Abstract
Recent studies have indicated that plant growth-promoting bacteria (PGPB) can improve revegetation of arid mine tailings as measured by increased biomass production. The goals of the present study were first to evaluate how mode of application of known PGPB affects plant growth, and second to evaluate the effect of this inoculation on rhizosphere microbial community structure. PGPB application strategies investigated include preliminary surface sterilization of seeds (a common practice in phytoremediation trials) followed by a comparison of two application methods; immersion and alginate encapsulation. Results with two native desert plant species, Atriplex lentiformis and Buchloe dactyloides, suggest that seed surface sterilization prior to inoculation is not necessary to achieve beneficial effects of introduced PGPB. Both PGPB application techniques generally enhanced plant growth although results were both plant and PGPB specific. These results demonstrate that alginate encapsulation, which allows for long-term storage and easier application to seeds, is an effective way to inoculate PGPB. In addition, the influence of PGPB application on B. dactyloides rhizosphere community structure was evaluated using PCR-DGGE (denaturing gradient gel electrophoresis) analysis of bacterial DNA extracted from rhizosphere samples collected 75 d following planting. A comparative analysis of DGGE profiles was performed using canonical correspondence analysis (CCA). DGGE-CCA showed that rhizosphere community profiles from PGPB-inoculated treatments are significantly different from both uninoculated tailings rhizosphere profiles and profiles from the compost used to amend the tailings. Further, community profiles from B. dactyloides inoculated with the best performing PGPB (Arthro mix) were significantly different from two other PGPB tested. These results suggest that introduced PGPB have the potential to influence the development of the rhizosphere community structure found in plants grown in mine tailings.
Keywords: mine tailings, PGPB, plant growth promoting bacteria, phytostabilization, DGGE, alginate encapsulation
1. Introduction
Mining tailings sites in arid and semi-arid environments remain barren of vegetation following deposition due to a combination of factors including metal toxicity, acidic pH, poor soil structure, low nutrient levels, and stressed microbial communities (Mendez and Maier, 2008). Phytostabilization, the establishment of a vegetative cap on mine tailings using plants that minimize metal accumulation into shoot tissues, is a remediation strategy being explored to ameliorate wind and water erosion of tailings in a cost-effective manner (Cunningham et al., 1995; Mendez and Maier, 2008). One way to overcome limitations to plant establishment is the addition of compost material which acts immediately to decrease bulk density, increase pH, mitigate metal toxicity, increase water retention, and add necessary nutrients. At heavily contaminated sites (high metal, low pH) high levels of compost material may be necessary to achieve plant establishment which increases remediation costs.
Plant growth-promoting bacteria (PGPB) have found wide use in agricultural applications and are now being explored for environmental applications (Bashan, 1998; Burd et al., 2000; Bashan et al., 2004; Carrillo et al., 2002). PGPB are introduced to seeds prior to planting to enhance one or more aspect of plant growth through a number of potential mechanisms which have been reviewed previously (Glick et al., 1998; Vazquez et al., 2000; Patten and Glick, 2002). Recently, we have demonstrated that PGPB can enhance plant biomass production in mine tailings at lower than optimal compost rates which represents a potential resource and cost savings (Grandlic et al., 2008).
The vast majority of previous studies have prepared seeds for inoculation by surface sterilization prior to introducing desired strains of PGPB (Bashan et al., 2000, 2002; Belimov et al., 2002, 2005; Donte-Correa et al., 2004; Reed and Glick, 2005). This is thought to give the introduced isolate a competitive advantage in colonizing the surface of the seed; however, it also represents an additional step to the inoculation procedure and may be potentially harmful to plant germination (Wilson, 1976). The two most common approaches used to introduce PGPB to seeds prior to planting are immersion which requires soaking seeds in PGPB suspensions immediately before planting, and alginate encapsulation which introduces PGPB to the seed surface in the form of alginate beads (Puente and Bashan, 1993; Bashan et al., 2002). The immersion method requires that PGPB be prepared at the time of use; however, alginate-encapsulated PGPB can be lyophilized and stored at high density for extended periods of time, making this method appealing for field-scale and commercial use (Reed and Glick, 2005). Alginate beads are also thought to offer protection to the PGPB in harsh environments by acting as a time-release coating that slowly disintegrates and releases PGPB to the germinating plant (Bashan et al., 2002).
One area that has received relatively little attention in the use of PGPB is their impact on the rhizosphere microbial community that supports plant establishment. Dynamic and large changes in this community have been shown to occur during phytostabilization even in the absence of PGPB. For example, a clone library analysis of the bacterial diversity in an acidic, high-metal-content, arid tailings prior to plant establishment showed low species richness and a dominance of acidophilic iron- and sulfur-oxidizers (Mendez et al., 2008). Enumeration of culturable members of this community revealed high numbers of iron- and sulfur-oxidizing autotrophs and low numbers of neutrophilic heterotrophs. Following plant establishment, dramatic shifts were observed in the numbers of these three communities with neutrophilic heterotrophs increasing by up to 5 logs, iron oxidizers decreasing by up to 5 logs and sulfur oxidizers decreasing by up to 2 logs (Mendez et al., 2007). Clearly these are microbial community changes that accompany, and are important for, plant establishment. Even though PGPB have been widely used in agriculture, there appears to be little information available concerning how PGPB participate in or influence the development of the rhizosphere community structure beyond contribution of activities such as siderophore or plant hormone production.
The objectives of the current study are (i) to investigate whether surface sterilization of seeds prior to inoculation is necessary for PGPB to enhance plant growth in mine tailings, (ii) to compare two inoculation methods; immersion versus alginate-encapsulation, and (iii) to investigate whether PGPB inoculation of seeds influences the structure of the rhizosphere bacterial community. The mine tailings used in this study were obtained from the Klondyke tailings site, an Arizona State Superfund site located in the southeast corner of Arizona (Tummala and Humble, 1998). This site is similar to many found in the southwestern United States in that it is acidic in nature and has elevated levels of metal contaminants. Previous research has indicated that a compost rate of 15% (w/w) is necessary to achieve plant growth in the Klondyke tailings that is statistically similar to offsite controls (Mendez et al., 2007). Additional research has identified PGPB that can significantly increase the growth of native desert plant species (Atriplex lentiformis and Buchloe dactyloides) in these tailings (Grandlic et al., 2008).
2. Materials and Methods
2.1. Site description
Tailings were collected and homogenized from the Klondyke mine tailings site located in Aravaipa Valley, Graham County, Arizona where a lead/zinc flotation mill was in operation from 1948 to 1952 (Wilson, 1959). Physical and chemical characteristics for the tailings used in this study have been previously described (Grandlic et al., 2008). Briefly, the tailings characteristics include: pH, 4.54 ± 0.02; TOC, 360 ± 68 mg kg−1; TN, 67 ± 12 mg kg−1; DOC, 38 ± 6.9 mg kg−1; DN, 20 ± 1.2 mg kg−1; and EC of 3.0 ± 0.12 dS m−1. The major metals in the tailings prior to compost amendment are (mg kg−1 ± standard deviation) As, 91 ± 9; Cd, 2.4 ± 0.02; Cu, 653 ± 7; Fe, 26,560 ± 270; Mn, 2,811 ± 280; Pb, 4,620 ± 46; Zn, 1,400 ± 14.
2.2. Native plants
Two plants native to the desert southwest were evaluated for growth in tailings in this study, the shrub Atriplex lentiformis (Torr.) S. Wats., commonly known as quailbush and the grass Buchloe dactyloides (Nutt.) Engelm., commonly known as buffalo grass. B. dactyloides seed was obtained from Western Native Seed, Coaldale, CO. A. lentiformis seed was obtained from Carter Seeds, Vista, CA.
2.3. Bacterial isolates
Three PGPB were used in this study; MTR-21A (Clavibacter sp.), MTR-45B (Rhodanobacter sp.) and K4-10/MTR-44, a mixture of two Arthrobacter sp. (K4-10C and MTR-44) referred to as Arthro Mix. All three inoculants have been previously characterized and have demonstrated the ability to enhance dry plant biomass production of A. lentiformis and B. dactyloides in the Klondyke tailings (Grandlic et al., 2008). Briefly, MTR-21A has the ability to grow at pH 4 and 5, is moderately tolerant to Zn, solubilizes phosphate, and produces small amounts of indole-3-acetic acid (IAA) and siderophores; MTR-45B grows at pH 4 and 5, is tolerant to both Pb and Zn, and produces IAA and siderophores; K4-10C has the ability to grow at pH 5, is tolerant to Zn and produces IAA and siderophores; MTR-44 has the ability to grow at pH 5, is tolerant to both Pb and Zn, solubilizes phosphate, has ACC-deaminase activity, and produces IAA and siderophores. All isolates used were maintained on R2A in a laboratory culture collection.
2.4. Experimental design and greenhouse conditions
A factorial study was designed to evaluate the effect of seed surface sterilization (sterile vs. non-sterile) and inoculation method (immersion vs. alginate-encapsulation) on plant biomass production. Experiments included (1) seeds that were surface sterilized (sterile) and inoculated using the immersion method, (2) seeds that were not surface sterilized (non-sterile) and inoculated using the immersion method, (3) sterile seeds that were inoculated using the alginate-encapsulation encapsulation method, and (4) non-sterile seeds that were inoculated using the alginate-encapsulation method. Following inoculation, seeds were transferred to 3 L pots filled with Klondyke tailings amended with 10% compost (w/w); tailings were pre-wetted to field capacity 48 h prior to planting. Fifteen seeds were planted in each pot and five replicates were used for each of the four experiments (sterile immersion, non-sterile immersion, sterile alginate, non-sterile alginate). Four controls were used; sterile and non-sterile seeds that were subjected to the immersion process with no PGPB and sterile and non-sterile seeds that were inoculated with PGPB-free alginate beads.
Following planting, pots were placed in a greenhouse located at the University of Arizona’s Controlled Environment Agriculture Center (Tucson, AZ) for 75 d (Dec. 2007 – Feb. 2008). Each pot was irrigated three times daily via a drip irrigation system distributing a total depth of 1.5 cm pot−1d−1. The greenhouse was maintained at high humidity with a constant temperature of 32°C. Fluorescent supplemental lighting (200 μm m−2 s−1) was used to extend the daily photoperiod to 13 h d−1 as necessary.
2.5. Seed surface preparation
Seeds were surface sterilized using the following procedure. Seeds were soaked in sterile distilled water for 10 min followed by a 1 min immersion in 95% ethanol. The seeds were then immediately rinsed with sterile distilled water, soaked for 10 min in a 2% sodium hypochlorite solution, rinsed three times with sterile distilled water, soaked for three min in a 0.1% sodium thiosulfate solution, and rinsed a final time. The effectiveness of the surface sterilization procedure was checked by placing sterilized seeds onto R2A plates and monitoring microbial growth. Non-sterilized seeds were prepared by soaking in sterile distilled water for 25 min. Following surface preparation, seeds were immediately inoculated and planted.
2.6. PGPB inoculation using the immersion method
PGPB cultures were prepared 48 h prior to inoculation by transferring single colonies from an R2A plate to 100 mL of R2B in a 250 mL Erlenmeyer flask and incubating on a rotary shaker (200 rpm) at 23°C. Immediately prior to inoculation, the cultures were centrifuged at 12,100 × g for 10 min, the culture supernatant was removed and cells were re-suspended in sterile PBS; (g L−1) 8.0 NaCl, 0.2 KCl, 1.44 Na2HPO4, 0.24 KH2PO4, adjusted to pH 7.4. Isolate suspensions were adjusted to an absorbance = 1 at 600 nm using PBS which was equivalent to an approximate concentration of 109 CFU mL−1. Counts were confirmed by enumerating all isolate suspensions used.
Approximately 100 surface sterilized seeds or non-sterilized seeds were aseptically transferred to each individual isolate suspension and allowed to incubate for 10 min with a 5 sec vortexing period every min. The suspension/seed mixtures were then subjected to a vacuum of 700 mm Hg for 5 min after which the vacuum was quickly removed. This step helps to force the bacteria into micropore spaces on the seed surface (Puente and Bashan, 1993).
2.7. Inoculation of alginate-encapsulated PGPB
To prepare alginate-encapsulated cells, individual isolates were grown at 23°C on a rotary shaker (200 rpm) in three 0.5 L volumes of 3X R2A; (g L−1) 1.5 yeast extract, 1.5 glucose, 1.5 casein hydrolysate, 1.5 soluble starch, 1.5 Proteose peptone No. 3, 0.9 K2HPO4, 0.9 sodium pyruvate, and 0.1 MgSO4·7H2O, adjusted to pH 7.4. After 48 – 72 hr (cell density of ~109 CFUs mL−1) cells were harvested and concentrated by centrifuging at 12,000 × g for 15 min. The supernatant was removed, discarded and the resulting cell pellets were re-suspended in 10 mL of 50 mM PIPES buffer (pH 7.4) to a cell density of approximately 1011 CFU mL−1. The cell suspension was then thoroughly mixed with an equal volume of a sterile 3.5% sodium alginate (Pfaltz and Bauer Inc, Waterbury, CT) solution and forced through a sterile 30.5 gauge syringe needle into chilled (4°C) 0.15 M CaCl2. Arthrobacter spp. mixture beads (MTR-44 and K4-10C) were produced by combining equal volumes of the two cultures after centrifugation prior to addition of the sodium alginate mixture. Alginate beads (approximately 300 – 500 μM) formed immediately upon contacting the CaCl2 solution and were allowed to set at a minimal stirring speed (100 rpm) for at least 2 hr. Polymerized alginate beads were collected by filtration through a sterile Buchner funnel and rinsed 3 times with sterile 50 mM PIPES buffer. Alginate-encapsulated cells were aseptically transferred to 50 mL plastic centrifuge bottles and lyophilized until completely dried for 48 – 72 hr. Cell densities (CFU gram−1 dry beads) were quantified by dissolving 0.05 g of beads in 5 mL of 0.25M sodium carbonate for 30 min, diluting in PBS and plating onto R2A agar.
Lyophilized beads for each individual inoculation were introduced to seeds at a concentration of 2 × 107 CFU seed−1. An appropriate volume of beads was added to inoculate 75 seeds per experiment. After inoculation the seed/bead mixture was aseptically homogenized allowing the dried beads to adhere to the seeds, and seeds were aseptically transferred to Klondyke tailings amended with 10% compost material (w/w) at a rate of 15 seeds per pot.
2.8. Plant biomass
Dry plant biomass was determined 75 d after planting. At this time each plant was carefully harvested (roots and shoots were separated) and rinsed gently under running water to remove all tailings and compost material. Plant roots and shoots were then placed in individual foil packets, dried for 96 h in a 65°C oven, and weighed.
2.9. Microbial community structure analysis
Bacterial community profiles associated with plant rhizospheres and mine tailings were evaluated by PCR-DGGE (denaturing gradient gel electrophoresis) analysis. DGGE analysis was used because it allows for the relatively rapid generation of “profiles” that represent the most abundant populations in a community (Muyzer et al., 1993). Rhizosphere samples were taken at the end of the experiment by randomly selecting three of the five replicate pots for each experiment and aseptically scraping approximately 2 g of substrate from plant root surfaces. Samples were transferred into sterile 1.8 mL Eppendorf tubes and stored at −20°C. Genomic DNA extractions from samples were done using the FastDNA SPIN Kit for Soil® (Bio 101, Inc., Vista, CA) following the instructions as recommended by the manufacturer. Genomic DNA extractions were quantified using PicoGreen (Invitrogen, Carlsbad, CA) and a TBS-380 fluorometer (Turner BioSystems, Sunnyvale CA).
The 16S rRNA gene fragments in DNA extracts were amplified using a modified protocol originally described by Colores et al. (2000) using the universal bacterial primers 1070f and 1406r-GC (Ferris et al, 1996). Each 50 μL reaction contained 1X buffer (10mM Tris-HCl, 50 mM KCl, 2.0 mM MgCl2 – pH 8.3), 0.5 μM each primer, 400 mg L−1 bovine serum albumin, 0.2 mM each dNTP, 5% DMSO, 1 U of Hot Start Taq DNA polymerase, and 2 ng of DNA template (genomic DNA extraction). The amplification protocol used was 95°C for 15 min followed by 30 cycles of 94°C, 55°C, 72°C for 45 sec each and a final extension at 72°C for 10 min followed by 4°C. After amplification, PCR products were visualized on a 1.5% agarose gel using an Alpha Imager (Alpha Innotech, San Leandro, CA). PCR products were quantified by comparison to a known standard, Mass Ruler™ (Fermentas Inc., Glen Burnie, MD), using the “density” feature on Quantity One® software (Bio-Rad Laboratories, Inc., Hercules, CA).
PCR products were separated on DGGE gels (500 ng loaded per lane) containing 7% acrylamide and a 40 – 80% urea-formamide denaturing gradient. Gels were run at 60°C and 60 V for 17 h, then stained in 1 X SYBR Green (Molecular Probes, Eugene, OR) for 20 min and imaged. Microbial community banding profiles on DGGE gels were analyzed using the Quantity One® software package (Bio-Rad Laboratories, Inc., Hercules, CA). Banding patterns were analyzed using Canonical Correspondence Analysis (CCA), a form of Correspondence Analysis widely used in community ecology (ter Braak, 1986; Palmer, 1993; and Legendre and Legendre, 1998). CCA finds axes of variation in banding patterns that are maximally related to explanatory variables or treatments (e.g., inoculum vs. no inoculum). CCA eigenvalues represent the strength of the relationship between DGGE profile bands and the treatment and are tested against the null model of no relationship using a permutation test (ter Braak and Wiertz, 1994). CCA also allows simultaneous visualization of different treatments, samples, and DGGE bands in few dimensions using a triplot. Treatments in CCA triplots are typically represented by centroids; i.e., points that reflect the average location of the treatment samples. According to the centroid rule, the proximity of a population (e.g., a DGGE band) to a centroid is directly related to the occurrence of that population in that centroid (treatment). PAST, version 1.90, was used for CCA (Hammer et al., 2001).
2.10. Statistical analysis
Statistical analysis of plant biomass data was conducted using SAS Version 9.1 (SAS Institute Inc. Cary, NC). Significant effects within each experiment for plant root, shoot and total biomass and root-to-shoot data were detected by employing a one-way ANOVA (p < 0.05). For each experiment, significant differences between means were determined by the Duncan’s Multiple Range Test (p < 0.05).
3. Results
3.1. Effect of surface sterilization
In the absence of PGPB inoculation (control treatments), surface sterilization of seeds did not affect the average total dry pot biomass of A. lentiformis for either the immersion (5.6 vs. 5.0 g dry biomass pot−1) or the alginate (2.9 vs. 3.0 g dry biomass pot−1) inoculation method (Table 1). In contrast, for B. dactyloides, surface sterilization of seeds resulted in a significant (2.6-fold) decrease in biomass production for both the immersion and alginate inoculation methods (Table 1). Surface sterilization of B. dactyloides plants also appeared to affect seed survival which decreased from 77 to 52% in immersion-treated controls and from 67 to 56% in alginate-treated controls when seeds were sterilized, respectively (Table 1).
Table 1.
| Experiment: | Sterile immersion | Non-Sterile Immersion | Sterile Alginate | Non-Sterile Alginate | ||||
|---|---|---|---|---|---|---|---|---|
| Survival1 | Biomass2 | Survival | Biomass | Survival | Biomass | Survival | Biomass | |
| Treatment | Atriplex lentiformis | |||||||
|
| ||||||||
| Control | 16 | 5.6 ± 2.8 a | 16 | 5.0 ± 4.0 a | 5 | 2.9 ± 3.6 a | 5 | 3.0 ± 2.9 a |
| Arthro Mix | 20 | 9.3 ± 4.6 a | 18 | 8.7 ± 2.7 a | 14 | 6.9 ± 4.3 a | 8 | 5.9 ± 3.6 a |
| MTR-21A | 13 | 7.1 ± 2.2 a | 16 | 5.3 ± 3.5 a | 12 | 9.4 ± 2.4 a | 12 | 6.3 ± 3.0 a |
| MTR-45B | 18 | 7.5 ± 5.1 a | 14 | 8.3 ± 3.6 a | 14 | 8.1 ± 3.6 a | 8 | 6.7 ± 1.3 a |
|
| ||||||||
| Buchloe dactyloides | ||||||||
|
| ||||||||
| Control | 39 | 1.5 ± 2.4 a | 58 | 3.9 ± 4.0 a | 42 | 2.1 ± 1.1 a | 50 | 5.6 ± 2.9 ab |
| Arthro Mix | 58 | 8.0 ± 1.3 b | 54 | 8.1 ± 1.9 a | 59 | 8.4 ± 3.3 b | 56 | 8.3 ± 1.8 b |
| MTR-21A | 42 | 5.3 ± 4.4 b | 33 | 4.6 ± 4.6 a | 33 | 1.4 ± 2.0 a | 48 | 7.3 ± 3.0 b |
| MTR-45B | 50 | 5.8 ± 2.0 b | 54 | 8.2 ± 3.7 a | 37 | 4.0 ± 2.1 a | 37 | 3.8 ± 1.8 a |
Survival is the number of seeds for each treatment that germinated and survived during the study. Each treatment had five replicate pots which each received 15 seeds. Therefore for each treatment the total possible number of surviving plants would be 75.
Biomass values are presented as the average and one standard deviation of 5 replicate pots for each treatment in g dry biomass pot−1. Within each treatment (e.g., sterile immersion) the four sample means were compared and those with different letters are significantly different in total dry pot biomass at p < 0.05.
Similar to the control treatments, surface sterilization of seeds in combination with PGPB inoculation also had no impact on A. lentiformis biomass production (Table 1). However, for B. dactyloides (which exhibited decreased biomass production when seeds were surface sterilized in control treatments), the seed sterilization effect was mitigated in most cases by the addition of PGPB. Specifically, the detrimental impact of seed sterilization was mitigated by all PGPB strains for the immersion method, and all except MTR-21A mitigated the effect for the alginate inoculation method (Table 1).
3.2. Effect of PGPB inoculation
In comparison to uninoculated controls, results show that the three PGPB either enhanced or did not affect plant biomass production regardless of whether immersion or alginate inoculation was used (Table 1). The predominantly positive response to alginate inoculation is important because this method allows the inoculum to be stored stably for long periods of time. In fact, we have stored lyophilized alginate encapsulated PGPB for periods exceeding one year and recovered normal levels of culturable counts (data not shown).
In looking more closely at individual results, both plant-dependent and PGPB-dependent differences were observed. Examining the results on a plant specific basis, the maximum biomass produced by A. lentiformis across all experiments was approximately 9 g pot−1. This level of biomass production was achieved only in the presence of PGPB and was 1.6 to 3-fold higher than uninoculated controls although these differences were not significant (Table 1). Interestingly, control A. lentiformis treatments that contained PGPB-free alginate beads had lower (but not significantly different) average dry pot biomass (2.9 and 3.0 g dry biomass pot−1) than control treatments treated with the immersion method (5.6 and 5.0 g dry biomass pot−1) suggesting that alginate itself may be detrimental to A. lentiformis biomass production (Table 1). However, when alginate-encapsulated PGPB were introduced, total pot biomass increased to levels consistent with good PGPB performance for all three PGPB tested (5.9 – 9.4 g dry biomass pot−1) (Table 1). These results suggest that potential deleterious effects of alginate alone are overcome when it is used to encapsulate PGPB.
For B. dactyloides, the maximum biomass produced was approximately 8 g pot−1. This level of production was achieved only with selected PGPB and was 1.5 to 5-fold higher (significant) than for uninoculated controls (Table 1). The Arthro Mix was the only PGPB to result in consistently high biomass production for all experiments, although MTR-45B performed well in the non-sterile immersion experiment and MTR-21A performed well in the non-sterile alginate experiment (Table 1).
Examining results on a PGPB-dependent basis shows that the Arthro Mix had the best performance in the greatest number of experiments for both plants. Although MTR-21A and MTR-45B also generally enhanced biomass production, each showed less consistent performance across experiments than the Arthro Mix. For example, MTR-21A performed less well than the uninoculated control for the B. dactyloides sterile alginate experiment (1.4 vs. 2.1 g dry biomass pot−1) and MTR-45B performed less well than the uninoculated control in the B. dactyloides non-sterile alginate experiment (3.8 vs. 5.6 g dry biomass pot−1) (Table 1).
3.3. Community structure analysis
Selected rhizosphere samples (three pots randomly selected from 5 replicate pots for each experiment) were collected following termination of the experiment at 75 d. These samples were analyzed using PCR-DGGE to determine whether PGPB inoculation has an effect on the rhizosphere bacterial community structure. We chose to focus this work on two experiments, sterile immersion and non-sterile immersion, in one plant, B. dactyloides. These experiments were chosen because they exhibited the greatest biomass differences between the uninoculated controls and inoculated treatments (Table 1). In addition, the bacterial community structure in the original compost used to amend the tailings was compared to each of the two experiments.
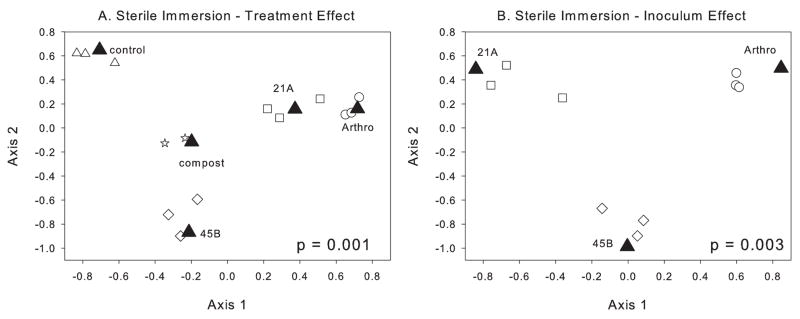
DGGE profiles were analyzed using CCA to determine whether there was a treatment (inoculum type vs. uninoculated vs. compost) effect and whether there was an inoculum (PGPB type) effect on the bacterial community structure observed at 75 d. CCA analysis shows that the DGGE profiles were significantly affected (p = 0.001) by treatment for the sterile immersion experiment (Fig. 1A). In this case five treatments were tested including: uninoculated control, compost, Artho Mix, MTR 21A and MTR 45B. The first (46%) and second (38%) axes explain 83% of the variance. Thus, rhizosphere samples from plants that received PGPB inoculation clustered separately from those did not receive PGPB and from the original compost community. This suggests that PGPB have an extended (at least 75 d) influence on the rhizosphere bacterial community structure that develops following plant establishment in mine tailings. Examination of the individual PGPB inoculants (Fig. 1B) shows a significant difference between the three PGPB tested (p = 0.003). In this case, the 65 and 35% of the variance is explained by the first and second axes respectively.
Fig. 1.
Canonical correspondence analysis of triplicate DGGE bacterial community profiles from the B. dactyloides sterile immersion experiment. This experiment (DGGE gel) included 14 samples representing five treatments: treatment 1, triplicate rhizosphere samples from plants inoculated with Arthro Mix (○ Arthro); treatment 2, triplicate rhizosphere samples from plants inoculated with MTR-21A (□ 21A); treatment 3, triplicate rhizosphere samples from plants inoculated with MTR-45B (◇45B); treatment 4, triplicate rhizosphere samples from uninoculated control plants (△ Control); and treatment 5, duplicate samples from the compost prior to mixing with the tailings (⋆Compost). The filled triangles (▲) represent the CCA centroid for each treatment. (A) Sterile immersion experiment examining treatment effects among all fourteen samples. (B) Sterile immersion experiment examining inoculum effects among the nine inoculated samples (Arthro-inoculated, 21A-inoculated, and 45B-inoculated) only. Following CCA, for each analysis, a permutation test was performed (1000 permutations) to test whether there was a significant treatment effect (panel A) or inoculum effect (panel B).
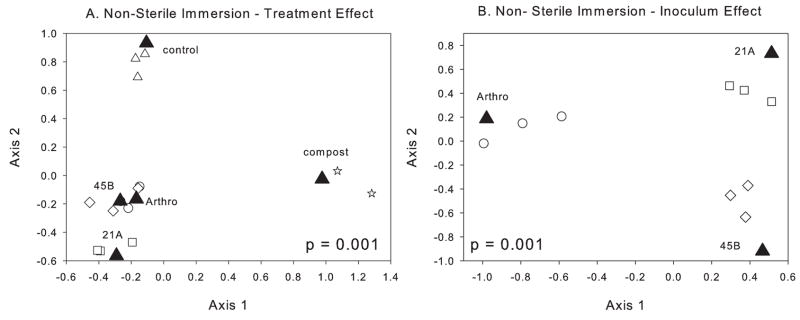
DGGE-CCA analysis of the B. dactyloides non-sterile immersion treatment revealed similar results showing both treatment (p = 0.001) and inoculum (p = 0.001) effects (Fig 2A and 2B). For the treatment effect, the first (46%) and second (35%) axes explain 81% of the variance while for the inoculum effect the first axis explains 82% of the variance and the second axis 18% of the variance. The combined results from the sterile and non-sterile experiments suggest that in mine tailings materials a bacterial inoculum has a distinct effect on the microbial community structure that develops. This leads to the intriguing idea that an inoculum may influence the development of a unique rhizosphere community that aids in the more effective establishment and growth of B. dactyloides.
Fig. 2.
Canonical correspondence analysis of triplicate DGGE bacterial community profiles from the B. dactyloides non-sterile immersion experiment. This experiment (DGGE gel) included 14 samples representing five treatments: treatment 1, triplicate rhizosphere samples from plants inoculated with Arthro Mix (○ Arthro); treatment 2, triplicate rhizosphere samples from plants inoculated with MTR-21A (□ 21A); treatment 3, triplicate rhizosphere samples from plants inoculated with MTR-45B (◇45B); treatment 4, triplicate rhizosphere samples from uninoculated control plants (△ Control); and treatment 5, duplicate samples from the compost prior to mixing with the tailings (⋆Compost). The filled triangles (▲) represent the CCA centroid for each treatment. (A) Non-sterile immersion experiment examining treatment effects among all fourteen samples. (B) Non-sterile immersion experiment examining inoculum effects among the nine inoculated samples (Arthro-inoculated, 21A-inoculated, and 45B-inoculated) only. Following CCA, for each analysis, a permutation test was performed (1000 permutations) to test whether there was a significant treatment effect (panel A) or inoculum effect (panel B).
Interestingly, the major difference between the sterile and non-sterile immersion experiments revealed by CCA analysis was in the relationship between the uninoculated control and the compost communities. In the sterile immersion experiment the control community structure was more similar to the initial compost community than in the non-sterile immersion experiment. Although this needs further exploration, these results suggest that surface sterilization of seeds alone has an effect on community development. This supported by the observation that plant biomass production was significantly lower in the uninoculated sterile versus non-sterile immersion treatment.
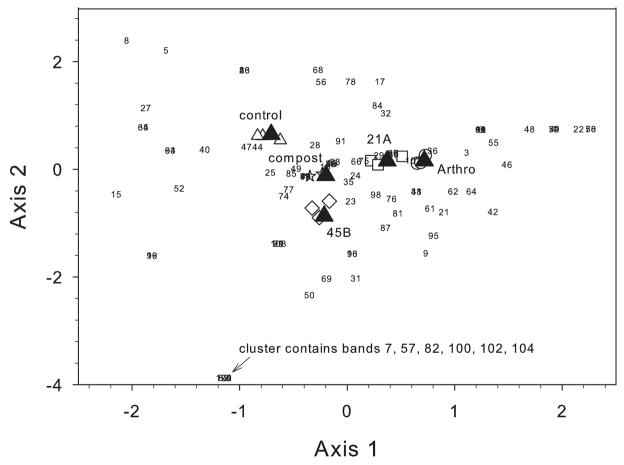
CCA also allows visualization of the distribution of specific bacterial populations (e.g., bands on the DGGE gel) among the treatments tested (Fig. 3). If one makes the assumption that each DGGE profile band generally represents a unique population within the community, then the CCA triplot provides information about the number of populations associated with specific treatments and the number of populations shared more equally between treatments. Figure 3 shows an example of this using the sterile immersion experiment from Fig. 1A. In this figure, each DGGE band beginning at the top of the gel was assigned a number (in numerical order) for each possible vertical location among the 14 lanes analyzed. Examination of the spatial relationship of each population to the five treatment centroids shows several populations that are associated primarily with the uninoculated control treatment, e.g., 5 and 8. Likewise, there are some populations that are found primarily in one of the inoculated treatments, for example the arrow on the figure points to a cluster of bands that are predominantly found in the MTR 45B inoculated treatment. In this manner CCA can serve as a tool to define the distribution of populations that have unique or shared distribution among treatments.
Fig. 3.
CCA of the DGGE bacterial community profile from the B. dactyloides sterile immersion experiment (see also Fig. 1, panel A). This triplot shows the relationship among the bands (represented by numbers in the plot) in the DGGE profiles and the five treatments tested (△ uninoculated control, ⋆ compost, ○ Arthro-inoculated, □ 21A-inoculated, and ◇45B-inoculated). In numerical order, beginning at the top of the gel, a number was assigned to each possible vertical location for a band among the 14 lanes analyzed (triplicate uninoculated controls, triplicate inoculated treatments for Arthro, 21A and 45B, and duplicate compost controls). The location of these numbers in relation to the treatment centroid (▲) can be used to distinguish the relative frequency of occurrence of a band in the different treatments.
4. Discussion
One question examined in this study is whether seed surface sterilization is a necessary step for successful application of PGPB in mine tailings revegetation. Our results suggest that surface sterilization of seeds prior to use in phytostabilization trials is not necessary whether or not a PGPB is used. In fact, surface sterilization may be potentially harmful to some plant species and this finding stresses the importance of investigating the effect of seed preparation on plant growth prior to conducting large-scale studies. This is consistent with previous work showing that seed surface sterilization using sodium hypochlorite was detrimental to wheat seed germination, however, had no effect on the germination of sorghum and soybean (Wilson, 1976). More recently, surface sterilization of seeds prior to introducing a PGPB was shown to have a negative effect on seed surface colonization (Miché and Balandreau, 2001).
A second focus of this study was whether alginate encapsulation of PGPB is compatible with revegetation success in mine tailings. Results show that alginate encapsulation of PGPB is generally compatible with plant growth in mine tailings. However, this study shows that some PGPB, in this case the Arthro Mix, are more robust than others across different plants (A. lentiformis and B. dactyloides), seed preparation (sterile vs. non-sterile), and inoculation methods (immersion vs. alginate encapsulation). Further, this work cautions that screening studies should be performed to demonstrate the efficacy of any given PGPB under the conditions that they will be applied.
Finally this study examined the influence of PGPB inoculation on the rhizosphere bacterial community structure after 75 d. Results reveal that introduced PGPB do influence the microbial structure and present the intriguing possibility that specific changes in community structure can be correlated both with the inoculum used and with enhanced plant biomass production. This is in contrast to a recent reciprocal planting study that was performed using healthy soils. This study compared samples from plant rhizospheres of the same plant (wild sand sedge, Carex arenaria) for which a rhizome was taken from 10 very different soil types and placed into the same soil. Following growth, DGGE profiles from rhizosphere samples were compared showing that the community structure was similar for each plant, regardless of the original soil that the rhizome was harvested from (de Ridder-Duine et al., 2005). Thus, in the case of a healthy soil, the rhizosphere community structure seems to be dictated by the bulk community found in the soil. This conclusion is supported by other investigations including one examining fatty acid profiles and substrate utilization in corn and soy bean rhizosphere samples from different agricultural soils (Buyer et al., 2002), and one examining Burkholderia cepacia populations on corn roots in three different agricultural soils (Dalmastri et al., 1999).
In contrast to the studies discussed above, our investigation suggests that PGPB do influence community structure in rhizosphere communities from mine tailings, at least within the first 75 d of B. dactyloides growth. This is likely because the mine tailings environment has severely impacted microbial communities (low numbers of neutrophilic heterotrophs and elevated numbers of iron- and sulfur-oxidizers) in comparison to healthy soils (Mendez et al., 2007). While further investigation into this phenomenon is necessary, the CCA analysis used suggests there is potential for identifying populations or consortia that will contribute to the development of desired, healthy rhizosphere communities during phytostabilization applications.
4. Conclusions
The use of PGPB to enhance plant establishment in mine tailings and metal contaminated soils has been previously demonstrated. Simplifying this process by removing unnecessary and potentially harmful steps such as surface sterilization and developing user-friendly methods such as alginate inoculation will make this technology more applicable to field-scale use. Our results show that surface sterilization is not necessary to achieve desirable results when applying PGPB to enhance plant establishment in mine tailings. The present study also demonstrates that the introduction of alginate-encapsulated PGPB is a viable method for enhancing plant growth in composted Klondyke tailings. Finally, investigation of the bacterial community associated with B. dactyloides rhizospheres suggests that introduced PGPB may have the potential to cause changes in microbial community structure that can be correlated with enhanced plant growth in metal-contaminated soils.
Acknowledgments
This research was supported by Grant 2 P42 ES04940-11 from the National Institute of Environmental Health Sciences Superfund Basic Research Program, NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bashan Y. Inoculants of plant growth-promoting bacteria for use in agriculture. Biotechnology Advances. 1998;16:729–770. [Google Scholar]
- Bashan Y, Moreno M, Troyo E. Growth promotion of the oilseed halophyte Salicornia bigelovii in seawater inoculated with mangrove rhizosphere bacteria and Azospirillum. Biology and Fertility of Soils. 2000;32:265–272. [Google Scholar]
- Bashan Y, Hernandez JP, Leyva LA, Bacilio M. Alginate microbeads as inoculant carriers for plant growth-promoting bacteria. Biology and Fertility of Soils. 2002;35:359–368. [Google Scholar]
- Bashan Y, Holguin G, de-Bashan LE. Azospirillum-plant relationships: physiological, molecular, agricultural, and environmental advances (1997–2003) Canadian Journal of Microbiology. 2004;50:521–577. doi: 10.1139/w04-035. [DOI] [PubMed] [Google Scholar]
- Belimov AA, Safronova VI, Mimura T. Response of spring rape to inoculation with plant growth-promoting rhizobacteria containing 1-aminocyclopropane-1-carboxylate deaminase dependes on nutrient status of the plant. Canadian Journal of Microbiology. 2002;48:189–199. doi: 10.1139/w02-007. [DOI] [PubMed] [Google Scholar]
- Belimov AA, Hontzeas N, Safronova VI, Demchinskaya SV, Piluzza G, Bullitta S, Glick BR. Cadmium-tolerant plant growth-promoting bacteria associated with the roots of Indian mustard (Brassica juncea L. Czern.) Soil Boilogy & Biochemistry. 2005;37:241–250. [Google Scholar]
- Burd GI, Dixon DG, Glick BR. Plant growth-promoting bacteria that decrease heavy metal toxicity in plants. Canadian Journal of Microbiology. 2000;46:237–245. doi: 10.1139/w99-143. [DOI] [PubMed] [Google Scholar]
- Buyer JS, Roberts DP, Russek-Cohen E. Soil and plant effects on microbial community structure. Canadian Journal of Microbiology. 2002;48:955–964. doi: 10.1139/w02-095. [DOI] [PubMed] [Google Scholar]
- Carrillo AE, Li CY, Bashan Y. Increased acidification in the rhizosphere of cactus seedlings induced by Azospirillum brasilense. Naturwissenschaften. 2002;89:428–432. doi: 10.1007/s00114-002-0347-6. [DOI] [PubMed] [Google Scholar]
- Cunningham SD, Betri WR, Huang JW. Phytoremediation of contaminated soils. Trends in Biotechnology. 1995;13:393–397. [Google Scholar]
- Dalmastri C, Chiarini L, Cantale C, Bevivino A, Tabacchioni S. Soil type and maize cultivar affect the genetic diversity of maize root-associated Burkholderia cepacia populations. Microbial Ecology. 1999;38:273–284. doi: 10.1007/s002489900177. [DOI] [PubMed] [Google Scholar]
- Donate-Correa J, Leon-Barrios M, Perez-Galdona R. Screening for plant growth-promoting rhizobacteria in Chamaecytisus proliferus (tagasaste), a forage tree-shrub legume endemic to the Canary Islands. Plant and Soil. 2004;266:261–272. [Google Scholar]
- Ferris MJ, Muyzer G, Ward DM. Denaturing gradient gel electrophoresis profiles of 16S rRNA-defined populations inhabiting a hot spring mat community. Applied and Environmental Microbiology. 1996;62:340–346. doi: 10.1128/aem.62.2.340-346.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick BR, Penrose DM, Li J. A model for the lowering of plant ethylene concentrations by plant growth-promoting bacteria. Journal of Theoretical Biology. 1998;190:63–68. doi: 10.1006/jtbi.1997.0532. [DOI] [PubMed] [Google Scholar]
- Grandlic CJ, Mendez MO, Chorover J, Machado B, Maier RM. Plant growth-promoting bacteria for phytostabilization of mine tailings. Environmental Science & Technology. 2008;42:2079–2084. doi: 10.1021/es072013j. [DOI] [PubMed] [Google Scholar]
- Legendre P, Legendre L. Numerical ecology. Elsevier; Amsterdam: 1998. [Google Scholar]
- Hammer Ø, Harper DAT, Ryan PD. PAST: Palaeontological Statistics software package for education and data analysis. Palaeontologia Electronica. 2001;4(1):9. [Google Scholar]
- Mendez MO, Glenn EP, Maier RM. Phytostabilization potential of quailbush for mine tailings: growth, metal accumulation, and microbial community changes. Journal of Environmental Quality. 2007;36:245–253. doi: 10.2134/jeq2006.0197. [DOI] [PubMed] [Google Scholar]
- Mendez MO, Maier RM. Phytostabilization of mine tailings in arid and semiarid environments – an emerging remediation technology. Environmental Health Perspectives. 2008;116:278–283. doi: 10.1289/ehp.10608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez MO, Neilson JW, Maier RM. Characterization of a Bacterial Community in an Abandoned Semiarid Lead-Zinc Mine Tailing Site. Applied and Environmental Microbiology. 2008;74:3899–3907. doi: 10.1128/AEM.02883-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miché L, Balandreau J. Effects of rice seed surface sterilization with hypochlorite on inoculated Burkholderia vietnamiensis. Applied and Environmental Microbiology. 2001;67:3046–3052. doi: 10.1128/AEM.67.7.3046-3052.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muyzer G, de Waal EC, Uitterlinden AG. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes encoding for 16S rRNA. Applied and Environmental Microbiology. 1993;59:695–700. doi: 10.1128/aem.59.3.695-700.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer MW. Putting things in even better order: the advantages of canonical correspondence analysis. Ecology. 1993;74:2215–2230. [Google Scholar]
- Patten CL, Glick BR. Role of Psuedomonas putida indoleacetic acid in development of the host plant root system. Applied and Environmental Microbiology. 2002;68:3795–3801. doi: 10.1128/AEM.68.8.3795-3801.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puente ME, Bashan Y. Effect of inoculation with Azospirillum brasilense strains on the germination and seedlings growth of the giant columnar cardon cactus (Pachycereus pringlei) Symbiosis. 1993;15:49–60. [Google Scholar]
- Reed MLE, Glick BR. Growth of canola (Brassica napus) in the presence of plant growth-promoting bacteria and either copper or polycyclic aromatic hydrocarbons. Canadian Journal of Microbiology. 2005;51:1061–1069. doi: 10.1139/w05-094. [DOI] [PubMed] [Google Scholar]
- de Ridder-Duine AS, Kowalchuk GA, Klein Gunnewiek PJA, Smant W, van Veen JA. Rhizosphere bacterial community composition in natural stands of Carex arenaria (sand sedge) is determined by bulk soil community composition. Soil Biology & Biochemistry. 2005;37:349–357. [Google Scholar]
- Rosario K, Iverson SL, Henderson DA, Chartrand S, McKeon C, Glenn EP, Maier RM. Bacterial community changes during plant establishment at the San Pedro River mine tailings site. Journal of Environmental Quality. 2007;36:1249–1259. doi: 10.2134/jeq2006.0315. [DOI] [PubMed] [Google Scholar]
- ter Braak CJF. Canonical correspondence analysis: a new eigenvector technique for multivariate direct gradient analysis. Ecology. 1986;67:1167–1179. [Google Scholar]
- ter Braak CJF, Wiertz J. On the statistical analysis of vegetation change: a wetland affected by water extraction and soil acidification. Journal of Vegetation Science. 1994;5:361–372. [Google Scholar]
- Tummala PSL, Humble W. Public health assessment; Klondyke mine tailings. Arizona Department of Health Services; 1998. [Google Scholar]
- Vazquez P, Holguin G, Puente ME, Lopez-Cortez A, Bashan Y. Phosphate-solubilizing microorganisms associated with the rhizosphere of mangroves in a semiarid coastal lagoon. Biology and Fertility of Soils. 2000;30:460–468. [Google Scholar]
- Venables WN, Ripley BD. Modern applied statistics with S, Forth Edition. Springer Statistics and Computing Series; New York, N.Y: 2002. pp. 385–389. [Google Scholar]
- Wilson DO. Evaluation of chemical seed coat sterilants. Plant and Soil. 1976;44:703–707. [Google Scholar]