Abstract
Surgery is the only curative option for patients with liver metastases of colorectal cancer, but few patients present with resectable hepatic lesions. Chemotherapy is increasingly used to downstage initially unresectable disease and allow for potentially curative surgery. Standard chemotherapy regimens convert 10%-20% of cases to resectable disease in unselected populations and 30%-40% of those with disease confined to the liver. One strategy to further increase the number of candidates eligible for surgery is the addition of active targeted agents such as cetuximab and bevacizumab to standard chemotherapy. Data from a phase III trial indicate that cetuximab increases the number of patients eligible for secondary hepatic resection, as well as the rate of complete resection when combined with first-line treatment with the FOLFIRI regimen. The safety profiles of preoperative cetuximab or bevacizumab have not been thoroughly assessed, but preliminary evidence indicates that these agents do not increase surgical mortality or exacerbate chemotherapy-related hepatotoxicity, such as steatosis (5-fluorouracil), steatohepatitis (irinotecan), and sinusoidal obstruction (oxaliplatin). Secondary resection is a valid treatment goal for certain patients with initially unresectable liver metastases and an important end point for future clinical trials.
Keywords: Colorectal cancer, Liver metastases, Liver resection, Cetuximab, Bevacizumab, Hepatotoxicity
INTRODUCTION
Globally, one half of the nearly 1 million patients diagnosed with colorectal cancer annually will develop liver metastases during the course of the disease[1,2]. Autopsy findings suggest that approximately 50% of patients who die of colorectal cancer have liver metastases, which are the only site of metastatic disease in approximately 20%-30% of patients[3] and the cause of death in most of these patients[4]. In other types of cancer, liver metastases are a sign of distant dissemination, and surgery is not a curative option[5]. In colorectal cancer, however, portal vein drainage from the gastrointestinal tract to the liver favors metastasis to the liver without systemic dissemination[5]. Surgery therefore provides a potentially curative treatment option for patients with resectable liver disease[6], in contrast with palliative chemotherapy for those with unresectable disease.
One of the most important recent advances in the management of advanced colorectal cancer is the concept of downstaging initially unresectable disease using chemotherapy so that more patients become eligible for potentially curative surgery. Current treatment guidelines[4] and substantial research highlight the importance of increasing the rates of secondary resection in initially unresectable disease. The addition of active targeted therapies, such as cetuximab and bevacizumab, to chemotherapy may further increase secondary resection rates. This article summarizes the current data on secondary resection of initially unresectable liver metastases of colorectal cancer using currently available systemic therapy regimens.
HOW RESECTABILITY MAY BE ACHIEVED
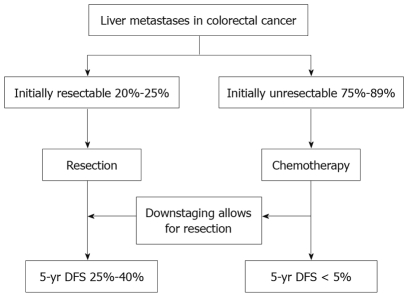
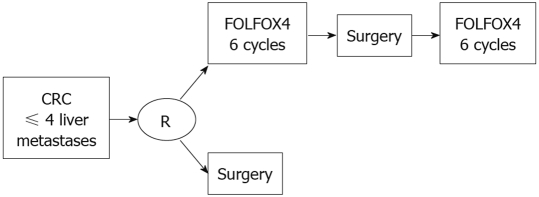
Reported 5-year survival rates following hepatic resection generally range from 25% to 40% (Figure 1)[7–9], though rates exceeding 50% have been observed in some studies[1,10,11], and these outcomes may be improved by the use of preoperative or postoperative chemotherapy or both[12,13]. In a recent phase III trial, hepatic resection was compared with resection plus perioperative chemotherapy (6 cycles of FOLFOX4 before and 6 cycles after surgery) (Figure 2). Perioperative chemotherapy increased 3-year survival rates significantly in eligible patients (36.2% vs 28.1%) and resected patients (42.4% vs 33.2%), compared with surgery alone[2].
Figure 1.
Breakdown of the incidence of liver metastases of colorectal cancer and expected outcomes with current treatment.
Figure 2.
Schema of the EORTC 40 983 of perioperative chemotherapy[2].
Unfortunately, 80%-90% of patients with liver metastases are considered to have unresectable disease at the time of diagnosis[4,7]. For them, modern chemotherapy and biologic agents afford median survival times hovering around 15 to 20 mo (Figure 1)[14–20].
Curative surgery remains an option if initially unresectable disease can be downstaged to allow for potentially definitive, ie, secondary, resection. Reported resectability rates after chemotherapy vary widely, depending on the patient population and definition of resectability. In unselected patients with unresectable liver metastases treated with chemotherapy, resection rates of 1% to 26% have been reported[21]. Higher resection rates (approximately 24%-54%) have been seen in patients with disease confined to the liver[21].
Outcomes following secondary resection are comparable to those observed after primary resection, making resectability a high-priority therapeutic goal. In a study of 872 patients with colorectal liver metastases, 701 (80%) were deemed to have unresectable disease. Of these patients, 95 (13%) ultimately underwent secondary resection after downstaging with chemotherapy. The 5-year survival rate in patients undergoing secondary resection was the same as that in patients undergoing primary resection at the same institution for initially resectable disease (34%)[7].
As investigators attempt to refine the use of resectability and collect information about resection as a clinical endpoint, a rough positive indicator of potential improvement in resectability rates is tumor shrinkage activity. It is therefore unsurprising that conversion to resectability correlates with response to chemotherapy[21]. A retrospective review of 305 patients treated with preoperative irinotecan- or oxaliplatin-based chemotherapy followed by resection of liver metastases showed that pathologic response to chemotherapy is an independent predictor of survival[22]. Importantly, these findings support an aggressive approach in which optimal management aims to put curative options within reach by tailoring systemic therapy to achieve the highest response rate possible, particularly in patients with borderline resectable disease.
SECONDARY RESECTION FOLLOWING STANDARD CHEMOTHERAPY
Oxaliplatin- and irinotecan-based regimens
Several combinations of 5-fluorouracil and leucovorin with either oxaliplatin or irinotecan have established efficacy and are accepted as standard treatment for advanced colorectal cancer[4,14,15,17,22,23]. These regimens can also lead to secondary resection in some patients with initially unresectable liver metastases. In unselected populations with advanced colorectal cancer, oxaliplatin-based therapy produces secondary resection rates of 15%-22% and complete resection rates (R0) of 9%-13% (Table 1)[14,23–25]. Among patients treated with irinotecan-based regimens, approximately 9% become eligible for surgery and 7% will achieve complete resection (Table 2)[14,23,26–28].
Table 1.
Outcomes related to secondary hepatic resection in patients treated with oxaliplatin-based chemotherapy
| Study | n | Regimen | Response rate (%) | Resection rate (%) | R0 rate (%) | MS (mo) |
| Unselected populations | ||||||
| GERCOR[14] | 111 | FOLFOX6 | 54.0 | 22.0 | 13.0 | NYR |
| Tournigand et al[24] | 311 | FOLFOX4 | 58.5 | 17.7 | 11.3 | 38.9 |
| Tournigand et al[24] | 309 | FOLFOX71 | 59.2 | 15.2 | 9.4 | 43.0 |
| Colucci et al[23] | 182 | FOLFOX4 | 34.0 | 4.4 | NR | NR |
| Liver metastases only | ||||||
| Alberts et al[25] | 44 | FOLFOX4 | 60.0 | 40.0 | 33.3 | NR |
FOLFOX7 × 6 cycles, followed by treatment without oxaliplatin × 12 cycles, followed by FOLFOX7 until PD. MS: Median survival in resected patients; FOLFOX: Oxaliplatin plus infusional 5-fluorouracil/leucovorin (5-FU/LV); NYR: Not yet reached; NR: Not reported.
Table 2.
Outcomes related to secondary hepatic resection in patients treated with irinotecan-based chemotherapy
| Study | n | Regimen | Response rate (%) | Resection rate (%) | R0 rate (%) | MS (mo) |
| Unselected populations | ||||||
| Tournigand et al[14] | 109 | FOLFIRI | 61.0 | 9.0 | 7.0 | 47 |
| Colucci et al[23] | 178 | FOLFIRI | 36.0 | 5.1 | NR | NR |
| Liver metastases only | ||||||
| Pozzo et al[26] | 40 | 5-FU/LV, irinotecan | 47.5 | 40.0 | 32.5 | NYR |
| Zelek et al[27] | 31 | 5-FU/LV, irinotecan, and HAI of pirarubicin | 48.0 | 35.0 | 29.0 | NYR |
| Ho et al[28] | 40 | 5-FU/LV, irinotecan | 55.0 | 10.0 | NR | NR |
HAI: Hepatic arterial infusion.
Comparative data from several studies suggest that oxaliplatin-based regimens may be more effective than irinotecan-based regimens in converting unresectable disease to resectable disease[14,29,30], although this finding has not always been consistent[23]. Importantly in these studies, median survival times for resected patients reach 42-47 mo.
Higher resection rates have been reported in studies of selected patients, such as those with liver metastases only. In a phase II trial evaluating FOLFOX4 in patients with unresectable metastases confined to the liver, response rate was 60% and surgery was possible in 40% of patients[25]. Of the 17 patients who underwent surgery, 14 were free of residual disease (R0). The median survival was 26 mo. Additional studies have evaluated irinotecan-based regimens in selected patient populations; resection rates were generally 30%-40%[26–28].
FOLFOXIRI
More recently, several groups have evaluated the combination of 5-fluorouracil, oxaliplatin, and irinotecan (FOLFOXIRI)[16,31,32], with the rationale of maximizing concurrent exposure to multiple active agents[14,33]. In a preliminary study in 39 patients with unresectable liver metastases, the response rate was 64% and secondary surgery was possible in 23 patients (59%)[32]. Of these patients, 84% achieved R0 resection.
Two randomized trials have compared FOLFOXIRI with FOLFIRI as first-line therapy for patients with initially unresectable advanced colorectal cancer[16,31]. Although the first study, of 283 patients, found no significant difference between the two treatment groups in response or survival[31], the second study, involving 244 patients, found that FOLFOXIRI significantly increased response rates (60% vs 34%) and median survival (22.6 mo vs 16.7 mo)[16]. Both studies, however, showed that FOLFOXIRI increased secondary resection rates compared with FOLFIRI. Significantly more patients treated with FOLFOXIRI in the first study were eligible for secondary resection (14 vs 6)[31]. This included 14 patients with liver metastases (11 vs 3), of whom 11 achieved R0 resection (9 vs 2). In the second study, the rate of R0 secondary resection was significantly greater with FOLFOXIRI (15% vs 6%) overall and in those with liver metastases only (36% vs 12%)[16].
Both randomized trials reported increased toxicity with FOLFOXIRI compared with FOLFIRI, including higher incidences of alopecia, diarrhea, neutropenia, and neurotoxicity, a factor to weigh against the benefits achieved[16,31]. Further investigation is needed to identify new strategies for increasing rates of secondary resection without increasing toxicity associated with standard chemotherapy agents.
IMPROVING ON STANDARD CHEMOTHERAPY: ADDITION OF BIOLOGICS
Two monoclonal antibodies are approved for use in advanced colorectal cancer that have been shown to improve outcomes when combined with chemotherapy. Combining these agents with standard chemotherapy may represent a safe and effective strategy for increasing the proportion of patients eligible for potentially curative surgery.
Cetuximab
Cetuximab blocks the activity of the epidermal growth factor receptor (EGFR) and is approved for use in the United States in combination with irinotecan in patients with irinotecan-refractory disease, or as monotherapy for patients who have failed both irinotecan- and oxaliplatin-based chemotherapy. Adam et al[34] assessed the ability of cetuximab-based therapy to downstage patients with unresectable disease and liver metastases refractory to prior chemotherapy to become eligible for surgery with curative intent. A total of 151 patients were treated either completely at the Paul Brousse Hospital (PBH, n = 133) or referred to PBH for hepatectomy after receiving cetuximab-based therapy elsewhere (n = 18). Of the 151 patients, 27 underwent surgery, of whom 20 had received cetuximab plus irinotecan; four, cetuximab plus oxaliplatin; and one, all three agents. Two-thirds of patients who underwent surgery had had at least two prior chemotherapy regimens. Of the 133 patients treated completely at PBH, 9 (7%) underwent surgery, which is encouraging, considering these were heavily pretreated and refractory patients.
In the first-line setting, an initial phase I/II trial of 21 patients with unresectable, EGFR-expressing disease who received cetuximab plus 5-fluorouracil, leucovorin, and irinotecan found a 67% response rate with median survival of 33 mo[19]. Five patients (24%) were eligible for secondary resection. A phase II study evaluated first-line cetuximab plus FOLFOX4 in 43 patients with unresectable, EGFR-expressing disease[35]. Confirmed responses were seen in 72% of patients and the disease control rate was 95%. Ten patients (23%) underwent potentially curative surgery and nine (21%) had no evidence of residual disease after surgery. Overall, the median survival was 30 mo.
A randomized phase II trial comparing FOLFOX4 with or without cetuximab (the OPUS trial) found that cetuximab significantly increases response rates in patients with good performance status when added to standard first-line chemotherapy (49.0% vs 36.8%)[36]. Resection rates were higher with cetuximab (6.5% vs 3.6%), as were R0 resection rates (4.7% vs 2.4%)[36]. Cetuximab was associated with an increase in skin reactions, infusion-related reactions, and hypomagnesemia, but no exacerbation of oxaliplatin-related toxicities.
The CRYSTAL study (n = 1198) investigating the addition of cetuximab to FOLFIRI was the first phase III trial to demonstrate that adding a therapeutic monoclonal antibody to standard chemotherapy may improve secondary resection rates (Table 3)[37–40]. Cetuximab significantly increased the overall secondary resection rate (6% vs 2.5%), and boosted the rate of complete resection (R0) 3-fold [4.3% vs 1.5%; odds ratio 3.0 (95% CI 1.4-6.5)]. As expected, higher rates of R0 resection were seen in the subgroup of patients with disease confined to the liver (approximately 20% of patients in each arm), for whom the addition of cetuximab also appeared to increase R0 resection rates compared with FOLFIRI alone (9.8% vs 4.5%) and was associated with a significant increase in progression-free survival (median, 11.4 mo vs 9.2 mo)[37].
Table 3.
Randomized trials comparing first-line chemotherapy with or without cetuximab or bevacizumab that report secondary resection rates
| Study | n | Regimen | Response rate (%) | Resection rate (%) | R0 rate (%) | R0 rate in liver-only disease (%) | PFS (mo) |
| Cetuximab | |||||||
| CRYSTAL[37] | 559 | FOLFIRI | 38.7 | 2.5 | 1.5 | 4.5 | 8.0 |
| 559 | FOLFIRI + cetuximab | 46.9 | 6.0 | 4.3 | 9.8 | 8.9 | |
| Bevacizumab | |||||||
| Hurwitz et al[38] | 411 | IFL | 34.8 | < 2% | NR | NR | 6.2 |
| 402 | IFL + bevacizumab | 44.8 | < 2% | NR | NR | 10.6 | |
| NO16966[39,40] | 701 | CT1 | 49.0 | 4.9 | NR | 11.5 | 8.0 |
| 699 | CT1 + bevacizumab | 47.0 | 6.3 | NR | 12.3 | 9.4 |
FOLFOX4 or XELOX. PFS: Progression-free survival in the study arm; FOLFIRI: Irinotecan plus infusional 5-fluorouracil/leucovorin (5-FU/LV); IFL: Irinotecan, fluorouracil, and leucovorin; CT: Chemotherapy.
The second key finding of CRYSTAL revealed that the benefit from cetuximab was concentrated in the subgroup of patients with wild-type K-RAS on their tumors (64.4% of the initial evaluable population); this population experienced a reduction in the risk of progression of 32% (HR = 0.68, P = 0.017), and 16% higher response rate (59.3% vs 43.2%, P = 0.0025)[42] compared with those with mutant K-RAS. The effect on patients with disease confined to the liver was also dramatic, with the response rate reaching 77% in the population with K-RAS wild type[41].
These promising observations have propelled more focused investigation on the role of cetuximab as a conversion or downstaging agent. The CELIM study was a randomized phase II study (n = 111) in which patients with unresectable liver metastases received cetuximab with either FOLFOX or FOLFIRI. Of 106 patients evaluable for efficacy, a response rate of 74% was reported in both arms combined, 79% in those with K-RAS wild-type tumors. Confirmed response (by second CT scan according to RECIST criteria or by resection) rate was 62% in both arms combined, 70% in those with K-RAS wild-type tumors. The rate of resection was an encouraging 46%, and 34% of patients achieved R0 resections. Nineteen of 75 patients (25%) with unresectable disease at enrollment had resectable disease after 16 wk of therapy (P = 0.021)[42].
Bevacizumab
Bevacizumab binds to vascular endothelial growth factor (VEGF) and is approved for use in combination with 5-fluorouracil-based chemotherapy as first- or second-line treatment for metastatic colorectal cancer. This agent has been shown to improve outcomes when added to first-line chemotherapy for advanced colorectal cancer, increasing response rates by approximately 10% compared with chemotherapy alone and producing median survival rates of approximately 17-24 mo[20,38,43]. Nonrandomized trials evaluating the combination of bevacizumab and oxaliplatin-based chemotherapy as neoadjuvant therapy in patients with resectable disease have also produced promising results[13,44].
Data from phase III trials suggest that bevacizumab does not increase secondary resection rates when added to standard chemotherapy (Table 3), both for irinotecan-based and oxaliplatin-based therapy, although the resected patients in bevacizumab are low to extract any firm conclusion[38,39].
A non-randomized, uncontrolled phase IV study (first BEAT) prospectively collected data on resection rates following bevacizumab plus various chemotherapy regimens as first-line treatment for advanced colorectal cancer. Of the 1914 evaluable patients, 215 (11.2%) underwent surgery after receiving systemic therapy and 170 (8.8%) achieved R0 resection. Preliminary data suggest a 2-year survival rate of 44% in the entire patient population and 82% in those who achieved R0 resection[40].
Based on the findings described above, it appears that bevacizumab does not compromise the feasibility of secondary resection of metastatic disease; it is unclear, however, whether adding bevacizumab has the potential to improve upon the resectability rates achieved with chemotherapy alone[40].
THE ROLE OF LOCAL THERAPIES IN THE MANAGEMENT OF LIVER METASTASES
Local treatments may be combined with surgery with the potential to improve upon the therapeutic benchmarks achieved with surgery alone. Abdalla et al[1] reported on the use of radiofrequency ablation (RFA), alone or in combination with surgery in 158 patients in whom R0 resection was not possible. Outcomes were far superior for the 190 patients who underwent complete resection, with roughly double survival rates at 4 years (65% vs 22%-36% with RFA alone or RFA plus surgery). RFA seemed to offer a modest advantage over palliative chemotherapy only.
Stereotactic body radiation (SBRT) is a local treatment approach that may enhance outcomes in patients with liver metastases[45], but it is too early to determine its relative efficacy compared with other modalities. A recent retrospective comparison of outcomes after surgery, RFA, or SBRT in patients whose liver disease recurred after initial partial resection, revealed equivalent outcomes across the three approaches[46]; well-controlled prospective comparisons are lagging.
Finally, selective internal radiation therapy (SIRT), using Yttrium-90 microspheres[47], is also a feasible technology awaiting validation in this disease setting.
TREATMENT DECISIONS, TOXICITY, FEASIBILITY AND OTHER CONCERNS
Considering the importance of achieving resectability as a therapeutic goal, its accurate assessment is key to delineate management strategies, and the availability of increasingly sophisticated diagnostic tests may help optimize evaluation approaches. Positron-emission tomography combined with computed tomography (PET-CT) with [18F]-fluoro-2’-deoxy-D-glucose (FDG) has an important role in staging patients with colorectal cancer, and in ruling out extrahepatic metastases in advanced disease, although prior chemotherapy may lower its sensitivity[48]. MRI is used for further evaluation of the liver in patients with metastatic colorectal cancer. A 2008 study of 20 consecutive patients with colorectal cancer compared whole-body MRI vs PET-CT for staging of lymph nodes and distant metastases. Results suggested a preference for PET-CT in diagnosing lymph nodes, but a trend toward superiority for whole-body MRI in detecting metastases in liver, brain, and bone[49]. Finally, intraoperative ultrasound (IOUS) is a standard method to determine resection margin and identify previously undetected tumors[50–52]. Contrast-enhanced IOUS offers greater sensitivity, increasing identification of new metastatic lesions by approximately 20%[50,53,54], and allows more time to find new metastases intraoperatively[51]. Zalinski et al[55] developed a new marking technique to help identify resection margins intraoperatively following preoperative chemotherapy: coils are placed behind the deep margin of lesions using a guide needle and CT or ultrasound guidance prior to systemic chemotherapy. The coils are then easily detected during surgery.
The relevance of resectability in disease prognosis recently became clearer. Patients with resectable metastases either hepatic or extrahepatic (but not both) had a 5-year overall survival rate of 54%, compared with 13% for patients with both resectable hepatic and extrahepatic disease and 0% for unresectable metastases, regardless of location[56]. The authors proposed the inclusion of resectability as a criterion to stratify stage IV colorectal cancer.
The degree of optimization of downstaging strategies in routine clinical practice, however, remains unclear. In a recent review of managed care records of nearly 500 patients with colorectal cancer who underwent hepatic resection, only 20% had received preoperative chemotherapy[57]. It is not known how many patients initially diagnosed with unresectable liver metastases are re-evaluated by a liver surgeon after first-line therapy to determine whether the patient’s resectability status has changed. Several authors have emphasized the importance of a multidisciplinary approach to the management of colorectal liver metastases and close cooperation among radiologists, medical oncologists, and surgeons with expertise in liver resection[4,7,58].
Chemotherapy-related hepatotoxicity
Due to recent advances in surgical techniques and postoperative care, the safety of hepatic resection for colorectal liver metastases has improved considerably[1]. Despite the trend toward increasing extensiveness of resection, surgical mortality rates remain less than 5%[8,59]. In addition, use of portal vein embolism (PVE), a well-established and well-tolerated procedure, has been shown to reduce the risk of postoperative liver failure, with a low rate of complications in resection of colorectal liver metastases[60,61].
However, systemic therapy may be associated with liver injury and affect surgical outcomes. Chemotherapy is known to cause pathologic changes in normal liver tissue, modifying gross appearance and impairing parenchymal hemostasis and regenerative capacity[2,12,62]. The impact of these effects on clinical outcomes in patients undergoing hepatic resection is unclear. In the EORTC 40 983 trial (Figure 2), six cycles of preoperative chemotherapy did not increase the rate of surgical mortality compared with surgery alone. Chemotherapy was associated with an increase in reversible postoperative morbidity that was generally within the expected range for hepatic resection (25% vs 16%)[2]. Karoui et al[63] retrospectively compared outcomes following hepatic resection in 45 patients who received preoperative chemotherapy and 22 who received no preoperative chemotherapy. Treatment with chemotherapy was associated with an increase in postoperative morbidity. Interestingly, morbidity correlated with the number of cycles of preoperative therapy given (≥ 6 cycles vs < 6 cycles), but not the type of chemotherapy used.
Others have reported that different chemotherapy regimens cause different types of liver injury, with varying effects on clinical outcomes (Table 4)[12]. Treatment with 5-fluorouracil-based treatment has been associated with an increase in steatosis, which is associated with increased postoperative morbidity[58,64]. In addition, longer duration of 5-fluorouracil-based therapy (≥ 9 cycles vs 1-8 cycles) was associated with significantly increased incidence of sinusoidal injury (42% vs 26%; P = 0.017) and postoperative liver insufficiency (11% vs 4%; P < 0.035), and was an independent predictor of postoperative liver insufficiency (P = 0.031; odds ratio, 3.90)[65]. Irinotecan has been associated with steatohepatitis, which in turn was correlated with increased mortality following hepatic resection, particularly due to liver failure[12]. Oxaliplatin therapy does not induce steatosis or steatohepatitis, but increases the risk of developing vascular lesions and sinusoidal obstruction syndrome; a clear link between these effects and postoperative morbidity or mortality rates has not been established[12,66].
Table 4.
Liver injury by chemotherapy regimen[12]
| Regimen |
Sinusoidal dilation1 (n = 22) |
Steatosis > 30% (n = 36) |
Steatohepatitis2 (n = 34) |
||||||
| Yes (%) | No (%) | P value3 | Yes (%) | No (%) | P value3 | Yes (%) | No (%) | P value3 | |
| No CT | 2 | 98 | 9 | 91 | 4 | 96 | |||
| FU | 0 | 100 | NS | 17 | 83 | NS | 5 | 95 | NS |
| IRI | 4 | 96 | NS | 11 | 89 | NS | 20 | 80 | 0.001 |
| OX | 19 | 81 | 0.00001 | 4 | 96 | NS | 6 | 94 | NS |
| Other | 0 | 100 | NS | 8 | 92 | NS | 0 | 100 | NS |
Rubbia: Brandt grade 2 or 3;
Kleiner score ≥ 4;
Presence of liver injury characteristic; comparison of each chemotherapy group vs no chemotherapy. FU: Fluorouracil; IRI: Irinotecan; OX: Oxaliplatin; NS: Not significant.
Given the varying effects of different chemotherapy agents on liver tissue, both the efficacy and safety of available regimens should be considered when selecting an appropriate regimen for use in an individual patient. It also is advisable limiting the number of cycles of preoperative chemotherapy in order to avoid extensive liver injury, and to perform surgery as soon as possible after achieving resectability.
Cetuximab and hepatic outcomes
The most common adverse event associated with cetuximab treatment is skin reactions. Less common but potentially serious adverse events include infusion reactions, cardiopulmonary arrest, and hypomagnesemia.
There is little data specifically on the effects of cetuximab on the liver in this setting. In Adam et al[34], nine patients who became eligible for surgery after cetuximab-based therapy had evidence of liver injury, but this was not attributable to cetuximab. Overall, one patient died postoperatively and approximately 50% of patients had surgical complications.
The addition of cetuximab does not appear to increase the incidence of adverse events typically associated with oxaliplatin-based therapy, such as neutropenia, diarrhea, fatigue, or neurotoxicity[35,36]. In the CRYSTAL trial, the addition of cetuximab to FOLFIRI in the first-line setting did not increase all-cause mortality rates, and there were no deaths attributed to cetuximab. Patients who received cetuximab and FOLFIRI had higher incidences of diarrhea and cetuximab-related skin reactions[37].
Bevacizumab and hepatic outcomes
Treatment with bevacizumab is associated with increased incidence of hypertension and relatively low rates of certain potentially serious events, such as bleeding, gastrointestinal perforation, and arterial thromboembolism[20,38,40]. Recent results have found that bevacizumab reduces the incidence of oxaliplatin-related sinusoidal injury, although the exact mechanism for this effect is unknown[44,65]. It has been suggested that the antiangiogenic effects of bevacizumab can interfere with hepatic regeneration and wound healing, which is relevant to its use in the perioperative setting[13,67]. To avoid surgical morbidity, it is currently recommended to stop bevacizumab therapy at least 6 wk before surgery and resume treatment 28 d or more after surgery, provided that all incisions have healed completely[4,13,67]. Reddy et al[68] reported that complications are more prevalent in patients who undergo surgery within 8 wk of stopping bevacizumab, compared with those who discontinue bevacizumab more than 8 wk before surgery (62.5% vs 30.4%). In a study of preoperative chemotherapy plus bevacizumab, Gruenberger et al[13] reported that when bevacizumab was discontinued about 5 wk before surgery, the resulting rates of complications were similar to that achieved in a historical control group of patients who received chemotherapy alone. Whether the need to discontinue bevacizumab for several weeks perioperatively hinders its ability to allow for complete resection is unknown.
CONCLUSION
The use of systemic therapy to downstage unresectable liver metastases to achieve resectability offers a curative option with long-term outcomes similar to those achieved with primary resection. Therefore, secondary resection is a valid treatment goal for certain patients with initially unresectable liver metastases and an important end point for future clinical trials (Table 5).
Table 5.
Secondary resection in patients with initially unresectable liver metastases
| Improve patient selection through early and continued consultation in a multidisciplinary team approach, including close cooperation among a radiologist, medical oncologist, and surgeon with experience in liver resection |
| Conduct surgical evaluation at baseline and, if disease is initially unresectable, reevaluation at intervals during therapy to determine if conversion to resectability has been achieved |
| Set appropriate goals of therapy (best response, conversion to resectable disease, or palliation) |
| Determine length of therapy, with consideration for the risk of potential toxicities |
| Consider the safety profile of individual agents and the risks of overtreatment, including hepatotoxicity |
| If the treatment goal is conversion to resection, treat to resectability and not to best response |
Refinement of available first-line treatment options may increase secondary resection rates. Because response rates correlate with secondary resection rates, aggressive approaches that increase the likelihood of preoperative response seem warranted. The blurring of first-line therapy and neoadjuvant therapy underscores the need for clear definitions of treatment approach, ie, curative versus palliative, and the goals of therapy, e.g. best response, conversion to resectable disease, and palliation. Multidisciplinary involvement in the management of the patient is essential to implement a continued surgical evaluation approach as patients with borderline resectable/unresectable disease undergo systemic treatment.
The benefits of aggressive chemotherapy to downstage liver metastases should be weighed against the potential increase in adverse events, particularly those related to liver injury and surgical outcomes. Different chemotherapy agents have different effects on the liver. To avoid excessive liver injury and postoperative complications, the safety profile of individual agents should be considered when selecting a preoperative regimen; the duration of preoperative chemotherapy should be kept to a minimum; and surgery should be performed as soon as resectability has been achieved.
The addition of targeted therapies to current first-line chemotherapy platforms may improve secondary resection rates. The two leading candidates for this approach are cetuximab and bevacizumab. Data from a randomized trial indicate that cetuximab increases secondary resection rates when added to irinotecan-based therapy. The addition of cetuximab to preoperative chemotherapy does not appear to increase hepatotoxicity or mortality compared with chemotherapy alone. When discontinued at least 6 wk before surgery and reinitiated at least 28 d after surgery, bevacizumab also appears to be a safe treatment option in this setting, and there is some evidence to suggest that bevacizumab may reduce the risk of sinusoidal injury. Current studies evaluating cetuximab, bevacizumab, and both in combination are underway and will help to further refine their role in the preoperative setting.
Acknowledgments
Dr. Saif was assisted by the Clinical Insights Inc. editorial team and supported by Bristol-Myers Squibb in researching references, preparing figures and tables, editing the draft, and formatting it for submission.
Peer reviewer: Hallgrimur Gudjonsson, MD, Gastroenterology, University Hospital, Landspitali, Hringbraut, Reykjavik 101, Iceland
S- Editor Tian L L- Editor O'Neill M E- Editor Ma WH
References
- 1.Abdalla EK, Vauthey JN, Ellis LM, Ellis V, Pollock R, Broglio KR, Hess K, Curley SA. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg. 2004;239:818–825; discussion 825-827. doi: 10.1097/01.sla.0000128305.90650.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P, Bechstein WO, Primrose JN, Walpole ET, Finch-Jones M, et al. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40 983): a randomised controlled trial. Lancet. 2008;371:1007–1016. doi: 10.1016/S0140-6736(08)60455-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weiss L, Grundmann E, Torhorst J, Hartveit F, Moberg I, Eder M, Fenoglio-Preiser CM, Napier J, Horne CH, Lopez MJ. Haematogenous metastatic patterns in colonic carcinoma: an analysis of 1541 necropsies. J Pathol. 1986;150:195–203. doi: 10.1002/path.1711500308. [DOI] [PubMed] [Google Scholar]
- 4.National Comprehensive Cancer Network. Colon cancer practice guidelines in oncology. Version 2. 2008. Available from: URL: http://www.nccn.org. [Google Scholar]
- 5.Borner MM. Neoadjuvant chemotherapy for unresectable liver metastases of colorectal cancer--too good to be true? Ann Oncol. 1999;10:623–626. doi: 10.1023/a:1008353227103. [DOI] [PubMed] [Google Scholar]
- 6.Adam R, Pascal G, Castaing D, Azoulay D, Delvart V, Paule B, Levi F, Bismuth H. Tumor progression while on chemotherapy: a contraindication to liver resection for multiple colorectal metastases? Ann Surg. 2004;240:1052–1061; discussion 1061-1064. doi: 10.1097/01.sla.0000145964.08365.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adam R. Chemotherapy and surgery: new perspectives on the treatment of unresectable liver metastases. Ann Oncol. 2003;14 Suppl 2:ii13–ii16. doi: 10.1093/annonc/mdg731. [DOI] [PubMed] [Google Scholar]
- 8.Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309–318; discussion 318-321. doi: 10.1097/00000658-199909000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wagner JS, Adson MA, Van Heerden JA, Adson MH, Ilstrup DM. The natural history of hepatic metastases from colorectal cancer. A comparison with resective treatment. Ann Surg. 1984;199:502–508. doi: 10.1097/00000658-198405000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choti MA, Sitzmann JV, Tiburi MF, Sumetchotimetha W, Rangsin R, Schulick RD, Lillemoe KD, Yeo CJ, Cameron JL. Trends in long-term survival following liver resection for hepatic colorectal metastases. Ann Surg. 2002;235:759–766. doi: 10.1097/00000658-200206000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pawlik TM, Scoggins CR, Zorzi D, Abdalla EK, Andres A, Eng C, Curley SA, Loyer EM, Muratore A, Mentha G, et al. Effect of surgical margin status on survival and site of recurrence after hepatic resection for colorectal metastases. Ann Surg. 2005;241:715–722, discussion 722-724. doi: 10.1097/01.sla.0000160703.75808.7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vauthey JN, Pawlik TM, Ribero D, Wu TT, Zorzi D, Hoff PM, Xiong HQ, Eng C, Lauwers GY, Mino-Kenudson M, et al. Chemotherapy regimen predicts steatohepatitis and an increase in 90-day mortality after surgery for hepatic colorectal metastases. J Clin Oncol. 2006;24:2065–2072. doi: 10.1200/JCO.2005.05.3074. [DOI] [PubMed] [Google Scholar]
- 13.Gruenberger B, Tamandl D, Schueller J, Scheithauer W, Zielinski C, Herbst F, Gruenberger T. Bevacizumab, capecitabine, and oxaliplatin as neoadjuvant therapy for patients with potentially curable metastatic colorectal cancer. J Clin Oncol. 2008;26:1830–1835. doi: 10.1200/JCO.2007.13.7679. [DOI] [PubMed] [Google Scholar]
- 14.Tournigand C, Andre T, Achille E, Lledo G, Flesh M, Mery-Mignard D, Quinaux E, Couteau C, Buyse M, Ganem G, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol. 2004;22:229–237. doi: 10.1200/JCO.2004.05.113. [DOI] [PubMed] [Google Scholar]
- 15.Goldberg RM, Sargent DJ, Morton RF, Fuchs CS, Ramanathan RK, Williamson SK, Findlay BP, Pitot HC, Alberts SR. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol. 2004;22:23–30. doi: 10.1200/JCO.2004.09.046. [DOI] [PubMed] [Google Scholar]
- 16.Falcone A, Ricci S, Brunetti I, Pfanner E, Allegrini G, Barbara C, Crino L, Benedetti G, Evangelista W, Fanchini L, et al. Phase III trial of infusional fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) compared with infusional fluorouracil, leucovorin, and irinotecan (FOLFIRI) as first-line treatment for metastatic colorectal cancer: the Gruppo Oncologico Nord Ovest. J Clin Oncol. 2007;25:1670–1676. doi: 10.1200/JCO.2006.09.0928. [DOI] [PubMed] [Google Scholar]
- 17.Seymour MT, for the UK NCRI Colorectal Clinical Studies Group. Fluorouracil, oxaliplatin and CPT-11 (irinotecan), use and sequencing (MRC FOCUS): a 2135-patient randomized trial in advanced colorectal cancer (ACRC) J Clin Oncol. 2005;23(16S suppl):A3518. [Google Scholar]
- 18.de Gramont A, Figer A, Seymour M, Homerin M, Hmissi A, Cassidy J, Boni C, Cortes-Funes H, Cervantes A, Freyer G, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18:2938–2947. doi: 10.1200/JCO.2000.18.16.2938. [DOI] [PubMed] [Google Scholar]
- 19.Folprecht G, Lutz MP, Schoffski P, Seufferlein T, Nolting A, Pollert P, Kohne CH. Cetuximab and irinotecan/5-fluorouracil/folinic acid is a safe combination for the first-line treatment of patients with epidermal growth factor receptor expressing metastatic colorectal carcinoma. Ann Oncol. 2006;17:450–456. doi: 10.1093/annonc/mdj084. [DOI] [PubMed] [Google Scholar]
- 20.Hochster HS, Hart LL, Ramanathan RK, Childs BH, Hainsworth JD, Cohn AL, Wong L, Fehrenbacher L, Abubakr Y, Saif MW, et al. Safety and efficacy of oxaliplatin and fluoropyrimidine regimens with or without bevacizumab as first-line treatment of metastatic colorectal cancer: results of the TREE Study. J Clin Oncol. 2008;26:3523–3529. doi: 10.1200/JCO.2007.15.4138. [DOI] [PubMed] [Google Scholar]
- 21.Folprecht G, Grothey A, Alberts S, Raab HR, Kohne CH. Neoadjuvant treatment of unresectable colorectal liver metastases: correlation between tumour response and resection rates. Ann Oncol. 2005;16:1311–1319. doi: 10.1093/annonc/mdi246. [DOI] [PubMed] [Google Scholar]
- 22.Blazer DG 3rd, Kishi Y, Maru DM, Kopetz S, Chun YS, Overman MJ, Fogelman D, Eng C, Chang DZ, Wang H, et al. Pathologic response to preoperative chemotherapy: a new outcome end point after resection of hepatic colorectal metastases. J Clin Oncol. 2008;26:5344–5351. doi: 10.1200/JCO.2008.17.5299. [DOI] [PubMed] [Google Scholar]
- 23.Colucci G, Gebbia V, Paoletti G, Giuliani F, Caruso M, Gebbia N, Cartene G, Agostara B, Pezzella G, Manzione L, et al. Phase III randomized trial of FOLFIRI versus FOLFOX4 in the treatment of advanced colorectal cancer: a multicenter study of the Gruppo Oncologico Dell’Italia Meridionale. J Clin Oncol. 2005;23:4866–4875. doi: 10.1200/JCO.2005.07.113. [DOI] [PubMed] [Google Scholar]
- 24.Tournigand C, Cervantes A, Figer A, Lledo G, Flesch M, Buyse M, Mineur L, Carola E, Etienne PL, Rivera F, et al. OPTIMOX1: a randomized study of FOLFOX4 or FOLFOX7 with oxaliplatin in a stop-and-Go fashion in advanced colorectal cancer--a GERCOR study. J Clin Oncol. 2006;24:394–400. doi: 10.1200/JCO.2005.03.0106. [DOI] [PubMed] [Google Scholar]
- 25.Alberts SR, Horvath WL, Sternfeld WC, Goldberg RM, Mahoney MR, Dakhil SR, Levitt R, Rowland K, Nair S, Sargent DJ, et al. Oxaliplatin, fluorouracil, and leucovorin for patients with unresectable liver-only metastases from colorectal cancer: a North Central Cancer Treatment Group phase II study. J Clin Oncol. 2005;23:9243–9249. doi: 10.1200/JCO.2005.07.740. [DOI] [PubMed] [Google Scholar]
- 26.Pozzo C, Basso M, Cassano A, Quirino M, Schinzari G, Trigila N, Vellone M, Giuliante F, Nuzzo G, Barone C. Neoadjuvant treatment of unresectable liver disease with irinotecan and 5-fluorouracil plus folinic acid in colorectal cancer patients. Ann Oncol. 2004;15:933–939. doi: 10.1093/annonc/mdh217. [DOI] [PubMed] [Google Scholar]
- 27.Zelek L, Bugat R, Cherqui D, Ganem G, Valleur P, Guimbaud R, Dupuis O, Aziza T, Fagniez PL, Auroux J, et al. Multimodal therapy with intravenous biweekly leucovorin, 5-fluorouracil and irinotecan combined with hepatic arterial infusion pirarubicin in non-resectable hepatic metastases from colorectal cancer (a European Association for Research in Oncology trial) Ann Oncol. 2003;14:1537–1542. doi: 10.1093/annonc/mdg404. [DOI] [PubMed] [Google Scholar]
- 28.Ho WM, Ma B, Mok T, Yeo W, Lai P, Lim R, Koh J, Wong YY, King A, Leow CK, et al. Liver resection after irinotecan, 5-fluorouracil, and folinic acid for patients with unresectable colorectal liver metastases: a multicenter phase II study by the Cancer Therapeutic Research Group. Med Oncol. 2005;22:303–312. doi: 10.1385/MO:22:3:303. [DOI] [PubMed] [Google Scholar]
- 29.Rubbia-Brandt L, Giostra E, Brezault C, Roth AD, Andres A, Audard V, Sartoretti P, Dousset B, Majno PE, Soubrane O, et al. Importance of histological tumor response assessment in predicting the outcome in patients with colorectal liver metastases treated with neo-adjuvant chemotherapy followed by liver surgery. Ann Oncol. 2007;18:299–304. doi: 10.1093/annonc/mdl386. [DOI] [PubMed] [Google Scholar]
- 30.Delaunoit T, Alberts SR, Sargent DJ, Green E, Goldberg RM, Krook J, Fuchs C, Ramanathan RK, Williamson SK, Morton RF, et al. Chemotherapy permits resection of metastatic colorectal cancer: experience from Intergroup N9741. Ann Oncol. 2005;16:425–429. doi: 10.1093/annonc/mdi092. [DOI] [PubMed] [Google Scholar]
- 31.Souglakos J, Androulakis N, Syrigos K, Polyzos A, Ziras N, Athanasiadis A, Kakolyris S, Tsousis S, Kouroussis Ch, Vamvakas L, et al. FOLFOXIRI (folinic acid, 5-fluorouracil, oxaliplatin and irinotecan) vs FOLFIRI (folinic acid, 5-fluorouracil and irinotecan) as first-line treatment in metastatic colorectal cancer (MCC): a multicentre randomised phase III trial from the Hellenic Oncology Research Group (HORG) Br J Cancer. 2006;94:798–805. doi: 10.1038/sj.bjc.6603011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De La Cámara J, Rodriguez J, Rotellar F, Viudez A, García-Foncillas J, Pardo F, Gíl-Bazo I, Chopitea A, Martín-Algarra S. Triplet therapy with oxaliplatin, irinotecan, 5-fluorouracil and folinic acid within a combined modality approach in patients with liver metastases from colorectal cancer. J Clin Oncol. 2004;22(14S suppl):A3593. [Google Scholar]
- 33.Grothey A, Sargent D, Goldberg RM, Schmoll HJ. Survival of patients with advanced colorectal cancer improves with the availability of fluorouracil-leucovorin, irinotecan, and oxaliplatin in the course of treatment. J Clin Oncol. 2004;22:1209–1214. doi: 10.1200/JCO.2004.11.037. [DOI] [PubMed] [Google Scholar]
- 34.Adam R, Aloia T, Levi F, Wicherts DA, de Haas RJ, Paule B, Bralet MP, Bouchahda M, Machover D, Ducreux M, et al. Hepatic resection after rescue cetuximab treatment for colorectal liver metastases previously refractory to conventional systemic therapy. J Clin Oncol. 2007;25:4593–4602. doi: 10.1200/JCO.2007.10.8126. [DOI] [PubMed] [Google Scholar]
- 35.Tabernero J, Van Cutsem E, Diaz-Rubio E, Cervantes A, Humblet Y, Andre T, Van Laethem JL, Soulie P, Casado E, Verslype C, et al. Phase II trial of cetuximab in combination with fluorouracil, leucovorin, and oxaliplatin in the first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2007;25:5225–5232. doi: 10.1200/JCO.2007.13.2183. [DOI] [PubMed] [Google Scholar]
- 36.Bokemeyer C, Bondarenko I, Makhson A, Hartmann JT, Aparicio J, de Braud F, Donea S, Ludwig H, Schuch G, Stroh C, et al. Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2009;27:663–671. doi: 10.1200/JCO.2008.20.8397. [DOI] [PubMed] [Google Scholar]
- 37.Van Cutsem E, Nowacki M, Lang I, Cascinu S, Shchepotin I, Maurel J, Rougier P, Cunningham D, Nippgen J, Köhne C. Randomized phase III study of irinotecan and 5-FU/FA with or without cetuximab in the first-line treatment of patients with metastatic colorectal cancer (mCRC): the CRYSTAL trial. J Clin Oncol. 2007;25(18S suppl):A4000. [Google Scholar]
- 38.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 39.Saltz L, Clarke S, Diaz-Rubio E, Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang T, Cassidy J. Bevacizumab (Bev) in combination with XELOX or FOLFOX4: updated efficacy results from XELOX-1/NO16966, a randomized phase III trial in first-line metastatic colorectal cancer. J Clin Oncol. 2007;25(18S suppl):A4028. [Google Scholar]
- 40.Cassidy J, Cunningham D, Berry SR, Rivera F, Clarke SJ, Kretzschmar A, Díaz-Rubio E, Van Cutsem E, Saltz LB. Surgery with curative intent in patients (pts) treated with first-line chemotherapy (CT) + bevacizumab (BEV) for metastatic colorectal cancer (mCRC): first BEAT and NO16966. J Clin Oncol. 2008;26(15S suppl):A4022. [Google Scholar]
- 41.Van Cutsem E, Lang I, D’Haens G, Moiseyenko V, Zaluski J. KRAS status and efficacy in the CRYSTAL study: first-line treatment of patients with metastatic colorectal cancer (mCRC) receiving FOLFIRI with or without cetuximab. Ann Oncol. 2008;19(Suppl 8):viii44–viii46. A710. [Google Scholar]
- 42.Folprecht G, Gruenberger T, Hartmann JT, Lordick F, Stoehlmacher J, Bechstein WO, Ockert D, Herrmann T, Liersch T, Köhne CH. Cetuximab plus FOLFOX6 or cetuximab plus FOLFIRI as neoadjuvant treatment of nonresectable colorectal liver metastases: A randomized multicenter study (CELIM-study). Abstract 296. Oral abstract presentation at 2009 Gastrointestinal Cancers Symposium of the American Society of Clinical Oncology; January 15-17, 2009; San Francisco, CA [Google Scholar]
- 43.Kabbinavar FF, Hambleton J, Mass RD, Hurwitz HI, Bergsland E, Sarkar S. Combined analysis of efficacy: the addition of bevacizumab to fluorouracil/leucovorin improves survival for patients with metastatic colorectal cancer. J Clin Oncol. 2005;23:3706–3712. doi: 10.1200/JCO.2005.00.232. [DOI] [PubMed] [Google Scholar]
- 44.Ribero D, Wang H, Donadon M, Zorzi D, Thomas MB, Eng C, Chang DZ, Curley SA, Abdalla EK, Ellis LM, et al. Bevacizumab improves pathologic response and protects against hepatic injury in patients treated with oxaliplatin-based chemotherapy for colorectal liver metastases. Cancer. 2007;110:2761–2767. doi: 10.1002/cncr.23099. [DOI] [PubMed] [Google Scholar]
- 45.Rusthoven KE, Kavanagh BD, Cardenes H, Stieber VW, Burri SH, Feigenberg SJ, Chidel MA, Pugh TJ, Franklin W, Kane M, et al. Multi-institutional phase I/II trial of stereotactic body radiation therapy for liver metastases. J Clin Oncol. 2009;27:1572–1578. doi: 10.1200/JCO.2008.19.6329. [DOI] [PubMed] [Google Scholar]
- 46.van der Pool AE, Lalmahomed ZS, de Wilt JH, Eggermont AM, Ijzermans JM, Verhoef C. Local treatment for recurrent colorectal hepatic metastases after partial hepatectomy. J Gastrointest Surg. 2009;13:890–895. doi: 10.1007/s11605-008-0794-2. [DOI] [PubMed] [Google Scholar]
- 47.Van Hazel G, Blackwell A, Anderson J, Price D, Moroz P, Bower G, Cardaci G, Gray B. Randomised phase 2 trial of SIR-Spheres plus fluorouracil/leucovorin chemotherapy versus fluorouracil/leucovorin chemotherapy alone in advanced colorectal cancer. J Surg Oncol. 2004;88:78–85. doi: 10.1002/jso.20141. [DOI] [PubMed] [Google Scholar]
- 48.Vriens D, de Geus-Oei LF, van der Graaf WT, Oyen WJ. Tailoring therapy in colorectal cancer by PET-CT. Q J Nucl Med Mol Imaging. 2009;53:224–244. [PubMed] [Google Scholar]
- 49.Squillaci E, Manenti G, Mancino S, Ciccio C, Calabria F, Danieli R, Schillaci O, Simonetti G. Staging of colon cancer: whole-body MRI vs. whole-body PET-CT--initial clinical experience. Abdom Imaging. 2008;33:676–688. doi: 10.1007/s00261-007-9347-5. [DOI] [PubMed] [Google Scholar]
- 50.Nakano H, Ishida Y, Hatakeyama T, Sakuraba K, Hayashi M, Sakurai O, Hataya K. Contrast-enhanced intraoperative ultrasonography equipped with late Kupffer-phase image obtained by sonazoid in patients with colorectal liver metastases. World J Gastroenterol. 2008;14:3207–3211. doi: 10.3748/wjg.14.3207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Torzilli G, Montorsi M, Donadon M, Palmisano A, Del Fabbro D, Gambetti A, Olivari N, Makuuchi M. “Radical but conservative” is the main goal for ultrasonography-guided liver resection: prospective validation of this approach. J Am Coll Surg. 2005;201:517–528. doi: 10.1016/j.jamcollsurg.2005.04.026. [DOI] [PubMed] [Google Scholar]
- 52.Jarnagin WR, Bach AM, Winston CB, Hann LE, Heffernan N, Loumeau T, DeMatteo RP, Fong Y, Blumgart LH. What is the yield of intraoperative ultrasonography during partial hepatectomy for malignant disease? J Am Coll Surg. 2001;192:577–583. doi: 10.1016/s1072-7515(01)00794-3. [DOI] [PubMed] [Google Scholar]
- 53.Torzilli G, Del Fabbro D, Palmisano A, Donadon M, Bianchi P, Roncalli M, Balzarini L, Montorsi M. Contrast-enhanced intraoperative ultrasonography during hepatectomies for colorectal cancer liver metastases. J Gastrointest Surg. 2005;9:1148–1153; discussion 1153-1154. doi: 10.1016/j.gassur.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 54.Leen E, Ceccotti P, Moug SJ, Glen P, MacQuarrie J, Angerson WJ, Albrecht T, Hohmann J, Oldenburg A, Ritz JP, et al. Potential value of contrast-enhanced intraoperative ultrasonography during partial hepatectomy for metastases: an essential investigation before resection? Ann Surg. 2006;243:236–240. doi: 10.1097/01.sla.0000197708.77063.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zalinski S, Abdalla EK, Mahvash A, Vauthey JN. A marking technique for intraoperative localization of small liver metastases before systemic chemotherapy. Ann Surg Oncol. 2009;16:1208–1211. doi: 10.1245/s10434-009-0328-5. [DOI] [PubMed] [Google Scholar]
- 56.Chun YS, Kopetz S, Palavecino M, Zorzi D, Curley SA, Abdalla EK, Vauthey JN. Proposal of new staging in advanced colorectal cancer. Abstract 304. Poster presented at 2009 Gastrointestinal Cancers Symposium of the American Society of Clinical Oncology; January 15-17, 2009; San Francisco, CA [Google Scholar]
- 57.Choti MA, Shetty S, Sullivan PA, Pawlik TM. Patterns of perioperative chemotherapy use in patients undergoing liver resection for colorectal metastases in a managed care setting. Abstract 287. Poster presented at 2008 Gastrointestinal Cancers Symposium of the American Society of Clinical Oncology; January 25-28, 2008; Orlando, FL [Google Scholar]
- 58.Zorzi D, Laurent A, Pawlik TM, Lauwers GY, Vauthey JN, Abdalla EK. Chemotherapy-associated hepatotoxicity and surgery for colorectal liver metastases. Br J Surg. 2007;94:274–286. doi: 10.1002/bjs.5719. [DOI] [PubMed] [Google Scholar]
- 59.Fong Y, Cohen AM, Fortner JG, Enker WE, Turnbull AD, Coit DG, Marrero AM, Prasad M, Blumgart LH, Brennan MF. Liver resection for colorectal metastases. J Clin Oncol. 1997;15:938–946. doi: 10.1200/JCO.1997.15.3.938. [DOI] [PubMed] [Google Scholar]
- 60.Donadon M, Ribero D, Morris-Stiff G, Abdalla EK, Vauthey JN. New paradigm in the management of liver-only metastases from colorectal cancer. Gastrointest Cancer Res. 2007;1:20–27. [PMC free article] [PubMed] [Google Scholar]
- 61.Abdalla EK, Barnett CC, Doherty D, Curley SA, Vauthey JN. Extended hepatectomy in patients with hepatobiliary malignancies with and without preoperative portal vein embolization. Arch Surg. 2002;137:675–680; discussion 680-681. doi: 10.1001/archsurg.137.6.675. [DOI] [PubMed] [Google Scholar]
- 62.Aloia T, Sebagh M, Plasse M, Karam V, Levi F, Giacchetti S, Azoulay D, Bismuth H, Castaing D, Adam R. Liver histology and surgical outcomes after preoperative chemotherapy with fluorouracil plus oxaliplatin in colorectal cancer liver metastases. J Clin Oncol. 2006;24:4983–4990. doi: 10.1200/JCO.2006.05.8156. [DOI] [PubMed] [Google Scholar]
- 63.Karoui M, Penna C, Amin-Hashem M, Mitry E, Benoist S, Franc B, Rougier P, Nordlinger B. Influence of preoperative chemotherapy on the risk of major hepatectomy for colorectal liver metastases. Ann Surg. 2006;243:1–7. doi: 10.1097/01.sla.0000193603.26265.c3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kooby DA, Fong Y, Suriawinata A, Gonen M, Allen PJ, Klimstra DS, DeMatteo RP, D’Angelica M, Blumgart LH, Jarnagin WR. Impact of steatosis on perioperative outcome following hepatic resection. J Gastrointest Surg. 2003;7:1034–1044. doi: 10.1016/j.gassur.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 65.Zorzi D, Kishi Y, Maru DM, Ribero D, Ravarino N, Risio M, Curley SA, Abdalla EK, Capussotti L, Vauthey JN. Effect of extended preoperative chemotherapy on pathologic response or postoperative liver insufficiency after hepatic resection for colorectal liver metastases. Abstract 295. Oral abstract presentation at 2009 Gastrointestinal Cancers Symposium of the American Society of Clinical Oncology; January 15-17, 2009; San Francisco, CA [Google Scholar]
- 66.Rubbia-Brandt L, Audard V, Sartoretti P, Roth AD, Brezault C, Le Charpentier M, Dousset B, Morel P, Soubrane O, Chaussade S, et al. Severe hepatic sinusoidal obstruction associated with oxaliplatin-based chemotherapy in patients with metastatic colorectal cancer. Ann Oncol. 2004;15:460–466. doi: 10.1093/annonc/mdh095. [DOI] [PubMed] [Google Scholar]
- 67.Chong G, Cunningham D. Improving long-term outcomes for patients with liver metastases from colorectal cancer. J Clin Oncol. 2005;23:9063–9066. doi: 10.1200/JCO.2005.04.4669. [DOI] [PubMed] [Google Scholar]
- 68.Reddy SK, Morse MA, Hurwitz HI, Bendell JC, Gan TJ, Hill SE, Clary BM. Addition of bevacizumab to irinotecan- and oxaliplatin-based preoperative chemotherapy regimens does not increase morbidity after resection of colorectal liver metastases. J Am Coll Surg. 2008;206:96–106. doi: 10.1016/j.jamcollsurg.2007.06.290. [DOI] [PubMed] [Google Scholar]