Abstract
Background
Total knee arthroplasty (TKA) relieves pain and improves quality of life for persons with advanced knee osteoarthritis. However, to our knowledge, the cost-effectiveness of TKA and the influences of hospital volume and patient risk on TKA cost-effectiveness have not been investigated in the United States.
Methods
We developed a Markov, state-transition, computer simulation model and populated it with Medicare claims data and cost and outcomes data from national and multinational sources. We projected lifetime costs and quality-adjusted life expectancy (QALE) for different risk populations and varied TKA intervention and hospital volume. Cost-effectiveness of TKA was estimated across all patient risk and hospital volume permutations. Finally, we conducted sensitivity analyses to determine various parameters’ influences on cost-effectiveness.
Results
Overall, TKA increased QALE from 6.822 to 7.957 quality-adjusted life years (QALYs). Lifetime costs rose from $37 100 (no TKA) to $57 900 after TKA, resulting in an incremental cost-effectiveness ratio of $18 300 per QALY. For high-risk patients, TKA increased QALE from 5.713 to 6.594 QALY, yielding a cost-effectiveness ratio of $28 100 per QALY. At all risk levels, TKA was more costly and less effective in low-volume centers than in high-volume centers. Results were insensitive to variations of key input parameters within policy-relevant, clinically plausible ranges. The greatest variations were seen for the quality of life gain after TKA and the cost of TKA.
Conclusions
Total knee arthroplasty appears to be cost-effective in the US Medicare-aged population, as currently practiced across all risk groups. Policy decisions should be made on the basis of available local options for TKA. However, when a high-volume hospital is available, TKAs performed in a high-volume hospital confer even greater value per dollar spent than TKAs performed in low-volume centers.
Knee osteoarthritis (OA) is a common and disabling condition. Approximately 12% of adults older than 60 years have symptomatic knee OA, with estimated direct medical costs ranging from $1000 to $4100 (in 2006 US dollars) per person-year.1–3 Since age and obesity are important OA risk factors, the prevalence of knee OA is rising rapidly in the United States due to both increased life expectancy and the growing obesity epidemic. 4,5 Total knee arthroplasty (TKA) is a frequently performed and effective procedure that relieves pain and improves functional status in patients with end-stage knee osteoarthritis.6
Almost 500 000 TKAs were performed in the United States in 2005 at a cost exceeding $11 billion.7 Projections indicate dramatic growth in the use of TKA over the next 2 decades.8 Because health care expenditures related to TKA are substantial, it is critical to understand the value obtained for the money spent on TKA. Although TKA is widely considered to be a beneficial intervention, its cost-effectiveness in the general US population of persons with end-stage knee OA has yet to be established. To our knowledge, the cost-effectiveness literature related to TKA has focused only on non-US populations,9 referral centers,10 specific prostheses, 10 or particular techniques11,12 such as computer-assisted surgery13 or unicompartmental knee arthroplasty. 14,15 Some of these studies were conducted over a short time frame.9–11
Outcomes after TKA are not uniformly excellent. Older age, black race, Hispanic ethnicity, female sex, poverty, and comorbidities are all associated with poorer TKA outcomes. 16,17 A growing body of evidence demonstrates that the annual volumes of TKA performed by the hospital and by the surgeon are inversely associated with perioperative complications, postoperative functional impairment levels, prosthesis failure rates, and procedure costs.16,18,19 Little is known about whether and how patient risk factors for poor surgical outcomes affect the cost-effectiveness of TKA compared with nonoperative management of end-stage knee OA. Likewise, to our knowledge the influence of hospital volume on the economic impact of TKA has not yet been researched.
In this study, we sought to examine whether TKA is cost-effective in the US Medicare population of persons 65 years or older with end-stage knee OA. We evaluated the cost-effectiveness of TKA for groups of patients with different risks for poor perioperative outcomes. Finally, to investigate the economic and quality of life (QOL) implications of the volume-outcomes relationship, we reported the long-term outcomes and cost-effectiveness of receiving TKA in high- and low-volume centers in the US Medicare population.
METHODS
ANALYTIC OVERVIEW
We developed a Markov, state-transition, computer-based simulation model of treatment choices for patients with end-stage knee OA. A state-transition Markov model characterizes the history of a specific condition in an individual patient as a sequence of transitions from one health state to another. Health states are defined so as to be descriptive of a person’s current health and prognostic of further disease progression20,21 and are characterized by QOL and resource utilization. The value assigned to health-related QOL as applied to economic evaluation of health-related interventions is formulated in terms of utility—the score ascribed to a given state of health to a patient, usually varying from perfect health (QOL value = 1.00) to death (QOL value=0.00). Utility values measure how a person is affected by a disease in his or her activities of everyday life.
We used the model to examine the incremental clinical impact and cost-effectiveness of 4 treatment strategies: (1) No TKA performed; (2) TKA performed in a low-volume hospital (1–25 TKAs performed per year on Medicare patients); (3) TKA performed in a medium-volume hospital (26–200 TKAs performed per year on Medicare patients); and (4) TKA performed in a high-volume hospital (>200 TKAs performed per year on Medicare patients). The choice of strata was guided by prior work indicating worse outcomes in hospitals with 25 or fewer cases per year in the Medicare population.16 In the national sample of Medicare beneficiaries who underwent TKA in 2000, 11% of patients had TKA in a low-volume center and 20% underwent TKA in a high-volume center.16 The analysis reported herein conformed to the reference case recommendations of the US Panel on Cost-Effectiveness in Health and Medicine.22 Outcome measures—including perioperative and longer-term clinical measures, quality-adjusted life expectancy, and direct medical cost (in 2006 US dollars)—were assessed from the societal perspective and reported on a present-value basis using a 3% annual discount rate. We expressed comparative value in dollars per quality-adjusted life-year (QALY) gained and evaluated the stability of findings to variations in the values of input parameters using both deterministic and probabilistic sensitivity analyses.23–25
MODEL STRUCTURE
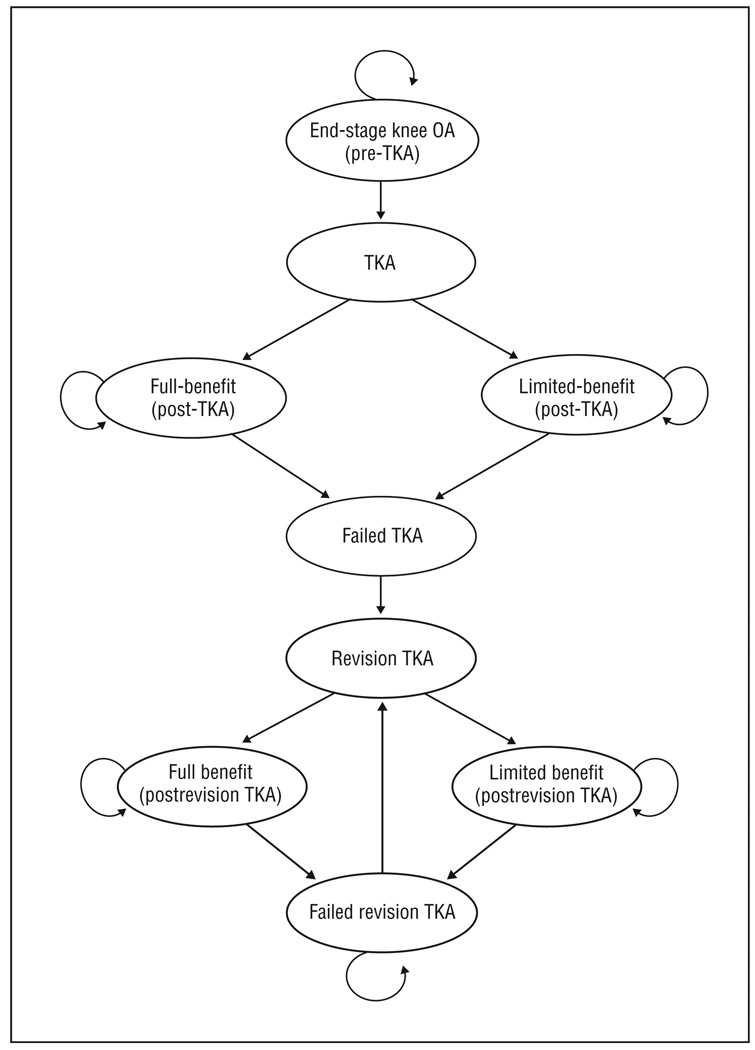
Figure 1 depicts the conceptual organization of 9 of the 10 health states of the model: 2 acute states (TKA and revision TKA) and 7 chronic states. After each evaluation cycle (1 year), subjects may either transition to another state or remain in the same chronic state for another year. Transition probabilities were derived from data from several national and multinational data sources.16 The model was developed and implemented using TreeAge Pro 8.0 software (TreeAge Pro Suite 2008, version 2.0, Williamstown, Massachusetts).
Figure 1.
Total knee arthroplasty (TKA) model structure not including the tenth and absorbing state, death. OA indicates osteoarthritis; straight arrows, transition from one state to another; curved arrows, no transition to a new state. A detailed explanation of each state and the movement between states is available in the “Methods” section.
The full model consists of 10 major health states: (1) end-stage knee OA (pre-TKA); (2) TKA; (3) full-benefit post-TKA (successful TKA); (4) limited-benefit post-TKA (unsuccessful TKA); (5) failed TKA; (6) revision TKA; (7) full-benefit postrevision TKA (successful revision); (8) limited-benefit postrevision TKA (unsuccessful revision); (9) failed revision TKA; and (10) death. Patients may transition from any health state to the absorbing death state (Figure 1).
Persons with end-stage knee OA may be unwilling to undergo TKA and therefore continue to experience the diminished QOL and costs associated with knee OA until death. Patients who undergo TKA transition from the end-stage knee OA state to the TKA state face the risk of postoperative complications and costs related to the surgery. After a year spent in the TKA state, patients transition to 1 of 2 postoperative states based on their outcomes as measured by their postoperative Western Ontario and McMaster Osteoarthritis Index (WOMAC)26 scores: full-benefit post-TKA (successful TKA, WOMAC score, ≥60), which indicates a large improvement in QOL; or limited-benefit post-TKA (unsuccessful TKA,WOMAC score, <60), which indicates little to no improvement in QOL compared with preoperative QOL.
Patients in both postoperative states are at risk of TKA failure. If this occurs, patients transition to the failed TKA state, and their diminished QOL will be similar to or worse than the pre-TKA state. These subjects, however, may choose to undergo revision TKA in a later cycle.
Patients with failed TKA who undergo TKA revision transition to the revision TKA state and thus have a chance of improving QOL. After a year in the revision TKA state, patients transition to 1 of 2 postoperative revision states: full-benefit (successful revision TKA) or limited-benefit (unsuccessful revision TKA) postrevision TKA. The model allows for multiple revision TKAs and for patients to transition back from postrevision states to the revision TKA state. Patients are always at risk of death from surgery-related and other-cause mortality.
POPULATIONS UNDER CONSIDERATION
In addition to analyzing the overall Medicare population with end-stage knee OA, we separately analyzed low-, medium-, and high-risk groups. Risk categories were developed based on the likelihood of perioperative complications after TKA and were derived as a function of age, comorbidities, and poverty status. 16 Details of risk group definition are provided in the eAppendix (http://www.archinternmed.com). The average ages for low-, medium-, and high-risk groups were 68, 75, and 79 years, respectively (Table 1).
Table 1.
Base Case Parameter Estimates for TKA Patients
| Primary TKA, Risk Category, Base Case | ||||||
|---|---|---|---|---|---|---|
| Characteristica | Overall | Low | Medium | High | Source | |
| Age, mean, y | 74 | 68 | 75 | 79 | Katz et al,16 2004 | |
| Annual Probability of Specific Perioperative Outcome Events | ||||||
| Mortality | 0.0063 | 0.0025 | 0.0062 | 0.0114 | Katz et al,16 2004 | |
| Low | 0.0037 | 0.0089 | 0.0145 | |||
| Medium | 0.0029 | 0.0059 | 0.0113 | |||
| High | 0.0007 | 0.0059 | 0.0087 | |||
| Surgical complications | 0.0037 | 0.0024 | 0.0037 | 0.0050 | Katz et al,16 2004 | |
| Low | 0.0037 | 0.0054 | 0.0050 | |||
| Medium | 0.0025 | 0.0039 | 0.0050 | |||
| High | 0.0014 | 0.0021 | 0.0050 | |||
| Medical complications | 0.0275 | 0.0160 | 0.0277 | 0.0412 | Katz et al,16 2004 | |
| Low | 0.0160 | 0.0383 | 0.0412 | |||
| Medium | 0.0160 | 0.0272 | 0.0412 | |||
| High | 0.0160 | 0.0237 | 0.0412 | |||
| Early failure | 0.0105 | 0.0115 | 0.0100 | 0.0115 | Losina et al,27 2006 | |
| Low | 0.0118 | 0.0118 | 0.0146 | |||
| Medium | 0.0105 | 0.0100 | 0.0109 | |||
| High | 0.0148 | 0.0094 | 0.0113 | |||
| Late failure | 0.0134 | 0.0172 | 0.0131 | 0.0103 | Losina et al,27 2006 | |
| Low | 0.0192 | 0.0152 | 0.0113 | |||
| Medium | 0.0172 | 0.0130 | 0.0104 | |||
| High | 0.0164 | 0.0126 | 0.0090 | |||
| Annual Probability of Specific Longer-term Outcome Events | ||||||
| WOMAC <60b | 0.1200 | 0.0938 | 0.1187 | 0.1622 | Katz et al,16 2007 | |
| Low | 0.1148 | 0.1797 | 0.1892 | |||
| Medium | 0.0840 | 0.0867 | 0.1351 | |||
| High | 0.0840 | 0.0867 | 0.1351 | |||
| Utility Score, Mean (SD) | ||||||
| WOMAC, pre-TKAb | 0.690 (0.120) | 0.690 (0.120) | 0.690 (0.120) | 0.690 (0.120) | Lingard et al,28 2004 | |
| WOMAC ≥60, post-TKAb | 0.835 (0.120) | 0.873 (0.147) | 0.832 (0.129) | 0.832 (0.129) | ||
| WOMAC <60, post-TKAb | 0.760 (0.143) | 0.760 (0.143) | 0.760 (0.143) | 0.760 (0.143) | ||
| Annual Costs, 2006 US$ | ||||||
| End-stage osteoarthritis | 3800 | 2000 | 2100 | 10 500 | Lanes et al,3 1997, and NHANES,29 2009 | |
| TKA and rehabilitation | 20 700 | 18 500 | 21 000 | 22 200 | eAppendix (http://www.archinternmed.com) | |
| Low | 19 200 | 21 300 | 21 400 | |||
| Medium | 18 500 | 21 100 | 21 800 | |||
| High | 18 100 | 20 200 | 24 600 | |||
| Perioperative complications | 12 600 | 12 600 | 12 600 | 12 600 | HCUP7 | |
| Revision TKA and rehabilitation | 24 500 | 22 200 | 24 700 | 26 000 | eAppendix | |
| Low | 22 900 | 25 000 | 25 100 | |||
| Medium | 22 200 | 24 800 | 25 500 | |||
| High | 21 800 | 23 900 | 28 300 | |||
Abbreviations: TKA, total knee arthroplasty; WOMAC, Western Ontario and McMaster Osteoarthritis Index.26
Low, medium, and high in this column refer to volume of TKA procedures (1–25, 26–100, and > 200) performed annually in the evaluated hospitals.
A WOMAC score of 60 or higher indicates a high level of functioning; a WOMAC score lower than 60 indicates worse functioning. We normalized WOMAC scores to a 0 to 100 range where 100 represents the best possible score.
ALTERNATIVE STRATEGIES FOR TKA DELIVERY—ROLE OF HOSPITAL VOLUME
We considered TKA delivery in low-, medium-, and high-volume centers (1–25, 26–200, and >200 TKAs per year, respectively).16 Risks of perioperative mortality and complications, likelihood of unsuccessful TKA, and costs of disease and primary and revision TKAs were stratified by hospital volume (Table 1).
CLINICAL DATA
Clinical data used in the model were derived from several population-based studies and included data on perioperative mortality and rates of complications, failure rates, functional status, and QOL. Mortality rates were obtained from the latest available US life tables.30
Perioperative Outcomes in Patients Undergoing TKA
We used the data from a national cohort of Medicare beneficiaries undergoing TKA in 2000 to derive input parameters on perioperative outcomes.16 The Medicare data contained information on comorbidities, age, poverty status, and hospital volume status, allowing us to stratify perioperative complications by hospital volume and patient risk group. For example, the perioperative mortality rates for medium-risk patients ranged from about 0.9% of TKAs performed at low-volume centers to about 0.6% for those performed at high-volume centers (Table 1). Medical complications, including myocardial infarction, pneumonia, and pulmonary embolism exhibited even stronger volume dependence.16 Medical complication rates for procedures performed on medium-risk patients at low-volume centers were about 1.6 times higher than those performed at high-volume centers (3.8% vs 2.4%).
Failure Rates in Patients Undergoing TKA
Early failures occurring in the same year as the index TKA and failures occurring in subsequent years were derived from the longitudinal data of a national cohort of Medicare beneficiaries undergoing TKA.16 Failure rates were stratified by the hospital volume and patient risk groups. For example, for medium-risk patients, failure rates ranged between 0.9% and 1.2% for early failure and between 1.3% and 1.5% for later failures depending on hospital volume (Table 1).
Functional Status After TKA
Data on long-term postoperative functional status in persons undergoing TKA were derived from the random sample of the national cohort of TKA recipients selected for more detailed study.16 The functional status was defined by WOMAC score: individuals achieving a WOMAC score of 60 or higher were assumed to experience a good outcome, and those with a WOMAC score lower than 60 were assumed to experience a suboptimal outcome. The proportion of persons with a WOMAC score of 60 or higher differed by hospital volume and patient risk group (Table 1).
QOL Estimates
The national Medicare study did not collect data on QOL; therefore, we derived these estimates from a separate multinational study of TKA recipients, where investigators collected data on the Short Form 36-Question Health Survey (SF-36) for both pre- and post-TKA. We transformed these SF-36 data to standard gamble (SG) utilities using the method proposed by Lingard et al28 and Brazier et al.31 Mean QOL prior to TKA had an overall value of 0.690. Post-TKA utilities for a successful procedure ranged from 0.832 to 0.837 for high- and low-risk populations, respectively, with an overall value of 0.835 (Table 1).
Costs
In the model we used 2 main cost domains: TKA-related costs and costs of living with end-stage knee OA. The TKA-related costs included hospital costs, physician costs, costs of complications, and costs of rehabilitation services following TKA discharge. Details of this cost derivation are outlined in the eAppendix. Recognizing that charges are an imperfect reflection of true economic resource consumption from the societal perspective,22,32 we converted charges to costs, using an overall ratio of costs to charges of 0.6 (unpublished data). All costs were updated to 2006 US dollars using the medical care component of the consumer price index.33 Further details on derivation of cost parameters and data sources used in the model are presented in the eAppendix.
MODEL ASSUMPTIONS
To ensure a conservative approach, where the data were limited, we used the following assumptions based on expert panel consensus: We assumed that a failed TKA (loosening of prosthesis) would result in a 25% reduction in QOL and a 50% increase in direct medical costs compared with pre-TKA (end-stage knee OA state). We assumed that 100% of patients with an early TKA failure (within the first postoperative year) would elect revision, while 50% of patients with a late TKA failure (after the first postoperative year) would elect revision. We also assumed that a successful TKA would reduce direct medical costs due to symptomatic OA by $512 (eAppendix). Sensitivity of our results to these assumptions was examined in sensitivity analyses with a wide variation in input parameters.
SENSITIVITY ANALYSES
We performed 2 types of sensitivity analysis: We used deterministic 1-way and 2-way sensitivity analysis (systematically varying 1 or 2 parameters at a time across wide intervals) to identify instances where small input changes produced large output swings and to illustrate the relative impact of each individual assumption on the cost-effectiveness of TKA.25 And we used probabilistic sensitivity analysis (varying multiple model parameters simultaneously using a second-order Monte Carlo technique23,24) to understand the aggregate impact of uncertainties in the model’s underlying parameters on overall estimates of cost-effectiveness. This second method involves the estimation of expected costs and effects for program alternatives using a random sampling of model parameter values drawn from joint probability distributions.34 The analysis is repeated over large numbers of samples to produce a distribution of cost-effectiveness ratios, thus permitting the analyst to estimate the probability that an intervention’s cost-effectiveness ratio will fall below any given threshold.35 The parameters to which the distributions were fitted included perioperative outcomes, cost of TKA, cost of living with end-stage OA, QOL improvements, and probability of TKA failure (eTable 5).
The study protocol was approved by the Partners Health-Care human subjects committee.
RESULTS
BASE CASE ANALYSIS: OVERALL MEDICARE POPULATION WITH END-STAGE KNEE OA
In the overall Medicare population with end-stage knee OA (average age, 74 years), TKA was associated with a projected discounted quality-adjusted life expectancy of 7.957 years compared with 6.822 years for patients not undergoing TKA. Lifetime costs varied from $37 100 per person for no TKA to $57 900 per person undergoing TKA. The incremental cost-effectiveness ratio of TKA was $18 300 per QALY (Table 2).
Table 2.
Cost-effectiveness of TKAa
| TKA Statusb | Cost | QALYs, No.c | ICER Compared With Next Least Expensive Strategyd |
ICER Compared With No TKAd |
|---|---|---|---|---|
| Overall Population | ||||
| No TKA | 37 100 | 6.822 | NA | NA |
| TKA | 57 900 | 7.957 | 18 300 | NA |
| Stratified by Riske of Perioperative Comorbidities | ||||
| Low-risk population | ||||
| No TKA | 25 800 | 8.716 | NA | NA |
| TKA | 44 000 | 10.589 | 9700 | NA |
| Medium-risk population | ||||
| No TKA | 19 800 | 6.574 | NA | NA |
| TKA | 39 900 | 7.649 | 18 700 | NA |
| High-risk population | ||||
| No TKA | 86 800 | 5.713 | NA | NA |
| TKA | 111 500 | 6.594 | 28 100 | NA |
| Stratified by Riske of Perioperative Comorbidities and Hospital Volume | ||||
| Low-risk population | ||||
| No TKA | 25 800 | 8.716 | NA | NA |
| High | 43 300 | 10.623 | 9200 | 9200 |
| Medium | 43 900 | 10.597 | Dominatedf | 9600 |
| Low | 45 500 | 10.537 | Dominatedf | 10 800 |
| Medium-risk population | ||||
| No TKA | 19 800 | 6.574 | NA | NA |
| High | 38 900 | 7.672 | 17 400 | 17 400 |
| Medium | 40 100 | 7.670 | Dominatedf | 18 500 |
| Low | 41 700 | 7.585 | Dominatedf | 21 700 |
| High-risk population | ||||
| No TKA | 86 800 | 5.713 | NA | NA |
| Medium | 110 600 | 6.608 | 26 600 | 26 600 |
| Low | 111 900 | 6.556 | Dominatedf | 29 800 |
| High | 113 600 | 6.630 | 135 700 | 29 200 |
Abbreviations: ICER, incremental cost-effectiveness ratio (ratio of additional costs to additional benefits); NA, not applicable; TKA, total knee arthroplasty; QALY, quality-adjusted life year.
All costs are reported as 2006 US dollars. All costs and QALYs are discounted at 3% annually.
Low, medium, and high in this column refer to volume of TKA procedures (1–25, 26–100, and >200, respectively) performed annually in the evaluated hospitals.
The QALY is a health outcome measure that combines quality of life, as determined by some preference-based valuation process, and length of life. One year in perfect health equals 1 QALY. One year in a health state rated as 70% of perfect health equals 0.7 QALY.
For analyses stratified by more than 1 strategy, we present ICERs that compare each strategy with the next less expensive strategy and with the no-TKA strategy.
Risk is defined as risk for complications.
By convention, a strategy that is both more costly and less effective than another strategy (or combination of other strategies) is called dominated.
COST-EFFECTIVENESS OF TKA AS A FUNCTION OF PATIENT RISK
Table 2 summarizes the results of the cost-effectiveness analyses for patients at increasing levels of risk for perioperative complications. Incremental cost-effectiveness estimates ranged from $9700 per QALY in the low-risk group to $28 100 per QALY in the high-risk group.
EFFECTS OF HOSPITAL VOLUME ON COST-EFFECTIVENESS OF TKA
We further stratified the perioperative risk categories by hospital volume (Table 2). For low- and medium-risk patients, cost-effectiveness findings for TKA in a high-volume center were $9200 per QALY and $17 400 per QALY, respectively, compared with no TKA. Incremental analysis revealed that delivery of TKA in either medium- or low-volume centers was a dominated strategy (higher costs and less health benefit) compared with TKA in high-volume centers for low- and medium-risk populations. Compared with no TKA, surgery in medium- and low-volume centers had cost-effectiveness ratios ranging between $9600 per QALY and $21 700 per QALY.
For high-risk patients, the cost-effectiveness ratio for TKA in a medium-volume center was $26 600 per QALY compared with no TKA. For these high-risk patients, TKA in a high-volume center compared with TKA in a medium-volume center increased quality-adjusted life expectancy from 6.608 to 6.630 years at an additional cost of $3000, resulting in an incremental cost-effectiveness ratio of $135 700 per QALY. For high-risk patients, performing TKA in low-volume centers cost more and produced worse outcomes than TKA performed in either high- or medium-volume centers. Compared with no TKA, surgery in low-volume centers had a cost-effectiveness ratio of $29 800 per QALY for high-risk persons.
SENSITIVITY ANALYSES
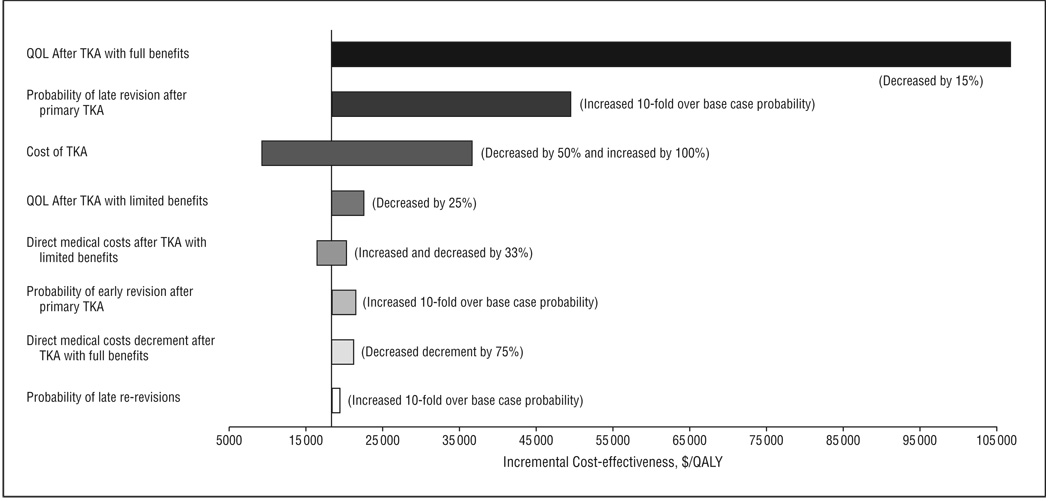
In general, results from the 1-way sensitivity analyses for the overall population were insensitive to variations within policy-relevant ranges ( ≤ $100 000 per QALY) (Figure2). Taking the cost-effectiveness ratio of the overall population as the baseline value ($18 300 per QALY), the ratio varied between $9200 per QALY and $106 700 per QALY. The greatest variation in the cost-effectiveness ratio was seen with changes in 2 parameters: the QOL gain following TKA and the cost of TKA. Reducing the base case QOL utility score for successful post-TKA from 0.835 to 0.710 (85% of the base case value) resulted in a cost-effectiveness ratio ranging from $18 300 per QALY (base case) to $106 700 per QALY. Varying the cost of TKA from $10 300 (half of the base case cost) to $41 400 (twice the base case cost) resulted in cost-effectiveness ratios ranging from $9200 per QALY to $36 500 per QALY. Varying other assumptions did not have substantial effects on the cost-effectiveness ratios (Figure 2).
Figure 2.
Sensitivity analysis of potentially important model parameters. The bars represent ranges of incremental cost-effectiveness ratio values when the value of indicated parameter is changed over the range shown in parentheses. QOL indicates quality of life; TKA, total knee arthroplasty; QALY, quality-adjusted life years. Varying the proportion of persons who lacked substantial functional improvement after revision (reduced by 50% and increased by 100%) did not have a significant impact on incremental cost-effectiveness ratios.
To further bias against TKA and to address the impact of subsequent revisions on the cost-effectiveness of TKA, we performed a 2-way sensitivity analysis varying revision rates and the proportion of patients undergoing revisions who will achieve satisfactory improvements in functional status (WOMAC >60). The cost-effectiveness of TKA was below $50 000 per QALY until the rates of failure were increased by more than 5-fold simultaneously with rates of satisfactory improvement in functional status decreased to as low as 10%. The ratios remained below $100 000 per QALY with up to 10-fold increases in failure rates and 10% improvement in functional status (eTable 1).
We also performed a 2-way sensitivity analysis examining the impact of pre-TKA QOL and post-TKA improvements in QOL on cost-effectiveness of TKA. We considered percentage improvement in QOL for TKA leading to satisfactory improvement in functional status ranging from 0.79 to 0.862 (0%–25% increase of base case). We also examined ranges in preoperative QOL varying from 25% reduction to 20% increases. The cost-effectiveness ratio of TKA remained below $50 000 per QALY for all considered scenarios if post-TKA QOL improvements reach at least a 15% increase from pre-TKA. For example, in the worst case scenario considered where pre-TKA QOL is reduced to 75% of the baseline value (0.518) and TKA yields only a 15% improvement in QOL (0.596), the cost-effectiveness of TKA is still below $50 000 per QALY (eFigure 2).
We conducted additional sensitivity analysis showing that delaying TKA in patients who have reached end-stage knee OA that severely limits their functions for any period is never efficient because it leads to a lesser value per dollar spent (eFigure 3).
Results of the probabilistic sensitivity analysis revealed that if willingness to pay (WTP) to improve QOL were set at $50 000 per QALY, TKA had a 93% chance of being the preferred choice (ie, TKA had the highest net benefit) compared with no TKA. Further analysis revealed that for low-risk patients, there was a 96% chance that TKA would be preferred to no TKA if the societal WTP were set at $50 000 per QALY. For high-risk patients, there was an 83% chance that TKA would be preferred to no TKA if societal WTP were $50 000 per QALY. If WTP were set at $100 000 per QALY, TKA had a greater than 97% chance of being the preferred choice; this value was insensitive to patient risk.
COMMENT
Our analyses showed that, at an incremental cost of $18 300 per QALY gained, TKA is a highly cost-effective procedure for management of end-stage knee OA among Medicare-aged persons compared with non-operative management. This result is robust across a broad range of assumptions regarding both patient risk and hospital volume. For patients who choose to undergo TKA, hospital volume plays an important role: regardless of patient risk level, higher-volume centers consistently deliver better outcomes. But the additional survival benefits associated with high-volume centers provide limited cost-effectiveness benefits for high-risk patients deliberating between medium- and high-volume centers. Across all levels of patient risk and hospital volume, the cost-effectiveness of TKA lies well within the range of accepted cost-effectiveness for other musculoskeletal procedures, such as lumbar diskectomy36 and fusion of the spine for spondylolisthesis (Table 3).37
Table 3.
Cost-effectiveness of Selected Health Care Interventionsa
| Condition | Treatment Method | Cost-effectiveness, $/QALY |
Sourceb |
|---|---|---|---|
| Lumbar disk protrusion | Lumbar diskectomy vs nonoperative management in patients with lower back pain |
20 000 | Malter et al,36 1996 |
| Degenerative spondylolisthesis |
Laminectomy with noninstrumental fusion vs nonoperative management in patients with lower back pain |
81 100 | Kuntz et al,37 2000 |
| Laminectomy with instrumental fusion vs laminectomy without instrumental fusion in patients with lower back pain |
4 460 900 | ||
| ACL tears | Reconstructive ACL surgery vs nonoperative management in patients with ACL tears |
8400 | Gottlob et al,38 1999 |
| Hip OA | THA vs nonoperative management for 60-year-old women with hip OA | Cost-saving | Chang et al,39 1996 |
| THA vs nonoperative management for 85-year-old men with hip OA | 8700 | ||
| Knee OA | TKA vs no TKA for low-risk patients with knee OA | 9700 | Present study |
| TKA vs no TKA for high-risk patients with knee OA | 28 100 |
Abbreviations: ACL, anterior cruciate ligament; OA, osteoarthritis; QALY, quality-adjusted life year; THA, total hip arthroplasty; TKA, total knee arthroplasty.
All costs are reported in 2006 US dollars.
All sources discounted the QALY at 3% except for Gottlob et al,38 who used a 5% discount.
Several studies have evaluated the cost-effectiveness of TKA, but none to our knowledge have used data from nationally representative US cohorts and evaluated cost-effectiveness over the long term. One single-center study estimated the cost-effectiveness of TKA over 1 year to be $14 000 per QALY (inflated to 2006 US dollars) for all TKA recipients.10 A study conducted in Finland also reached favorable conclusions about the cost-effectiveness of TKA, though differences in method and setting make the study difficult to compare with ours.9 It is notable, however, that our general conclusions regarding cost-effectiveness of TKA are consistent with these prior findings.
For patients at highest risk, shifting from medium- to high-volume hospitals had a cost-effectiveness ratio of $135 700 per QALY. This comparatively unfavorable figure arises from the increased rates at which high-risk patients undergo TKA in high-volume centers, potentially distant from their places of residence, and the patients are then referred to costly inpatient rehabilitation centers. By contrast, low- and medium-risk patients who are shifted to hospitals with greater volume experience reduced costs and lower complication rates. Discharge to costly inpatient rehabilitation facilities is less common for these patients.
Our study had several limitations. We found no published literature on costs of TKA that provided estimates stratified by disease severity and patient risk. Thus, we used data from NHANES III29 to assist in building our cost estimates. Notably, the results of our sensitivity analyses suggest that TKA remains cost-effective under wide variations in costs and disease severity. We could not incorporate willingness to undergo TKA as a model input and did not consider the disutility of having surgery in an unfamiliar high-volume center vs a familiar low-volume center. We estimated the rate of TKA failure by using data on revisions of TKA. We acknowledge that revision may be an insensitive proxy for prosthesis failure because patients with a failed TKA may not be offered or may decline revision. Our sensitivity analyses showed that cost-effectiveness estimates were not sensitive to the proportion of persons with failed TKA who underwent revision. We also note that the QOL and annual cost associated with the no-TKA option did not account for continuing worsening of functional limitations related to end-stage knee OA. This assumption biased results against TKA, making the analysis more conservative.
For patients with symptomatic end-stage knee OA, TKA was very cost-effective. This finding applied even to the highest-risk patients. While having TKA in low-volume centers cost more and produced worse outcomes than having TKA in higher-volume centers, having TKA even in low-volume centers was cost-effective compared with no TKA for patients at all levels of risk for perioperative complications. On a societal level, it is more cost-effective for the population with end-stage knee OA to undergo TKA than to not have TKA, regardless of hospital TKA volume.
Clinicians, patients, and policy makers should consider the relative cost-effectiveness of TKA in making decisions about who should undergo TKA, where, and when. Cost-effectiveness analysis is useful in estimating the value of medical practices when randomized controlled trials are logistically or ethically difficult to implement. Lack of physician and patient equipoise regarding randomization would make the conduct of a trial of TKA difficult, and blinding would be nearly impossible. Cost-effectiveness analysis in this setting is particularly valuable in informing policy and practice.
Based on the data derived from TKA recipients of various risk groups, we showed that TKA is effective and cost-effective across all risk groups.40,41 Further analysis on the timing of TKA is necessary. While regionalization efforts to consolidate TKAs in high-volume centers are currently under consideration, our analysis showed that hospital volume above 25 TKA per year is sufficient to assure cost-effective delivery of TKA in the situations where there is a choice among different hospital settings. Even in the absence of such choice, TKA remains a cost-effective treatment compared with no TKA for patients with end-stage knee OA, regardless of setting and patient risk for complications and postoperative mortality.
Supplementary Material
Acknowledgments
Funding/Support: This research was supported in part by National Institutes of Health, National Institute of Arthritis and Musculoskeletal and Skin Diseases grants R01 AR053112, P60 AR 47782, and K24 AR 02123, and an Arthritis Foundation Innovative Research Grant.
Footnotes
Financial Disclosure: None reported.
Additional Information: An eAppendix containing eTables 1, 2, 3, 4, and 5 and eFigures 1, 2, and 3 is available at http://www.archinternmed.com.
REFERENCES
- 1.Dillon CF, Rasch EK, Gu Q, Hirsch R. Prevalence of knee osteoarthritis in the United States: arthritis data from the Third National Health and Nutrition Examination Survey 1991–94. J Rheumatol. 2006;33(11):2271–2279. [PubMed] [Google Scholar]
- 2.Gabriel SE, Crowson CS, Campion ME, O’Fallon WM. Direct medical costs unique to people with arthritis. J Rheumatol. 1997;24(4):719–725. [PubMed] [Google Scholar]
- 3.Lanes SF, Lanza LL, Radensky PW, et al. Resource utilization and cost of care for rheumatoid arthritis and osteoarthritis in a managed care setting: the importance of drug and surgery costs. Arthritis Rheum. 1997;40(8):1475–1481. doi: 10.1002/art.1780400816. [DOI] [PubMed] [Google Scholar]
- 4.Clarfield AM. Teaching public health related to the elderly. Public Health Rev. 2002;30(1–4):271–276. [PubMed] [Google Scholar]
- 5.Elders MJ. The increasing impact of arthritis on public health. J Rheumatol Suppl. 2000;60:6–8. [PubMed] [Google Scholar]
- 6.Harris WH, Sledge CB. Total hip and total knee replacement (1) N Engl J Med. 1990;323(11):725–731. doi: 10.1056/NEJM199009133231106. [DOI] [PubMed] [Google Scholar]
- 7.HCUPnet. [Accessed January 20, 2009];National statistics on all stays:2005 outcomes by patient and hospital characteristics for ICD-9-CM principal procedure code 81.54 total knee replacement. http://hcupnet.ahrq.gov/HCUPnet.jsp.
- 8.Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780–785. doi: 10.2106/JBJS.F.00222. [DOI] [PubMed] [Google Scholar]
- 9.Rissanen P, Aro S, Sintonen H, Asikainen K, Slatis P, Paavolainen P. Costs and cost-effectiveness in hip and knee replacements: a prospective study. Int J Technol Assess Health Care. 1997;13(4):575–588. doi: 10.1017/s0266462300010059. [DOI] [PubMed] [Google Scholar]
- 10.Lavernia CJ, Guzman JF, Gachupin-Garcia A. Cost effectiveness and quality of life in knee arthroplasty. Clin Orthop Relat Res. 1997;(345):134–139. [PubMed] [Google Scholar]
- 11.Ikejiani CE, Leighton R, Petrie DP. Comparison of patellar resurfacing versus non-resurfacing in total knee arthroplasty. Can J Surg. 2000;43(1):35–38. [PMC free article] [PubMed] [Google Scholar]
- 12.Burns AW, Bourne RB, Chesworth BM, MacDonald SJ, Rorabeck CH. Cost effectiveness of revision total knee arthroplasty. Clin Orthop Relat Res. 2006;446:29–33. doi: 10.1097/01.blo.0000214420.14088.76. [DOI] [PubMed] [Google Scholar]
- 13.Dong H, Buxton M. Early assessment of the likely cost-effectiveness of a new technology: a Markov model with probabilistic sensitivity analysis of computer-assisted total knee replacement. Int J Technol Assess Health Care. 2006;22(2):191–202. doi: 10.1017/S0266462306051014. [DOI] [PubMed] [Google Scholar]
- 14.Slover J, Espehaug B, Havelin LI, et al. Cost-effectiveness of unicompartmental and total knee arthroplasty in elderly low-demand patients: a Markov decision analysis. J Bone Joint Surg Am. 2006;88(11):2348–2355. doi: 10.2106/JBJS.E.01033. [DOI] [PubMed] [Google Scholar]
- 15.Soohoo NF, Sharifi H, Kominski G, Lieberman JR. Cost-effectiveness analysis of unicompartmental knee arthroplasty as an alternative to total knee arthroplasty for unicompartmental osteoarthritis. J Bone Joint Surg Am. 2006;88(9):1975–1982. doi: 10.2106/JBJS.E.00597. [DOI] [PubMed] [Google Scholar]
- 16.Katz JN, Barrett J, Mahomed NN, Baron JA, Wright RJ, Losina E. Association between hospital and surgeon procedure volume and the outcomes of total knee replacement. J Bone Joint Surg Am. 2004;86(9):1909–1916. doi: 10.2106/00004623-200409000-00008. [DOI] [PubMed] [Google Scholar]
- 17.Mahomed NN, Barrett J, Katz JN, Baron JA, Wright J, Losina E. Epidemiology of total knee replacement in the United States Medicare population. J Bone Joint Surg Am. 2005;87(6):1222–1228. doi: 10.2106/JBJS.D.02546. [DOI] [PubMed] [Google Scholar]
- 18.Taylor HD, Dennis DA, Crane HS. Relationship between mortality rates and hospital patient volume for Medicare patients undergoing major orthopaedic surgery of the hip, knee, spine, and femur. J Arthroplasty. 1997;12(3):235–242. doi: 10.1016/s0883-5403(97)90018-8. [DOI] [PubMed] [Google Scholar]
- 19.Hervey SL, Purves HR, Guller U, Toth AP, Vail TP, Pietrobon R. Provider volume of total knee arthroplasties and patient outcomes in the HCUP-Nationwide inpatient sample. J Bone Joint Surg Am. 2003;85(9):1775–1783. doi: 10.2106/00004623-200309000-00017. [DOI] [PubMed] [Google Scholar]
- 20.Beck JR, Pauker SG. The Markov process in medical prognosis. Med Decis Making. 1983;3(4):419–458. doi: 10.1177/0272989X8300300403. [DOI] [PubMed] [Google Scholar]
- 21.Holloway C. Decision Making Under Uncertainty: Models and Choices. Engle-wood Cliffs, NJ: Prentice-Hall; 1979. [Google Scholar]
- 22.Weinstein MC, Siegel JE, Gold MR, Kamlet MS, Russell LB. Recommendations of the Panel on Cost-effectiveness in Health and Medicine. JAMA. 1996;276(15):1253–1258. [PubMed] [Google Scholar]
- 23.Briggs AH. Handling uncertainty in cost-effectiveness models. Pharmacoeconomics. 2000;17(5):479–500. doi: 10.2165/00019053-200017050-00006. [DOI] [PubMed] [Google Scholar]
- 24.Halpern EF, Weinstein MC, Hunink MG, Gazelle GS. Representing both first- and second-order uncertainties by Monte Carlo simulation for groups of patients. Med Decis Making. 2000;20(3):314–322. doi: 10.1177/0272989X0002000308. [DOI] [PubMed] [Google Scholar]
- 25.Paltiel AD, Fuhlbrigge AL, Kitch BT, et al. Cost-effectiveness of inhaled corticosteroids in adults with mild-to-moderate asthma: results from the asthma policy model. J Allergy Clin Immunol. 2001;108(1):39–46. doi: 10.1067/mai.2001.116289. [DOI] [PubMed] [Google Scholar]
- 26.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15(12):1833–1840. [PubMed] [Google Scholar]
- 27.Losina E, Emrani PS, Wright EA, Kessler CL, Katz JN. Cost-effectiveness of TKR: economic side of the volume-outcome relationship; Paper presented at: Osteoarthritis Research Society Internationale Congress on Osteoarthritis; December 7–10, 2006; Prague, Czech Republic. [Google Scholar]
- 28.Lingard EA, Katz JN, Wright EA, Sledge CB. Kinemax Outcomes Group. Predicting the outcome of total knee arthroplasty. J Bone Joint Surg Am. 2004;86(10):2179–2186. doi: 10.2106/00004623-200410000-00008. [DOI] [PubMed] [Google Scholar]
- 29.National Center for Health Statistics. NHANES III, Series 11 Data Files. 1A—Interview and Exam Components. [Accessed January 23, 2009];Household Adult File. http://www.cdc.gov/nchs/about/major/nhanes/nh3data.htm#NHANES%20III%20Series%2011,%20No.%201a.
- 30.Arias E. [Accessed February 10, 2009];National Vital Statistics Reports: United States Life Tables. http://www.cdc.gov/nchs/products/pubs/pubd/lftbls/life/1966.htm.
- 31.Brazier J, Roberts J, Deverill M. The estimation of a preference-based measure of health from the SF-36. J Health Econ. 2002;21(2):271–292. doi: 10.1016/s0167-6296(01)00130-8. [DOI] [PubMed] [Google Scholar]
- 32.Finkler SA. The distinction between cost and charges. Ann Intern Med. 1982;96(1):102–109. doi: 10.7326/0003-4819-96-1-102. [DOI] [PubMed] [Google Scholar]
- 33.Bureau of Labor Statistics. [Accessed January 20, 2009];Consumer Price Index—All Urban Consumers. US City Average, Medical Care, Not Seasonally Adjusted. http://data.bls.gov/PDQ/outside.jsp?survey=cu.
- 34.Groot Koerkamp B, Hunink MG, Stijnen T, Hammitt JK, Kuntz KM, Weinstein MC. Limitations of acceptability curves for presenting uncertainty in cost-effectiveness analysis. Med Decis Making. 2007;27(2):101–111. doi: 10.1177/0272989X06297394. [DOI] [PubMed] [Google Scholar]
- 35.van Hout BA, Al MJ, Gordon GS, Rutten FF. Costs, effects and C/E ratios along-side a clinical trial. Health Econ. 1994;3(5):309–319. doi: 10.1002/hec.4730030505. [DOI] [PubMed] [Google Scholar]
- 36.Malter AD, Larson EB, Urban N, Deyo RA. Cost-effectiveness of lumbar discectomy for the treatment of herniated intervertebral disc. Spine. 1996;21(9):1048–1055. doi: 10.1097/00007632-199605010-00011. [DOI] [PubMed] [Google Scholar]
- 37.Kuntz KM, Snider RK, Weinstein JN, Pope MH, Katz JN. Cost-effectiveness of fusion with and without instrumentation for patients with degenerative spondy-lolisthesis and spinal stenosis. Spine. 2000;25(9):1132–1139. doi: 10.1097/00007632-200005010-00015. [DOI] [PubMed] [Google Scholar]
- 38.Gottlob CA, Baker CL, Jr, Pellissier JM, Colvin L. Cost effectiveness of anterior cruciate ligament reconstruction in young adults. Clin Orthop Relat Res. 1999;(367):272–282. [PubMed] [Google Scholar]
- 39.Chang RW, Pellisier JM, Hazen GB. A cost-effectiveness analysis of total hip arthroplasty for osteoarthritis of the hip. JAMA. 1996;275(11):858–865. [PubMed] [Google Scholar]
- 40.Fortin PR, Clarke AE, Joseph L, et al. Outcomes of total hip and knee replacement: preoperative functional status predicts outcomes at six months after surgery. Arthritis Rheum. 1999;42(8):1722–1728. doi: 10.1002/1529-0131(199908)42:8<1722::AID-ANR22>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 41.Fortin PR, Penrod JR, Clarke AE, et al. Timing of total joint replacement affects clinical outcomes among patients with osteoarthritis of the hip or knee. Arthritis Rheum. 2002;46(12):3327–3330. doi: 10.1002/art.10631. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.