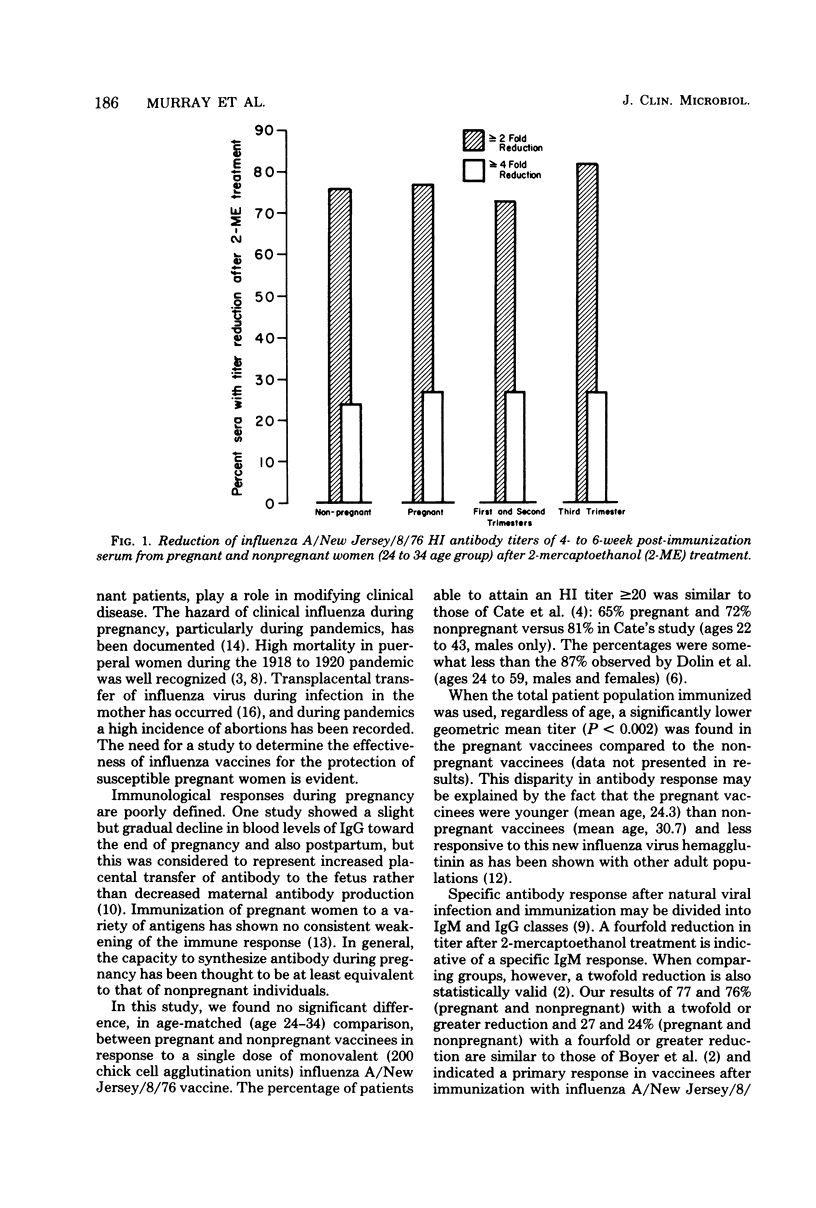
Abstract
The decision to implement a mass immunization program with A/New Jersey/8/76 (Hsw1N1) influenza vaccine provided a unique opportunity to evaluate immunological responses during pregnancy. Fifty-nine pregnant and 27 nonpregnant women participated in this study. Influenza virus hemagglutination-inhhibition antibody titers were determined to A/New Jersey/8/76 (Hsw1N1), A/Japan/305/57 (H2N2), and A/Hong Kong/8/68 (H3N2) before and after a single dose of monovalent (200 chick cell agglutination units) influenza A/New Jersey/8/76 (Hsw1N1) vaccine. The difference in titers between pregnant and nonpregnant women was insignificant. Treatment of the sera with 2-mercaptoethanol disclosed a similar immunoglobulin M response to the vaccine in both groups. The mean fold rise in heterologous antibody titer was similar in pregnant and nonpregnant women. This study demonstrated that pregnant women were able to respond to an original myxovirus antigen, influenza A/New Jersey/8/76, in a manner equivalent to nonpregnant, age-matched controls.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amstey M. S. Immunization in pregnancy. Clin Obstet Gynecol. 1976 Mar;19(1):47–54. doi: 10.1097/00003081-197603000-00006. [DOI] [PubMed] [Google Scholar]
- Boyer K. M., Cherry J. D., Welliver R. C., Dudley J. P., Deseda-Tous J., Zahradnik J. M., Krause P. J., Spencer M. J., Bryson Y. J., Garakian A. J. IgM and IgG antibody responses after immunization of children with inactivated monovalent (A/New Jersey/76) and bivalent (A/New Jersey/76-A/Victoria/75) influenza virus vaccines. J Infect Dis. 1977 Dec;136 (Suppl):S665–S671. doi: 10.1093/infdis/136.supplement_3.s665. [DOI] [PubMed] [Google Scholar]
- Broun G. O., Sr, O'Connor D., Schmidt R. R., Puder B. Factors involved in immunization program for swine influenza. Am J Med. 1976 Dec;61(6):925–931. doi: 10.1016/0002-9343(76)90416-2. [DOI] [PubMed] [Google Scholar]
- Cate T. R., Couch R. B., Kasel J. A., Six H. R. Clinical trials of monovalent influenza A/New Jersey/76 virus vaccines in adults: reactogenicity, antibody response, and antibody persistence. J Infect Dis. 1977 Dec;136 (Suppl):S450–S455. doi: 10.1093/infdis/136.supplement_3.s450. [DOI] [PubMed] [Google Scholar]
- Cherry J. D., Feigin R. D., Lobes L. A., Jr, Hinthorn D. R., Shackelford P. G., Shirley R. H., Lins R. D., Choi S. C. Urban measles in the vaccine era: a clinical, epidemiologic, and serologic study. J Pediatr. 1972 Aug;81(2):217–230. doi: 10.1016/s0022-3476(72)80287-7. [DOI] [PubMed] [Google Scholar]
- Dolin R., Wise T. G., Mazur M. H., Tuazon C. U., Ennis F. A. Immunogenicity and reactogenicity of influenza A/New Jersey/76 virus vaccines in normal adults. J Infect Dis. 1977 Dec;136 (Suppl):S435–S442. doi: 10.1093/infdis/136.supplement_3.s435. [DOI] [PubMed] [Google Scholar]
- HARDY J. M., AZAROWICZ E. N., MANNINI A., MEDEARIS D. N., Jr, COOKE R. E. The effect of Asian influenza on the outcome of pregnancy, Baltimore, 1957-1958. Am J Public Health Nations Health. 1961 Aug;51:1182–1188. doi: 10.2105/ajph.51.8.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffner R. R., Jr, Schluederberg A. Specificity of the primary and secondary antibody responses to myxoviruses. J Immunol. 1967 Apr;98(4):668–672. [PubMed] [Google Scholar]
- Marolis G. B., Buckley R. H., Younger J. B. Serum immunogbulin cocentrations during normal pregnancy. Am J Obstet Gynecol. 1971 Apr 1;109(7):971–976. doi: 10.1016/0002-9378(71)90275-4. [DOI] [PubMed] [Google Scholar]
- Noble G. R., Kaye H. S., Kendal A. P., Dowdle W. R. Age-related heterologous antibody responses to influenza virus vaccination. J Infect Dis. 1977 Dec;136 (Suppl):S686–S692. doi: 10.1093/infdis/136.supplement_3.s686. [DOI] [PubMed] [Google Scholar]
- Parkman P. D., Hopps H. E., Rastogi S. C., Meyer H. M., Jr Summary of clinical trials of influenza virus vaccines in adults. J Infect Dis. 1977 Dec;136 (Suppl):S722–S730. doi: 10.1093/infdis/136.supplement_3.s722. [DOI] [PubMed] [Google Scholar]
- WILSON M. G., HEINS H. L., IMAGAWA D. T., ADAMS J. M. Teratogenic effects of Asian influenza. J Am Med Assoc. 1959 Oct 10;171:638–641. doi: 10.1001/jama.1959.03010240006003. [DOI] [PubMed] [Google Scholar]
- Yawn D. H., Pyeatte J. C., Joseph J. M., Eichler S. L., Garcia-Bunuel R. Transplacental transfer of influenza virus. JAMA. 1971 May 10;216(6):1022–1023. [PubMed] [Google Scholar]


