Abstract
Descending monoaminergic systems modulate spinal cord function, yet spinal dopaminergic actions are poorly understood. Using the in vitro lumbar cord, we studied the effects of dopamine and D2-like receptor ligands on spinal reflexes in wild-type (WT) and D3-receptor knock-out mice (D3KO).
Low dopamine levels (1 μm) decreased the monosynaptic “stretch” reflex (MSR) amplitude in WT animals and increased it in D3KO animals. Higher dopamine concentrations (10-100 μm) decreased MSR amplitudes in both groups, but always more strongly in WT. Like low dopamine, the D3 receptor agonists pergolide and PD 128907 reduced MSR amplitude in WT but not D3KO mice. Conversely, D3 receptor antagonists (GR 103691 and nafadotride) increased the MSR in WT but not in D3KO mice. In comparison, D2-preferring agonists bromocriptine and quinpirole depressed the MSR in both groups. Low dopamine (1-5 μm) also depressed longer-latency (presumably polysynaptic) reflexes in WT but facilitated responses in D3KO mice. Additionally, in some experiments (e.g., during 10 μm dopamine or pergolide in WT), polysynaptic reflexes were facilitated in parallel to MSR depression, demonstrating differential modulatory control of these reflex circuits. Thus, low dopamine activates D3 receptors to limit reflex excitability. Moreover, in D3 ligand-insensitive mice, excitatory actions are unmasked, functionally converting the modulatory action of dopamine from depression to facilitation.
Restless legs syndrome (RLS) is a CNS disorder involving abnormal limb sensations. Because RLS symptoms peak at night when dopamine levels are lowest, are relieved by D3 agonists, and likely involve increased reflex excitability, the D3KO mouse putatively explains how impaired D3 activity could contribute to this sleep disorder.
Keywords: spinal cord reflex, monoamine, neuromodulation, restless legs syndrome, monosynaptic, mouse model
Introduction
Spinal cord function is strongly modulated by monoamines (serotonin, dopamine, noradrenaline) (Baldissera et al., 1981; Jordan et al., 1992; Tanaka et al., 1997; Kiehn and Katz, 1999); however, despite a wide body of literature on the roles of serotonin and noradrenaline, there are only few studies on the modulatory actions of dopamine in the spinal cord (Garraway and Hochman, 2001). The sole source for spinal dopamine is the A11 cell group in the dorsal posterior hypothalamus (Skagerberg et al., 1982; Lindvall et al., 1983; Skagerberg and Lindvall, 1985; Holstege et al., 1996). A11 dopaminergic neurons send collaterals throughout most of the spinal cord, but mostly exclude the substantia gelatinosa. Dopaminergic synapses in spinal cord are both synaptic and nonsynaptic, suggesting that dopaminergic signaling is via both classic synapses and volume transmission (Ridet et al., 1992).
There is extensive evidence for the existence of D1, D2, and D3 receptors in the spinal cord (van Dijken et al., 1996; Gladwell and Coote, 1999a,b; Gladwell et al., 1999; Levant and McCarson, 2001); however, the contribution of these receptors to spinal reflex excitability is not well known, except that D2-like receptor agonists depress monosynaptic “stretch” reflexes (MSRs) in cat and rat (Carp and Anderson, 1982; Gajendiran et al., 1996). We studied the effects of dopamine and D2-like receptor agonists on spinal cord excitability as measured by electrophysiological recordings of spinal reflexes in the mouse, and we compared the effects of dopamine and D3 receptor-specific drugs in wild-type (WT) and D3 receptor knock-out mice (D3KO) to determine the contribution of spinal D3 receptors to dopamine-evoked modulatory actions.
The D3KO mouse chosen phenotypically displays hyperactivity, increased locomotor activity, and hypertension (Accili et al., 1996; Asico et al., 1998). This phenotype resembles features of patients with restless legs syndrome (RLS) that express a locomotor-like activity during sleep (termed periodic leg movements) and commonly have hypertension (Ali et al., 1991; Espinar-Sierra et al., 1997). RLS is a CNS disorder that manifests itself with abnormal sensations in the limbs that are reduced during motor activity and with a circadian pattern that peaks at night. Intriguingly, hypothalamic dopamine has a circadian rhythm with lowest concentrations observed at night when RLS emerges (Carlsson et al., 1980), and primary treatment for RLS involves agonists with high affinities to D3 receptors, implicating deficits in D3 signaling in the expression of this sleep disorder (Montplaisir et al., 2000; Allen and Earley, 2001; Stiasny et al., 2002).
Here we show that, at low doses, the modulatory response of dopamine on spinal reflexes is converted from depressant in WT to facilitatory in D3KO animals because of the loss of D3 receptor function. Thus, D3 receptors are involved in limiting spinal cord excitability. Given our observations in D3KO mice, their phenotype, and its correspondence to the RLS phenotype in patients, it is an intriguing possibility that a similar conversion in modulatory actions occurs in patients suffering from RLS.
Some of these data have been published previously in abstract form (Clemens et al., 2003).
Materials and Methods
All experimental procedures complied with the National Institutes of Health guidelines for animal care and the Emory Institutional Animal Care and Use Committee. D3KO mice (B6.129S4-Drd3tm1Dac/J; Jackson Laboratory, Bar Harbor, ME), and their associated wild types (C57BL/6 mice) were anesthetized with 10% urethane (2 mg/kg, i.p., body weight) and decapitated. Mice ranged in age from postnatal day 5 to 17. The spinal cord was carefully dissected out of the body cavity and placed in a Sylgard-lined (Dow Corning, Midland, MI) Petri dish in cooled (<4°C) artificial CSF (ACSF) containing (in mm): 125 NaCl, 2.5 KCl, 2 CaCl2, 1 MgCl2, 25 glucose, 1.25 NaH2PO4, and 26 NaHCO3 at a pH of 7.4, oxygenated with 95% O2/5% CO2. After the dura mater was opened and removed to facilitate access of ACSF to the cord, dorsal and ventral roots were identified and pinned out with small insect pins. Preparations were left to recover at room temperature for 30-60 min before the onset of experimentation.
To record reflexes, spinal cord preparations were either left intact (in animals <7 d old) or hemisected midsagittally (in animals >1 week old). The hemisection in the older animals served to better oxygenate the tissue and allow for better access of ACSF. We did not observe any differences in the modulatory actions reported here when comparing these two approaches. Glass suction electrodes were placed on the distal parts of dorsal and ventral roots of lumbar segments L2-L5. After the stability of the electrode connections to the roots was established, dorsal roots were stimulated with current pulses of 500 μA, 100-500 μsec, to achieve a maximal reflex response, at interstimulus intervals of 30-180 sec. Reflexes were recorded from the corresponding ventral roots, amplified, and digitized with a Digidata 1322A using pClamp 9 software (Axon Instruments, Union City, CA).
To allow comparisons, reflex responses were rectified, and the calculated integrals of these responses were measured and compared between epochs of identical duration before and after drug application. Reflex amplitudes were normalized to the mean of the control values and are reported here as percentage changes from the control (predrug) conditions. Comparisons were made between the averaged amplitudes of the last 10 consecutive reflex responses measured before drug application and 10 consecutive reflex responses during the application, starting at ∼10 min after the drug was added.
Advantage of the transgenic D3 receptor knock-out mouse strain used. Several different transgenic forms of D3KO mice have been developed using slightly different approaches (Accili et al., 1996; Xu et al., 1997; Jung and Schmauss, 1999), leading to subtle differences in the phenotype of the null-mutants. For instance, the D3KO developed by Xu et al. (1997) expresses only a temporary increase in locomotor behavior in a novel environment; thus compensatory mechanisms may have developed in those mutants to counter the lack of D3 receptor function (Jung et al., 1999). In contrast, the D3KO mice used here (Accili et al., 1996) maintain an increased locomotor behavior for extended time periods, are hypertensive (Asico et al., 1998), and consequently may not possess such a mechanism to compensate for the functional loss of the D3 receptor. Therefore, these mice might be better suited to potentially unravel the mechanisms that underlie D3 receptor-dependent actions normally occurring in the spinal cord.
Pharmacology. After a stable response in the ventral roots recordings was established, generally 30-60 min after onset of the stimulation protocol (at interstimulus intervals of 30-180 sec), drugs were bath applied in general for durations of 30 min in their respective carrier substance. Tests for each of these carrier substances (ethanol, DMSO, and HCl) in their final concentrations used in the experiments did not lead to any of the effects on the reflex amplitude observed with the drugs (data not shown). Dopamine was bath applied in concentrations ranging from 1 to 100 μm, whereas the dopamine receptor selective ligands pergolide, bromocriptine, and quinpirole (RBI/Sigma, Natick, MA) were bath applied at concentrations of 1-2 μm. The D3 selective agonist PD 128907 (Tocris, Ellisville, MO) was bath applied at a concentration of 10 μm. In contrast, the highly selective D3 receptor selective antagonists GR 103691 and nafadotride (both from Tocris) were applied at concentrations of 100 and 50 nm, respectively. Several drugs were often compared in the same animal. In these cases, after drug applications, we interrupted the stimulation protocol and washed the preparations carefully and thoroughly (three to four times the bath volume over a 3-5 min period) with ACSF and subsequently let the preparation recover from the drug application for an additional 30 min. During this time, we reinstated the stimulation protocol to assess the recovery of the reflex amplitudes. In general, reflex responses returned to predrug amplitudes, and these “recovered” reflex amplitudes in turn became the predrug control of the subsequent drug application. With this protocol we were able to test several drugs per experiment. To exclude potential lingering effects of the drug applications within the spinal cord, we specifically tested whether the order in which the drugs were applied might play a role in the modulatory effects observed; however, we did not find any correlation between the order of the drugs tested and the modulatory effects induced by these drugs (data not shown).
Data analysis. All values are given as mean ± SE. We used SigmaPlot and SigmaStat (SPSS Science, Chicago, IL) to analyze the data and test for significant differences in the course of an experiment using parametric or nonparametric tests where appropriate. Differences were considered significant if p < 0.05.
Results
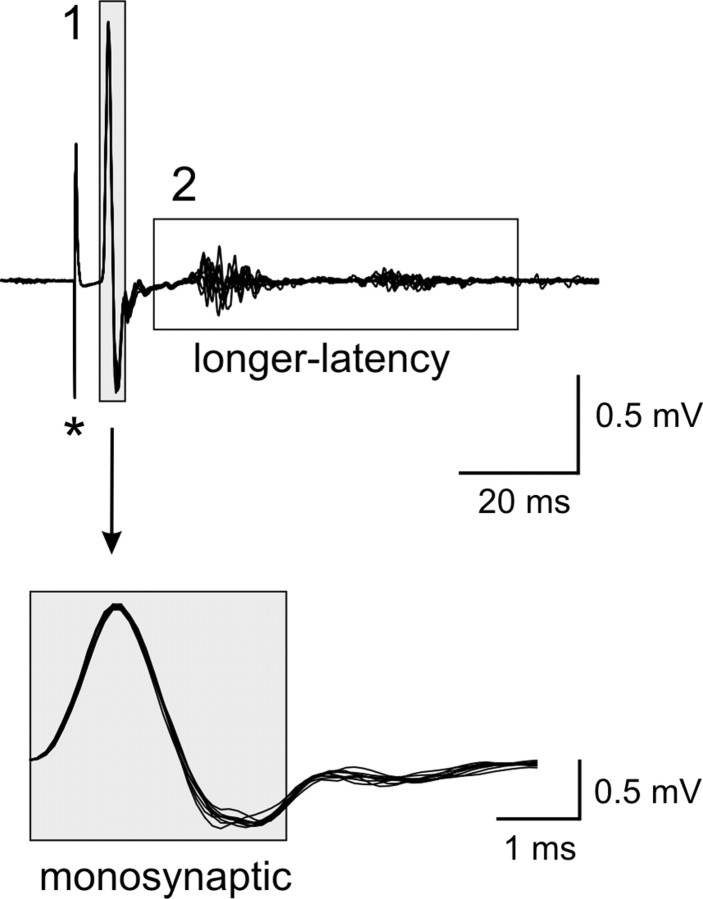
Reflexes were analyzed from a total of 68 preparations (35 WT and 33 D3KO mice). Stimulation of the lumbar dorsal roots generally evoked short-latency monosynaptic and longer-latency reflexes (Fig. 1). Up to three different reflex periods could be distinguished based on the start of the monosynaptic (MSR) component: at 0-3 msec, at ∼15-30 msec, and a third epoch starting at ∼30 msec. In some preparations, however, the longer-latency reflex components overlapped and could not be easily distinguished. Therefore, in this study we chose to divide the reflex response into two epochs only: the monosynaptic reflex at 0-3 msec (Pinco and Lev-Tov, 1993, 1994; Jonas et al., 1998) corresponding to the group I muscle spindle afferent-evoked stretch reflex, and a second epoch from 5-65 msec after the onset of the MSR response, comprising longer-latency and presumably including polysynaptic reflex pathways.
Figure 1.
Example of a reflex response recorded from the ventral roots (10 consecutive sweeps superimposed). Stimulation of an L5 dorsal root (500 μA, 500 μsec, at asterisk) induced a reflex response in the corresponding ventral root that consisted of a monosynaptic (1) and a longer-latency reflex response (2). Note that the longer-latency response consists here of two epochs that can be easily distinguished, at ∼15-30 and ∼40-60 msec after the onset of the MSR, respectively. The boxed regions identify those epochs chosen to calculate the amplitude of monosynaptic and longer-latency reflexes. The MSR component is expanded horizontally to highlight the 3 msec period and show its stability.
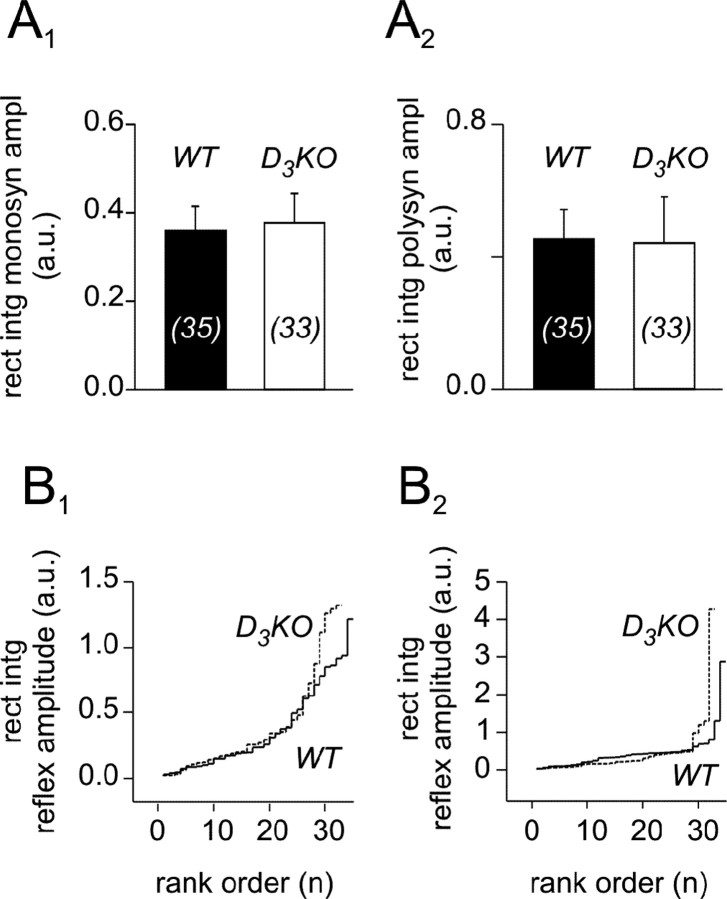
To establish a baseline of the amplitudes of the monosynaptic stretch and longer-latency reflexes in WT and D3KO mice, we first compared the amplitudes under control conditions. We found that the mean amplitudes of both MSR and the longer-latency reflexes were similar between WT and D3KO mice (Fig. 2A1,A2). A rank order comparison revealed that low- and median-reflex amplitudes of WT and D3KO responses were similar. In contrast, we observed more large-reflex amplitudes in D3KO animals for both MSR and longer-latency reflex responses when compared with the WT (Fig. 2B1,B2).
Figure 2.
The MSR and longer-latency reflex amplitudes of WT and D3KO mice have a similar average but a slightly different distribution pattern. A, Comparison of the rectified and integrated amplitudes. A1, Monosynaptic reflex amplitudes of wild-type and D3KO animals under controlconditions. In WT animals, the average is 0.36 ± 0.05 mV (n = 35), and in D3KO animals it is 0.38 ± 0.07 mV (n = 33). The populations are not different (p = 0.932). A2, Longer-latency reflex amplitudes of WT and D3KO animals under control conditions. In WT, the average is 0.44 ± 0.08 mV, and in D3KO animals it is 0.43 ± 0.13 mV. Again, there is no significant difference between the two populations (p = 0.948). Sample sizes are indicated within histogram bars in brackets. B, Rank ordered histograms of WT and D3KO reflex responses. Data are from the same pool as in A and plotted as a function of their rank. B1, Monosynaptic reflex amplitudes. WT (solid line) and D3KO (dashed line) reflex amplitudes are similar for low and median ranks, but there are more larger-reflex amplitudes in D3KO than in WT. B2, Longer-latency reflex amplitudes. Here again, WT (solid line) and D3KO (dashed line) reflex amplitudes are similar for low and median ranks, and there are more larger-reflex amplitudes in D3KO than in WT animals. rect in t monosyn ampl, Rectified and integrated monosynaptic reflex amplitude; polysyn ampl, rectified and integrated polysynaptic reflex amplitude; a.u., artificial units.
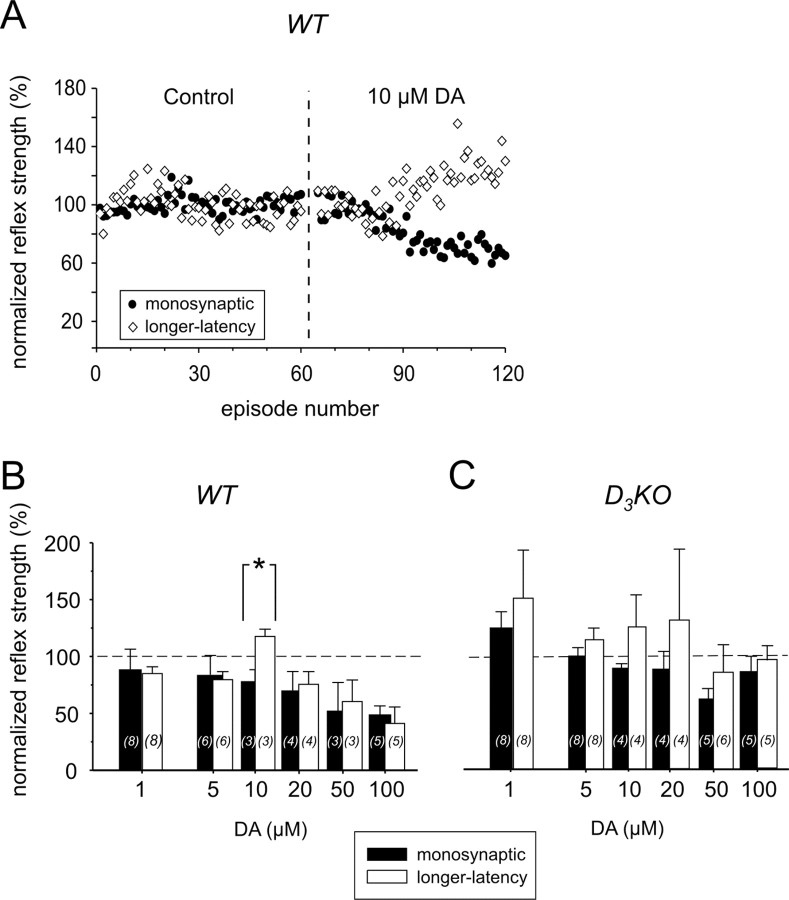
Effects of dopamine on the monosynaptic reflex strength in WT and D3KO mice
In WT mice, application of 1 μm dopamine to the bath generally led to a decrease in the MSR amplitudes (Fig. 3A1). Specifically, in four of eight experiments, dopamine depressed the reflex amplitude (to 75 ± 6% of control) and weakly facilitated it in two experiments (109 ± 5% of control), leading to an overall decrease to 89 ± 6% (Fig. 3B). In contrast, in D3KO mice, application of 1 μm dopamine generally led to an increase in MSR amplitude (Fig. 3A2). There, in five of eight experiments, the size of the MSR increased to 145 ± 13% of the control with a decrease observed in only a single experiment. The difference in modulatory actions between WT and D3KO animal populations was significant (Fig. 3B).
Figure 3.
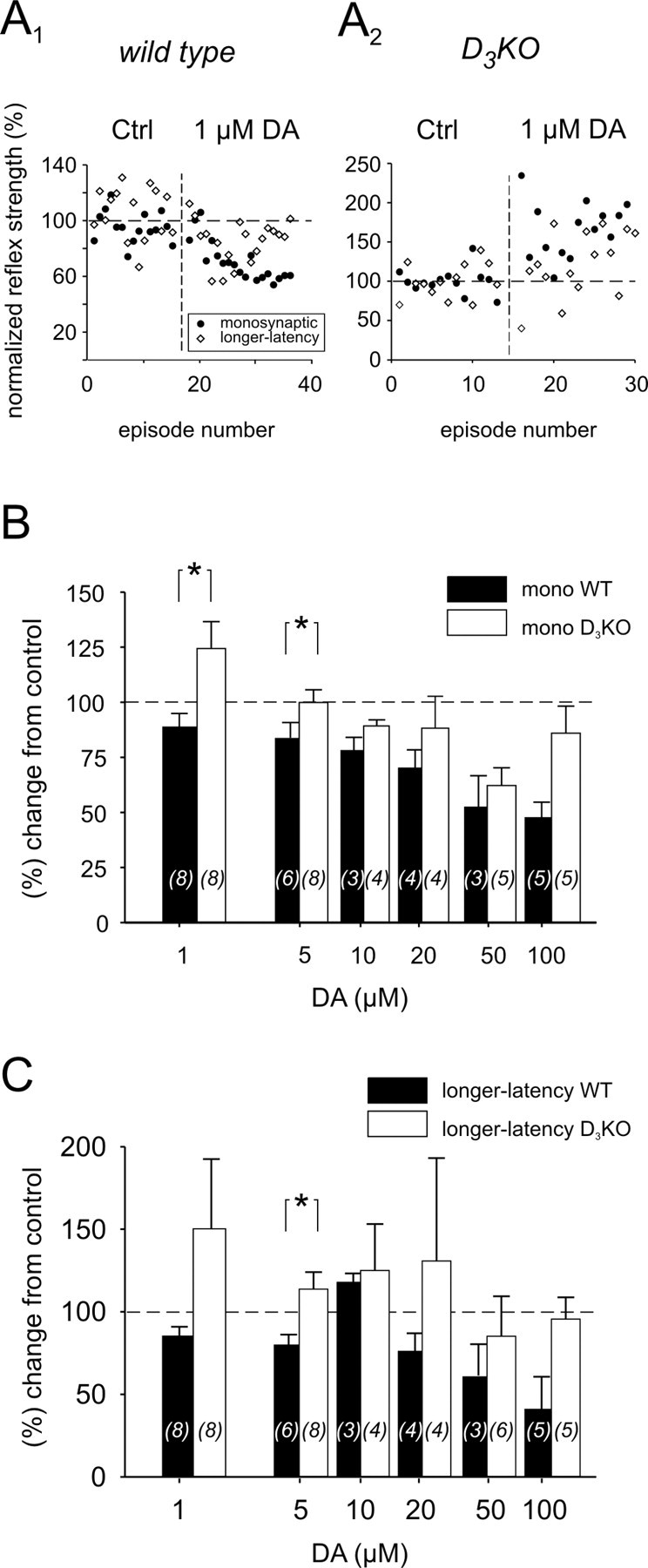
Effects of dopamine (DA) on MSR (mono) and longer-latency reflex amplitudes in WT and D3KO animals. A1, Typical example of a WT animal, in which application of 1 μm dopamine to the bath led to a depression of monosynaptic and longer-latency reflex amplitudes. A2, Example of the effect of bath application of 1 μm dopamine to a D3KO animal preparation. Here, MSR and longer-latency amplitude increased during dopamine application. B, Comparison of the dopamine effects on the MSR amplitude at increasing concentrations. At 1 μm dopamine, the MSR amplitude of WT animals decreased, whereas it increased in D3KO animals. The difference between WT and D3KO mice was significant (p = 0.021). At 5 μm dopamine, there was still a significant difference between the two groups (p = 0.013); however, the average amplitude in the D3KO animals was no longer facilitated over the control response. At 10-50 μm dopamine, the reflex amplitudes of both animal types were depressed similarly, although the WT continued to show a slightly stronger depression than the D3KO animals. At 100 μm dopamine, however, D3KO animals showed a noticeably smaller depression than the WT animals. C, Comparison of the dopamine effects on longer-latency reflex amplitudes at increasing dopamine concentrations. At 1 and 5 μm dopamine, the reflex amplitude of WT animals generally decreased, whereas the amplitude of D3KO animals increased. This difference between WT and D3KO mice was significant at 5 μm (p = 0.026). At 10-100 μm dopamine, the depression observed in WT animals was consistently slightly stronger than in the D3KO animals. In this and the following figures, sample sizes are indicated within histogram bars in brackets. All comparisons are based on ANOVA with Tukey or Dunn's post hoc comparison. Asterisks denote significant differences.
To examine whether the differences in the modulation of the reflex response between WT and D3KOs continued to persist at higher concentrations, we next tested the effects of dopamine applications at 5, 10, 20, 50, and 100 μm (Fig. 3B). Application of 5 μm dopamine in WT preparations consistently led to a significant depression of the MSR amplitude to 84 ± 7% of the control. In contrast, in the D3KO animals, in five of eight preparations, dopamine had no effect on the MSR amplitude; it lead to an increase in two additional experiments, and it decreased in one experiment. Here again, the difference between WT and D3KO animals was significant. At higher concentrations of dopamine, WT animals continued to show a trend to a stronger modulation by dopamine.
Effects of dopamine on the longer-latency reflex strength in WT and D3KO mice
The longer-latency reflex amplitudes were similarly affected by dopamine (Fig. 3C). Application of 1 μm dopamine in WT preparations generally led to a depression of the longer-latency reflex amplitude (85 ± 6% of control). In contrast, in the D3KO animals, dopamine generally induced an increase in reflex strength (150 ± 42% of control); however, because of the high variability in the D3KO dataset, the difference between WT and D3KO animals was not significant (p = 0.065). At 5 μm dopamine, however, at a lower variability of responses from the D3KO animals, the longer-latency reflex amplitudes of WT and D3KO animals were significantly different, with WT amplitudes depressed to 80 ± 7% and D3KO amplitudes increased to 114 ± 10%. At 10-100 μm dopamine, as with the MSR, WT animals continued to show a trend to a stronger reflex depression by dopamine than D3KO animals.
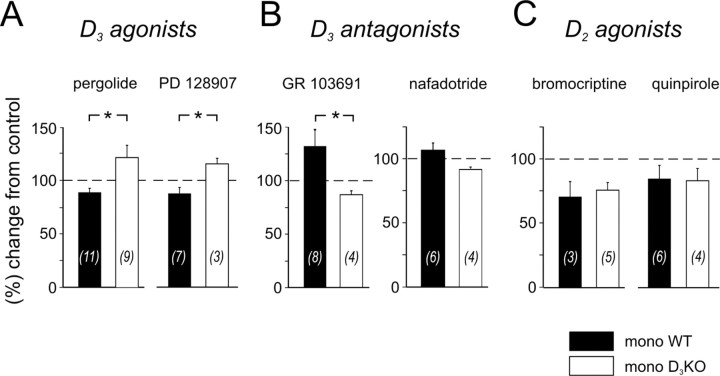
D3 receptor agonists depress the monosynaptic reflex strength in WT but not in D3KO mice
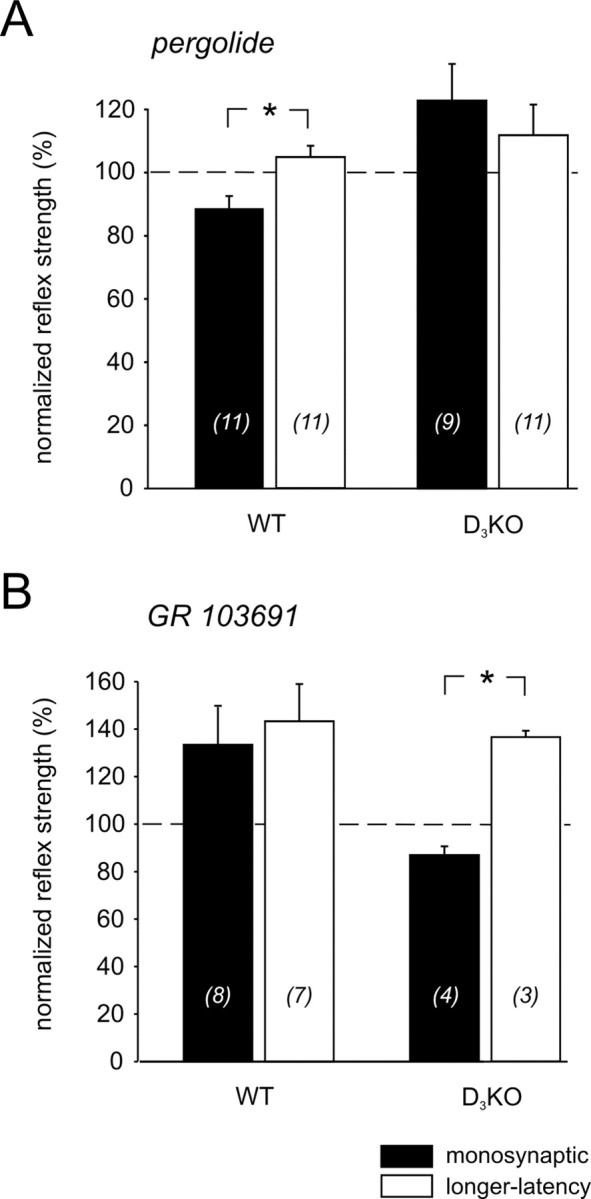
Pergolide is a dopamine receptor agonist with a higher affinity for D3 receptors than for D2 or D4 receptors (Sokoloff et al., 1990, 1992; Millan et al., 2002). In 6 of 11 experiments, application of pergolide to WT mice induced a depression of the MSR amplitude. The overall amplitude decreased to 89 ± 4% of the control (Fig. 4A). In D3KO animals, application of pergolide did not depress the MSR amplitude, but led in four of nine experiments to a significant facilitation, with an overall increase of 121 ± 12%. Moreover, the difference in the amplitude of the MSR between WT and D3KO was significant.
Figure 4.
Differential actions of D3 receptor ligands on the MSR amplitudes in WT and D3KO animals. A, D3 receptor agonists. Pergolide (left) induced a depression of the amplitude in WT animals (to 89 ± 4%) but led to a facilitation of the reflex amplitude in the D3KO animals (to 121 ± 12%), which was significantly different (p = 0.012). Similarly, PD 128907 (right) also induced a depression of the monosynaptic amplitude in the WT animals (to 88 ± 6%) and a facilitation in the D3KO animals (to 123 ± 16%). Here again, the differences between the modulatory effects was significant (p = 0.033). B, D3 receptor antagonists. GR 103691 (left) induced a facilitation of the amplitude in WT animals (to 134 ± 15% of control) but led to a depression of the reflex amplitude in the D3KO animals (to 87 ± 4% of control). This difference was significant (p = 0.029). Similarly, nafadotride (right) also induced a facilitation of the monosynaptic amplitude in the WT animals (to 107 ± 6%), and a depression in the D3KO animals (to 92 ± 2%); however, the overall difference was not significant (p = 0.067). C, D2 receptor agonists. Bromocriptine (left) induced a depression of the amplitude in WT animals (to 70.1 ± 11.8% of control) and D3KO animals (to 75.4 ± 5.9% of control) alike (p = 1.0). Similarly, quinpirole (right) also induced a depression of the monosynaptic amplitude in both WT animals (to 84.3 ± 10.4%) and D3KO animals (to 82.8 ± 9.5%), which again was not different (p = 0.914).
Bath application of a second D3 agonist, PD 128907, modulated the reflex amplitude in a manner similar to pergolide. PD 128907 has a higher affinity to D3 than D2 receptors (Pugsley et al., 1995; Sautel et al., 1995; Cussac et al., 2000), and in WT animals it induced a depression of MSR amplitudes to 88 ± 6%. In contrast, in the D3KO animals, application of PD 128907 led to an overall increase of reflex strength response to 115 ± 5%. These differences in reflex amplitudes between WT and D3KO animals were also significant (Fig. 4A).
D3 receptor antagonists increase the monosynaptic reflex strength in WT but not in D3KO mice
GR 103691 is a potent D3 receptor antagonist (Hurley et al., 1996), with a >100-fold selectivity over D4 receptor sites (Audinot et al., 1998). When applied to the WT spinal cord, it greatly facilitated MSR responses in five of eight experiments, leading to an overall increase to 134 ± 15% (Fig. 4B). In contrast, in D3KO animals, GR 103691 did not facilitate the reflex, but rather led to decrease of the amplitude to 87 ± 4%. The differences in reflex amplitudes between WT and D3KO animals were significant (Fig. 4B).
A second D3 antagonist tested, nafadotride, evoked effects in the modulation of the reflex responses similar to the ones observed during application with GR 103691. Nafadotride is a preferential D3 receptor antagonist, with a 10 and 500 times higher affinity over D2 and D4 receptors, respectively (Sautel et al., 1995; Audinot et al., 1998). In three of six WT experiments, nafadotride weakly facilitated the MSR response, leading to an overall increase to 107 ± 6%. In contrast, and similar to the results obtained during application of GR 103691, nafadotride did not increase the MSR amplitude in any of the four experiments in D3KO mice (92 ± 2%) (Fig. 4B).
D2 receptor agonists decrease the monosynaptic reflex strength in WT and D3KO mice similarly
The D3 receptor is part of the D2-like receptor family, which consists of D2, D3, and D4 receptor subtypes. Therefore, to compare the results observed in the D3KO mouse during the application of dopamine with another D2-like receptor, we tested the effects of two D2-preferring agonists, bromocriptine and quinpirole, on spinal reflex amplitudes. Bromocriptine is a D2/D3 receptor agonist that expresses a two to three times higher affinity for D2 than D3 receptors when tested with [125I]iodosulpride (Sokoloff et al., 1990; Freedman et al., 1994; Sautel et al., 1995) and an approximately six times higher affinity to D2 than D3 receptors when tested with [3H]thymidine (Sautel et al., 1995). Quinpirole has been shown to have a 30 times higher affinity to D2 over D3 receptors when cloned from human tissue (Sokoloff et al., 1990; Freedman et al., 1994; Sautel et al., 1995) and a 10 times higher affinity to D2 over D3 receptors when cloned from rat tissue (Sokoloff et al., 1990; Sautel et al., 1995).
Application of bromocriptine consistently led to a decrease in the reflex amplitude in WT (to 70 ± 12% of control) and D3KO mice (75 ± 6%). Bath application of quinpirole led to results similar to those observed during the bromocriptine experiments. Quinpirole depressed the reflex amplitude to 84 ± 10% in WT and 83 ± 10% in D3KO mice. There was no statistical difference in the datasets between WT and D3KO animals for either drug (Fig. 4C).
Monosynaptic and longer-latency reflexes can be modulated differentially by dopaminergics
We observed in a number of experiments (n = 22) that the monosynaptic and longer-latency reflexes were modulated differently by the dopaminergics, or even in an opposite manner (Fig. 5A). Longer-latency reflexes include a contribution from polysynaptic pathways that involve recruitment of other afferent modalities and interposed interneurons (e.g., those involved in generating the flexion reflex). It is possible that dopaminergic modulatory actions differ between MSR and longer-latency reflex pathways. Generally, in these cases the MSR was depressed, whereas the longer-latency reflex was facilitated. The variability of these results was quite high and not statistically significant at most of the dopamine concentrations tested; however, at 10 μm dopamine, opposite modulatory actions were consistent and significant in WT (Fig. 5B). In D3KO mice, however, we did not observe any consistent differences in the dopaminergic modulation of monosynaptic and longer-latency reflexes, although the longer-latency reflex displayed a tendency toward facilitation rather than depression in the range from 1 to 20 μm dopamine (Fig. 5C). The D2 receptor-preferring agonists bromocriptine and quinpirole did not induce any differential modulation of monosynaptic and longer-latency reflexes in WT or D3KO mice (data not shown); however, such differential actions were again evident with the use of D3 receptor-selective ligands. In WT mice, the D3 receptor agonist pergolide depressed monosynaptic but facilitated longer-latency reflexes (Fig. 6 A), whereas in D3KO animals a similar result was obtained with the D3 antagonist GR 103691 (Fig. 6 B).
Figure 5.
Monosynaptic and longer-latency reflex responses can be modulated differently by dopamine. A, Example of a differential modulation of monosynaptic and longer-latency reflex responses in the presence of 10 μm dopamine. Dopamine induced a depression of the MSR, yet at the same time also evoked a facilitation of the longer-latency reflex. B, In WT animals, the differential modulation of monosynaptic and longer-latency reflex amplitude was significant only at a concentration of 10 μm dopamine (p = 0.01; ANOVA with Tukey post hoc comparison), but not at the lower and higher concentrations tested. C, In contrast, in D3KO animals, we did not observe a similar differential modulation of monosynaptic and longer-latency reflexes within the range of dopamine concentrations tested. Note, however, the comparatively greater variability of reflex amplitudes in the D3KO animals. DA, Dopamine.
Figure 6.
Differential actions of D3 receptor-specific drugs on the monosynaptic and longer-latency reflex amplitudes in WT and D3KO animals. A, The D3 receptor agonist pergolide induced a depression of the monosynaptic amplitude (black) in WT animals (to 89 ± 4% of control), but also led to a facilitation of the longer-latency reflex amplitude (white) (to 105 ± 4% of control; p = 0.007). There was no such difference in the effects of pergolide in D3KO animals. B, Conversely, in D3KO but not WT animals, the D3 receptor antagonist GR 103691 induced a depression of the monosynaptic amplitude (to 87 ± 4%) and a facilitation of the longer-latency reflex amplitude (to 137 ± 3%), which also was different (p < 0.001).
Discussion
This study examined the concentration-dependent actions of dopamine as well as the actions of selective ligands for D2 and D3 receptors on monosynaptic and longer-latency reflexes in WT and D3KO mice. Receptor identity of pharmacological actions was verified by testing two different drugs each for D3 agonists, antagonists, and D2 agonists. Overall, our results demonstrate that D2 and D3 receptors provide considerable modulatory control of spinal cord reflex excitability. Furthermore, loss of D3 receptor activity can result in a conversion of the modulatory actions of dopamine from depression to facilitation.
Modulatory effects of dopaminergics on spinal reflex amplitudes
Our data indicate that dopamine as low as 1 μm can depress the monosynaptic stretch reflex in WT animals. This depression is likely attributable to D3 receptor activation because (1) dopamine binds to D3 receptors with very high affinity (Sokoloff et al., 1992; Freedman et al., 1994; Sautel et al., 1995), (2) D3 agonists depress reflexes, and (3) reflex depression at low dopamine or with D3 agonists is lost in the D3KO animals. Thus, D3 receptors may tonically control spinal dopaminergic modulatory actions during conditions of minimal activity.
Although we did not try doses of dopamine <1 μm, dopamine has highest affinity for the D3 receptor with reported Kd values averaging ∼25 nm (Sokoloff et al., 1992; Freedman et al., 1994; Sautel et al., 1995). Interestingly, microdialysis data suggest that dopamine is found in the extracellular space in the ∼10 nm range (Smith et al., 1992), supporting the possibility of a tonic control of reflex strength via D3 receptor activation. Evidence that D3 receptors are tonically modulating spinal reflexes is provided by the following two observations. First, under control conditions, we observed a greater incidence of large-reflex amplitudes in D3KO than in WT animals. Second, in the absence of exogenously applied dopamine, D3 receptor antagonists facilitate reflex strength in WT but not D3KO mice. Tonic D3 receptor activity could occur either by constitutive activity in the absence of ligand (Tiberi and Caron, 1994) or by continued dopamine release from descending terminals after the isolation of the spinal cord. Hadjiconstantinou et al. (1984) demonstrated that another descending monoamine, serotonin, remains in the spinal cord for >1 week after spinal transection. Thus, low levels of endogenously released dopamine could serve to tonically regulate synaptic gain of primary afferent input.
In contrast to the depression observed in WT at low dopamine concentrations, modulatory actions were transformed into facilitation in D3KO mice. This change was attributable to the lack of a functional D3 receptor because D3 receptors ligand actions were altered concomitantly. In the absence of functional D3 receptors in D3KO mice, the observed net facilitatory effect at low dopamine concentrations might be channeled via an unmasking of facilitatory actions of D1-like receptor activity (Barasi and Roberts, 1977; Smith et al., 1995; Mizuo et al., 2004). For example, D1 and D3 receptors are coexpressed in neurons of the nucleus accumbens and the islands of Calleja (Le Moine and Bloch, 1996; Ridray et al., 1998; Schwartz et al., 1998), can form heterodimers that coregulate each other (Karasinska et al., 2000), and can induce opposite actions within the same cell (Ridray et al., 1998; Schwartz et al., 1998). Thus, although dopamine has higher affinity for D3 over D1 receptors when tested in binding studies (Billard et al., 1984; Sokoloff et al., 1992), it might be possible that, in the absence of D3 receptors, low dopamine (1 and 5 μm) activates a sufficient number of D1-like receptors to exert facilitatory physiological actions.
At higher dopamine concentrations, other receptor activity, including the D2 receptor, might contribute to an overall reflex depression observed in WT and D3KO. Dopamine has on average a >10 times lower affinity to D2 over D3 receptors (Imafuku, 1987; Freedman et al., 1994). Thus, at higher dopamine concentrations (>10-100 μm), D2 receptors are likely contributing to the reflex depression observed in WT and D3KO mice, which would be expected to be similar between the two mice groups. This hypothesis is validated by the fact that D2 receptor agonists depress the monosynaptic reflexes in WT and D3KO mice correspondingly.
For longer-latency reflexes, dopamine also predominantly depressed reflex amplitude in WT, whereas in D3KO mice, lower dopamine actions (1-20 μm) were generally facilitatory. Thus, D3 receptors also reduce longer-latency reflex strength, and in their absence, facilitatory actions are unmasked. In contrast, D2 receptor agonists depressed MSR and longer-latency reflex components similarly in WT and D3KO animals.
Longer-latency responses may be attributable to (1) activation of other afferent fibers including Aδ and C fibers (Lozier and Kendig, 1995; Hedo and Lopez-Garcia, 2002), (2) repetitive firing in motoneuron pools, and/or (3) activation of interneuronal pathways. Because we observed a differential modulation of short- and longer-latency reflexes, we assume that at least some of the longer-latency actions are via intercalated interneurons; however, because there are multiple spinal interneuronal populations that can be responsible for these actions (Baldissera et al., 1981; Jankowska, 1992), their identity cannot be determined with any certainty. In those cases in which we were able to clearly distinguish two longer-latency reflex components, no differential modulatory responses were observed (data not shown).
Opposite modulatory actions, even within the same experiment, were observed between MSR (depression) and longer-latency reflexes (facilitation) with 10 μm dopamine and with D3 but not D2 receptor ligands. Thus, dopaminergics appear to exert a differential control on reflex pathways at least partly via actions at D3 but not D2 receptors. These differences may be the result of different distributions of the dopamine receptor subtypes in conjunction with different affinities for dopamine. Thus, spinal cord reflex function may be modified in different ways depending on receptor activation identity (Joyce et al., 2002). For example, differential actions of dopaminergics on spinal circuitry have been observed previously in the lamprey cord, in which dopamine depressed polysynaptic IPSPs, whereas polysynaptic excitatory potentials were unaltered (Kemnitz, 1997).
Previous studies on actions of dopamine related to spinal cord reflex function
In comparison with serotonin and noradrenaline, the modulatory actions of dopamine in the spinal cord are modest (Garraway and Hochman, 2001). This could explain the variability and often weak modulatory actions in some of the datasets reported here. Dopamine has been shown to directly increase the excitability of rat motoneurons (Barasi and Roberts, 1977), and in cultured embryonic chick motoneurons, it enhances kainate-evoked currents by a D1-like, PKA-sensitive mechanism (Smith et al., 1995). In cat and rat it has been shown that D2-like receptors depress the amplitude of muscle spindle activation-evoked monosynaptic reflexes (Carp and Anderson, 1982; Gajendiran et al., 1996), but the effects of longer-latency reflex responses were not reported. The reported actions may take place on the primary afferents themselves, because dopamine has direct actions in primary afferents in frog (Ryan et al., 1985) and can depolarize dorsal root ganglia (DRG) neurons (Gallagher et al., 1980) via D1-like receptors and hyperpolarize via D2-like receptors (Abramets and Samoilovich, 1991). Moreover, dopamine depresses Ca2+ currents in isolated DRG neurons (Formenti et al., 1998), an observation that may support a dopamine-induced reduction in sensory input by presynaptic mechanisms.
Hypothalamic dopamine, circadian variation, and RLS
The A11 region provides the only dopaminergic input to the rat spinal cord (Skagerberg and Lindvall, 1985) with very similar projections in rat, cat, and monkey (Holstege et al., 1996) and levels in cat (Fleetwood-Walker and Coote, 1981) and human (Commissiong and Sedgwick, 1975). Hypothalamic dopamine content has a strong circadian variation (Huie et al., 1989) already observed in the neonatal rat (Macho et al., 1986). In human tissue it was shown that hypothalamic dopamine peaks between 3 and 6 P.M. and then drops continuously to reach its nadir in early morning (Carlsson et al., 1980). Thus, superimposed on circadian fluctuations in dopamine release, low dopamine could preferentially activate high-affinity D3 receptors at night, to reduce sensory responsiveness and motoneuron excitability. In contrast, during daytime with dopamine levels increased, facilitatory D1 receptor activity (Barasi and Roberts, 1977; Smith et al., 1995) could overwhelm the depressant actions and exert a net increase in spinal reflex gain (as observed with 10 μm dopamine) (Fig. 3C). Circadian cycling of D2-like and D1-like receptor gene expression has been observed recently in motor regions of the rat brain (Weber et al., 2004), and if also present in the spinal cord it might be timed to complement changes in concentrations of hypothalamic dopamine release. A functional consequence of such a coordinated action would be an optimized sensorimotor responsiveness tuned to the behavioral state of the animal.
Our results suggest that D3 receptors are involved in tonically limiting spinal cord excitability and may provide insight into the consequences of D3 receptor dysfunction. For example, D3 receptor dysfunction is implicated in individuals suffering from RLS. RLS is a CNS disorder involving abnormal limb sensations that peak at night when dopamine levels are at their lowest. RLS likely involves increased spinal cord reflex excitability (Bara-Jimenez et al., 2000) and is relieved best by D3 receptor-preferring agonists (Montplaisir et al., 2000). Intriguingly, however, there exists no animal model for RLS. Because the modulatory action of low dopamine is converted from depression to facilitation in an animal that lacks a functional D3 receptor, the D3KO mouse identifies one explanation of how reduced D3 activity could contribute to the increased reflex excitability seen in RLS, thus representing a relevant model to address these questions in more detail. It is important to be aware, however, that in patients with RLS, D3 receptor agonists commonly have a therapeutic benefit, suggesting that RLS is not caused by D3 receptor dysfunction but rather by reduced D3 receptor activation.
In summary, the D3KO mouse identifies the role and the importance of D3 receptors in modulating spinal cord excitability. Spinal dopamine actions originate from the hypothalamus and so are expected to vary with a circadian profile. Because dopamine projections can be found in autonomic, sensory, and motor systems, it is likely that dopamine regulates excitability in an integrative manner, to adjust spinal cord function within a physiological range appropriate for the animal's state.
Footnotes
S.C. was supported by a Fellowship award from the Christopher Reeve Paralysis Foundation (CB2-0205-1B). S.H. was supported by National Institute of Neurological Disorders and Stroke Grant NS045248. We thank Dr. David Rye (Department of Neurology, Emory University School of Medicine, Atlanta, GA) for providing wild-type and D3KO mice, Gillian Hue for initiating the breeding colonies, and Dr. Peter Wenner, Kim Dougherty, and David Machacek for comments on this manuscript. We also thank Maggie Hatcher and Megan Daugherty for expert technical assistance.
Correspondence should be addressed to Shawn Hochman, Department of Physiology, Emory University School of Medicine, 615 Michael Street, Atlanta, GA 30322. E-mail: shochman@physio.emory.edu.
Copyright © 2004 Society for Neuroscience 0270-6474/04/2411337-09$15.00/0
References
- Abramets II, Samoilovich IM (1991) Analysis of two types of dopaminergic responses of neurons of the spinal ganglia of rats. Neurosci Behav Physiol 21: 435-440. [DOI] [PubMed] [Google Scholar]
- Accili D, Fishburn CS, Drago J, Steiner H, Lachowicz JE, Park BH, Gauda EB, Lee EJ, Cool MH, Sibley DR, Gerfen CR, Westphal H, Fuchs S (1996) A targeted mutation of the D3 dopamine receptor gene is associated with hyperactivity in mice. Proc Natl Acad Sci USA 93: 1945-1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali NJ, Davies RJ, Fleetham JA, Stradling JR (1991) Periodic movements of the legs during sleep associated with rises in systemic blood pressure. Sleep 14: 163-165. [PubMed] [Google Scholar]
- Allen RP, Earley CJ (2001) Restless legs syndrome: a review of clinical and pathophysiologic features. J Clin Neurophysiol 18: 128-147. [DOI] [PubMed] [Google Scholar]
- Asico LD, Ladines C, Fuchs S, Accili D, Carey RM, Semeraro C, Pocchiari F, Felder RA, Eisner GM, Jose PA (1998) Disruption of the dopamine D3 receptor gene produces renin-dependent hypertension. J Clin Invest 102: 493-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audinot V, Newman-Tancredi A, Gobert A, Rivet JM, Brocco M, Lejeune F, Gluck L, Desposte I, Bervoets K, Dekeyne A, Millan MJ (1998) A comparative in vitro and in vivo pharmacological characterization of the novel dopamine D3 receptor antagonists (+)-S 14297, nafadotride, GR 103,691 and U 99194. J Pharmacol Exp Ther 287: 187-197. [PubMed] [Google Scholar]
- Baldissera F, Hultborn H, Illert M (1981) Integration in spinal neuronal systems. In: Handbook of physiology—the nervous system II, Vol 2, pp 509-595. Baltimore: Williams and Wilkins. [Google Scholar]
- Bara-Jimenez W, Aksu M, Graham B, Sato S, Hallett M (2000) Periodic limb movements in sleep: state-dependent excitability of the spinal flexor reflex. Neurology 54: 1609-1616. [DOI] [PubMed] [Google Scholar]
- Barasi S, Roberts MT (1977) Responses of motoneurones to electrophoretically applied dopamine. Br J Pharmacol 60: 29-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billard W, Ruperto V, Crosby G, Iorio LC, Barnett A (1984) Characterization of the binding of 3H-SCH 23390, a selective D-1 receptor antagonist ligand, in rat striatum. Life Sci 35: 1885-1893. [DOI] [PubMed] [Google Scholar]
- Carlsson A, Svennerholm L, Winblad B (1980) Seasonal and circadian monoamine variations in human brains examined post mortem. Acta Psychiatr Scand [Suppl] 280: 75-85. [PubMed] [Google Scholar]
- Carp JS, Anderson RJ (1982) Dopamine receptor-mediated depression of spinal monosynaptic transmission. Brain Res 242: 247-254. [DOI] [PubMed] [Google Scholar]
- Clemens S, Hue G, Sawchuk M, Zhu H, Hochman S (2003) Expression of dopamine D2 and D3 receptors and actions of dopaminergics on spinal circuits in wild-type and D3 knock-out mice. Soc Neurosci Abstr 29: 186.3. [Google Scholar]
- Commissiong JW, Sedgwick EM (1975) Letter: dopamine and noradrenaline in human spinal cord. Lancet 1: 347. [DOI] [PubMed] [Google Scholar]
- Cussac D, Newman-Tancredi A, Sezgin L, Millan MJ (2000) [3H]S33084: a novel, selective and potent radioligand at cloned, human dopamine D3 receptors. Naunyn Schmiedebergs Arch Pharmacol 361: 569-572. [DOI] [PubMed] [Google Scholar]
- Espinar-Sierra J, Vela-Bueno A, Luque-Otero M (1997) Periodic leg movements in sleep in essential hypertension. Psychiatry Clin Neurosci 51: 103-107. [DOI] [PubMed] [Google Scholar]
- Fleetwood-Walker SM, Coote JH (1981) Contribution of noradrenaline-, dopamine- and adrenaline-containing axons to the innervation of different regions of the spinal cord of the cat. Brain Res 206: 95-106. [DOI] [PubMed] [Google Scholar]
- Formenti A, Martina M, Plebani A, Mancia M (1998) Multiple modulatory effects of dopamine on calcium channel kinetics in adult rat sensory neurons. J Physiol (Lond) 509: 395-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman SB, Patel S, Marwood R, Emms F, Seabrook GR, Knowles MR, McAllister G (1994) Expression and pharmacological characterization of the human D3 dopamine receptor. J Pharmacol Exp Ther 268: 417-426. [PubMed] [Google Scholar]
- Gajendiran M, Seth P, Ganguly DK (1996) Involvement of the presynaptic dopamine D2 receptor in the depression of spinal reflex by apomorphine. NeuroReport 7: 513-516. [DOI] [PubMed] [Google Scholar]
- Gallagher JP, Inokuchi H, Shinnick-Gallagher P (1980) Dopamine depolarisation of mammalian primary afferent neurones. Nature 283: 770-772. [DOI] [PubMed] [Google Scholar]
- Garraway SM, Hochman S (2001) Modulatory actions of serotonin, norepinephrine, dopamine, and acetylcholine in spinal cord deep dorsal horn neurons. J Neurophysiol 86: 2183-2194. [DOI] [PubMed] [Google Scholar]
- Gladwell SJ, Coote JH (1999a) Fast excitatory postsynaptic potentials and their response to catecholaminergic antagonists in rat sympathetic preganglionic neurones in vitro. Neurosci Lett 268: 89-92. [DOI] [PubMed] [Google Scholar]
- Gladwell SJ, Coote JH (1999b) Inhibitory and indirect excitatory effects of dopamine on sympathetic preganglionic neurones in the neonatal rat spinal cord in vitro. Brain Res 818: 397-407. [DOI] [PubMed] [Google Scholar]
- Gladwell SJ, Pyner S, Barnes NM, Coote JH (1999) D(1)-like dopamine receptors on retrogradely labeled sympathoadrenal neurones in the thoracic spinal cord of the rat. Exp Brain Res 128: 377-382. [DOI] [PubMed] [Google Scholar]
- Hadjiconstantinou M, Panula P, Lackovic Z, Neff NH (1984) Spinal cord serotonin: a biochemical and immunohistochemical study following transection. Brain Res 322: 245-254. [DOI] [PubMed] [Google Scholar]
- Hedo G, Lopez-Garcia JA (2002) 5-HT(1B) but not 5-HT(6) or 5-HT(7) receptors mediate depression of spinal nociceptive reflexes in vitro. Br J Pharmacol 135: 935-942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holstege JC, van Dijken H, Bujis RM, Goedkengt H, Gosenes T, Bongers CMH (1996) Distribution of dopamine immunoreactivity in the rat, cat, and monkey spinal cord. J Comp Neurol 376: 631-652. [DOI] [PubMed] [Google Scholar]
- Huie JM, Sharma RP, Coulombe Jr RA (1989) Diurnal alterations of catecholamines, indoleamines and their metabolites in specific brain regions of the mouse. Comp Biochem Physiol C 94: 575-579. [DOI] [PubMed] [Google Scholar]
- Hurley MJ, Stubbs CM, Jenner P, Marsden CD (1996) Dopamine D3 receptors are not involved in the induction of c-fos mRNA by neuroleptic drugs: comparison of the dopamine D3 receptor antagonist GR103691 with typical and atypical neuroleptics. Eur J Pharmacol 318: 283-293. [DOI] [PubMed] [Google Scholar]
- Imafuku J (1987) The characterization of [3H]sulpiride binding sites in rat striatal membranes. Brain Res 402: 331-338. [DOI] [PubMed] [Google Scholar]
- Jankowska E (1992) Interneuronal relay in spinal pathways from proprioceptors. Prog Neurobiol 38: 335-378. [DOI] [PubMed] [Google Scholar]
- Jonas P, Bischofberger J, Sandkuhler J (1998) Corelease of two fast neuro-transmitters at a central synapse. Science 281: 419-424. [DOI] [PubMed] [Google Scholar]
- Jordan LM, Brownstone RM, Noga BR (1992) Control of functional systems in the brainstem and spinal cord. Curr Opin Neurobiol 2: 794-801. [DOI] [PubMed] [Google Scholar]
- Joyce JN, Ryoo HL, Beach TB, Caviness JN, Stacey M, Gurevich EV, Reiser M, Adler CH (2002) Loss of response to levodopa in Parkinson's disease and co-occurrence with dementia: role of D3 and not D2 receptors. Brain Res 955: 138-152. [DOI] [PubMed] [Google Scholar]
- Jung MY, Schmauss C (1999) Decreased c-fos responses to dopamine D(1) receptor agonist stimulation in mice deficient for D(3) receptors. J Biol Chem 274: 29406-29412. [DOI] [PubMed] [Google Scholar]
- Jung MY, Skryabin BV, Arai M, Abbondanzo S, Fu D, Brosius J, Robakis NK, Polites HG, Pintar JE, Schmauss C (1999) Potentiation of the D2 mutant motor phenotype in mice lacking dopamine D2 and D3 receptors. Neuroscience 91: 911-924. [DOI] [PubMed] [Google Scholar]
- Karasinska JM, George SR, El-Ghundi M, Fletcher PJ, O'Dowd BF (2000) Modification of dopamine D(1) receptor knockout phenotype in mice lacking both dopamine D(1) and D(3) receptors. Eur J Pharmacol 399: 171-181. [DOI] [PubMed] [Google Scholar]
- Kemnitz CP (1997) Dopaminergic modulation of spinal neurons and synaptic potentials in the lamprey spinal cord. J Neurophysiol 77: 289-298. [DOI] [PubMed] [Google Scholar]
- Kiehn O, Katz P (1999) Making circuits dance: neuromodulation of motor systems. In: Beyond neurotransmission (Katz PS, ed), pp 275-317. Oxford: Oxford UP.
- Le Moine C, Bloch B (1996) Expression of the D3 dopamine receptor in peptidergic neurons of the nucleus accumbens: comparison with the D1 and D2 dopamine receptors. Neuroscience 73: 131-143. [DOI] [PubMed] [Google Scholar]
- Levant B, McCarson KE (2001) D(3) dopamine receptors in rat spinal cord: implications for sensory and motor function. Neurosci Lett 303: 9-12. [DOI] [PubMed] [Google Scholar]
- Lindvall O, Bjorklund A, Skagerberg G (1983) Dopamine-containing neurons in the spinal cord: anatomy and some functional aspects. Ann Neurol 14: 255-260. [DOI] [PubMed] [Google Scholar]
- Lozier AP, Kendig JJ (1995) Long-term potentiation in an isolated peripheral nerve-spinal cord preparation. J Neurophysiol 74: 1001-1009. [DOI] [PubMed] [Google Scholar]
- Macho L, Kvetnansky R, Culman J, Fickova M (1986) Neurotransmitter levels in the hypothalamus during postnatal development of rats. Exp Clin Endocrinol 88: 142-150. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Maiofiss L, Cussac D, Audinot V, Boutin JA, Newman-Tancredi A (2002) Differential actions of antiparkinson agents at multiple classes of monoaminergic receptor. I. A multivariate analysis of the binding profiles of 14 drugs at 21 native and cloned human receptor subtypes. J Pharmacol Exp Ther 303: 791-804. [DOI] [PubMed] [Google Scholar]
- Mizuo K, Narita M, Miyatake M, Suzuki T (2004) Enhancement of dopamine-induced signaling responses in the forebrain of mice lacking dopamine D3 receptor. Neurosci Lett 358: 13-16. [DOI] [PubMed] [Google Scholar]
- Montplaisir J, Denesle R, Petit D (2000) Pramipexole in the treatment of restless legs syndrome: a follow-up study. Eur J Neurol 1[Suppl 7]: 27-31. [DOI] [PubMed] [Google Scholar]
- Pinco M, Lev-Tov A (1993) Modulation of monosynaptic excitation in the neonatal rat spinal cord. J Neurophysiol 70: 1151-1158. [DOI] [PubMed] [Google Scholar]
- Pinco M, Lev-Tov A (1994) Synaptic transmission between ventrolateral funiculus axons and lumbar motoneurons in the isolated spinal cord of the neonatal rat. J Neurophysiol 72: 2406-2419. [DOI] [PubMed] [Google Scholar]
- Pugsley TA, Davis MD, Akunne HC, Mackenzie RG, Shih YH, Damsma G, Wikstrom H, Whetzel SZ, Georgic LM, Cooke LW, Demattos SB, Corbin AE, Glase SA, Wise LD, Dijkstra D, Heffner TG (1995) Neurochemical and functional characterization of the preferentially selective dopamine D3 agonist PD 128907. J Pharmacol Exp Ther 275: 1355-1366. [PubMed] [Google Scholar]
- Ridet JL, Sandillon F, Rajaofetra N, Geffard M, Privat A (1992) Spinal dopaminergic system of the rat: light and electron microscopic study using an antiserum against dopamine, with particular emphasis on synaptic incidence. Brain Res 598: 233-241. [DOI] [PubMed] [Google Scholar]
- Ridray S, Griffon N, Mignon V, Souil E, Carboni S, Diaz J, Schwartz JC, Sokoloff P (1998) Coexpression of dopamine D1 and D3 receptors in islands of Calleja and shell of nucleus accumbens of the rat: opposite and synergistic functional interactions. Eur J Neurosci 10: 1676-1686. [DOI] [PubMed] [Google Scholar]
- Ryan GP, Hackman JC, Wohlberg CJ, Davidoff RA (1985) Potential changes of frog afferent terminals in response to dopamine. Brain Res 328: 283-290. [DOI] [PubMed] [Google Scholar]
- Sautel F, Griffon N, Levesque D, Pilon C, Schwartz JC, Sokoloff P (1995) A functional test identifies dopamine agonists selective for D3 versus D2 receptors. NeuroReport 6: 329-332. [DOI] [PubMed] [Google Scholar]
- Schwartz JC, Diaz J, Bordet R, Griffon N, Perachon S, Pilon C, Ridray S, Sokoloff P (1998) Functional implications of multiple dopamine receptor subtypes: the D1/D3 receptor coexistence. Brain Res Brain Res Rev 26: 236-242. [DOI] [PubMed] [Google Scholar]
- Skagerberg G, Lindvall O (1985) Organization of diencephalic dopamine neurones projecting to the spinal cord in the rat. Brain Res 342: 340-351. [DOI] [PubMed] [Google Scholar]
- Skagerberg G, Bjorklund A, Lindvall O, Schmidt RH (1982) Origin and termination of the diencephalo-spinal dopamine system in the rat. Brain Res Bull 9: 237-244. [DOI] [PubMed] [Google Scholar]
- Smith AD, Olson RJ, Justice Jr JB (1992) Quantitative microdialysis of dopamine in the striatum: effect of circadian variation. J Neurosci Methods 44: 33-41. [DOI] [PubMed] [Google Scholar]
- Smith DO, Lowe D, Temkin R, Jensen P, Hatt H (1995) Dopamine enhances glutamate-activated currents in spinal motoneurons. J Neurosci 15: 3905-3912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokoloff P, Giros B, Martres MP, Bouthenet ML, Schwartz JC (1990) Molecular cloning and characterization of a novel dopamine receptor (D3) as a target for neuroleptics. Nature 347: 146-151. [DOI] [PubMed] [Google Scholar]
- Sokoloff P, Andrieux M, Besancon R, Pilon C, Martres MP, Giros B, Schwartz JC (1992) Pharmacology of human dopamine D3 receptor expressed in a mammalian cell line: comparison with D2 receptor. Eur J Pharmacol 225: 331-337. [DOI] [PubMed] [Google Scholar]
- Stiasny K, Oertel WH, Trenkwalder C (2002) Clinical symptomatology and treatment of restless legs syndrome and periodic limb movement disorder. Sleep Med Rev 6: 253-265. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Takahashi S, Oki J (1997) Developmental regulation of spinal motoneurons by monoaminergic nerve fibers. J Peripher Nerv Syst 2: 323-332. [PubMed] [Google Scholar]
- Tiberi M, Caron MG (1994) High agonist-independent activity is a distinguishing feature of the dopamine D1B receptor subtype. J Biol Chem 269: 27925-27931. [PubMed] [Google Scholar]
- van Dijken H, Dijk J, Voom P, Holstege JC (1996) Localization of dopamine D2 receptor in rat spinal cord identified with immunocytochemistry and in situ hybridization. Eur J Neurosci 8: 621-628. [DOI] [PubMed] [Google Scholar]
- Weber M, Lauterburg T, Tobler I, Burgunder JM (2004) Circadian patterns of neurotransmitter related gene expression in motor regions of the rat brain. Neurosci Lett 358: 17-20. [DOI] [PubMed] [Google Scholar]
- Xu M, Koeltzow TE, Santiago GT, Moratalla R, Cooper DC, Hu XT, White NM, Graybiel AM, White FJ, Tonegawa S (1997) Dopamine D3 receptor mutant mice exhibit increased behavioral sensitivity to concurrent stimulation of D1 and D2 receptors. Neuron 19: 837-848. [DOI] [PubMed] [Google Scholar]