Abstract
AIM: To clarify the clinicopathological significance of laminin-5 γ2 (LNγ2) and β3 (LNβ3) chains and MMP7 expression in biliary tract cancer.
METHODS: We analyzed the association between immunohistochemically detected LNγ2, LNβ3, and MMP7 expression in biliary tract cancer and clinicopathological characteristics. Activity of MMP7 was analyzed by casein zymography. An in vitro invasion assay after treatment with MMP7-specific siRNA was performed.
RESULTS: LNγ2 expression was predominantly observed in carcinoma cells at the invasive front. LNγ2 expression was seen in 57% of patients with biliary tract cancer, and was associated with depth of invasion, histologic type, and advanced stage. The expression pattern of LNβ3 was classified into two types: invasive front dominant type (38%) and diffuse type (28%). The invasive front dominant type was associated with histologic type and advanced stage. MMP7 positivity was correlated with LNγ2 or LNβ3 expression but not with clinicopathological characteristics. Active MMP7 detected by casein zymography was correlated with depth of invasion and advanced stage. Downregulation of MMP7 expression by siRNA resulted in a significant decrease in biliary tract cancer cell invasion in vitro.
CONCLUSION: Our results suggest that LNγ2 and LNβ3, in conjunction with MMP7, play a key role in the progression of biliary tract cancer.
Keywords: Biliary tract cancer, Laminin-5, Laminin γ2, Laminin β3, MMP7
INTRODUCTION
Despite recent advances in diagnosis and treatment, the prognosis of patients with biliary tract cancer is still poor. Surgical resection is possible in only a small proportion of patients[1,2]. Consequently, elucidating the biological characteristics of these carcinomas has become necessary to improve the prognosis of patients and to devise better treatment strategies.
Laminins are components of the extracellular matrix (ECM) that contribute to the architecture of the basal lamina surrounding the epithelial cells and mediate cell adhesion, growth, migration, proliferation, and differentiation. Laminins are heterotrimeric glycoproteins composed of three different polypeptide chains (α, β and γ) arranged in a cruciform structure. A separate gene encodes each polypeptide chain and different combinations of these chains lead to the 15 different laminin isoforms[3–5]. Laminin-5/laminin-332 (LN5), consists of α3, β3, and γ2 chains, which are encoded by three distinct genes (LAMA3, LAMB3, and LAMC2, respectively)[6]. LN5 has been shown to promote the adhesion, migration, and scattering of a variety of cultured cells, mainly through integrin α3β1, more strongly than other ECM proteins[7]. Moreover, in hepatocellular carcinoma (HCC), LN5 reportedly plays an important role in epithelial mesenchymal transition through down-regulation of E-cadherin and translocation of β-catenin into the nuclei[8].
Expression of the three subunits of LN5 is regulated differentially in cancer cell lines and in normal and malignant tissues including HCC[9–11]. Indeed, LNγ2 has been shown to be secreted as a single subunit in gastric cancer[12]. Several lines of evidence suggest that the tumor-derived LNγ2 contributes to invasion of tumor cells. LNγ2 expression has been immunohistochemically detected in various types of carcinomas, such as HCC, colorectum, stomach, and esophagus[9,12–16]. It is notable that LNγ2 has been predominantly detected at the invasive front, where tumor cells with the most aggressive phenotype are localized[17].
Degradation of ECM components is mostly controlled by proteolytic enzymes called matrix metalloproteinases (MMPs)[18]. Specific cleavage of LN5 (γ2 subunit at residue 587) by MMP2 has been shown to induce migration of breast epithelial cells[19]. This altered form of LN5 was found in tumors and in tissues undergoing remodeling, but not in quiescent tissues. LN5 is also converted into a migration-promoting substrate by MT1-MMP[20]. MMP7, also known as matrilysin, is a ‘‘minimal domain MMP’’ that exhibits broad proteolytic activity against components of the ECM and non-ECM[18]. MMP7 is often overexpressed at the invasive front in various types of human cancer and is associated with cancer progression[21,22]. Both LNγ2 and MMP7 are targets of the Wnt/β-catenin pathway[23,24].
Although there are only a few reports regarding LNβ3 expression in human cancer, coexpression of LNγ2 and LNβ3 has been reported in HCC, squamous cell carcinoma of the tongue and colorectal carcinoma and basal cell carcinoma of the skin[9,25,26]. It has recently been reported that human LN5 is a ligand for MMP7 and that a specific cleavage occurs in its β3 chain[27]. These results are interesting because MMP7 is overexpressed in HCC and colorectal carcinoma[18,28]. However, expression of LN5 and MMP7 in biliary tract cancer has not been clearly addressed.
To clarify the possible involvement of LN5 and MMP7 in the progression of biliary tract cancer, we immunohistochemically analyzed these expressions in 61 primary biliary tract cancer. Activity of MMP7 was analyzed by casein zymography. An in vitro invasion assay of biliary tract cancer cell lines after treatment with MMP7-specific siRNA was performed.
MATERIALS AND METHODS
Cell lines and tissue samples
Human bile duct cancer cell lines (TFK-1, HuH-28, and MEC) were obtained from Cell Resource Center for Biomedical Research, Tohoku University. Bile duct cancer cell line TKKK and gallbladder carcinoma cell lines TGBC1TKB, TGBC2TKB, and TGBC14TKB were purchased from Riken Cell Bank (Tsukuba, Japan). Cells were cultured in RPMI1640 or DMEM supplemented with 10% fetal bovine serum. Formalin-fixed, paraffin-embedded sections of 61 biliary tract carcinomas (30 extrahepatic bile duct carcinomas, 18 gallbladder carcinomas, 13 carcinomas of the ampulla of Vater) were used for immunohistochemically. Sections containing the most invasive part of each tumor were used. Fresh specimens of extrahepatic bile duct carcinoma (n = 10), gallbladder carcinoma (n = 7), and carcinoma of the ampulla of Vater (n = 3) were obtained from patients who had undergone surgical treatment. Specimens were immediately frozen in liquid nitrogen at the time of surgery and stored at -80°C. Each tissue specimen was used for casein zymography. Histopathological features of the specimens were classified according to the pathological tumor-node-metastasis (TNM) classification system of the International Union Against Cancer. Informed consent was obtained from each subject and the institutional review committee approved the experiments.
Semi-quantitative reverse transcriptase-PCR (RT-PCR) and real-time RT-PCR
Semi-quantitative RT-PCR was carried out as described previously[29]. Total RNA was extracted from cell lines using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s instructions. cDNA was synthesized from 1 μg of total RNA using SuperScript III reverse transcriptase (Invitrogen) with random hexamers. PCR was performed using primers specific for the LAMA3, LAMB2 and LAMC2 gene and the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene. GAPDH served as an internal control of the reaction. Standard curves for semiquantitative RT-PCR were drawn as described previously[30]. All reactions were carried out at least in duplicate and controlled without reverse transcriptase. PCR products were electrophoresed in 2% agarose gels. Real-time RT-PCR was performed using the TaqMan real-time PCR system as described previously[31].
Immunohistochemistry
Immunohistochemistry was carried out as described previously[32]. The antibodies used were as follows: anti-LNα3 rabbit polyclonal antibody (1/100 dilution, Santa Cruz, CA, USA), anti-laminin5 (γ2 chain) mouse monoclonal antibody (1/50 dilution, Chemicon, Temecula, CA, USA), anti-LNβ3 rabbit polyclonal antibody (1/100 dilution, Santa Cruz), and anti-MMP7 mouse monoclonal antibody (1/50 dilution, Daiichi Fine Chemical, Takaoka, Japan). Normal mouse or rabbit immunoglobulins were substituted for each primary antibody as negative controls. Cytoplasmic expression was defined as positive when immunoreactivity was observed in more than 10% of carcinoma cells. We defined the cells at the deepest invading part of the tumor as the invasive front.
Casein zymography
Casein zymography was performed as previously described with some modifications[28]. Tissue extracts were electrophoresed on 8% polyacrylamide gel containing 1 mg/mL casein. After electrophoresis, gels were washed in 2.5% Triton-X 100 and incubated for 48 h at 37°C in 50 mmol/L Tris-HCl (pH 7.4), 10 mmol/L CaCl2, 1 mmol/L ZnCl2, and 0.02% NaN3, followed by staining with 0.1% Coomassie brilliant blue.
siRNA transfection
siRNA transfection was performed as described previously[33]. Levels of MMP7 inhibition were analyzed by RT-PCR and Western blotting. siRNA-transfected cells were used for the in vitro invasion assay.
In vitro invasion assay
Assays were performed by the modified Boyden Chamber method as described previously[34]. Assays were also performed with 250 ng/mL of TIMP1, an MMP inhibitor. The results were presented as means ± SD for each sample.
Statistical analysis
Expression was assessed for associations with clinicopathological parameters using the chi-square two-tailed test or Fisher’s exact test. A P value < 0.05 was considered statistically significant. A P value between 0.05 and 0.10 was considered as a trend toward an association.
RESULTS
Expression of LNα3, LNβ3 and LNγ2, and MMP7 in cell lines
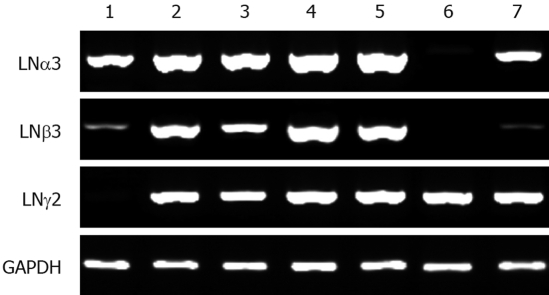
Expression levels of LNα3, LNβ3 and LNγ2, and MMP7 in cancer cell lines were analyzed using semi-quantitative RT-PCR (Figure 1). LNα3 mRNA was detected in all seven cell lines but at very low levels in TGBC-14TKB. LNβ3 mRNA was detected in six cell lines but at very low levels in MEC and TKKK. LNγ2 mRNA was detected in all seven cell lines but at very low levels in MEC. TFK-1, HuH-28, TGBC-1TKB, and TGBC-2TKB expressed considerable amounts of all 3 chains of LN5. MMP7 mRNA was detected in all seven cell lines. There were no significant correlations between these expression patterns and characteristics of the cell lines. Similar data were observed by real-time RT-PCR (data not shown).
Figure 1.
RT-PCR analysis of the LNα3, LNβ3, LNγ2 genes in biliary tract cancer cell lines. 1: MEC; 2: TFK-1; 3: HuH28; 4: TGBC1TKB; 5: TGBC2TKB; 6: TGBC14TKB; 7: TKKK.
Overexpression of LNγ2, LNβ3, and MMP7 in biliary tract cancer tissues
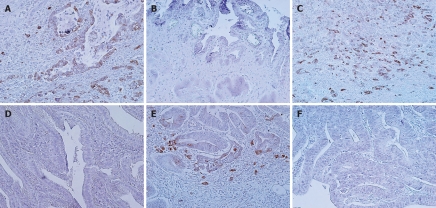
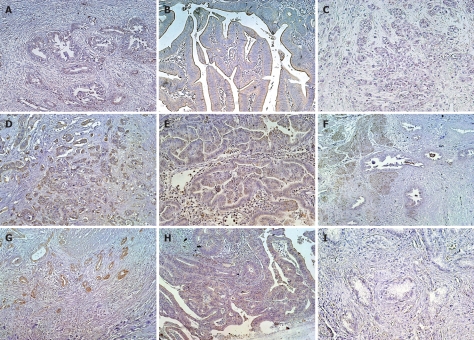
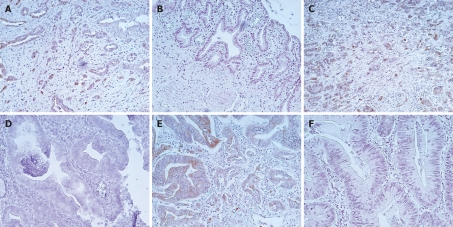
Expression of LNα3 was detected in normal basement membranes but not in carcinoma cells (data not shown). Figure 2 shows representative results of immunohistochemistry for LNγ2 in biliary tract cancer. In carcinoma tissues, the cytoplasm of carcinoma cells was stained for LNγ2 at levels much stronger than those in normal basement membranes. The cytoplasmic immunoreactivity was often more intense at the invasive front. Cancer cells budding or dissociating from the tumor nests showed intense cytoplasmic staining. Sections with immunostaining signals in over 10% of carcinoma cells, which were observed in 35 (57%) of 61 cases, were judged to be positive for LNγ2. LNγ2 positivity was 67% in extrahepatic bile duct cancer, 50% in gallbladder cancer, and 46% in carcinoma of the ampulla of Vater. Figure 3 shows representative results of immunohistochemistry for LNβ3 in biliary tract cancer. In carcinoma tissues, the cytoplasm of carcinoma cells was stained for LNβ3 at levels much stronger than those in normal basement membranes. The expression pattern of LNβ3 was classified into 2 patterns: invasive front dominant pattern and diffuse pattern. The invasive front dominant pattern and diffuse pattern were observed in 23 (38%) and 17 (28%) of 61 cases, respectively. Positivity for invasive front dominant pattern and diffuse pattern was 47% and 17% in extrahepatic bile duct cancer, 28% and 39% in gallbladder cancer, and 31% and 38% in carcinoma of the ampulla of Vater. Figure 4 shows representative results of immunohistochemistry for MMP7 in biliary tract cancer tissues. MMP7 immunoreactivity was intense at the invasive front in several cases. In general, MMP7 immunoreactivity was diffuse rather than invasive front dominant like LNγ2. Sections with immunostaining signals in over 10% of carcinoma cells, which were observed in 42 (69%) of 61 cases, were judged to be positive for MMP7. MMP7 positivity was 80% in extrahepatic bile duct cancer, 50% in gallbladder cancer, and 69% in carcinoma of the ampulla of Vater.
Figure 2.
LNγ2 expression in biliary tract cancer tissues. A, B: Extrahepatic bile duct cancer tissues; A: Moderately differentiated tubular adenocarcinoma positive for staining. Note that LNγ2 is strongly positive in tumor cells at the invasive front; B: Papillary adenocarcinoma negative for staining; C, D: Gallbladder cancer tissues; C: Moderately differentiated tubular adenocarcinoma positive for staining; D: Well differentiated tubular adenocarcinoma negative for staining; E, F: Carcinoma tissues of the ampulla of Vater; E: Moderately differentiated tubular adenocarcinoma positive for staining. Note that LNγ2 is strongly positive in tumor cells at the invasive front; F: Well differentiated tubular adenocarcinoma negative for staining.
Figure 3.
LNβ3 expression in biliary tract cancer tissues. A-C: Extrahepatic bile duct cancer tissues; A: Well differentiated tubular adenocarcinoma positive for invasive front dominant staining; B: Papillary adenocarcinoma positive for diffuse staining; C: Moderately differentiated tubular adenocarcinoma negative for staining; D-F: Gallbladder cancer tissues; A-C: Extrahepatic bile duct cancer tissues; D: Moderately differentiated tubular adenocarcinoma positive for invasive front dominant staining; E: Papillary adenocarcinoma positive for diffuse staining; G-I: Carcinoma tissues of the ampulla of Vater; G: Well differentiated tubular adenocarcinoma positive for invasive front dominant staining; H: Well differentiated tubular adenocarcinoma positive for diffuse staining; I: Well differentiated tubular adenocarcinoma negative for staining.
Figure 4.
MMP7 expression in biliary tract cancer tissues. A, B: Extrahepatic bile duct cancer tissues; A: Moderately differentiated tubular adenocarcinoma positive for staining. Note that MMP7 is strongly positive in tumor cells at the invasive front; B: Papillary adenocarcinoma negative for staining; C, D: Gallbladder cancer tissues; C: Moderately differentiated tubular adenocarcinoma positive for staining; D: Well differentiated tubular adenocarcinoma negative for staining; E, F: Carcinoma tissues of the ampulla of Vater; E: Moderately differentiated tubular adenocarcinoma positive for staining. Note that MMP7 is strongly positive in tumor cells at the invasive front; F: Well differentiated tubular adenocarcinoma negative for staining.
Association of LNγ2, LNβ3, and MMP7 expression with clinicopathological characteristics
The relationship between LNγ2 positivity and clinicopathological characteristics is summarized in Table 1. LNγ2 positivity was significantly correlated with histologic type (less differentiated type), depth of invasion (invasion into serosa), and advanced stage. The relationship between LNβ3 positivity and clinicopathological characteristics is summarized in Table 2. LNβ3 invasive front dominant pattern was significantly correlated with histologic type (less differentiated type) and advanced stage. There was a tendency that lymph node metastasis was more frequently observed in cases with LNβ3 invasive front dominant pattern than in other cases (P = 0.063). The relationship between MMP7 positivity and clinicopathological characteristics is summarized in Table 3. MMP7 positivity was not significantly correlated with clinicopathological characteristics. The expression of LNγ2, LNβ3 invasive front dominant pattern, and MMP7 were correlated with each other (Tables 456).
Table 1.
Correlation between laminin γ2 staining and clinicopathologic factors n (%)
| Case (n = 61) |
Laminin γ2 |
P value | ||
| Positive (n = 35) | Negative (n = 26) | |||
| Age (yr) | ||||
| ≤ 65 | 25 (41) | 15 (60) | 10 (40) | |
| > 65 | 36 (59) | 20 (56) | 16 (44) | 0.47 |
| Gender | ||||
| Male | 41 (67) | 25 (61) | 16 (39) | |
| Female | 20 (33) | 10 (50) | 10 (50) | 0.29 |
| Location | ||||
| Bile duct | 30 (49) | 20 (67) | 10 (33) | |
| Gall bladder | 18 (30) | 9 (50) | 9 (50) | |
| Ampulla of Vater | 13 (21) | 6 (46) | 7 (54) | 0.34 |
| Maximum diameter (mm) | ||||
| < 30 | 41 (67) | 25 (61) | 16 (39) | |
| ≥ 30 | 20 (33) | 10 (50) | 10 (50) | 0.29 |
| Histologic type | ||||
| Tub1 & pap | 35 (57) | 16 (46) | 19 (54) | |
| Others | 26 (43) | 19 (73) | 7 (27) | 0.03 |
| Depth | ||||
| Serosa negative | 37 (61) | 17 (46) | 20 (54) | |
| Serosa positive | 24 (39) | 18 (75) | 6 (25) | 0.02 |
| Lymph node metastasis | ||||
| Absent | 29 (48) | 16 (55) | 13 (45) | |
| Present | 27 (44) | 17 (63) | 10 (37) | 0.37 |
| Stage | ||||
| < IV | 43 (70) | 21 (49) | 22 (51) | |
| ≥ IV | 17 (28) | 13 (76) | 4 (24) | 0.046 |
Table 2.
Correlation between parameters and the pattern of laminin β3 expression n (%)
| Total (n = 61) |
Laminin β3 |
P value | |||
| Invasive (n = 23) | Diffuse (n = 17) | Negative (n = 21) | |||
| Age (yr) | |||||
| ≤ 65 | 25 (41) | 9 (36) | 8 (32) | 8 (32) | |
| > 65 | 36 (59) | 14 (39) | 9 (25) | 13 (36) | 0.83 |
| Gender | |||||
| Male | 41 (67) | 17 (41) | 8 (20) | 16 (39) | |
| Female | 20 (33) | 6 (30) | 9 (45) | 5 (25) | 0.11 |
| Location | |||||
| Bile duct | 30 (49) | 14 (47) | 5 (17) | 11 (37) | |
| Gall bladder | 18 (30) | 5 (28) | 7 (39) | 6 (33) | |
| Ampulla of Vater | 13 (21) | 4 (31) | 5 (38) | 4 (31) | 0.40 |
| Maximum diameter (mm) | |||||
| < 30 | 40 (66) | 18 (45) | 10 (25) | 12 (30) | |
| ≥ 30 | 21 (34) | 5 (24) | 7 (33) | 9 (43) | 0.27 |
| Histologic type | |||||
| Tub1 & pap | 35 (57) | 9 (26) | 14 (40) | 12 (34) | |
| Others | 26 (43) | 14 (54) | 3 (12) | 9 (35) | 0.02 |
| Depth | |||||
| Serosa negative | 37 (61) | 11 (30) | 13 (35) | 13 (35) | |
| Serosa positive | 24 (39) | 12 (50) | 4 (17) | 8 (33) | 0.18 |
| Lymph node metastasis | |||||
| Absent | 29 (48) | 8 (28) | 11 (38) | 10 (34) | |
| Present | 27 (44) | 14 (52) | 5 (19) | 8 (30) | 0.13 |
| Stage | |||||
| < IV | 43 (70) | 10 (23) | 15 (35) | 18 (42) | |
| ≥ IV | 17 (28) | 12 (71) | 2 (12) | 3 (18) | 0.003 |
Table 3.
Correlation between MMP7 staining and clinicopathologic factors n (%)
| Case (n = 61) |
MMP7 |
P value | ||
| Positive (n = 42) | Negative (n = 19) | |||
| Age (yr) | ||||
| ≤ 65 | 25 (42) | 17 (68) | 8 (32) | |
| > 65 | 36 (58) | 25 (69) | 11 (31) | 0.66 |
| Gender | ||||
| Male | 41 (67) | 30 (73) | 11 (27) | |
| Female | 20 (33) | 12 (60) | 8 (40) | 0.23 |
| Location | ||||
| Bile duct | 30 (49) | 24 (80) | 6 (20) | |
| Gall bladder | 18 (30) | 9 (50) | 9 (50) | |
| Ampulla of Vater | 13 (21) | 9 (69) | 4 (31) | 0.09 |
| Maximum diameter (mm) | ||||
| < 30 | 41 (67) | 29 (71) | 12 (29) | |
| ≥ 30 | 20 (33) | 13 (65) | 7 (35) | 0.43 |
| Histologic type | ||||
| Tub1 & pap | 35 (57) | 23 (66) | 12 (34) | |
| Others | 26 (43) | 19 (73) | 7 (27) | 0.37 |
| Depth | ||||
| Serosa negative | 37 (61) | 25 (68) | 12 (32) | |
| Serosa positive | 24 (39) | 17 (71) | 7 (29) | 0.58 |
| Lymph node metastasis | ||||
| Absent | 29 (48) | 21 (72) | 8 (28) | |
| Present | 27 (44) | 18 (67) | 9 (33) | 0.43 |
| Stage | ||||
| < IV | 43 (70) | 31 (72) | 12 (28) | |
| ≥ IV | 17 (28) | 10 (59) | 7 (41) | 0.24 |
Table 4.
Expression of laminin γ2 and MMP7 in biliary tract carcinoma n (%)
| MMP7 expression |
Laminin γ2 |
P value | |
| Positive (n = 42) | Negative (n = 19) | ||
| Positive (n = 34) | 28 (46) | 6 (10) | |
| Negative (n = 27) | 14 (23) | 13 (21) | 0.01 |
Table 5.
Expression of laminin β3 and MMP7 in biliary tract carcinoma n (%)
| MMP7 expression |
Laminin β3 |
P value | ||
| Invasive (n = 23) | Diffuse (n = 17) | Negative (n = 21) | ||
| Positive (n = 34) | 17 (28) | 6 (10) | 14 (23) | |
| Negative (n = 27) | 6 (10) | 11 (18) | 7 (11) | 0.037 |
Table 6.
Expression of laminin β3 and laminin γ2 in biliary tract carcinoma n (%)
| Laminin γ2 expression |
Laminin β3 |
P value | ||
| Invasive (n = 23) | Diffuse (n = 17) | Negative (n = 21) | ||
| Positive (n = 42) | 18 (30) | 8 (13) | 9 (15) | |
| Negative (n = 19) | 5 (8) | 9 (15) | 12 (20) | 0.036 |
MMP7 activity detected by zymography
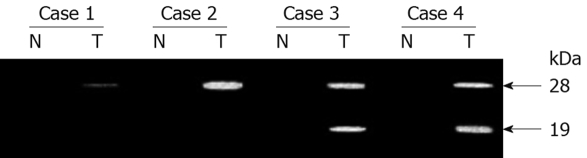
Using casein zymography, the levels of secreted matrilysin were analyzed (Figure 5). Nontumorous tissues secreted neither latent (28 kDa) nor activated (19 kDa) MMP7 activity. Latent and activated forms of MMP7 were detected in 16 (80%) and 12 (60%) of 20 carcinoma tissues, respectively. The activity was eliminated by the addition of the metalloproteinase inhibitor EDTA (data not shown). The activated form but not the latent form was correlated with depth of invasion and advanced stage.
Figure 5.
Casein zymography of surgical specimen pairs of biliary tract carcinoma and adjacent nontumor tissue. N and T: Matched samples from nontumor and tumor tissue, respectively.
Suppression of cancer cell invasiveness by MMP7 siRNA treatment
In vitro invasion assays after treatment with specific siRNA for the MMP7 gene were carried out to assess the direct role of the expression of MMP7 in cancer cell invasiveness. Transfection efficiency determined by fluorescein isothiocyanate-labeled oligonucleotide uptake was 84% ± 6% in TFK-1 cells and 86% ± 5% in TGBC-2TKB cells (data not shown). Transfection with siRNA resulted in over 80% inhibition of mRNA and protein expression (data not shown). Transfection with MMP7-specific siRNA decreased invasiveness of TFK-1 cells compared with control siRNA-transfected counterparts (P < 0.01, Figure 6). This difference was significantly diminished by the addition of TIMP1. Similar results were observed in MMP7-specific siRNA-transfected TGBC-2TKB cells (data not shown).
Figure 6.
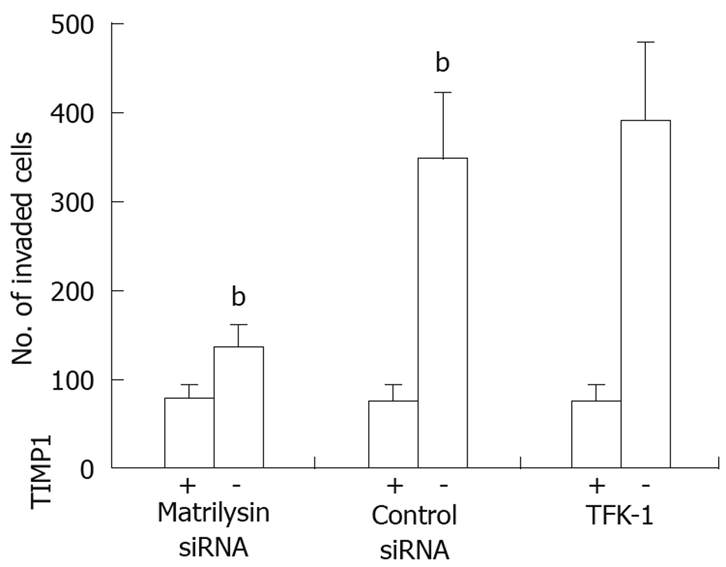
In vitro invasion assay with or without TIMP1 (250 ng/mL) in TFK-1 and siRNA transfectants. Each column indicates the means of three experiments; bP < 0.01.
DISCUSSION
In the current study, LNγ2 positivity in carcinoma cells at the invasive front was immunohistochemically observed in 57% of patients with biliary tract cancer, and was associated with histologic type (less differentiated type), depth of invasion, and advanced stage. LNβ3 invasive front dominant pattern was immunohistochemically observed in 38% of patients with biliary tract cancer, and was associated with histologic type (less differentiated type) and advanced stage. These results suggest that LNγ2 and LNβ3 expression in carcinoma cells at the invasive front contributes to the more aggressive phenotype of carcinoma cells, resulting in the progression of biliary tract cancer.
Preferential expression of LNγ2 and LNβ3 in carcinoma cells at the invasive front and its correlation with tumor progression suggest that these molecules play a role in the acquisition of a migrating and invading epithelial cell phenotype that is a prerequisite for malignancy[13]. Also in metastatic HCC, LN5 was mainly distributed along the tumoral advancing edge[9]. The mechanism underlying the preferential distribution of LNγ2 and LNβ3 at the invasive front in cancer is not known. It is known that activation of cancer-related genes in carcinoma cells affects their associated stromal cells. Certain stromal cell populations lying close to carcinoma cells may be induced to assist the invasion process by signals sent out by the cancer cells, stimulating the synthesis of gene products that facilitate cancer cell invasion and migration[35]. Interactions of carcinoma cells with stromal cells or with the surrounding extracellular matrix at the invasive front may result in an accumulation of LNγ2 and LNβ3 at the invasive front, where they may play a direct role in tumor invasion processes[19].
Although there are only a few reports regarding LNβ3 expression in human cancer, coexpression of LNγ2 and LNβ3 has been reported in HCC, squamous cell carcinoma of the tongue, colorectal carcinoma and basal cell carcinoma of the skin[9,25,26]. Sordat et al[14] reported that the heterodimer of the LNγ2 and LNβ3 chains is accumulated in the cytoplasm of dissociating (or budding) tumor cells from neoplastic tubules of colon carcinomas. Since LNγ2 and LNβ3 were not always coexpressed in biliary tract cancer, further analysis is necessary to elucidate the mechanism of overexpression and localization of LNγ2 and LNβ3 in biliary tract cancer. LN5 reportedly plays an important role in epithelial mesenchymal transition through down-regulation of E-cadherin and translocation of β-catenin into the nuclei[8]. It will be interesting to address this issue in biliary tract cancer in the near future.
In contrast to preferential expression of MMP7 in carcinoma cells at the invasive front in various carcinomas, the expression pattern of MMP7 was diffuse in biliary tract cancer. The mechanism underlying the differential distribution of MMP7 in carcinomas needs to be further analyzed. MMP7 positivity was not significantly correlated with clinicopathological characteristics. However, the expression of LNγ2, LNβ3 invasive front dominant pattern, and MMP7 were correlated with each other. It has been suggested that the controlled up-regulation of gene products is one of the characteristics of invading cancer cells and that these gene products have functions crucial for the invasive phenotype of cancer cells[13]. It is notable that limited proteolysis of LNβ3 by MMP7 increases the cell motility activity of LN5 in colon carcinoma cells[27].
The activated form but not the latent form of MMP7 was correlated with depth of invasion and advanced stage, suggesting that active MMP7 plays an important role in the progression of biliary tract cancer. The implication of up-regulation of MMP7 expression in tumor progression was further substantiated by the in vitro invasion analysis. We revealed that down-regulation of MMP7 expression by siRNA resulted in a significant decrease in biliary tract cancer cell invasion in vitro, suggesting that up-regulation of MMP7 contributes to the more invasive phenotype of biliary tract cancer cells. Taken together, our results suggest that LNγ2 and LNβ3, in conjunction with MMP7, play a key role in the progression of biliary tract cancer.
COMMENTS
Background
Biliary tract cancers are relatively rare human malignancies involving the gallbladder and/or the bile ducts, but the prognosis is poor. Understanding the molecular biological features of biliary tract cancer progression is necessary for improving the prognosis. The potential role of laminin-5 (LN5) and MMP7 in human cancer is receiving increasing attention.
Research frontiers
Altered expression patterns of LN5, especially the LNγ2 chain, and MMP7 have been correlated with tumor behavior, such as invasiveness, vascularization, metastatic potential, and patients’ poor prognosis. However, expression of LN5 and MMP7 in biliary tract cancer has not been clearly addressed. In this study, the authors demonstrate that LNγ2, LNβ3, and active MMP7 play a key role in the progression of biliary tract cancer.
Innovations and breakthroughs
This is the first study to report that invasive front dominant expression of LNγ2 and LNβ3, and active MMP7 play a key role in the progression of biliary tract cancer. Furthermore, our in vitro studies suggest that MMP7 plays an important role in biliary tract cancer cell invasion.
Applications
Detection of LNγ2, LNβ3, and active MMP7 could be molecular markers for tumor aggressiveness in biliary tract cancer. Understanding how LNγ2, LNβ3, and active MMP7 are induced and how their expression is blocked may represent a future strategy for therapeutic intervention in the treatment of patients with biliary tract cancer.
Terminology
Laminin: A heterotrimeric glycoprotein composed of three different polypeptide chains (α, β and γ), is a component of the extracellular matrix (ECM) that contributes to the architecture of the basal lamina surrounding the epithelial cells and mediates cell adhesion, growth, migration, proliferation, and differentiation. LN-5/LN-332: LN5, consists of α3, β3 and γ2 chains, and is involved in cell adhesion, migration, and scattering. Altered expression of LN-5 plays an important role in cancer. MMP7: Degradation of ECM components is mostly controlled by proteolytic enzymes called MMP. MMP7, also known as matrilysin, is a minimal domain MMP that exhibits broad proteolytic activity against components of the ECM and non-ECM.
Peer review
This paper reports the expression of LN-5 chains and MMP-7 in biliary cancer. The authors showed that LNγ2 and LNβ3, in conjunction with MMP7, play a key role in the progression of biliary cancer. The study sounds interesting and confirms the role of LN-5 and MMP7 in human cancer.
Supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (H.Y. and K.I.) and Grants-in-Aid for Cancer Research and for the Third Term Comprehensive 10-Year Strategy for Cancer Control from the Ministry of Health, Labor and Welfare of Japan (H.Y. and K.I.)
Peer reviewer: Gianluigi Giannelli, MD, Dipartimento di Clinica Medica, Immunologia e Malattie Infettive, Sezione di Medicina Interna, Policlinico, Piazza G. Cesare 11, 70124 Bari, Italy
S- Editor Li LF L- Editor Webster JR E- Editor Zheng XM
References
- 1.Cleary SP, Dawson LA, Knox JJ, Gallinger S. Cancer of the gallbladder and extrahepatic bile ducts. Curr Probl Surg. 2007;44:396–482. doi: 10.1067/j.cpsurg.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 2.Thomas MB. Biological characteristics of cancers in the gallbladder and biliary tract and targeted therapy. Crit Rev Oncol Hematol. 2007;61:44–51. doi: 10.1016/j.critrevonc.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 3.Hao J, Jackson L, Calaluce R, McDaniel K, Dalkin BL, Nagle RB. Investigation into the mechanism of the loss of laminin 5 (alpha3beta3gamma2) expression in prostate cancer. Am J Pathol. 2001;158:1129–1135. doi: 10.1016/s0002-9440(10)64060-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calaluce R, Kunkel MW, Watts GS, Schmelz M, Hao J, Barrera J, Gleason-Guzman M, Isett R, Fitchmun M, Bowden GT, et al. Laminin-5-mediated gene expression in human prostate carcinoma cells. Mol Carcinog. 2001;30:119–129. doi: 10.1002/1098-2744(200102)30:2<119::aid-mc1020>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 5.Patarroyo M, Tryggvason K, Virtanen I. Laminin isoforms in tumor invasion, angiogenesis and metastasis. Semin Cancer Biol. 2002;12:197–207. doi: 10.1016/S1044-579X(02)00023-8. [DOI] [PubMed] [Google Scholar]
- 6.Korang K, Christiano AM, Uitto J, Mauviel A. Differential cytokine modulation of the genes LAMA3, LAMB3, and LAMC2, encoding the constitutive polypeptides, alpha 3, beta 3, and gamma 2, of human laminin 5 in epidermal keratinocytes. FEBS Lett. 1995;368:556–558. doi: 10.1016/0014-5793(95)00740-z. [DOI] [PubMed] [Google Scholar]
- 7.Carter WG, Ryan MC, Gahr PJ. Epiligrin, a new cell adhesion ligand for integrin alpha 3 beta 1 in epithelial basement membranes. Cell. 1991;65:599–610. doi: 10.1016/0092-8674(91)90092-d. [DOI] [PubMed] [Google Scholar]
- 8.Giannelli G, Bergamini C, Fransvea E, Sgarra C, Antonaci S. Laminin-5 with transforming growth factor-beta1 induces epithelial to mesenchymal transition in hepatocellular carcinoma. Gastroenterology. 2005;129:1375–1383. doi: 10.1053/j.gastro.2005.09.055. [DOI] [PubMed] [Google Scholar]
- 9.Giannelli G, Fransvea E, Bergamini C, Marinosci F, Antonaci S. Laminin-5 chains are expressed differentially in metastatic and nonmetastatic hepatocellular carcinoma. Clin Cancer Res. 2003;9:3684–3691. [PubMed] [Google Scholar]
- 10.Mizushima H, Miyagi Y, Kikkawa Y, Yamanaka N, Yasumitsu H, Misugi K, Miyazaki K. Differential expression of laminin-5/ladsin subunits in human tissues and cancer cell lines and their induction by tumor promoter and growth factors. J Biochem. 1996;120:1196–1202. doi: 10.1093/oxfordjournals.jbchem.a021541. [DOI] [PubMed] [Google Scholar]
- 11.Virtanen I, Tani T, Bäck N, Häppölä O, Laitinen L, Kiviluoto T, Salo J, Burgeson RE, Lehto VP, Kivilaakso E. Differential expression of laminin chains and their integrin receptors in human gastric mucosa. Am J Pathol. 1995;147:1123–1132. [PMC free article] [PubMed] [Google Scholar]
- 12.Koshikawa N, Moriyama K, Takamura H, Mizushima H, Nagashima Y, Yanoma S, Miyazaki K. Overexpression of laminin gamma2 chain monomer in invading gastric carcinoma cells. Cancer Res. 1999;59:5596–5601. [PubMed] [Google Scholar]
- 13.Pyke C, Rømer J, Kallunki P, Lund LR, Ralfkiaer E, Danø K, Tryggvason K. The gamma 2 chain of kalinin/laminin 5 is preferentially expressed in invading malignant cells in human cancers. Am J Pathol. 1994;145:782–791. [PMC free article] [PubMed] [Google Scholar]
- 14.Sordat I, Bosman FT, Dorta G, Rousselle P, Aberdam D, Blum AL, Sordat B. Differential expression of laminin-5 subunits and integrin receptors in human colorectal neoplasia. J Pathol. 1998;185:44–52. doi: 10.1002/(SICI)1096-9896(199805)185:1<44::AID-PATH46>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 15.Pyke C, Salo S, Ralfkiaer E, Rømer J, Danø K, Tryggvason K. Laminin-5 is a marker of invading cancer cells in some human carcinomas and is coexpressed with the receptor for urokinase plasminogen activator in budding cancer cells in colon adenocarcinomas. Cancer Res. 1995;55:4132–4139. [PubMed] [Google Scholar]
- 16.Yamamoto H, Itoh F, Iku S, Hosokawa M, Imai K. Expression of the gamma(2) chain of laminin-5 at the invasive front is associated with recurrence and poor prognosis in human esophageal squamous cell carcinoma. Clin Cancer Res. 2001;7:896–900. [PubMed] [Google Scholar]
- 17.Hase K, Shatney C, Johnson D, Trollope M, Vierra M. Prognostic value of tumor "budding" in patients with colorectal cancer. Dis Colon Rectum. 1993;36:627–635. doi: 10.1007/BF02238588. [DOI] [PubMed] [Google Scholar]
- 18.Ii M, Yamamoto H, Adachi Y, Maruyama Y, Shinomura Y. Role of matrix metalloproteinase-7 (matrilysin) in human cancer invasion, apoptosis, growth, and angiogenesis. Exp Biol Med (Maywood) 2006;231:20–27. doi: 10.1177/153537020623100103. [DOI] [PubMed] [Google Scholar]
- 19.Giannelli G, Falk-Marzillier J, Schiraldi O, Stetler-Stevenson WG, Quaranta V. Induction of cell migration by matrix metalloprotease-2 cleavage of laminin-5. Science. 1997;277:225–228. doi: 10.1126/science.277.5323.225. [DOI] [PubMed] [Google Scholar]
- 20.Koshikawa N, Giannelli G, Cirulli V, Miyazaki K, Quaranta V. Role of cell surface metalloprotease MT1-MMP in epithelial cell migration over laminin-5. J Cell Biol. 2000;148:615–624. doi: 10.1083/jcb.148.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamamoto H, Adachi Y, Itoh F, Iku S, Matsuno K, Kusano M, Arimura Y, Endo T, Hinoda Y, Hosokawa M, et al. Association of matrilysin expression with recurrence and poor prognosis in human esophageal squamous cell carcinoma. Cancer Res. 1999;59:3313–3316. [PubMed] [Google Scholar]
- 22.Adachi Y, Yamamoto H, Itoh F, Arimura Y, Nishi M, Endo T, Imai K. Clinicopathologic and prognostic significance of matrilysin expression at the invasive front in human colorectal cancers. Int J Cancer. 2001;95:290–294. doi: 10.1002/1097-0215(20010920)95:5<290::aid-ijc1050>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 23.Brabletz T, Jung A, Dag S, Hlubek F, Kirchner T. beta-catenin regulates the expression of the matrix metalloproteinase-7 in human colorectal cancer. Am J Pathol. 1999;155:1033–1038. doi: 10.1016/s0002-9440(10)65204-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hlubek F, Jung A, Kotzor N, Kirchner T, Brabletz T. Expression of the invasion factor laminin gamma2 in colorectal carcinomas is regulated by beta-catenin. Cancer Res. 2001;61:8089–8093. [PubMed] [Google Scholar]
- 25.Akimoto S, Nakanishi Y, Sakamoto M, Kanai Y, Hirohashi S. Laminin 5 beta3 and gamma2 chains are frequently coexpressed in cancer cells. Pathol Int. 2004;54:688–692. doi: 10.1111/j.1440-1827.2004.01681.x. [DOI] [PubMed] [Google Scholar]
- 26.Svensson Månsson S, Reis-Filho J, Landberg G. Transcriptional upregulation and unmethylation of the promoter region of p16 in invasive basal cell carcinoma cells and partial co-localization with the gamma 2 chain of laminin-332. J Pathol. 2007;212:102–111. doi: 10.1002/path.2152. [DOI] [PubMed] [Google Scholar]
- 27.Remy L, Trespeuch C, Bachy S, Scoazec JY, Rousselle P. Matrilysin 1 influences colon carcinoma cell migration by cleavage of the laminin-5 beta3 chain. Cancer Res. 2006;66:11228–11237. doi: 10.1158/0008-5472.CAN-06-1187. [DOI] [PubMed] [Google Scholar]
- 28.Yamamoto H, Itoh F, Adachi Y, Sakamoto H, Adachi M, Hinoda Y, Imai K. Relation of enhanced secretion of active matrix metalloproteinases with tumor spread in human hepatocellular carcinoma. Gastroenterology. 1997;112:1290–1296. doi: 10.1016/s0016-5085(97)70143-4. [DOI] [PubMed] [Google Scholar]
- 29.Hirata T, Yamamoto H, Taniguchi H, Horiuchi S, Oki M, Adachi Y, Imai K, Shinomura Y. Characterization of the immune escape phenotype of human gastric cancers with and without high-frequency microsatellite instability. J Pathol. 2007;211:516–523. doi: 10.1002/path.2142. [DOI] [PubMed] [Google Scholar]
- 30.Taniguchi H, Yamamoto H, Hirata T, Miyamoto N, Oki M, Nosho K, Adachi Y, Endo T, Imai K, Shinomura Y. Frequent epigenetic inactivation of Wnt inhibitory factor-1 in human gastrointestinal cancers. Oncogene. 2005;24:7946–7952. doi: 10.1038/sj.onc.1208910. [DOI] [PubMed] [Google Scholar]
- 31.Hafner C, Schmitz G, Meyer S, Bataille F, Hau P, Langmann T, Dietmaier W, Landthaler M, Vogt T. Differential gene expression of Eph receptors and ephrins in benign human tissues and cancers. Clin Chem. 2004;50:490–499. doi: 10.1373/clinchem.2003.026849. [DOI] [PubMed] [Google Scholar]
- 32.Miyamoto N, Yamamoto H, Taniguchi H, Miyamoto C, Oki M, Adachi Y, Imai K, Shinomura Y. Differential expression of angiogenesis-related genes in human gastric cancers with and those without high-frequency microsatellite instability. Cancer Lett. 2007;254:42–53. doi: 10.1016/j.canlet.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 33.Taniguchi H, Yamamoto H, Akutsu N, Nosho K, Adachi Y, Imai K, Shinomura Y. Transcriptional silencing of hedgehog-interacting protein by CpG hypermethylation and chromatic structure in human gastrointestinal cancer. J Pathol. 2007;213:131–139. doi: 10.1002/path.2216. [DOI] [PubMed] [Google Scholar]
- 34.Yamamoto H, Vinitketkumnuen A, Adachi Y, Taniguchi H, Hirata T, Miyamoto N, Nosho K, Imsumran A, Fujita M, Hosokawa M, et al. Association of matrilysin-2 (MMP-26) expression with tumor progression and activation of MMP-9 in esophageal squamous cell carcinoma. Carcinogenesis. 2004;25:2353–2360. doi: 10.1093/carcin/bgh270. [DOI] [PubMed] [Google Scholar]
- 35.Dano K, Behrendt N, Brunner N, Eliis V, Ploug M, Pyke C. The urokinase receptor: protein structure and role in plasminogen activation and cancer invasion. Fibrinolysis. 1994;8:189–203. [Google Scholar]