gastric carcinoma. Ann Surg Oncol. 2002;9:775–784. doi: 10.1007/BF02574500. [DOI] [PubMed] [Google Scholar]
- 25.Liu C, Lu P, Lu Y, Xu H, Wang S, Chen J. Clinical implications of metastatic lymph node ratio in gastric cancer. BMC Cancer. 2007;7:200. doi: 10.1186/1471-2407-7-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu CW, Hsieh MC, Lo SS, Tsay SH, Lui WY, P’eng FK. Relation of number of positive lymph nodes to the prognosis of patients with primary gastric adenocarcinoma. Gut. 1996;38:525–527. doi: 10.1136/gut.38.4.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marchet A, Mocellin S, Ambrosi A, Morgagni P, Garcea D, Marrelli D, Roviello F, de Manzoni G, Minicozzi A, Natalini G, et al. The ratio between metastatic and examined lymph nodes (N ratio) is an independent prognostic factor in gastric cancer regardless of the type of lymphadenectomy: results from an Italian multicentric study in 1853 patients. Ann Surg. 2007;245:543–552. doi: 10.1097/01.sla.0000250423.43436.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nitti D, Marchet A, Olivieri M, Ambrosi A, Mencarelli R, Belluco C, Lise M. Ratio between metastatic and examined lymph nodes is an independent prognostic factor after D2 resection for gastric cancer: analysis of a large European monoinstitutional experience. Ann Surg Oncol. 2003;10:1077–1085. doi: 10.1245/aso.2003.03.520. [DOI] [PubMed] [Google Scholar]
- 29.Takagane A, Terashima M, Abe K, Araya M, Irinoda T, Yonezawa H, Nakaya T, Inaba T, Oyama K, Fujiwara H, et al. Evaluation of the ratio of lymph node metastasis as a prognostic factor in patients with gastric cancer. Gastric Cancer. 1999;2:122–128. doi: 10.1007/s101200050034. [DOI] [PubMed] [Google Scholar]
- 30.Hyung WJ, Noh SH, Yoo CH, Huh JH, Shin DW, Lah KH, Lee JH, Choi SH, Min JS. Prognostic significance of metastatic lymph node ratio in T3 gastric cancer. World J Surg. 2002;26:323–329. doi: 10.1007/s00268-001-0227-9. [DOI] [PubMed] [Google Scholar]
- 31.Kim DY, Joo JK, Ryu SY, Park YK, Kim YJ, Kim SK. Clinicopathological characteristics and prognosis of carcinoma of the gastric cardia. Dig Surg. 2006;23:313–318. doi: 10.1159/000097895. [DOI] [PubMed] [Google Scholar]
- 32.Volpe CM, Driscoll DL, Miloro SM, Douglass HO Jr. Survival benefit of extended D2 resection for proximal gastric cancer. J Surg Oncol. 1997;64:231–236. doi: 10.1002/(sici)1096-9098(199703)64:3<231::aid-jso10>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 33.Barbour AP, Rizk NP, Gonen M, Tang L, Bains MS, Rusch VW, Coit DG, Brennan MF. Lymphadenectomy for adenocarcinoma of the gastroesophageal junction (GEJ): impact of adequate staging on outcome. Ann Surg Oncol. 2007;14:306–316. doi: 10.1245/s10434-006-9166-x. [DOI] [PubMed] [Google Scholar]
- 34.Gee DW, Rattner DW. Management of gastroesophageal tumors. Oncologist. 2007;12:175–185. doi: 10.1634/theoncologist.12-2-175. [DOI] [PubMed] [Google Scholar]
- 35.McKee MD, Posner MC. The staging of gastric cancer: nothing novel but perhaps better. Ann Surg Oncol. 2003;10:1005–1006. doi: 10.1245/aso.2003.09.914. [DOI] [PubMed] [Google Scholar]


