Abstract
AIM: To evaluate in bile duct ligated rats whether there were progressive alterations of renal function without changes in histopathology.
METHODS: Male Wistar rats were submitted to sham-surgery or bile duct ligation (BDL) and divided according to the post-procedure time (2, 4 and 6-wk). To determine renal function parameters, rats were placed in metabolic cages and, at the end of the experiment, blood and urine samples were obtained. Histology and hydroxyproline content were analyzed in liver and renal tissue.
RESULTS: Rats with 2 wk of BDL increased free water clearance (P = 0.02), reduced urinary osmolality (P = 0.03) and serum creatinine (P = 0.01) in comparison to the sham group. In contrast, rats at 6 wk of BDL showed features of HRS, including significant increase in serum creatinine and reductions in creatinine clearance, water excretion and urinary sodium concentration. Rats with 4 wk of BDL exhibited an intermediate stage of renal dysfunction. Progressive hepatic fibrosis according to post-procedure time was confirmed by histology. The increased levels of liver hydroxyproline contrasted with the absence of structural changes in the kidney, as assessed by histology and unchanged hydroxyproline content in renal tissue.
CONCLUSION: Our data show that BDL produced progressive renal dysfunction without structural changes in the kidney, characterizing HRS. The present model will be useful to understand the pathophysiology of HRS.
Keywords: Hepatorenal syndrome, Bile duct ligation, Renal function, Renin angiotensin system
INTRODUCTION
Hepatorenal syndrome (HRS) has been defined as a progressive renal failure that occurs in patients with chronic liver disease and advanced hepatic failure in the absence of any apparent clinical cause for renal insufficiency[1,2]. HRS represents the final stage of a process that gradually reduces the renal blood flow and the glomerular filtration rate (GFR) due to a marked renal vasoconstriction[1–4]. Despite the severity of renal failure, no significant histological abnormalities are found in the kidneys.
There are many experimental models to induce hepatic fibrosis[5]. However, none of them has been evaluated systematically as a model of hepatorenal syndrome. The two most frequently used experimental models of liver disease are the administration carbon tetrachloride, and the common bile duct ligation (BDL)[6]. The main advantage of BDL is to allow the study of renal function alterations in a short period of time with lower mortality rates than the administration of carbon tetrachloride[6]. In addition, this model mimics clinical conditions characterized by obstructive jaundice, such as biliary atresia and choledocal cysts[5,6]. In this study, we aimed to systematically evaluate renal function parameters, renal histology and tissue hydroxyproline content at different time-points of BDL.
MATERIALS AND METHODS
Animals and experimental design
Male Wistar rats weighing 220 to 300 g were maintained under temperature controlled conditions with an artificial 12-h light-dark cycle, and were allowed standard chow and water ad libitum. Hepatic fibrosis was induced by BDL. Briefly, the animals were anesthetized with intraperitoneal administration of 2.5% tribromoethanol (1 mL/100 g). A 1.5 cm midline incision was made and the common bile duct was located, double ligated with 4-0 silk and sectioned as previously described[7]. Our Ethics Committee approved all animal procedures.
Experimental protocol
Animals were randomized into the following groups: sham-operated and those that underwent BDL. Sham-operated rats (n = 17) underwent a midline incision and manipulation of the bile duct without ligation and were evaluated at various times following sham-surgery: 2-wk (n = 5), 4-wk (n = 7), and 6-wk (n = 5). Bile duct ligated rats were also evaluated at the same post-procedure times: 2-wk (n = 8), 4-wk (n = 7) and 6-wk (n = 7). Three days before blood sampling, all rats were placed in metabolic cages to measure urinary volume, water and food intake. At the end of the experiment, animals were weighed and blood samples were collected by decapitation to determine renal function parameters. Liver and renal tissue fragments were also obtained for histology and hydroxyproline determination.
Biochemical parameters
Serum and urinary levels of creatinine (Jaffe method) were measured using Katal Kit and a semi-automatic analyzer BIO 2000. Urinary and serum osmolality were determined using a freezing point osmometer (Fiske Osmometer, Fiske Ass. Inc., MA, USA). Serum and urinary levels of sodium and potassium were measured by flame photometry (Corning 400, Corning Inc., NY, USA).
Hydroxyproline determination
Fragments (200 mg) of liver and renal tissue were removed for hydroxyproline determination as an indirect measure of tissue collagen content, as described by Reddy & Enwemeka[8]. Briefly, tissue fragments were homogenized in saline 0.9%, frozen and lyophilized. The assay was performed with 40 mg of the lyophilized tissue that was subjected to alkaline hydrolysis in 300 μL plus 75 μL NaOH 10 mol/L at 120°C for 20 min. An aliquot of 50 μL of the hydrolysed tissue was added to 450 μL of chloramine T oxidizing reagent (Chloramine T 0.056 mol/L, n-propanol 10% in acetate/citrate buffer pH 6.5) and allowed to react for 20 min. A hydroxyproline standard curve with the highest concentration of 400 μg was prepared likewise. Colour was developed by the addition of 500 μL of the Ehrlich reagent (p-dimethylamine-benzaldehyde, 1 mol/L) diluted in n-propanol/perchloric acid, 2:1 v/v). The samples then were centrifuged for 1500 g for 10 min at 4°C. An aliquot of 200 μL of the supernatant was transferred to 96-well plates and the absorbance was read at 550 nm.
Five-micrometer sections of formalin-fixed and paraffin-embedded right liver lobes and kidney slices were processed routinely with hematoxylin-eosin, Masson’s trichrome and ammoniac silver of Gomori. A single pathologist, blinded to experimental protocol, analyzed all liver and kidney fragments using light microscopy. The degree of liver fibrosis was measured based on the semi-quantitative scoring described by Ishak et al[9].
Statistical analysis
The data are expressed as mean ± SD. Analysis of variance followed by Student Newman Keuls test was used to compare the differences between groups. Values of P < 0.05 were considered significant.
RESULTS
Morphological studies
Because there did not appear to be any difference in the many variables studied in the sham group at two, four and 6 wk after sham operation, results from all the sham-operated groups were pooled for ease of presentation.
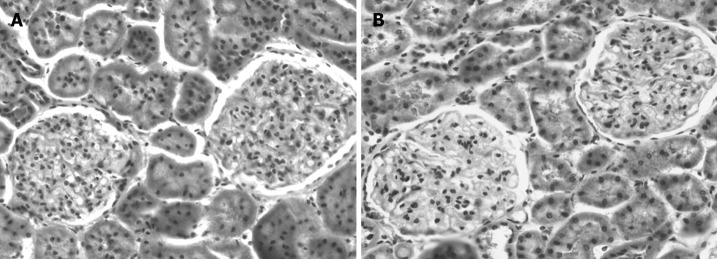
Hepatic fibrosis progressed over the time after BDL. Based on Ishak’s score, the following score values for each group of BDL rats were obtained: sham operated rats scored 0 (normal hepatic architecture), 2-wk rats scored 3 (fibrous expansion of portal tracts with occasional portal-to-portal bridging), 4-wk rats scored 4 (fibrous expansion of portal tracts with marked portal-to-portal and portal-to-central bridging) and, at 6-wk, definite cirrhosis occurred (Score 6). In sharp contrast, as shown on Figure 1, no alterations in renal histology were observed in any bile duct ligated rats when compared to sham-operated animals.
Figure 1.
Representative micrographs of the renal slices from bile duct ligated (BDL) and sham operated rats (HE, × 100). A: Sham operated rat with normal kidney; B: BDL rat at 6-wk also showing the absence of kidney histological alterations.
Hydroxyproline determination
The progression of collagen deposition in liver tissue was also confirmed by the measurement of tissue hydroxyproline content at different time-points after bile duct liagation. Sham-operated rats represented the basal values of hydroxyproline content from a normal liver (235 ± 45 μg/mg of liver tissue). As expected, according to the time after BDL, hydroxyproline content progressively increased in liver tissue, reaching values significantly higher than the control group in all time-points (2 wk: 540 ± 60 μg/mg; 4 wk: 863 ± 57 μg/mg; 6 wk: 1735 ± 73 μg/mg; P = 0.0001 for all comparisons, Figure 2A). The highest amount of liver hydroxyproline was detected in animals at 6 wk of BDL, indicating the significant degree of liver fibrosis (Figure 2A). Hydroxyproline content in renal tissue remained unchanged in sham-operated animals (288 ± 31 μg/mg of kidney tissue) as well as in all groups of bile duct ligated rats (2 wk: 298 ± 55 μg/mg; 4 wk: 300 ± 73 μg/mg; 6 wk: 294 ± 39 μg/mg; P > 0.05 for all comparisons, Figure 2B). The results obtained with histological analysis and tissue hydroxyproline determinations showed the absence of structural changes in renal tissue during the development of liver fibrosis.
Figure 2.
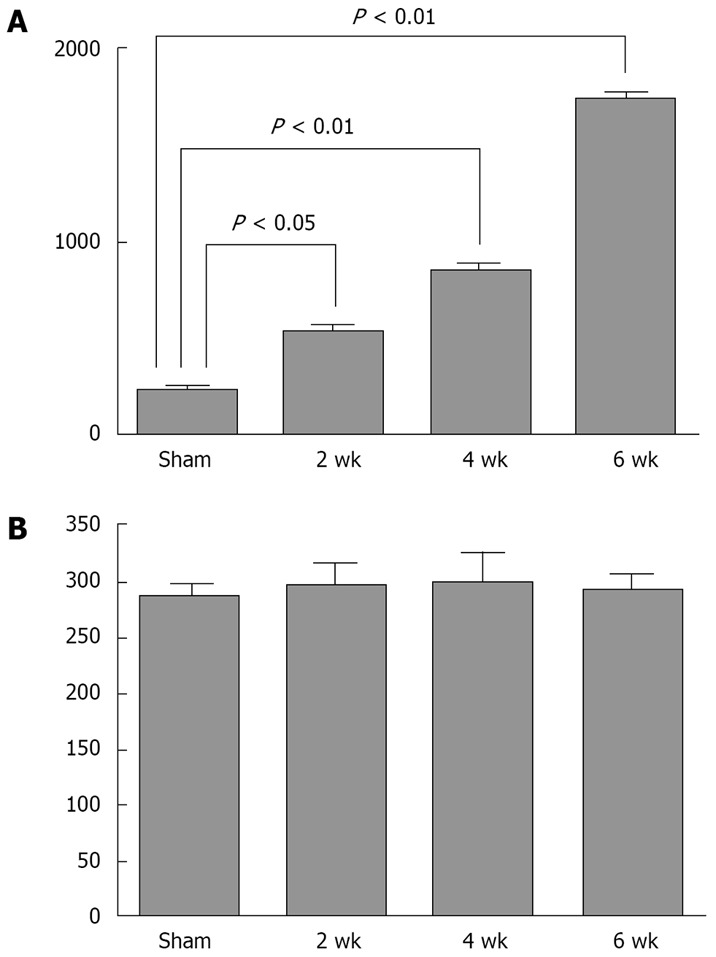
Hydroxyproline determinations in the liver and renal tissue from bile duct ligated and sham operated rats. A: Hydroxyproline content in the liver tissue of sham operated rats (sham), and animals with 2-wk, 4-wk and 6-wk of bile duct ligation; B: Hydroxyproline content in renal tissue of sham operated rats (sham), and animals with 2 wk, 4 wk and 6 wk of bile duct ligation.
Renal function parameters
Despite the well-preserved renal structure, important changes in renal function were clearly evidenced in bile duct ligated rats, as shown in Table 1 and Figure 3. As shown in Table 1, the 24-h urinary volume was significantly higher in animals with 4 and 6 wk of BDL compared to sham group. On the other hand, the 24-h urinary volume of 2 wk animals did not differ from sham group. Despite having the same urinary volume as sham-operated animals, rats with 2 wk of BDL exhibited an attempt to compensate the hydroelectrolyte imbalance produced by hepatic dysfunction. These animals significantly increased water excretion (Figure 3A, P = 0.02), and reduced the urinary osmolality (P = 0.03) and serum creatinine levels (P = 0.01) in comparison to sham-operated rats (Table 1). An elevation in potassium excretion was also observed. However, the fractional excretion of this ion was unchanged in comparison with the sham group. Rats with 4 wk of BDL presented a progression in renal dysfunction as shown by a significant increase in serum creatinine (P = 0.01) and a reduction in urinary sodium concentration (P = 0.02) when compared to sham-operated animals (Table 1). Rats with 6 wk of BDL clearly developed hepatorenal syndrome as revealed by a complete deterioration in renal compensatory mechanisms. These animals presented high levels of serum creatinine, a pronounced decrease in creatinine clearance (Figure 3B, P = 0.01), and an important impairment in water excretion (Figure 3A, P = 0.02) when compared to sham operated and 2 wk of BDL animals (P < 0.05 for all comparisons, Table 1). Rats with 4 and 6 wk of BDL also presented dilutional hyponatremia and an elevation of fractional excretion of potassium when compared to sham group and animals with 2-wk of BDL (P < 0.05 for both comparisons, Table 1). It should be pointed out that body weights were similar in all groups at the beginning of the experimental protocol and no differences were observed in water and food intake (data not shown). No ascites was observed in the rats at 2-wk after BDL. In contrast, animals at 4 wk and 6 wk clearly exhibited ascites, also indicating the presence of water retention.
Table 1.
Renal function parameters in sham-operated (Sham) and bile duct ligated rats at 2-wk, 4-wk and 6-wk (mean ± SD)
| Sham (n = 17) | 2-wk (n = 8) | 4-wk (n = 7) | 6-wk (n = 7) | |
| Urinary volume (mL/24 h) | 12 ± 0.5 | 14.2 ± 1.0 | 19.4 ± 1.7a | 20.4 ± 2.8a |
| Serum creatinine (mg/dL) | 0.60 ± 0.10 | 0.28 ± 0.05a | 1.21 ± 0.25a | 2.50 ± 0.40a |
| Creatinine clearance (mL/min) | 1.14 ± 0.19 | 1.31 ± 0.11 | 0.97 ± 0.43 | 0.47 ± 0.25a |
| Serum osmolality (mOsm/kg) | 292 ± 2 | 289 ± 4 | 280 ± 14a | 282 ± 3a |
| Urinary osmolality (mOsm/kg) | 2147 ± 115 | 1578 ± 76a | 1499 ± 117a | 1745 ± 73a |
| Osmolal clearance (mL/min) | 0.061 ± 0.003 | 0.049 ± 0.002 | 0.071 ± 0.009 | 0.088 ± 0.013 |
| Free water clearance (mL/min) | -0.052 ± 0.003 | -0.040 ± 0.002a | -0.056 ± 0.008 | -0.074 ± 0.011a |
| Serum [Na+] (mEq/L) | 137 ± 1 | 138 ± 3 | 125 ± 3a | 126 ± 2a |
| Urinary [Na+] (mEq/L) | 111 ± 13 | 102 ± 10 | 57 ± 15a | 50 ± 15a |
| Na+ excreted (mEq) | 1.47 ± 0.15 | 1.59 ± 0.19 | 1.20 ± 0.33 | 1.09 ± 0.34 |
| Fractional Na+ excreted (%) | 0.65 ± 0.17 | 0.63 ± 0.13 | 0.91 ± 0.40 | 1.72 ± 0.61 |
| Serum [K+] (mEq/L) | 4.5 ± 0.3 | 5.1 ± 0.4 | 4.0 ± 0.5 | 4.3 ± 0.1 |
| Urinary [K+] (mEq/L) | 281 ± 12 | 269 ± 5 | 199 ± 21a | 180 ± 27a |
| K+ excreted (mEq) | 3.35 ± 0.17 | 4.12 ± 0.31a | 3.65 ± 0.56 | 3.38 ± 0.39 |
| Fractional K+ excreted (%) | 38 ± 6 | 43 ± 8 | > 100a | > 100a |
[Na+], sodium concentration; [K+], potassium concentration.
P < 0.05 vs sham group.
Figure 3.
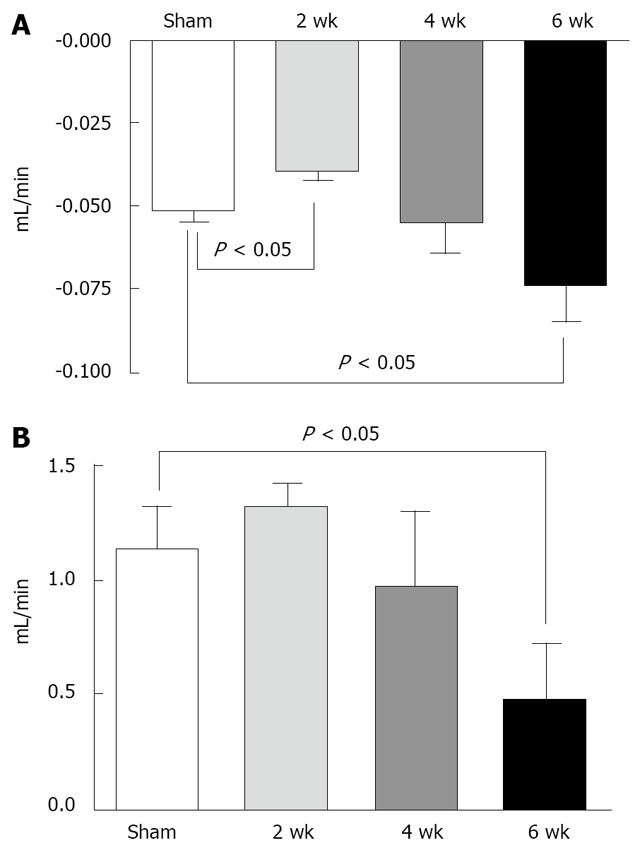
Free water and creatinine clearance in bile duct ligated (BDL) and sham operated rats. A: Free water clearance of sham operated rats (sham), and animals with 2-wk, 4-wk and 6 of bile duct ligation; B: Creatinine clearance of sham operated rats (sham), and animals with 2 wk, 4 wk and 6 wk of bile duct ligation.
DISCUSSION
This study supports the concept that the progression of hepatic damage promotes the manifestation of HRS. Indeed, the duration of BDL was positively correlated with renal function disarrangement without alterations in renal histology.
Animals at 2 wk of BDL seemed to be in a compensated state of hepatic injury, without ascites and alterations in water balance. These rats exhibited well-preserved renal function, suggesting that the homeostatic compensatory mechanisms remained intact at this moment of hepatic damage. Of note, serum creatinine was reduced in this group even when compared to sham operated animals. The creatinine clearance was slightly higher than in sham group, but significantly increased when compared to rats at 6 wk of BDL. These animals were also able to excrete water by increasing free water clearance. For this reason, serum osmolality remained at normal range and urinary osmolality was reduced when compared to sham. It has been reported that, at early stages of hepatic injury, as observed in animals with 2 wk of BDL, renal compensatory mechanisms against fluid retention still remain operating[1,3,4]. However, the progression of the process culminates in a non-compensated state by compromising the negative feedback loops of different regulatory systems[1,3,4]. Consistent with this, 4 wk after BDL, the rats already presented ascites, changes in water balance and an initial disturbance in renal function, revealed by sodium retention and an increase of the serum creatinine levels. After 6 wk of BDL, the hepatic damage evolved into a non-compensated stage with features of HRS[1,3,4], including reduction in creatinine clearance and an evident fluid retention associated with significant reductions of the serum osmolality and of the free water clearance.
Clinical studies have attempted to delineate the natural history of cirrhotic patients with ascites with respect to the development of HRS. Factors predictive for the development of HRS include intense urinary sodium retention, dilutional hyponatremia, and increased activity of systemic vasoconstrictors[10]. These features were clearly evidenced in our bile duct ligated rats, mostly at 6 wk. However, some characteristics of bile duct ligated rats are not typically observed in HRS seen in clinical medicine. While patients with HRS are usually oliguric[1,3], our bile duct ligated animals at 4 wk and 6 wk increased the urinary volume when compared to the sham-operated group. It should be mentioned that, despite the elevation in the urinary volume, these animals still presented water and sodium retention, according to the observed reductions in free water clearance and in urinary sodium concentration. The so-called polyuria was not enough to excrete the whole amount of water and sodium retained by rats with 4 wk and 6 wk of BDL. Indeed, most investigators have used the BDL model to study pathological sodium retention[6,11], which occurs in liver disease. Another possible explanation for this apparently elevated urinary volume is the well-known effect of circulating bile acids in kidney function during obstructive jaundice[12,13]. Acute cholaemia may cause volume depletion by increasing urinary salt loss, which, in turn, may aggravate the direct nephrotoxicity of circulating bile compounds[12]. In vitro addition of bile acids or bilirubin at concentrations comparable to those found in the plasma of BDL rats, to a mixture of reactive enzymes strongly inhibited most, particularly mitochondrial oxidative phosphorylation[13]. Thus, high concentrations of these substances in the blood may explain the development of renal failure during liver disease, and its reversibility when liver function returns to normal[13]. It also should be noted that the normal kidney histology and the unchanged levels of renal hydroxyproline content also favors the existence of HRS, a syndrome characterized by functional rather than structural disarrangement of the kidneys in presence of progressive liver disease[1,4]. Despite the differences in renal parameters in HRS in our system and in HRS observed in humans, this model seems to be very useful to evaluate the progression of renal dysfunction in hepatic diseases, since BDL rats are normally able to maintain a residual diuresis, probably allowing their long-lasting survival[6].
The pathophysiology of HRS is still poorly understood. Hypoperfusion of the kidney due to active renal vasoconstrictors has been considered the hallmark of HRS[1–4]. In this context, Ozdogan and co-workers[14] conducted an elegant study that evidenced the role of endothelin-1, a potent vasoconstrictor, in an experimental model of HRS, which was induced by endotoxin administration to carbon tetrachloride-treated rats. In addition, the renin-angiotensin system (RAS) and the sympathetic nervous system, some of the major systems with a vasoconstrictor effect in the renal circulation, have been suggested as potential mediators of renal vasoconstriction in HRS[4,11,15]. During hepatic damage, systemic vasodilation and hyperdynamic circulation have been observed[16], which in turn promote an increase in sympathetic nervous activity, plasma renin activity (PRA), angiotensin II and aldosterone levels, especially in the presence of HRS[3,4]. It is well known that angiotensin II is one of the most powerful regulators of sodium excretion, operating through extrarenal as well as intrarenal mechanisms[17–19]. Some authors believe that, at the early stages of hepatic injury, the renal effects of angiotenisin II represent a compensatory mechanism against the drop in organ perfusion pressure[3,4]. However, the development of renal impairment leading to HRS would occur as a result of an uncontrolled activation of systemic vasoconstrictor factors such as angiotensin II, sympathetic nervous system, endothelin and others that could not be counteracted by vasodilators such as nitric oxide, prostaglandins, bradykinin and maybe angiotensin-(1-7)[3,4].
In this regard, we recently have shown that bile duct ligated rats presented different profiles of circulating RAS expression according to the progress of hepatic damage[20]. At early stages (1 wk and 2 wk of BDL), animals exhibited an elevation of angiotensin II and angiotensin-(1-7) levels, without concomitant changes in PRA and angiotensinI. With the progression of liver fibrosis (4 wk and 6 wk of BDL), RAS profile changed toward an overall enhancement of the PRA and the circulating levels of angiotensinI, angiotensin II and angiotensin-(1-7)[20]. According to these data[20], we hypothesize that not only angiotensin II, but also angiotensin-(1-7) may possibly participate in the regulation of renal blood flow, glomerular filtration, and tubular transport in liver diseases. However, we still do not know how angiotensin-(1-7) could affect renal function in BDL rats. It has been clearly demonstrated that angiotensin-(1-7) also exerts complex renal actions[19,21,22]. Our group and others detected in vivo and in vitro antidiuretic effects of angiotensin-(1-7) by increasing fluid reabsorption[23–26]. These renal actions could contribute to sodium and water retention observed in bile duct ligated rats. In contrast, other studies showed that angiotensin-(1-7) has natriuretic and diuretic effects by inhibiting sodium reabsorption[27,28]. In addition, angiotensin-(1-7) seems to be involved in renal hemodynamic regulation by opposing the vasoconstrictive effects of angiotensin II in glomerular vessels[29,30]. However, it is difficult to know if the changes in the components of the RAS preceded or were caused by the decline in renal function. The liver, or maybe also the kidney, could produce angiotensin peptides, which, in turn, act either as systemic hormones or as locally generated factors. Accordingly, Paizis et al[31] detected an up-regulation of angiotensin converting enzyme 2 (ACE2), the main enzyme responsible for angiotensin-(1-7) synthesis[22], in liver tissue from cirrhotic patients and bile duct ligated rats. Herath et al[32] showed increased expression of angiotensin-(1-7) receptor, the Mas receptor[33], in experimental billiary fibrosis, suggesting a role for ACE2-angiotensin-(1-7)-Mas axis in liver injury.
Finally, the overall state of sodium and water balance and the effect of many circulating and/or local regulators may influence the direction of the observed renal actions in bile duct ligated rats. Further studies are necessary to clarify the mechanisms involved in the development of HRS in experimental cirrhosis. However, our data indicate that BDL emerges as a good model for the study of HRS.
COMMENTS
Background
Hepatorenal syndrome (HRS) has been defined as a progressive renal failure that occurs in patients with chronic liver disease and advanced hepatic failure in the absence of any apparent clinical cause for renal insufficiency. HRS corresponds to a functional alteration without histological changes in kidney tissue. There are many experimental models to induce hepatic fibrosis. However, none of them has been systematically evaluated as a model of hepatorenal syndrome.
Research frontiers
In this study, we aimed to systematically evaluate in bile duct ligated rats at different post-procedure time-points, whether there were alterations of renal function without changes in histopathology.
Innovations and breakthroughs
Renal dysfunction without histological changes occurred according to the duration of bile duct ligation (BDL) in the absence of any additional treatment. Animals at 2 wk of BDL exhibited a well-preserved renal function, suggesting that the renal homeostatic compensatory mechanisms remained intact at this moment of hepatic damage. However, the progression of the process culminates in a non-compensated state, as already shown by rats at 4 wk of BDL with ascites, changes in water balance, sodium retention and increased serum creatinine levels. After 6 wk of BDL, features of hepatorenal syndrome (HRS) became evident, including reduction in creatinine clearance and fluid retention without alterations in renal histology and renal tissue collagen content. Our data showed that BDL seems to be a helpful model for the study of HRS, since it mimics clinical conditions characterized by obstructive jaundice, such as biliary atresia and choledochal cysts.
Applications
The mechanisms for the renal changes observed in BDL animals remain unclear; however, this study indicates that BDL emerges as a good model for further studies of HRS and its treatment.
Peer review
This study is a well-designed experimental work, which tries to define an experimental model for hepatorenal syndrome.
Supported by FAPEMIG (Fundação de Amparo à Pesquisa do Estado de Minas Gerais), CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) and PRONEX (Grupos de Excelãncia)
Peer reviewer: Osman C Ozdogan, Associate Professor, Department of Gastroenterology, Liver Unit, Marmara University School of Medicine, Istanbul 34662, Turkey
S- Editor Zhong XY L- Editor Li M E- Editor Ma WH
References
- 1.Bataller R, Sort P, Gines P, Arroyo V. Hepatorenal syndrome: definition, pathophysiology, clinical features and management. Kidney Int Suppl. 1998;66:S47–S53. [PubMed] [Google Scholar]
- 2.Cardenas A, Gines P. Hepatorenal syndrome. Clin Liver Dis. 2006;10:371–385, ix-x. doi: 10.1016/j.cld.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 3.Arroyo V, Jimenez W. Complications of cirrhosis. II. Renal and circulatory dysfunction. Lights and shadows in an important clinical problem. J Hepatol. 2000;32:157–170. doi: 10.1016/s0168-8278(00)80423-7. [DOI] [PubMed] [Google Scholar]
- 4.Gentilini P, Vizzutti F, Gentilini A, Zipoli M, Foschi M, Romanelli RG. Update on ascites and hepatorenal syndrome. Dig Liver Dis. 2002;34:592–605. doi: 10.1016/s1590-8658(02)80094-9. [DOI] [PubMed] [Google Scholar]
- 5.Oberti F, Vuillemin E, Fort J, Cales P. [Experimental models of portal hypertension] Gastroenterol Clin Biol. 2000;24:896–901. [PubMed] [Google Scholar]
- 6.Poo JL, Estanes A, Pedraza-Chaverri J, Cruz C, Perez C, Huberman A, Uribe M. [Chronology of portal hypertension, decreased sodium excretion, and activation of the renin-angiotensin system in experimental biliary cirrhosis] Rev Invest Clin. 1997;49:15–23. [PubMed] [Google Scholar]
- 7.Kountouras J, Billing BH, Scheuer PJ. Prolonged bile duct obstruction: a new experimental model for cirrhosis in the rat. Br J Exp Pathol. 1984;65:305–311. [PMC free article] [PubMed] [Google Scholar]
- 8.Reddy GK, Enwemeka CS. A simplified method for the analysis of hydroxyproline in biological tissues. Clin Biochem. 1996;29:225–229. doi: 10.1016/0009-9120(96)00003-6. [DOI] [PubMed] [Google Scholar]
- 9.Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, Denk H, Desmet V, Korb G, MacSween RN. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696–699. doi: 10.1016/0168-8278(95)80226-6. [DOI] [PubMed] [Google Scholar]
- 10.Cardenas A, Gines P. Therapy insight: Management of hepatorenal syndrome. Nat Clin Pract Gastroenterol Hepatol. 2006;3:338–348. doi: 10.1038/ncpgasthep0517. [DOI] [PubMed] [Google Scholar]
- 11.Jonassen TE, Brond L, Torp M, Graebe M, Nielsen S, Skott O, Marcussen N, Christensen S. Effects of renal denervation on tubular sodium handling in rats with CBL-induced liver cirrhosis. Am J Physiol Renal Physiol. 2003;284:F555–F563. doi: 10.1152/ajprenal.00258.2002. [DOI] [PubMed] [Google Scholar]
- 12.Alon U, Berant M, Mordechovitz D, Better OS. The effect of intrarenal infusion of bile on kidney function in the dog. Clin Sci (Lond) 1982;62:431–433. doi: 10.1042/cs0620431. [DOI] [PubMed] [Google Scholar]
- 13.Israeli BA, Bogin E. Biochemical changes in liver, kidney and blood associated with common bile duct ligation. Clin Chim Acta. 1986;160:211–221. doi: 10.1016/0009-8981(86)90144-0. [DOI] [PubMed] [Google Scholar]
- 14.Ozdogan O, Goren M, Ratip S, Giral A, Moini H, Enc F, Birsel S, Berkman K, Tozun N. Role of endothelin-1 in a cirrhotic rat model with endotoxin induced acute renal failure. Hepatol Res. 2002;24:114. doi: 10.1016/s1386-6346(02)00082-7. [DOI] [PubMed] [Google Scholar]
- 15.Aliaga L, Zozoya JM, Omar M, Mediavilla JD, Prieto J. Interrelationships between systemic hemodynamics, urinary sodium excretion, and renin-angiotensin system in cirrhosis. Acta Gastroenterol Belg. 1995;58:213–221. [PubMed] [Google Scholar]
- 16.Blendis L, Wong F. The hyperdynamic circulation in cirrhosis: an overview. Pharmacol Ther. 2001;89:221–231. doi: 10.1016/s0163-7258(01)00124-3. [DOI] [PubMed] [Google Scholar]
- 17.Kim S, Iwao H. Molecular and cellular mechanisms of angiotensin II-mediated cardiovascular and renal diseases. Pharmacol Rev. 2000;52:11–34. [PubMed] [Google Scholar]
- 18.Ichihara A, Kobori H, Nishiyama A, Navar LG. Renal renin-angiotensin system. Contrib Nephrol. 2004;143:117–130. doi: 10.1159/000078716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Souza Dos Santos RA, Passaglio KT, Pesquero JB, Bader M, Simoes E Silva AC. Interactions between angiotensin-(1-7), kinins, and angiotensin II in kidney and blood vessels. Hypertension. 2001;38:660–664. doi: 10.1161/01.hyp.38.3.660. [DOI] [PubMed] [Google Scholar]
- 20.Pereira RM, Dos Santos RA, Teixeira MM, Leite VH, Costa LP, da Costa Dias FL, Barcelos LS, Collares GB, Simoes e Silva AC. The renin-angiotensin system in a rat model of hepatic fibrosis: evidence for a protective role of Angiotensin-(1-7) J Hepatol. 2007;46:674–681. doi: 10.1016/j.jhep.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 21.Simoes e Silva AC, Pinheiro SV, Pereira RM, Ferreira AJ, Santos RA. The therapeutic potential of Angiotensin-(1-7) as a novel Renin-Angiotensin System mediator. Mini Rev Med Chem. 2006;6:603–609. doi: 10.2174/138955706776876203. [DOI] [PubMed] [Google Scholar]
- 22.Chappell MC, Modrall JG, Diz DI, Ferrario CM. Novel aspects of the renal renin-angiotensin system: angiotensin-(1-7), ACE2 and blood pressure regulation. Contrib Nephrol. 2004;143:77–89. doi: 10.1159/000078713. [DOI] [PubMed] [Google Scholar]
- 23.Santos RA, Simoes e Silva AC, Magaldi AJ, Khosla MC, Cesar KR, Passaglio KT, Baracho NC. Evidence for a physiological role of angiotensin-(1-7) in the control of hydroelectrolyte balance. Hypertension. 1996;27:875–884. doi: 10.1161/01.hyp.27.4.875. [DOI] [PubMed] [Google Scholar]
- 24.Garcia NH, Garvin JL. Angiotensin 1-7 has a biphasic effect on fluid absorption in the proximal straight tubule. J Am Soc Nephrol. 1994;5:1133–1138. doi: 10.1681/ASN.V541133. [DOI] [PubMed] [Google Scholar]
- 25.Vallon V, Richter K, Heyne N, Osswald H. Effect of intratubular application of angiotensin 1-7 on nephron function. Kidney Blood Press Res. 1997;20:233–239. doi: 10.1159/000174151. [DOI] [PubMed] [Google Scholar]
- 26.Magaldi AJ, Cesar KR, de Araujo M, Simoes e Silva AC, Santos RA. Angiotensin-(1-7) stimulates water transport in rat inner medullary collecting duct: evidence for involvement of vasopressin V2 receptors. Pflugers Arch. 2003;447:223–230. doi: 10.1007/s00424-003-1173-1. [DOI] [PubMed] [Google Scholar]
- 27.Andreatta-van Leyen S, Romero MF, Khosla MC, Douglas JG. Modulation of phospholipase A2 activity and sodium transport by angiotensin-(1-7) Kidney Int. 1993;44:932–936. doi: 10.1038/ki.1993.334. [DOI] [PubMed] [Google Scholar]
- 28.Handa RK. Angiotensin-(1-7) can interact with the rat proximal tubule AT(4) receptor system. Am J Physiol. 1999;277:F75–F83. doi: 10.1152/ajprenal.1999.277.1.F75. [DOI] [PubMed] [Google Scholar]
- 29.Ren Y, Garvin JL, Carretero OA. Vasodilator action of angiotensin-(1-7) on isolated rabbit afferent arterioles. Hypertension. 2002;39:799–802. doi: 10.1161/hy0302.104673. [DOI] [PubMed] [Google Scholar]
- 30.Sampaio WO, Nascimento AA, Santos RA. Systemic and regional hemodynamic effects of angiotensin-(1-7) in rats. Am J Physiol Heart Circ Physiol. 2003;284:H1985–H1994. doi: 10.1152/ajpheart.01145.2002. [DOI] [PubMed] [Google Scholar]
- 31.Paizis G, Tikellis C, Cooper ME, Schembri JM, Lew RA, Smith AI, Shaw T, Warner FJ, Zuilli A, Burrell LM, et al. Chronic liver injury in rats and humans upregulates the novel enzyme angiotensin converting enzyme 2. Gut. 2005;54:1790–1796. doi: 10.1136/gut.2004.062398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herath CB, Warner FJ, Lubel JS, Dean RG, Jia Z, Lew RA, Smith AI, Burrell LM, Angus PW. Upregulation of hepatic angiotensin-converting enzyme 2 (ACE2) and angiotensin-(1-7) levels in experimental biliary fibrosis. J Hepatol. 2007;47:387–395. doi: 10.1016/j.jhep.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Santos RA, Simoes e Silva AC, Maric C, Silva DM, Machado RP, de Buhr I, Heringer-Walther S, Pinheiro SV, Lopes MT, Bader M, et al. Angiotensin-(1-7) is an endogenous ligand for the G protein-coupled receptor Mas. Proc Natl Acad Sci USA. 2003;100:8258–8263. doi: 10.1073/pnas.1432869100. [DOI] [PMC free article] [PubMed] [Google Scholar]