Abstract
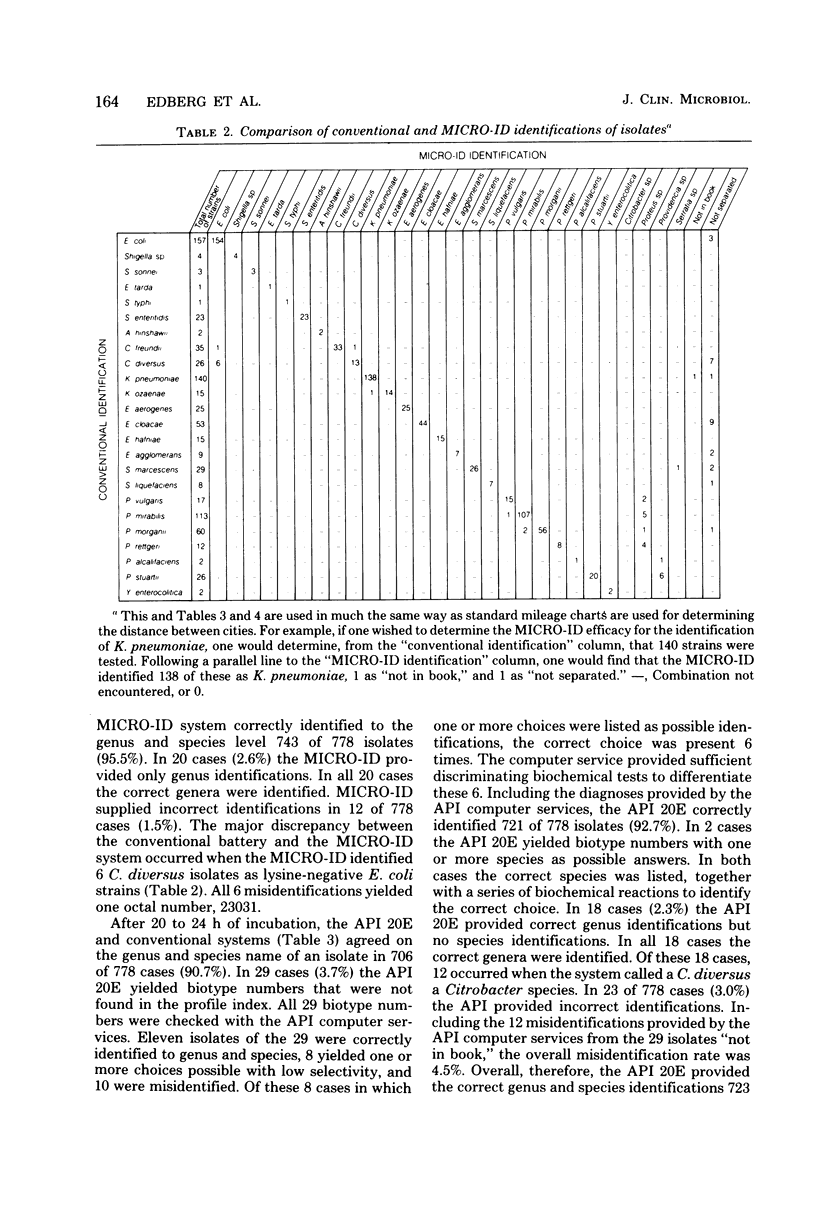
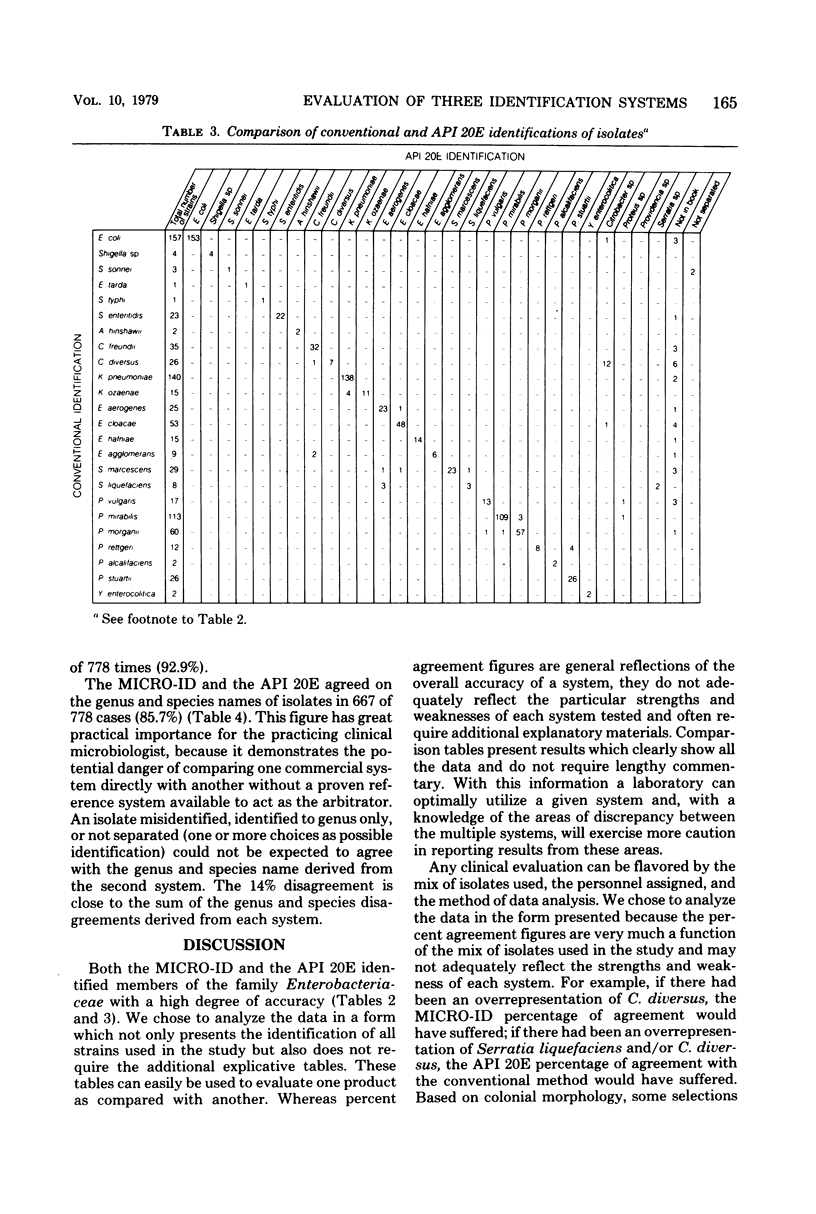
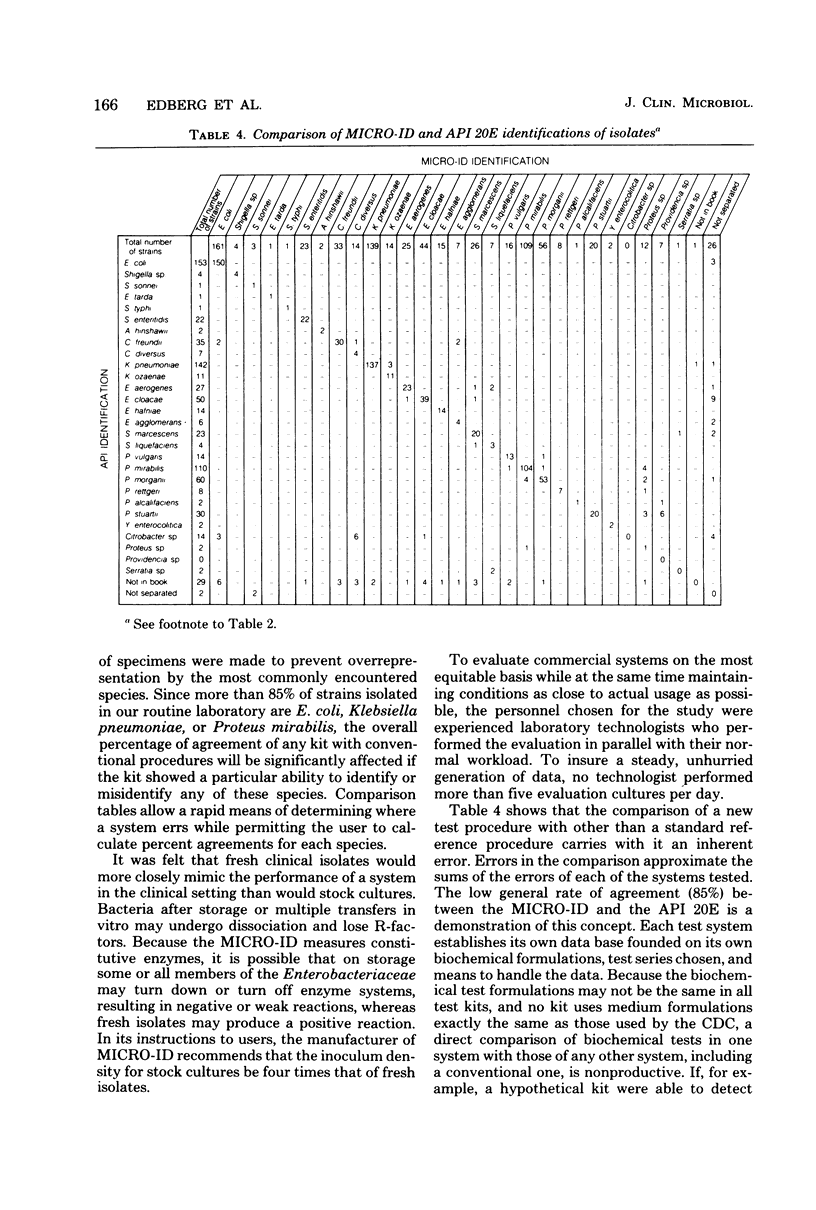
MICRO-ID (General Diagnostics, Morris Plains, N.J.) is a new kit system designed for the identification of Enterobacteriaceae in 4 h. It consists of 15 biochemical tests of paper disks. Each test is in its own compartment in a molded plastic tray. Only one reagent need be added to the system (2 drops of 20% KOH, which is added to the Voges-Proskauer test). Based on the pattern of positive and negative biochemical test results, a five-digit octal code number is calculated. An identification is derived from a computer-generated identification manual. A study was conducted to compare three systems—the MICRO-ID 4-h and the API 20E (Analytab Products Inc., Plainview, N.Y.) 18- to 24-h systems and a conventional media system—to measure the ability of each to identify members of the family Enterobacteriaceae. Comparison tables, rather than simple percentage agreement tables, were generated to define the particular strengths and weaknesses of each system and allow the laboratory to best use the data. The MICRO-ID compared quite favorably with conventional media. MICRO-ID yielded incorrect identifications with 1.5% of the isolates tested (API 20E, 4.7% misidentification rate). Half the MICRO-ID misidentifications occurred when the system identified a Citrobacter diversus as a lysine-negative Escherichia coli; all gave one octal number. A direct comparison of the MICRO-ID and API 20E was of limited value because percentage agreements were merely the sums of the errors of each. The ease of inoculation, the requirement for the addition of only one reagent, and the 4-h capability make the MICRO-ID system an extremely attractive development in the field of bacterial identification.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldridge K. E., Gardner B. B., Clark S. J., Matsen J. M. Comparison of micro-ID, API 20E, and conventional media systems in identification of Enterobacteriaceae. J Clin Microbiol. 1978 Jun;7(6):507–513. doi: 10.1128/jcm.7.6.507-513.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nord C. E., Lindberg A. A., Dahlbäck A. Evaluation of five test-kits-API, AuxoTab, Enterotube, PathoTec and R-B-for identification of Enterobacteriaceae. Med Microbiol Immunol. 1974 Mar 22;159(3):211–220. doi: 10.1007/BF02121337. [DOI] [PubMed] [Google Scholar]
- Smith P. B., Rhoden D. L., Tomfohrde K. M. Evaluation of the pathotec Rapid I-D system for identification of Enterobacteriaceae. J Clin Microbiol. 1975 Apr;1(4):359–362. doi: 10.1128/jcm.1.4.359-362.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. B., Tomfohrde K. M., Rhoden D. L., Balows A. API system: a multitube micromethod for identification of Enterobacteriaceae. Appl Microbiol. 1972 Sep;24(3):449–452. doi: 10.1128/am.24.3.449-452.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]




