Abstract
AIM: To evaluate the long-term risk of gastroduodenal ulcer and cardiovascular events induced by celecoxib in a population-based, randomized, double-blind, placebo-controlled study.
METHODS: From 2004 to 2006, a total of 1024 Chinese patients (aged 35 to 64 years) with severe chronic atrophic gastritis, intestinal metaplasia or dysplasia were randomly assigned to receive 200 mg of celecoxib twice daily or placebo in Linqu County (Shandong Province, China), a high-risk area of gastric cancer. All gastroduodenal ulcer and cardiovascular events occurred were recorded and the patients were followed up for 1.5 years after treatment. At the end of the trial, a systematic interview survey about other adverse events was conducted.
RESULTS: Gastroduodenal ulcer was detected in 19 of 463 (3.72%) patients who received celecoxib and 17 of 473 (3.31%) patients who received placebo, respectively (odds ratio = 1.13, 95% CI = 0.58-2.19). Cardiovascular (CV) events occurred in 4 patients who received celecoxib and in 5 patients who received placebo, respectively. Compared with those who received placebo, patients who received celecoxib had no significant increase in occurrence of CV events (hazard ratio = 0.84, 95% CI = 0.23-3.15). Among the adverse events acquired by interview survey, only the frequency of bloating was significantly higher in patients treated with celecoxib than in those treated with placebo.
CONCLUSION: Treatment of gastric cancer with celecoxib is not associated with increased risk of gastroduodenal ulcer and cardiovascular events.
Keywords: Celecoxib, Gastroduodenal ulcer, Cardiovascular diseases, Adverse effects, Epidemiology, Randomized controlled trial
INTRODUCTION
Celecoxib, approved by the US Food and Drug Administration (FDA) in 1998 for osteoarthritis and rheumatoid arthritis, is a cyclooxygenase-2 (COX-2) inhibitor. Owing to the selective inhibition of COX-2, this drug provides similar anti-inflammatory effects and a reduced risk of gastrointestinal complications in osteoarthritis and rheumatoid arthritis patients compared with nonsteroidal anti-inflammatory drugs (NSAIDs)[1,2], which inhibit both COX-1 and COX-2. In addition, celecoxib, a selective inhibitor of COX-2, can block tumor growth by its antiangiogenic and proapoptotic effects, suggesting that it can be used in the prevention and treatment of cancers[3–5].
However, it was reported that rofecoxib, also a COX-2 inhibitor, is associated with gastrointestinal toxic effects and cardiovascular (CV) events[6,7]; But, it has no gastrointestinal toxicity[8]. The conflicting results have raised the concern about the safety of celecoxib[9,10]. In 2005, the FDA Advisory Committee concluded that the adverse events of celecoxib are less than those of rofecoxib[11]. Therefore, we studied the safety issue of celecoxib. Gastroduodenal ulcer and CV events induced by celecoxib are reported in this paper.
MATERIALS AND METHODS
Study population
In 2004, a total of 1024 subjects, randomly selected from 12 villages of Linqu County (Shandong Province, China), participated in this study. Their age was 35-64 years. All subjects received a brief physical examination and their medical history was recorded. Subjects were ineligible if they had a history of stroke within two years, angina or congestive heart failure or myocardial infarction within one year, neoplastic diseases in the previous 10 years, esophageal or gastric surgery, inflammatory bowel disease, or bleeding diathesis, paracetamol allergy or hypersensitivity to aspirin, or other life-threatening illness. The remainders received 13C-urea breath test (13C-UBT) and gastroscopic examination with biopsies from 5 standard sites of the stomach. Only those who had Helicobacter pylori (H pylori) infection and a histological diagnosis of severe chronic atrophic gastritis (CAG), intestinal metaplasia (IM) or dysplasia (DYS) were enrolled in the intervention trial. A written informed consent was obtained from each participant and the trial was approved by the Institutional Review Board (IRB) of Peking University School of Oncology (PUSO).
Study design and randomization
Subjects were randomly assigned to received antibiotics and/or celecoxib or their placebo in a 2 × 2 factorial design. Finally, the subjects were divided into four groups. Group 1 received anti-H pylori treatment in the first week followed by 200 mg celecoxib twice daily for 24 mo, group 2 received anti-H pylori treatment in the first week followed by a look-alike celecoxib placebo for 24 mo, group 3 received a look-alike anti-H pylori placebo in the first week followed by celecoxib twice daily for 24 mo, group 4 received a look-alike anti-H pylori placebo in the first week followed by a look-alike celecoxib placebo for 24 mo. We only observed and evaluated the risk of cardiovascular and other adverse events in the celecoxib and placebo groups (Figure 1). Both the participants and investigators were blinded to the treatment. Randomization of treatment assignments was generated at Westat Inc. in the US after eligibility was determined.
Figure 1.
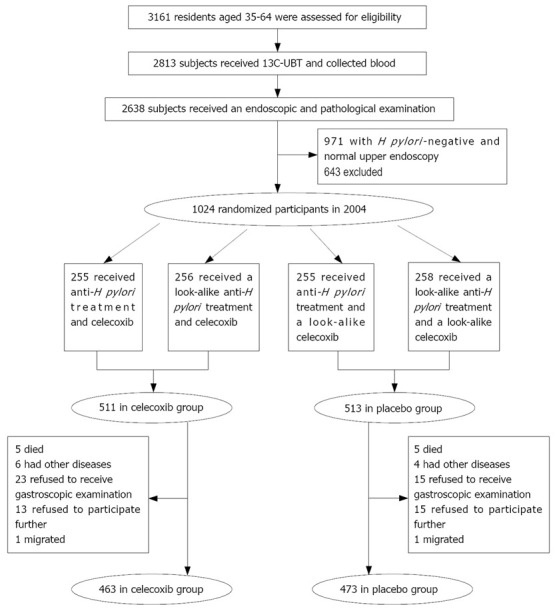
Trial profile.
From March 16 to 30, 2004, the eligible participants were given a triple therapy with 20 mg omeprazole, 1 g amoxicillin and 500 mg clarithromycin or placebo twice daily for 7 d to eradicate their H pylori infection. Then 200 mg of celecoxib or placebo twice daily was given orally from April 8, 2004 to May 6, 2006, except for April 2005 because of the interim gastroscopic examination.
Follow-up
During the period of study, labeled pill bottles of celecoxib or placebo were distributed to participants in each village by PUSO staff and trained field staff each month. The field staff visited each participant twice a month to monitor treatment-related events and to promote pill compliance in the entire duration of the study. The staff counted and recorded the number of pills remaining in each bottle before the new pill bottles were distributed each month. If a subject was not at home during the staff visit, an evening visit was scheduled. A subject was considered compliant if the pill bottle was empty at the end of that month. If a subject was unable to be contacted at the time of counting pills, he or she was considered non-compliant.
Adverse events
Gastroduodenal ulcer was detected in 2005 and 2006 by the same group of PUSO physicians and gastroenterologists. Gastroscopic procedures, including biopsy samples taken from seven standard sites of stomach and histopathologic criteria, have been described elsewhere[12]. The gastroenterologist and pathologist were blinded to the subjects’ intervention.
The CV events were defined as fatal or nonfatal myocardial infarction, ischemic and hemorrhagic stroke as previously described[13]. When visiting the participants, investigators recorded the CV events and other complaints of the participants. While investigators were absent, participants-reported symptoms were recorded by doctors in village clinics. All the CV events were diagnosed in local hospitals.
Other non-adjudicated adverse events were acquired by an interview among all the subjects at the end of the trial in May 2006. All the subjects’ symptoms in the past two years were inquired and recorded by the trained interviewers, checked and categorized by two physicians in a blinded fashion after completion of the survey.
If the symptoms were related to treatment, PUSO physicians and field staff paid a close attention to the subjects for at least 2 mo and these subjects received continuous treatment if the symptoms were aggravated.
Statistical analysis
This study was designed to achieve a significant level of 95% (< 0.05) and a power of 90% to detect a 20% regression of pre-malignant lesions, based on the background of 80% prevalence of gastric atrophy. At least 120 subjects were required in each group in order to detect a significant difference between the different treatment groups.
All data analyses were performed in a blinded fashion. The relative risks (with 95% confidence intervals) of gastroduodenal ulcer were analyzed using logistic regression by adjusting gender, age, smoking and drinking. The rate of CV events was determined and multivariate hazard ratio (HR) was calculated using the Cox proportional-hazard model. All P values were two-sided, and P < 0.05 was considered statistically significant. All analyses were performed with SAS software, version 8.2.
RESULTS
The 1024 participants were divided into celecoxib treatment group (n = 511) and placebo treatment group (n = 513). The baseline characteristics were balanced between the two groups (Table 1). During the two-year period of treatment, 88 participants who were relatively evenly distributed between the two groups withdrew from the study (Figure 1). The compliance rate was 90.61% in the celecoxib treatment group and 92.20% in the placebo treatment group, respectively.
Table 1.
Baseline characteristics of 1024 participants
| Celecoxib, n (%) | Placebo, n (%) | P | |
| Male | 238 (46.58) | 235 (45.81) | 0.81 |
| Age (yr, means ± SD) | 52.94 ± 6.51 | 52.93 ± 6.48 | 0.97 |
| Smoking | 146 (28.57) | 142 (27.68) | 0.75 |
| Drinking | 172 (33.66) | 175 (34.11) | 0.88 |
| Hypertension | 155 (30.33) | 160 (31.19) | 0.77 |
From April 2004 to May 2006, gastroduodenal ulcer was detected in 19 of 463 (3.72%) participants of the celecoxib treatment group and in 17 of 473 (3.31%) participants of the placebo treatment group, respectively. The odds ratio (OR) was 1.13 (95% CI = 0.58-2.19, Table 2).
Table 2.
Incidence and risk of side effects in two groups
| Celecoxib, n (%) | Placebo, n (%) | OR (95% CI) | |
| Gastroduodenal ulcer | 19 (3.72) | 17 (3.31) | 1.13 (0.58-2.22) |
| CV events | 4 (0.86) | 5 (1.06) | 0.84 (0.23-3.15) |
| Main nonadjudicated side effects | |||
| Abdominal pain | 8 (1.73) | 13 (2.75) | 0.62 (0.26-1.52) |
| Bloating | 19 (4.10) | 7 (1.48) | 2.85 (1.19-6.84) |
| Constipation | 9 (1.94) | 15 (3.17) | 0.61 (0.26-1.40) |
| Diarrhea | 24 (5.18) | 24 (5.07) | 1.02 (0.57-1.83) |
| Dizziness | 25 (5.40) | 36 (7.61) | 0.69 (0.41-1.17) |
| Gastric spasmus | 15 (3.24) | 15 (3.17) | 1.02 (0.49-2.12) |
| Headache | 26 (5.62) | 23 (4.86) | 1.16 (0.65-2.07) |
| Heartburn | 29 (6.26) | 23 (4.86) | 1.31 (0.75-2.30) |
| Loss of appetite | 25 (5.40) | 16 (3.38) | 1.63 (0.86-3.10) |
| Muscle pain | 55 (11.88) | 70 (14.80) | 0.78 (0.53-1.13) |
| Nausea | 14 (3.02) | 17 (3.59) | 0.84 (0.41-1.72) |
| Pain in the chest | 16 (3.46) | 17 (3.59) | 0.96 (0.48-1.92) |
| Palpitations | 22 (4.75) | 16 (3.38) | 1.43 (0.74-2.75) |
During the entire period of follow-up, CV events occurred in 4 participants of the celecoxib treatment group and in 5 participants of the placebo treatment group, respectively (Table 2). Compared with the placebo treatment group, the celecoxib treatment group had no significant increase in occurrence of CV events (HR = 0.84, 95% CI = 0.23-3.15).
The main nonadjudicated side effects are listed in Table 2. Except for bloating (OR = 2.85, 95% CI = 1.19-6.84), there were no significant differences in the frequency of other nonadjudicated adverse events between the two groups.
DISCUSSION
In this study, gastroduodenal ulcer and CV events occurred in the subjects who took 200 mg celecoxib twice daily.
Two previous trials addressed the possibility that celecoxib has a lower rate of gastrointestinal complications than NSAIDs[14,15]. It was reported that the annual incidence rate of upper gastrointestinal complications and symptomatic ulcers is significantly lower in the celecoxib treatment group than in the combined diclofenac and ibuprofen treatment group (2.08% vs 3.54%; P = 0.02) after 6 mo of treatment[14]. It has been shown that the incidence rate of gastric ulcer in the celecoxib treatment group and diclofenac treatment group is 18% and 34%, respectively (P < 0.001), and the incidence rate of duodenal ulcer is 5% and 11%, in the celecoxib treatment group and diclofenac treatment group, respectively (P < 0.009)[15].
Although the distinct role of celecoxib in ulcer is still unclear[16], most studies suggested that celecoxib is not associated with gastric or duodenal ulcer[17–19]. Our trial compared the effects of celecoxib and placebo on gastroduodenal ulcer, and the risk of gastroduodenal ulcer was not increased after treatment with 200 mg celecoxib daily compared with placebo.
The association between celecoxib and CV events was still debatable in our study. It was reported that a single dose of 400 mg celecoxib daily and placebo does not induce excess CV risk[20]. However, it was reported that 800 mg celecoxib increases the risk of death due to cardiovascular disease, myocardial infarction, stroke, or heart failure[21].
The mechanism underlying the potential cardiovascular risk of COX-2 inhibitors is not fully understood. Although the imbalance caused by COX-2 inhibitors suppressing the COX-2 dependent prostacyclin production in endothelial cells without affecting the synthesis of platelet-derived thromboxane A2, may promote thrombosis and increase the risk of CV events[22–24], the extent of instability to serum thromboxane and platelet function can be influenced by many factors, such as different doses of COX-2 inhibitors, variability among patients[16].
In this study, different dose-effects of celecoxib on cardiovascular risk were observed. The dose of 800 mg celecoxib daily could increase the CV risk. However, 400 mg celecoxib daily did not increase the CV risk, suggesting that it can be used in the treatment of gastric ulcer.
In the present study, the frequency of bloating was higher in the celecoxib treatment group than in the placebo treatment group. However, the CV events were mild and tolerable, and none of the participants withdrew from this trial.
In conclusion, increases in gastroduodenal ulcer and CV events do not occur in subjects who take 200 mg celecoxib twice daily for two years. Celecoxib can be used in prevention and treatment of gastric cancer.
COMMENTS
Background
Celecoxib, a cyclooxygenase-2 inhibitor, is widely used as an analgesic and anti-inflammatory agent. In addition, it can prevent cancer. However, it is necessary to evaluate the risk of gastroduodenal ulcer and cardiovascular events, particularly in population-based studies.
Research frontiers
No increase in gastroduodenal ulcer and cardiovascular (CV) events were found in the subjects who took 200 mg celecoxib twice daily for two years.
Innovations and breakthroughs
This paper firstly reports an assessment of celecoxib-related gastroduodenal ulcer and cardiovascular events in Chinese population.
Applications
Celecoxib (200 mg twice daily for two years) can prevent and treat gastric cancer in Chinese population.
Peer review
The authors documented the absence of adverse effects of prolonged celecoxib administration at gastroduodenal and cardiovascular level in Chinese patients. The study was well designed and the results are reliable.
Acknowledgments
The authors thank the residents, field staff, and governments of Linqu County for supporting this long-term trial.
Supported by (in part) Grants from National High Technology R&D Program (No. 2002BA711A06), National “211” Project in Peking University 529 and 533, Beijing Municipal Commission of Science and Technology (No. H209-20030130), National Natural Science Foundation of China (No. 30471957), Research Grant Council Earmarked Grant (HKU 7256/01M) of the Hong Kong Special Administration Region, and Research Grant from Peking University School of Oncology, Beijing Cancer Hospital & Institute, China
Peer reviewer: Francesco Feo, Professor, Dipartimento di Scienze Biomediche, Sezione di Patologia Sperimentale e Oncologia, Università di Sassari, Via P, Manzella 4, Sassari 07100, Italy
S- Editor Zhong XY L- Editor Wang XL E- Editor Lin YP
References
- 1.Bombardier C, Laine L, Reicin A, Shapiro D, Burgos-Vargas R, Davis B, Day R, Ferraz MB, Hawkey CJ, Hochberg MC, et al. Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. VIGOR Study Group. N Engl J Med. 2000;343:1520–1528, 2 p following 1528. doi: 10.1056/NEJM200011233432103. [DOI] [PubMed] [Google Scholar]
- 2.Singh G, Fort JG, Goldstein JL, Levy RA, Hanrahan PS, Bello AE, Andrade-Ortega L, Wallemark C, Agrawal NM, Eisen GM, et al. Celecoxib versus naproxen and diclofenac in osteoarthritis patients: SUCCESS-I Study. Am J Med. 2006;119:255–266. doi: 10.1016/j.amjmed.2005.09.054. [DOI] [PubMed] [Google Scholar]
- 3.Gasparini G, Longo R, Sarmiento R, Morabito A. Inhibitors of cyclo-oxygenase 2: a new class of anticancer agents? Lancet Oncol. 2003;4:605–615. doi: 10.1016/s1470-2045(03)01220-8. [DOI] [PubMed] [Google Scholar]
- 4.Steinbach G, Lynch PM, Phillips RK, Wallace MH, Hawk E, Gordon GB, Wakabayashi N, Saunders B, Shen Y, Fujimura T, et al. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N Engl J Med. 2000;342:1946–1952. doi: 10.1056/NEJM200006293422603. [DOI] [PubMed] [Google Scholar]
- 5.Harris RE, Beebe-Donk J, Alshafie GA. Reduction in the risk of human breast cancer by selective cyclooxygenase-2 (COX-2) inhibitors. BMC Cancer. 2006;6:27. doi: 10.1186/1471-2407-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Langman MJ, Jensen DM, Watson DJ, Harper SE, Zhao PL, Quan H, Bolognese JA, Simon TJ. Adverse upper gastrointestinal effects of rofecoxib compared with NSAIDs. JAMA. 1999;282:1929–1933. doi: 10.1001/jama.282.20.1929. [DOI] [PubMed] [Google Scholar]
- 7.Bresalier RS, Sandler RS, Quan H, Bolognese JA, Oxenius B, Horgan K, Lines C, Riddell R, Morton D, Lanas A, et al. Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. N Engl J Med. 2005;352:1092–1102. doi: 10.1056/NEJMoa050493. [DOI] [PubMed] [Google Scholar]
- 8.Bertagnolli MM, Eagle CJ, Zauber AG, Redston M, Solomon SD, Kim K, Tang J, Rosenstein RB, Wittes J, Corle D, et al. Celecoxib for the prevention of sporadic colorectal adenomas. N Engl J Med. 2006;355:873–884. doi: 10.1056/NEJMoa061355. [DOI] [PubMed] [Google Scholar]
- 9.Psaty BM, Potter JD. Risks and benefits of celecoxib to prevent recurrent adenomas. N Engl J Med. 2006;355:950–952. doi: 10.1056/NEJMe068158. [DOI] [PubMed] [Google Scholar]
- 10.Graham DJ. COX-2 inhibitors, other NSAIDs, and cardiovascular risk: the seduction of common sense. JAMA. 2006;296:1653–1656. doi: 10.1001/jama.296.13.jed60058. [DOI] [PubMed] [Google Scholar]
- 11.Okie S. Raising the safety bar--the FDA's coxib meeting. N Engl J Med. 2005;352:1283–1285. doi: 10.1056/NEJMp058055. [DOI] [PubMed] [Google Scholar]
- 12.You WC, Li JY, Blot WJ, Chang YS, Jin ML, Gail MH, Zhang L, Liu WD, Ma JL, Hu YR, et al. Evolution of precancerous lesions in a rural Chinese population at high risk of gastric cancer. Int J Cancer. 1999;83:615–619. doi: 10.1002/(sici)1097-0215(19991126)83:5<615::aid-ijc8>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 13.Collaborative overview of randomised trials of antiplatelet therapy--I: Prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. Antiplatelet Trialists' Collaboration. BMJ. 1994;308:81–106. [PMC free article] [PubMed] [Google Scholar]
- 14.Silverstein FE, Faich G, Goldstein JL, Simon LS, Pincus T, Whelton A, Makuch R, Eisen G, Agrawal NM, Stenson WF, et al. Gastrointestinal toxicity with celecoxib vs nonsteroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis: the CLASS study: A randomized controlled trial. Celecoxib Long-term Arthritis Safety Study. JAMA. 2000;284:1247–1255. doi: 10.1001/jama.284.10.1247. [DOI] [PubMed] [Google Scholar]
- 15.Emery P, Zeidler H, Kvien TK, Guslandi M, Naudin R, Stead H, Verburg KM, Isakson PC, Hubbard RC, Geis GS. Celecoxib versus diclofenac in long-term management of rheumatoid arthritis: randomised double-blind comparison. Lancet. 1999;354:2106–2111. doi: 10.1016/S0140-6736(99)02332-6. [DOI] [PubMed] [Google Scholar]
- 16.Brinkmann G, Burkhart C, Clausen M, Henze E. [The effect of Belzer and Bretschneider cardioplegia solutions on myocardial energy metabolism. A study with 31phosphorus magnetic resonance spectroscopy in an animal model] Z Kardiol. 1992;81:339–344. [PubMed] [Google Scholar]
- 17.Varas-Lorenzo C, Maguire A, Castellsague J, Perez-Gutthann S. Quantitative assessment of the gastrointestinal and cardiovascular risk-benefit of celecoxib compared to individual NSAIDs at the population level. Pharmacoepidemiol Drug Saf. 2007;16:366–376. doi: 10.1002/pds.1299. [DOI] [PubMed] [Google Scholar]
- 18.Kasliwal R, Layton D, Harris S, Wilton L, Shakir SA. A comparison of reported gastrointestinal and thromboembolic events between rofecoxib and celecoxib using observational data. Drug Saf. 2005;28:803–816. doi: 10.2165/00002018-200528090-00005. [DOI] [PubMed] [Google Scholar]
- 19.Deeks JJ, Smith LA, Bradley MD. Efficacy, tolerability, and upper gastrointestinal safety of celecoxib for treatment of osteoarthritis and rheumatoid arthritis: systematic review of randomised controlled trials. BMJ. 2002;325:619. doi: 10.1136/bmj.325.7365.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arber N, Eagle CJ, Spicak J, Racz I, Dite P, Hajer J, Zavoral M, Lechuga MJ, Gerletti P, Tang J, et al. Celecoxib for the prevention of colorectal adenomatous polyps. N Engl J Med. 2006;355:885–895. doi: 10.1056/NEJMoa061652. [DOI] [PubMed] [Google Scholar]
- 21.Solomon SD, McMurray JJ, Pfeffer MA, Wittes J, Fowler R, Finn P, Anderson WF, Zauber A, Hawk E, Bertagnolli M. Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. N Engl J Med. 2005;352:1071–1080. doi: 10.1056/NEJMoa050405. [DOI] [PubMed] [Google Scholar]
- 22.Fitzgerald GA. Coxibs and cardiovascular disease. N Engl J Med. 2004;351:1709–1711. doi: 10.1056/NEJMp048288. [DOI] [PubMed] [Google Scholar]
- 23.Hawkey CJ. COX-2 inhibitors. Lancet. 1999;353:307–314. doi: 10.1016/s0140-6736(98)12154-2. [DOI] [PubMed] [Google Scholar]
- 24.Antman EM, DeMets D, Loscalzo J. Cyclooxygenase inhibition and cardiovascular risk. Circulation. 2005;112:759–770. doi: 10.1161/CIRCULATIONAHA.105.568451. [DOI] [PubMed] [Google Scholar]


