Abstract
Diffuse large B-cell lymphoma (DLBCL) is the most common histologic subtype of the non-Hodgkin’s lymphoma (NHL) accounting for about 40% of all NHLs. This is a case report about the endoscopic appearance of a DLBCL with infiltration to the stomach in a 39-year-old female. She had a 6-mo history of lumbar and left upper quadrant pain with intermittent episodes of melena. A computer tomography (CT) scan showed mural thickening of the gastric antrum. Endoscopic examination revealed multiple gastric ulcers. Definite diagnosis could be made by endoscopic biopsies and the patient had a good response to chemotherapy. This response correlated well with a further endoscopic follow-up. A follow-up endoscopic examination could be considered to evaluate a good response to chemotherapy in DLBCL patients with secondary gastric dissemination.
Keywords: Diffuse large B-cell lymphoma, Non-Hodgkin’s lymphoma, Gastric infiltration
INTRODUCTION
Diffuse large B-cell lymphoma (DLBCL) is the most common histologic subtype of non-Hodgkin’s lymphoma (NHL) accounting for about 40% of all NHLs[1,2]. Tumors may be localized or confined to one side of the diaphragm in 20% to 40% of cases (Stage I or II). Stage IV or disseminated disease is observed in approximately 40% of patients and is usually characterized by extranodal extramedullary infiltration[3,4]. Sites of extranodal involvement in DLBCL can include the stomach/gastrointestinal system among others[5–7]. In this report, we describe a patient with a stage IV DLBCL infiltrating the stomach diagnosed at endoscopic examination and an excellent response after 6 cycles of chemotherapy.
CASE REPORT
A 39-year-old female was referred for an upper endoscopy because of melena, weight loss and a retroperitoneal mass. She had a 6-mo history of lumbar and left upper quadrant pain. Two months before admission she presented with nausea, early satiety, and intermittent episodes of melena and malaise. At admission she also reported a 5 kg weight loss and nocturnal diaphoresis. Physical examination revealed bilateral supraclavicular lymphadenopathy, hepatosplenomegaly and a palpable tender epigastric mass. A computer tomography (CT) scan showed cervical and mediastinal lymphadenopathy, bilateral pleural effusion, two pulmonary nodules in the upper right lobe, hepatosplenomegaly and a retroperitoneal mass with mural thickening of the gastric antrum. A cervical lymph node biopsy was obtained and the patient underwent an upper endoscopic examination. At endoscopy, we observed multiple gastric ulcers without active bleeding. The ulcers had elevated margins, ranging from 5 to 15 mm in diameter and their base was covered by fibrin and/or necrotic material. The margins of the ulcers showed a characteristic erythematous, congestive, mosaic-like pattern suggestive of infiltration (Figure 1). Multiple biopsies were taken from the ulcer margins. Histopathologic analysis revealed a centroblastic DLBCL positive for CD20 at immunohistochemical analysis (Figures 2 and 3). The histologic results from the lymph node biopsy confirmed the diagnosis (Figure 4). The patient received rituximab, cyclophosphamide, adriamycin, vincristine and prednisone (R-CHOP) chemotherapy and omeprazole (20 mg po twice a day). Her clinical symptoms improved dramatically after 2 cycles of chemotherapy. After 3 cycles of chemotherapy she underwent a follow-up upper endoscopy (6 wk after the first endoscopy) that showed the presence of scarring tissue in majority of the ulcer sites and improvement at the sites where the ulcers with a bigger size were located. A final upper endoscopy performed after 6 cycles of chemotherapy (12 wk after the first endoscopy), showed almost complete resolution of the lesions (Figure 5). She denied early satiety or pain and had no signs of gastrointestinal hemorrhage.
Figure 1.
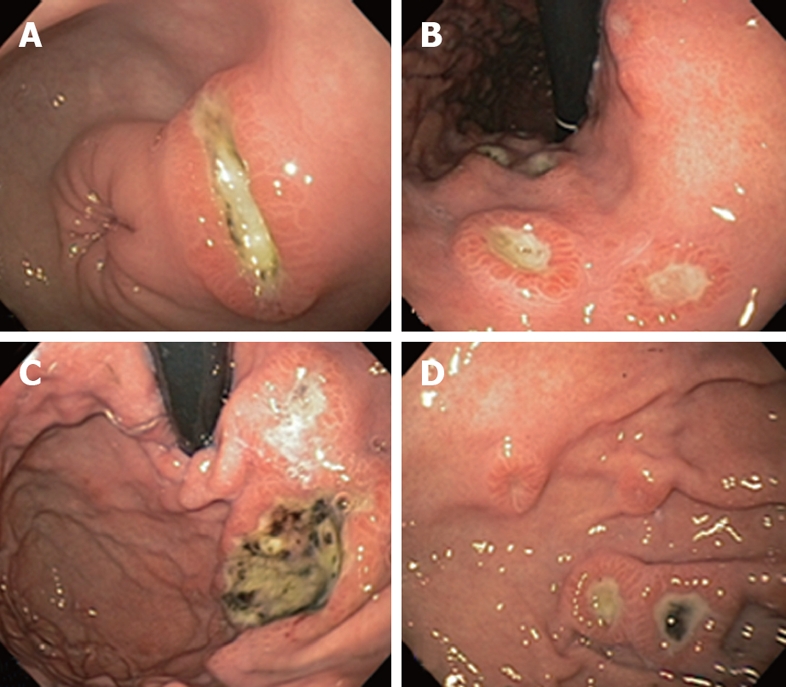
Endoscopic appearance of DLBCL infiltration of gastric antrum (A), lesser curvature (B), fundus (C), and body (D).
Figure 2.
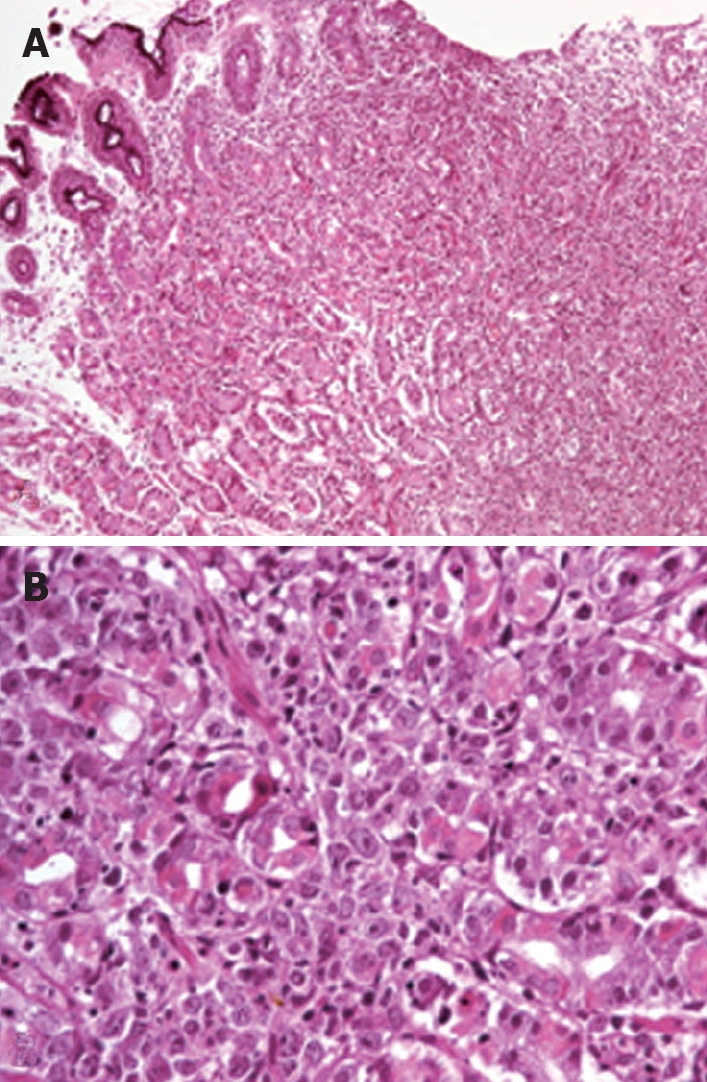
Gastric mucosa biopsy showing diffuse infiltration of lamina propria with distortion of the glandular architecture (A) and diffuse infiltration by large lymphoid cells (centroblast-type) that surround and destroy the gastric glands (B).
Figure 3.
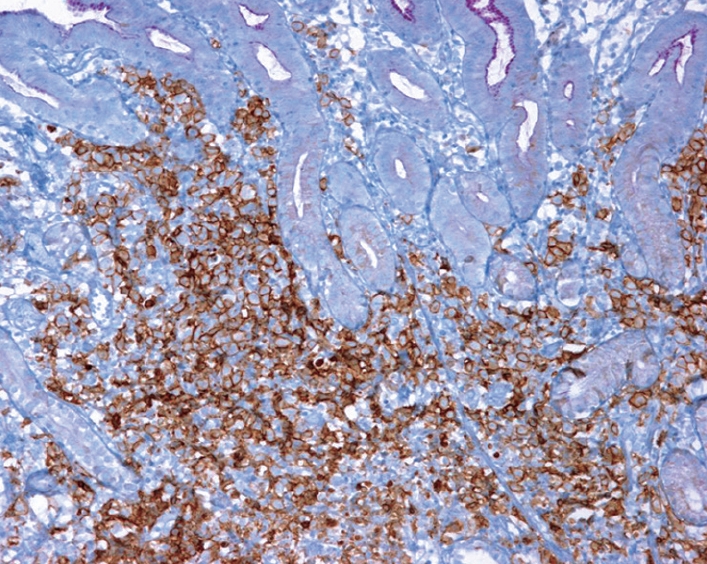
Gastric biopsy showing infiltration by atypical lymphoid cells in the gastric mucosa with intense positivity for CD20 at immunohistochemical analysis.
Figure 4.
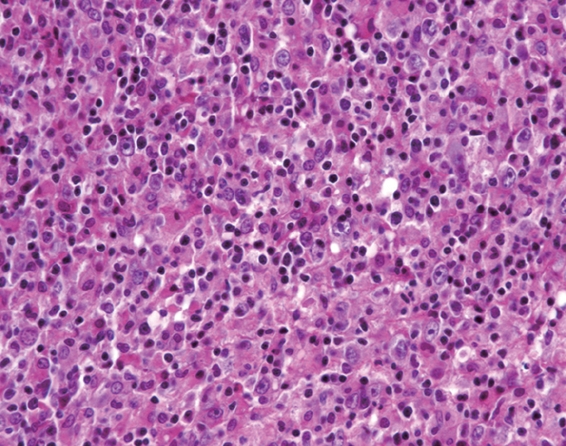
Lymph node biopsy showing neoplastic lymphocytes with fine nuclear chromatin, some of which have 2 or 3 peripheral nucleoli (centroblastic type) and others have prominent central nucleoli (immunoblastic type).
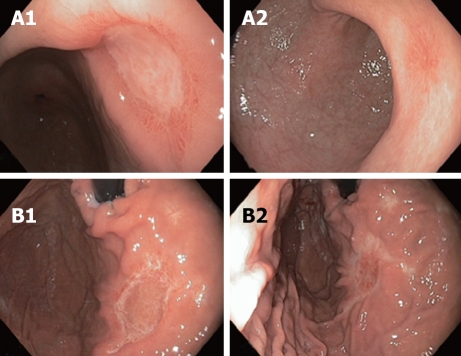
Figure 5.
Endoscopic appearances of gastric antrum (A1, A2) and fundus (B1, B2) after 3 and 6 cycles of chemotherapy respectively.
DISCUSSION
Disseminated DLBCL (stage IV) is seen in approximately 40% of patients and the gastrointestinal tract is the most common site of extranodal NHL accounting for 20% to 60% of newly diagnosed cases[8–10]. Disseminated nodal disease requires systemic chemotherapy as opposed to localized primary gastric NHL that is potentially curable with local radical treatment[11]. Thereby, it is crucial to make an early and reliable identification of the disease to initiate a correct therapy. The characteristics of the lesions at endoscopic examination can differ between primary and secondary gastric NHL. Kolve et al[12] described the endoscopic differences between 176 patients with primary NHL and 29 with secondary NHL, the lesions had macroscopical polypoid or exulcerative infiltrating changes. Primary low-grade gastric NHL was mainly characterized by diffuse infiltration and unifocal growth pattern with bulky disease in 80% of the cases with high-grade malignancy, whereas the lesions with secondary involvement showed a multifocal growth pattern in 66% of cases with bulky disease in 35%[12]. Our case showed a multifocal pattern of ulcerative lesions with elevated margins. Infiltrative disease was suspected on the basis of her clinical presentation, the number of ulcers and the erythematous mosaic-like pattern at margins of the lesions. Biopsies taken at the margin sites were diagnostic. This description of gastric infiltration by this specific type of neoplasia could help the endoscopist to suspect or identify this entity since appearance of the ulcers at endoscopic examination was very characteristic. The reason to perform the second endoscopic examination was to correlate the endoscopic findings with the improvement in the clinical picture and the radiological follow-up. Obviously, omeprazole treatment could also improve gastric ulcers, but we consider that in such a short period of time and considering the nature of the disease, it is highly improbable that without chemotherapy, we would have observed the same results. This shows that a follow-up endoscopic examination could also be taken into consideration to evaluate a good response to chemotherapy in DLBCL patients with secondary gastric dissemination.
Peer reviewer: Yusuf Bayraktar, Professor, Department of Gastroenterology, School of Medicine, Hacettepe University, Ankara 06100, Turkey
S- Editor Li DL L- Editor Wang XL E- Editor Yin DH
References
- 1.Harris NL, Jaffe ES, Stein H, Banks PM, Chan JK, Cleary ML, Delsol G, De Wolf-Peeters C, Falini B, Gatter KC. A revised European-American classification of lymphoid neoplasms: a proposal from the International Lymphoma Study Group. Blood. 1994;84:1361–1392. [PubMed] [Google Scholar]
- 2.Anderson JR, Armitage JO, Weisenburger DD. Epidemiology of the non-Hodgkin's lymphomas: distributions of the major subtypes differ by geographic locations. Non-Hodgkin's Lymphoma Classification Project. Ann Oncol. 1998;9:717–720. doi: 10.1023/a:1008265532487. [DOI] [PubMed] [Google Scholar]
- 3.Paryani S, Hoppe RT, Burke JS, Sneed P, Dawley D, Cox RS, Rosenberg SA, Kaplan HS. Extralymphatic involvement in diffuse non-Hodgkin's lymphoma. J Clin Oncol. 1983;1:682–688. doi: 10.1200/JCO.1983.1.11.682. [DOI] [PubMed] [Google Scholar]
- 4.Reddy S, Pellettiere E, Saxena V, Hendrickson FR. Extranodal non-Hodgkin's lymphoma. Cancer. 1980;46:1925–1931. doi: 10.1002/1097-0142(19801101)46:9<1925::aid-cncr2820460906>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 5.Daum S, Ullrich R, Heise W, Dederke B, Foss HD, Stein H, Thiel E, Zeitz M, Riecken EO. Intestinal non-Hodgkin's lymphoma: a multicenter prospective clinical study from the German Study Group on Intestinal non-Hodgkin's Lymphoma. J Clin Oncol. 2003;21:2740–2746. doi: 10.1200/JCO.2003.06.026. [DOI] [PubMed] [Google Scholar]
- 6.Ferrucci PF, Zucca E. Primary gastric lymphoma pathogenesis and treatment: what has changed over the past 10 years? Br J Haematol. 2007;136:521–538. doi: 10.1111/j.1365-2141.2006.06444.x. [DOI] [PubMed] [Google Scholar]
- 7.Vitolo U, Ferreri AJ, Zucca E. Primary testicular lymphoma. Crit Rev Oncol Hematol. 2008;65:183–189. doi: 10.1016/j.critrevonc.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 8.Otter R, Gerrits WB, vd Sandt MM, Hermans J, Willemze R. Primary extranodal and nodal non-Hodgkin's lymphoma. A survey of a population-based registry. Eur J Cancer Clin Oncol. 1989;25:1203–1210. doi: 10.1016/0277-5379(89)90416-1. [DOI] [PubMed] [Google Scholar]
- 9.Ferreri AJ, Montalban C. Primary diffuse large B-cell lymphoma of the stomach. Crit Rev Oncol Hematol. 2007;63:65–71. doi: 10.1016/j.critrevonc.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 10.López-Guillermo A, Colomo L, Jiménez M, Bosch F, Villamor N, Arenillas L, Muntañola A, Montoto S, Giné E, Colomer D, et al. Diffuse large B-cell lymphoma: clinical and biological characterization and outcome according to the nodal or extranodal primary origin. J Clin Oncol. 2005;23:2797–2804. doi: 10.1200/JCO.2005.07.155. [DOI] [PubMed] [Google Scholar]
- 11.Koch P, Probst A, Berdel WE, Willich NA, Reinartz G, Brockmann J, Liersch R, del Valle F, Clasen H, Hirt C, et al. Treatment results in localized primary gastric lymphoma: data of patients registered within the German multicenter study (GIT NHL 02/96) J Clin Oncol. 2005;23:7050–7059. doi: 10.1200/JCO.2005.04.031. [DOI] [PubMed] [Google Scholar]
- 12.Kolve M, Fischbach W, Greiner A, Wilms K. Differences in endoscopic and clinicopathological features of primary and secondary gastric non-Hodgkin's lymphoma. German Gastrointestinal Lymphoma Study Group. Gastrointest Endosc. 1999;49:307–315. doi: 10.1016/s0016-5107(99)70006-4. [DOI] [PubMed] [Google Scholar]