Abstract
Selective cyclooxygenase-2 (COX-2) inhibitors are widely used due to their efficacy and good safety profile. However, recent case reports have described varying degrees of liver injuries associated with the use of COX-2 inhibitors. We report the case of a patient who developed acute cholestatic hepatitis progressing to hepatic failure requiring liver transplantation, following a 3-d course of celecoxib for treatment of generalized muscle aches and pains. The clinical presentation, the laboratory data, as well as the liver histopathology were supportive of the putative diagnosis of drug induced liver injury.
Keywords: Celecoxib, Cholestatic hepatitis, Liver failure, Liver transplantation
INTRODUCTION
The primary effect of non-selective non-steroidal anti-inflammatory drugs (NSAIDs) is to inhibit cyclooxygenase (prostaglandin synthase), thereby impairing the ultimate transformation of arachidonic acid to prostaglandins, prostacyclin, and thromboxanes. The extent of enzyme inhibition varies among the different NSAIDs. At least two isoforms of cyclooxygenase enzymes have been described. While cyclooxygenase-1 (COX-1) is constitutively expressed in most normal tissues, cyclooxygenase-2 (COX-2) expression is predominantly induced during inflammation and tissue injury[1]. Most of the side effects associated with the use of non-selective NSAIDs are thought to be due to inhibition of COX-1. Therefore, selective COX-2 inhibitors have been developed in order to minimize some of the NSAID-associated adverse effects. Celecoxib is a widely used COX-2 inhibitor with high levels of patient acceptability and satisfaction, which may result from its combination of efficacy and relatively benign adverse effect profile. The daily recommended adult dose ranges between 100 and 800 mg/d for various clinical indications including osteoarthritis, ankylosing spondylitis, rheumatoid arthritis, chemoprevention of familial polyposis, primary dysmenorrhea, and acute pain[2].
Recent reports described liver injuries in association with COX-2 inhibitors ranging from acute liver failure[3–6] to varying degrees of transient cholestatic[7–10] and/or hepatocellular injuries[11,12]. We report a case of celecoxib-associated acute cholestatic hepatitis progressing to liver failure requiring transplantation.
CASE REPORT
A 52-year-old Caucasian female was in her usual state of health until she developed generalized muscle aches and pains 1 d after performing yard work. Over the next 3 d, she took a total of eight 200 mg-tablet of the prescription drug Celecoxib. On the 3rd day, she developed fatigue, loss of appetite, intense pruritus and dark-brown (“coke”) colored urine. The patient’s symptoms progressively worsened over the next 3 d, and 1 wk after the use of celecoxib, she presented to the local emergency room. She denied abdominal pain, nausea, vomiting, but did endorse a weight loss of five pounds.
The patient worked as a registered nurse. Past medical history was positive for a needle stick injury from an HIV/HCV co-infected patient one year earlier, for which she was evaluated at employee health. Her liver function tests (LFTs) were normal (Table 1), and the serologies for HIV, hepatitis B and C were negative at the time of needle stick and at subsequent follow-up visits at three, seven and 10 mo. The patient was single and not sexually active. She denied any alcohol intake, smoking, use of aspirin, or over the counter medications, herbals, and illicit drugs. She denied recent travel or sick contacts.
Table 1.
Laboratory values
| AST | ALT | AP | GGT | Bilirubin | INR | |
| One year before | 22 | 18 | 78 | 33 | 0.4 | 1.0 |
| D 1-3 | Celecoxib (eight 200 mg-tablet) | |||||
| D 10 | 104 | 258 | 700 | 262 | 10.8 | 1.0 |
| D 24 | 220 | 297 | 889 | 711 | 15 | 1.0 |
| D 48 | 442 | 509 | 1427 | 895 | 35 | 1.0 |
| D 51 | 152 | 167 | 1024 | 573 | 38 | 2.6 |
| D 54 | Orthotopic liver transplantation | |||||
| 1 mo (post OLTx) | 16 | 27 | 570 | 116 | 1.7 | 1.1 |
| 6 mo (post OLTx) | 36 | 28 | 204 | 109 | 0.3 | 0.9 |
AST: Aspartate aminotransferase (17-59 IU/L); ALT: Alanine amino-transferase (21-72 IU/L); AP: Alkaline phosphatase (38-126 IU/L); GGT: Gamma glutamyl transpeptidase (0-65 IU/L); Bil: Total bilirubin (0.2-1.3 mg/dL); INR: International normalized ratio (0.8-1.2).
On physical exam, she was afebrile and jaundiced with mild right upper quadrant tenderness. Initial laboratory studies revealed abnormal LFTs with predominantly cholestatic pattern (Table 1). Serum urea nitrogen and creatinine levels were normal. Blood count differential was notable for peripheral eosinophilia with an absolute eosinophil count of 760/mL. Platelets count, INR and serum albumin were within normal limits.
Repeat testing at a 2-wk follow-up revealed worsening LFTs (Table 1). Antibodies to hepatitis A, B and C and Epstein-Barr virus, Cytomegalovirus and Herpes simplex virus were negative. Iron studies, an autoimmune panel (Anti-nuclear antibodies, anti-smooth muscle antibodies, liver-kidney-microsomal antibodies and immunoglobulins), anti-mitochondrial antibodies, alpha-one anti-trypsin, and ceruloplasmin were within normal limits. A CT scan of abdomen with intravenous contrast revealed normal liver morphology with no focal lesions and no biliary duct dilatation; the hepatic and portal veins were patent. Ultrasound guided liver biopsy showed ductopenia with lobular foam cell change and cholestasis along with periportal fibrosis and no evidence of bridging fibrosis (Figure 1A and B). The patient was started on 500 mg of ursodiol twice daily, oral fat-soluble vitamins, and prednisone 40 mg daily, tapered progressively over 2 wk for presumed drug induced liver injury (DILI).
Figure 1.
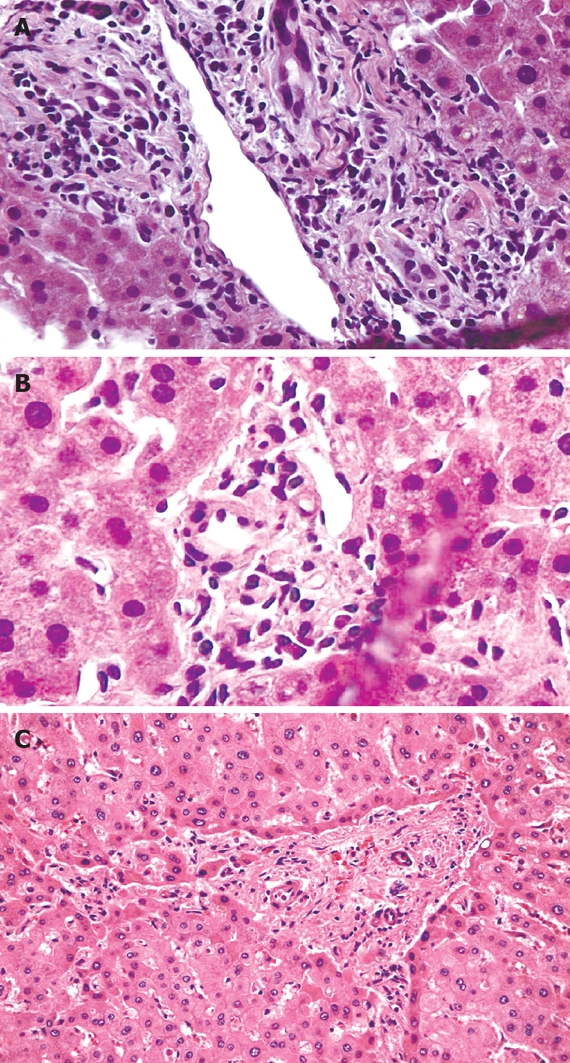
Hepatic histology. A: Relatively large portal tract containing bile ducts with increased nuclear to cytoplasmic ratio, eosinophilic transformation of the cytoplasm, nuclear hyperchromasia, and uneven nuclear spacing; B: Small portal tract containing the hepatic artery and portal vein, but there is no bile duct. Five of twelve interlobular portal tracts in this biopsy lacked bile ducts; C: Hepatectomy specimen: loss of interlobular bile ducts in most of the small portal tracts, more advanced portal and periportal fibrosis with short fibrous septa. (Haematoxylin & Eosin stain, × 100).
On a return visit 2 wk later, the patient showed no improvement in her symptoms and was complaining of worsening pruritus and an additional 10-pound weight loss. Lab tests at this time revealed a total bilirubin of 35 mg/dL and acute renal failure with BUN of 89 and creatinine of 7.5 (Table 1). The patient was admitted to the medical intensive care unit. She was challenged with intravenous fluids with improvement in her creatinine to 3.9 mg/dL. However, the patient’s clinical condition continued to deteriorate with a new development of lethargy. The INR increased to 3.4 despite subcutaneous injections of Vitamin K, and the total bilirubin peaked at 51 mg/dL. The patient underwent orthotopic liver transplantation, 54 d after the initial ingestion of celecoxib.
Sections through the hepatectomy specimen showed severe intrahepatic cholestasis, prominent ductopenia, and degenerative changes of the duct epithelial cells involving most of the small bile duct branches; the large bile ducts showed little to no evidence of duct injury. More advanced portal and periportal fibrosis was noted (Figure 1C). The postoperative course was uneventful. On follow-up visits at 1 and 6 mo, the patient remained clinically stable and had normal LFTs (Table 1).
DISCUSSION
Many NSAIDs and COX-2 inhibitors have been associated with hepatotoxicity varying from transient cholestatic[7–10] and hepatocellular injury[11,12] to fulminant hepatic failure[3–6]. The incidence of COX-2-associated hepatotoxicity is extremely difficult to estimate accurately and the current literature is based on case reports of side effects. Several cases of liver injury associated with celecoxib use have been reported. Self-limited cholestatic hepatitis was reported in most of the cases with resolution of symptoms after stopping celecoxib[7–10].
Liver failure associated with COX-2 inhibitor use has been reported with Nimesulide and rofecoxib[3–6]. Fulminant liver failure requiring orthotopic liver transplantation after celecoxib use has been recently reported in one case[3]. To our knowledge, our case is the second documented case of celecoxib-induced liver failure requiring liver transplantation. Interestingly, our patient developed a liver injury after a 3-d course of celecoxib when compared to the 2-wk course in the other reported case of celecoxib-induced liver failure[3].
Several factors support the diagnosis of drug-induced liver injury (DILI) secondary to celecoxib as the most likely etiology for our patient’s liver failure. These include: the acute presentation following celecoxib use; the significant eosinophilia in the blood and on the liver biopsy; and finally, the exclusion of other etiologies for liver failure.
To further assess the likelihood of DILI, we used the Roussel Uclaf Causality Assessment Method/Council for International Organizations of Medical Sciences (RUCAM/CIOMS) scoring system[13]. The criteria for scoring include: time to onset of the reaction, course of the reaction, risk factor(s) for drug reaction, concomitant drug(s), non drug-related causes, previous information on the drug, and response to re-administration. Using this system, the causality of the drug in our case is classified as “probable”, with a score of 8. A more recent DILI diagnostic scale uses temporal relationship between drug intake and the onset of clinical picture, exclusion of alternative causes, extrahepatic manifestations, intentional or accidental re-exposure to the drug, previous reports in the literature of cases of DILI associated with the drug[14]. On a scale of -6 to 20, the probability of the diagnosis of DILI is expressed as: definite ≥ 17, probable = 14-17, possible = 10-13, unlikely = 6-9, and excluded ≤ 6. Our patient scored 15, hence the likelihood of DILI is “probable”.
The underlying mechanisms for NSAIDs-induced liver injury are poorly understood. The adverse effects are due to either host dependent idiosyncratic reactions or dose dependent intrinsic reactions. Idiosyncratic reaction, which is the most common type, is mediated by either an immunological mechanism or abnormalities in drug metabolism[15]. The etiology of DILI in our patient is most likely an idiosyncratic reaction as she developed her symptoms after taking a relatively short course of celecoxib, well within its recommended daily adult dose.
In conclusion, we present a case of cholestatic hepatic injury following a short course of celecoxib progressing to liver failure and requiring liver transplantation. Clinicians should be aware that despite its better safety profile for gastrointestinal side effects compared to nonselective NSAIDs, celecoxib may be associated with severe hepatotoxicity.
Peer reviewers: Paul E Sijens, PhD, Associate Professor, Radiology, UMCG, Hanzeplein 1, 9713GZ Groningen, The Netherlands; James M Millis, Professor, University of Chicago, Section of Transplantation, MC 5027, 5841 S. Maryland Avenue, Chicago, IL 60637, United States
S- Editor Cheng JX L- Editor Stewart GJ E- Editor Ma WH
References
- 1.Brooks PM, Day RO. Nonsteroidal antiinflammatory drugs--differences and similarities. N Engl J Med. 1991;324:1716–1725. doi: 10.1056/NEJM199106133242407. [DOI] [PubMed] [Google Scholar]
- 2.Antoniou K, Malamas M, Drosos AA. Clinical pharmacology of celecoxib, a COX-2 selective inhibitor. Expert Opin Pharmacother. 2007;8:1719–1732. doi: 10.1517/14656566.8.11.1719. [DOI] [PubMed] [Google Scholar]
- 3.Dastis SN, Rahier J, Lerut J, Geubel AP. Liver transplantation for nonsteroidal anti-inflammatory drug-induced liver failure: nimesulide as the first implicated compound. Eur J Gastroenterol Hepatol. 2007;19:919–922. doi: 10.1097/MEG.0b013e3282eeb4cc. [DOI] [PubMed] [Google Scholar]
- 4.Andrade RJ, Lucena MI, Fernandez MC, Gonzalez M. Fatal hepatitis associated with nimesulide. J Hepatol. 2000;32:174. doi: 10.1016/s0168-8278(00)80208-1. [DOI] [PubMed] [Google Scholar]
- 5.McCormick PA, Kennedy F, Curry M, Traynor O. COX 2 inhibitor and fulminant hepatic failure. Lancet. 1999;353:40–41. doi: 10.1016/s0140-6736(05)74867-4. [DOI] [PubMed] [Google Scholar]
- 6.Papachristou GI, Demetris AJ, Rabinovitz M. Acute cholestatic hepatitis associated with long-term use of rofecoxib. Dig Dis Sci. 2004;49:459–461. doi: 10.1023/b:ddas.0000020503.92146.8b. [DOI] [PubMed] [Google Scholar]
- 7.O'Beirne JP, Cairns SR. Drug Points: Cholestatic hepatitis in association with celecoxib. BMJ. 2001;323:23. doi: 10.1136/bmj.323.7303.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galan MV, Gordon SC, Silverman AL. Celecoxib-induced cholestatic hepatitis. Ann Intern Med. 2001;134:254. doi: 10.7326/0003-4819-134-3-200102060-00028. [DOI] [PubMed] [Google Scholar]
- 9.Grieco A, Miele L, Giorgi A, Civello IM, Gasbarrini G. Acute cholestatic hepatitis associated with celecoxib. Ann Pharmacother. 2002;36:1887–1889. doi: 10.1345/aph.1C110. [DOI] [PubMed] [Google Scholar]
- 10.Alegria P, Lebre L, Chagas C. Celecoxib-induced cholestatic hepatotoxicity in a patient with cirrhosis. Ann Intern Med. 2002;137:75. doi: 10.7326/0003-4819-137-1-200207020-00030. [DOI] [PubMed] [Google Scholar]
- 11.Zinsser P, Meyer-Wyss B, Rich P. Hepatotoxicity induced by celecoxib and amlodipine. Swiss Med Wkly. 2004;134:201. doi: 10.4414/smw.2004.10479. [DOI] [PubMed] [Google Scholar]
- 12.Nachimuthu S, Volfinzon L, Gopal L. Acute hepatocellular and cholestatic injury in a patient taking celecoxib. Postgrad Med J. 2001;77:548–550. doi: 10.1136/pmj.77.910.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Danan G, Benichou C. Causality assessment of adverse reactions to drugs--I. A novel method based on the conclusions of international consensus meetings: application to drug-induced liver injuries. J Clin Epidemiol. 1993;46:1323–1330. doi: 10.1016/0895-4356(93)90101-6. [DOI] [PubMed] [Google Scholar]
- 14.Maria VA, Victorino RM. Development and validation of a clinical scale for the diagnosis of drug-induced hepatitis. Hepatology. 1997;26:664–669. doi: 10.1002/hep.510260319. [DOI] [PubMed] [Google Scholar]
- 15.Boelsterli UA, Zimmerman HJ, Kretz-Rommel A. Idiosyncratic liver toxicity of nonsteroidal antiinflammatory drugs: molecular mechanisms and pathology. Crit Rev Toxicol. 1995;25:207–235. doi: 10.3109/10408449509089888. [DOI] [PubMed] [Google Scholar]


