Abstract
Human and animal laboratory studies show that females and males respond differently to drugs and that drug administration during adolescence leads to different behavioral effects than during adulthood. Adult female rats are more sensitive to the behavioral effects of cocaine than adult males, but it is not known if the same effect of sex exists during adolescence. In the present study, sensitivity to the conditioned reward of cocaine was evaluated using a conditioned place preference (CPP) paradigm where adolescent (PND 34) and adult (PND 66) male and female rats were trained and tested for the development of CPP to multiple doses of cocaine. Female rats developed CPP at lower doses than males, regardless of age. In addition, adolescent male and female rats established a CPP at lower doses of cocaine than adult male and female rats, respectively. Thus, both age and sex altered cocaine conditioned reward with the order of sensitivity being adolescent females > adult females > adolescent males > adult males. These data show that adolescents are more sensitive to the conditioned rewarding properties of cocaine than adults and that females respond to lower doses of cocaine compared to males regardless of age.
Keywords: adolescent, male, female, sex differences, conditioned place preference, cocaine
INTRODUCTION
In 2006, an estimated 2.8 million people aged 12 or older used an illicit drug for the first time (NSDUH, 2007). Most treatment programs and research on drug abuse focus on the male population even though greater than 50 million women reported using illegal drugs in their lifetime and approximately 15 million women reported using illicit drugs in the past year, according to the latest statistics (NSDUH, 2007). In adolescence, 3.9 million males and 3.6 million females ages 12-17 reported using illicit drugs (NSDUH, 2007). Thus, the number of teenage females using illicit drugs is approaching that of males.
Adolescence is a critical period for the initiation of illicit drug use and according to the National Survey on Drug Use and Health, approximately 33.7 million Americans age 12 and older had tried cocaine at least once in their lifetimes, representing 13.8% of the population ages 12 and older (NSDUH, 2007). Adolescents under age 18 accounted for 34% of new cocaine initiates, which amounted to 918 adolescents per day who tried cocaine for the first time during 2006 (NSDUH, 2007).
Women cocaine users demonstrate different abuse patterns than males (Griffin et al., 1989), and the prevalence of cocaine dependence is higher in adolescent females than males (Kandel et al., 1997). There have been a number of studies showing differences in the effects of cocaine in female and male adult rats, with the predominant finding being that adult female rats are more sensitive to cocaine than adult males. For example, there was a greater increase in striatal dopamine in response to cocaine (Walker et al., 2006) and amphetamine (Castner et al., 1993) administration in females than in males. There was a greater rise in ACTH in response to cocaine in females than in males (Kuhn and Francis, 1997), and there were sex differences in self-administered cocaine (Lynch et al., 2000). In addition, female rats are more sensitive to treatment with a cannabinoid agonist during adolescence, in that they subsequently exhibit an increase in cocaine self-administration, an effect that is not seen in males (Higuera et al., 2005; Higuera-Matas et al., 2008). Ovariectomized female rats receiving estrogen had a greater response to cocaine and a more rapid development of sensitization to its psychomotor effects than males (Becker et al., 2001).
In addition to sex differences, it has been shown that adolescent male rats approached a novel object more rapidly than adult male rats (Stansfield and Kirstein, 2005) and that cocaine conditioned place preference was greater in adolescent than in adult male rats in response to a low dose of cocaine (Badanich et al., 2006). Adolescent female rats developed sensitization to the locomotor-activating effects of cocaine by the second day of administration, while adolescent male rats did not become sensitized over a seven-day administration regimen (Collins and Izenwasser, 2002). In contrast, in adult males and females, a significant sensitization was observed after five days of treatment. In addition, it has been shown that dopamine D2 receptor pruning is greater in male than female rats during adolescence (Andersen and Teicher, 2000) and that prenatal exposure to cocaine produced lower levels of prodynorphin mRNA in female than in male adolescent rats (Torres-Reveron et al., 2007). Thus, there are differences between male and female rats in both the behavioral response and in neurochemical adaptations to repeated cocaine administration and these differences vary across development.
Since there are no reports showing full dose-effect curves for CPP in adult and adolescent male and female rats, the goal of this study was to evaluate and compare sensitivity to the conditioned-reward effects of multiple doses of cocaine in four rat sex/age groups: male adolescents, female adolescents, male adults, and female adults using a conditioned place preference (CPP) paradigm.
METHODS
Subjects
Male and female Sprague-Dawley adolescent and adult rats (Charles River, MA) were used in all studies. Rats were housed two per cage with the same-sex cage mate in a temperature and humidity-controlled environment under a 12 h light/dark cycle with lights on at 7 a.m. and off at 7 p.m.
All behavioral tests were done during the light schedule between 9 a.m. and 4 p.m. with each group tested at the same hour every day and the groups randomized over the course of the day. Food and water were available ad libitum. The animals used in this study were maintained and the studies were conducted in accordance to the guidelines of the Guide for Care and Use of Laboratory Animals, National Research Council, Department of Health, Education and Welfare, NIH Publication 85-23, revised 1996.
Chemicals
Cocaine HCl was obtained from the National Institute on Drug Abuse (Rockville, MD) and was dissolved in saline.
Conditioned place preference
The apparatus consisted of an acrylic box (40.64×40.64 cm) with a removable center barrier. On one side, the walls and the lid were white and the floor was smooth. On the other side, the walls and the lid were black and white striped, and the bottom was covered with a textured floor. A pretest was done on postnatal day (PND) 34 (adolescents) or PND 66 (adults), followed by three days of training and a post-test on PND 38 or PND 70, respectively. Thus, the entire experiment was conducted during the periadolescent stage of adolescence, which is the period immediately prior to puberty (Spear and Brake, 1983). There were 8-12 rats/group for each dose of cocaine tested. On PND 35 (the first day of drug administration) the male adolescent rats weighed 122 ± 4, the female adolescents weighed 107 ± 1, the adult male rats weighed 298 ± 15, and the adult female rats weighed 226 ± 2 gms. During the pretest, the rats were placed into the CPP test chamber with the center barrier removed so that the rats were able to move freely to both sides and the amount of time spent in each side was recorded for 30 min. During the conditioning phase, the rats were trained in the morning with saline and in the afternoon with cocaine and each training session lasted 30 min. The doses of cocaine tested were 3, 5, 7.5, 10 or 20 mg/kg i.p. and different groups of rats were used for each dose. This training schedule was used instead of training saline and drug on separate days because of the constraints involved in doing developmental studies as has been described elsewhere (e.g. Badanich et al., 2006; Balda et al., 2006; Brenhouse and Andersen, 2008). To ensure that the entire experiment could be completed within the periadolescent period, it was important to keep the CPP training period as short as possible. The post-test was done in the middle of the day. During this test, rats were placed in the chamber with the center barrier removed and they were able to move freely to both sides, as during the pretest. The amount of time spent in each side was recorded for 30 min by observers blind as to which side had been paired with drug.
Data analysis
CPP data for each drug were analyzed using a three-way (sex x dose x age) analysis of variance (ANOVA). Post hoc analysis using Fisher`s Protected Least Significant Difference (PLSD) was used when warranted. In addition, data were analyzed using t-tests to compare the amount of time spent in the cocaine-paired side during the post-test minus time spent in the cocaine-paired side during the pretest to 0 to determine whether a significant change in behavior had occurred in each group. A value of 0 would indicate that there was no change in preference, whereas a positive value would mean that more time was spent in the cocaine-paired side after training than during the pretest. P values less than or equal to 0.05 were considered significant for all tests.
RESULTS
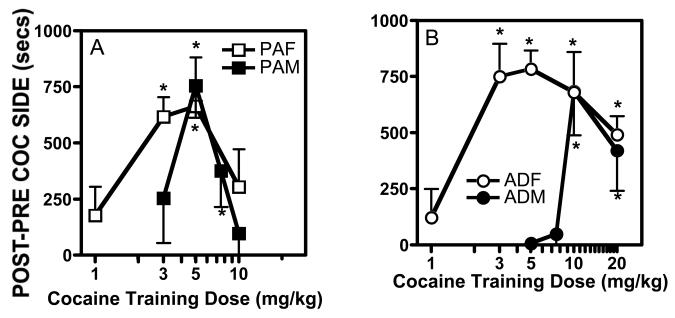
After three days of training, the periadolescent male (PAM) rats exhibited a significant CPP to both 5.0 (t(11)=5.27, P ≤ 0.0003) and 7.5 (t(11)=2.34, P ≤ 0.039) mg/kg cocaine, as shown by a significant increase in the amount of time spent in the cocaine-paired chamber during the post-test compared to the pretest (Fig. 1A). Neither 3 nor 10 mg/kg cocaine produced a significant CPP in the adolescent male rats. The periadolescent female (PAF) rats spent significantly more time in the compartment associated with either 3 (t(9)=7.219, P ≤ 0.0001) or 5 (t(7)=3.038, P ≤ 0.019) mg/kg of cocaine during the post-test, compared to the pretest (Fig. 1A). There was not a significant CPP for the adolescent female rats in response to training with either 1 or 10 mg/kg cocaine.
Fig.1.
Dose-effect curves for cocaine CPP in (A) periadolescent female (PAF) vs periadolescent male (PAM) and (B) adult female (ADF) vs adult male (ADM). Data are presented as mean ± SEM post-pre values (time spent in cocaine-paired side during post-test minus pretest in seconds). N=8-12 rats/group. * significant difference from 0 (p<0.05).
In contrast to the adolescents, adult male (ADM) rats did not develop a significant CPP to 5 or 7.5 mg/kg cocaine, but did spend significantly more time in the cocaine-paired chamber during the post-test after training with either 10 (t(7)=3.50, P ≤ 0.009) or 20 (t(9)=2.34, P ≤ 0.044) mg/kg cocaine (Fig. 1B). The adult female (ADF) rats were more sensitive to cocaine than the adult males in that they developed a significant CPP to a dose of 3 (t(7) = 5.121, P ≤ 0.001) mg/kg. In addition to the responsiveness of the adult females to a low dose of cocaine, they exhibited a significant CPP to a wider range of doses than any of the other groups with a significant CPP developing in response to all but the lowest dose (1 mg/kg) of cocaine (Fig. 1B).
A three-way ANOVA (sex x age x dose) was significant (F(1,141)=4.654, P ≤ 0.033). In addition, there were significant effects of sex (F(1,141)=6.037, P ≤ 0.015), of dose (F(5,141)= 2.420, P ≤ 0.039), and of dose x age (F(1,141)= 2.832, P ≤ 0.027). It is interesting that although the dose-response curves for the female rats are shifted to the left compared to the male rats in both age groups, the maximal level of CPP achieved in all groups is equal. Thus, all of the groups are able to develop a CPP to cocaine to the same extent under these conditions.
DISCUSSION
The dose-effect curves for cocaine CPP are shifted to the left in periadolescent male and female rats compared to adult male and female rats, respectively. The entire dose-response curve for the PAM was shifted to the left compared to the ADM, while for the PAF only the descending part of the curve was shifted to the left compared to the ADF. In addition, the ascending parts of the dose-response curves for the females were shifted to the left compared to the males such that female rats developed CPP at lower doses of cocaine than males, regardless of age. It is interesting to note that under these conditions, the maximum levels of CPP were the same regardless of age or sex, suggesting that all of the groups had the same ability to learn, although the sensitivity to cocaine reward differed. A recent study showed that adolescent male rats that have developed a CPP to cocaine are more resistant to extinction of the CPP, and show a greater preference for the previously cocaine paired chamber after drug-primed reinstatement than are adult male rats (Brenhouse and Andersen, 2008). Together, these findings support the idea that cocaine is more rewarding to the adolescent rats than to the adult male rats.
Previous studies have shown that there are higher cocaine-induced activity levels in female than male adult rats (Craft and Stratmann, 1996; Festa et al., 2004; Laviola et al., 1995; Sell et al., 2000; Walker et al., 2001a; Walker et al., 2001b). In response to chronic cocaine, female rats exhibited more stereotypy than males during a 14-day cocaine treatment regimen (Chin et al., 2001), and female rats also developed greater sensitization to the behavioral effects of cocaine after the initial treatment period compared to male rats (Becker et al., 2001; Bowman and Kuhn, 1996; Chin et al., 2001; Griffin et al., 1989). In addition, adult female rats developed CPP to low doses of cocaine, to which the males did not respond (Russo et al., 2003), a finding that was replicated in the present study.
Females in the estrus stage of their estrous cycle lever pressed to increase the dose of cocaine to the maximum available when given a choice of doses (Lynch et al., 2000) and reached higher breaking points on a cocaine progressive ratio schedule (Roberts et al., 1989) than rats in other stages. Together, these studies suggest that hormones play a role in the unique behavioral effects of cocaine in females. In the present study, there was no relationship between estrous cycle and the development of CPP in the adult rats or the adolescent rats, who were just at the point of vaginal opening during these studies.
There are numerous reported sex differences in dopamine neurochemistry. For example, it has been shown that dopamine uptake and release in the striatum are up to 40% greater in female compared to male rats (Walker et al., 2000). Further, dopamine transporter density in the striatum is higher in females compared to males, as is dopamine transporter mRNA in the substantia nigra (Rivest et al., 1995). In addition, dopamine D1 and D2 receptor density in the striatum increased more between adolescence (PND 25) and puberty (PND 40) and then decreased more between puberty (PND 40) and adulthood (PND 120) in male rats compared to female rats (Andersen and Teicher, 2000). This suggests more overproduction and elimination of dopamine receptors in the striatum in male rats. In the nucleus accumbens, dopamine D1 receptor density was higher in males than females from adolescence (PND 25) through adulthood (PND 120) and dopamine D2 receptor density is greatly increased in males compared to females between the onset of adolescence (PND 25) and puberty (PND 40) (Andersen and Teicher, 2000). Overall, the literature suggests that there are differences in dopamine neurochemistry in female and male rats during periadolescence that continue through adulthood. Therefore, the differential behavioral effects of cocaine in females and males may be due in part to sex differences in the dopaminergic system.
In summary, both age and sex altered the conditioned reward associated with cocaine with the order of sensitivity being adolescent females > adult females > adolescent males > adult males. In adults, there was a greater shift to the left in the dose effect curve for cocaine CPP in females compared to males during adulthood than during adolescence. Similarly, there was a shift to the left of the entire curve in adolescent males compared to adults, whereas in the females, the ascending limb of the dose-response curve was the same regardless of age. It is interesting to note that the descending limb of the dose-effect curve in the female rats is steeper in the adolescents than in the adults. While both the adult and adolescent rats exhibit a significant CPP to 3 or 5 mg/kg cocaine, the adults also have significant effects at both 10 and 20 mg/kg cocaine while the adolescents do not. Thus, the female adolescent rats seem to have a narrower range of doses than the adults to which there is a significant conditioned reward. It is not clear what the implications of this finding are, but it does suggest that perhaps the adolescents are more sensitive to the aversive effects of cocaine.
Thus, sex and age are factors that predetermine sensitivity to the rewarding effects of cocaine, however the magnitude of differences between males and females differs depending upon age. A better understanding of this differential regulation may lead to sex- and age-specific preventions and treatments for cocaine abuse. In addition, it is important to not assume that the same doses used to test male rats should be used in studies of female rats regardless of age.
ACKNOWLEDGMENTS
This work was supported by the National Institute on Drug Abuse and the NIH Office of Research on Women's Health (grants DA 015119 and DA 024584).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Andersen SL, Teicher MH. Sex differences in dopamine receptors and their relevance to ADHD. Neuroscience and Biobehavioral Reviews. 2000;24:137–41. doi: 10.1016/s0149-7634(99)00044-5. [DOI] [PubMed] [Google Scholar]
- Badanich KA, Adler KJ, Kirstein CL. Adolescents differ from adults in cocaine conditioned place preference and cocaine-induced dopamine in the nucleus accumbens septi. Eur J Pharmacol. 2006;550:95–106. doi: 10.1016/j.ejphar.2006.08.034. [DOI] [PubMed] [Google Scholar]
- Balda MA, Anderson KL, Itzhak Y. Adolescent and adult responsiveness to the incentive value of cocaine reward in mice: role of neuronal nitric oxide synthase (nNOS) gene. Neuropharmacology. 2006;51:341–9. doi: 10.1016/j.neuropharm.2006.03.026. [DOI] [PubMed] [Google Scholar]
- Becker JB, Molenda H, Hummer DL. Gender differences in the behavioral responses to cocaine and amphetamine: implications for mechanisms mediating gender differences in drug abuse. Annals New York Academy of Sciences. 2001;937:172–87. doi: 10.1111/j.1749-6632.2001.tb03564.x. [DOI] [PubMed] [Google Scholar]
- Bowman BP, Kuhn CM. Age-related differences in the chronic and acute response to cocaine in the rat. Developmental Psychobiology. 1996;29:597–611. doi: 10.1002/(SICI)1098-2302(199611)29:7<597::AID-DEV4>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Brenhouse HC, Andersen SL. Delayed extinction and stronger reinstatement of cocaine conditioned place preference in adolescent rats, compared to adults. Behav Neurosci. 2008;122:460–5. doi: 10.1037/0735-7044.122.2.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castner SA, Xiao L, Becker JB. Sex differences in striatal dopamine: in vivo microdialysis and behavioral studies. Brain Research. 1993;610:127–34. doi: 10.1016/0006-8993(93)91225-h. [DOI] [PubMed] [Google Scholar]
- Chin J, Sternin O, Wu HB, Fletcher H, Perrotti LI, Jenab S, Quinones-Jenab V. Sex differences in cocaine-induced behavioral sensitization. Cellular and Molecular Biology. 2001;47:1089–95. [PubMed] [Google Scholar]
- Collins SL, Izenwasser S. Cocaine differentially alters behavior and neurochemistry in periadolescent versus adult rats. Developmental Brain Research. 2002;138:27–34. doi: 10.1016/s0165-3806(02)00471-6. [DOI] [PubMed] [Google Scholar]
- Craft RM, Stratmann JA. Discriminative stimulus effects of cocaine in female versus male rats. Drug and Alcohol Dependence. 1996;42:27–37. doi: 10.1016/0376-8716(96)01259-8. [DOI] [PubMed] [Google Scholar]
- Festa ED, Russo SJ, Gazi FM, Niyomchai T, Kemen LM, Lin S-N, Foltz R, Jenab S, Quinones-Jenab V. Sex differences in cocaine-induced behavioral responses, pharmacokinetics, and monoamine levels. Neuropharmacology. 2004;46:672–87. doi: 10.1016/j.neuropharm.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Griffin ML, Weiss RD, Mirin SM, Lange U. A comparison of male and female cocaine abusers. Archives of General Psychiatry. 1989;46:122–6. doi: 10.1001/archpsyc.1989.01810020024005. [DOI] [PubMed] [Google Scholar]
- Higuera A, Biscaia M, Fernández B, Miguéns M, Olmo Nd, Torres I, García-Lecumberri C, Viveros MP, Ambrosio E. Pre-exposure to cannabinoid agonist CP 55,940 during rat early adolescence facilitates acquisition of cocaine self-administration behavior in the adulthood. CPDD. 2005 abstract. [Google Scholar]
- Higuera-Matas A, Soto-Montenegro ML, del Olmo N, Miguens M, Torres I, Vaquero JJ, Sanchez J, Garcia-Lecumberri C, Desco M, Ambrosio E. Augmented acquisition of cocaine self-administration and altered brain glucose metabolism in adult female but not male rats exposed to a cannabinoid agonist during adolescence. Neuropsychopharmacology. 2008;33:806–13. doi: 10.1038/sj.npp.1301467. [DOI] [PubMed] [Google Scholar]
- Kandel D, Chen K, Warner LA, Kessler RC, Grant B. Prevalence and demographic correlates of symptoms of last year dependence on alcohol, nicotine, marijuana and cocaine in the U.S. population. Drug and Alcohol Dependence. 1997;44:11–29. doi: 10.1016/s0376-8716(96)01315-4. [DOI] [PubMed] [Google Scholar]
- Kuhn C, Francis R. Gender differences in cocaine-induced HPA axis activation. Neuropsychopharmacology. 1997;16:399–407. doi: 10.1016/S0893-133X(96)00278-3. [DOI] [PubMed] [Google Scholar]
- Laviola G, Wood RD, Kuhn C, Francis R, Spear LP. Cocaine sensitization in periadolescent and adult rats. journal of Pharmacology and Experimental Therapeutics. 1995;275:345–57. [PubMed] [Google Scholar]
- Lynch WJ, Arizzi MN, Carroll ME. Efffects of sex and the estrous cycle on regulation of intravenously self-administered cocaine in rats. Psychopharmacology. 2000;152:132–9. doi: 10.1007/s002130000488. [DOI] [PubMed] [Google Scholar]
- NSDUH. Substance Abuse and Mental Health Services Administration . Results from the 2006 National Survey on Drug Use and Health: National Findings. Rockville: 2007. (Office of Applied Studies, NSDUH Series H-30, DHHS Publication No. SMA 06-4194). MD. 2007. [Google Scholar]
- Rivest R, Falardeau P, Paolo TD. Brain dopamine transporter: gender differences and effect of chronic haloperidol. Brain Research. 1995;692:269–72. doi: 10.1016/0006-8993(95)00611-s. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Bennett SA, Vickers GJ. The estrous cycle affects cocaine self-administration on a progressive ratio schedule in rats. Psychopharmacology(Berlin) 1989;98:408–11. doi: 10.1007/BF00451696. [DOI] [PubMed] [Google Scholar]
- Russo SJ, Jenab S, Fabian SJ, Festa ED, Kemen LM, Quinones-Jenab V. Sex differences in the conditioned rewarding effects of cocaine. Brain Research. 2003;970:214–20. doi: 10.1016/s0006-8993(03)02346-1. [DOI] [PubMed] [Google Scholar]
- Sell SL, Scalzitti JM, Thomas ML, Cunningham KA. Influence of ovarian hormones and estrous cycle on the behavioral response to cocaine in female rats. Journal of Pharmacology and Experimental Therapeutics. 2000;293:879–86. [PubMed] [Google Scholar]
- Spear LP, Brake SC. Periadolescence: age-development behavior and psychopharmacological responsivity in rats. Developmental Psychobiology. 1983;16:83–109. doi: 10.1002/dev.420160203. [DOI] [PubMed] [Google Scholar]
- Stansfield KH, Kirstein CL. Neurochemical effects of cocaine in adolescence compared to adulthood. Brain Res Dev Brain Res. 2005;159:119–25. doi: 10.1016/j.devbrainres.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Torres-Reveron A, Hurd YL, Dow-Edwards DL. Gender differences in prodynorphin but not proenkephalin mRNA expression in the striatum of adolescent rats exposed to prenatal cocaine. Neurosci Lett. 2007;421:213–7. doi: 10.1016/j.neulet.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker QD, Cabassa J, Kaplan KA, Li S-T, Haroon J, spohr HA, Kuhn CM. Sex differences in cocaine-stimulated motor behavior: disparate efects of gonadectomy. Neuropsychopharmacology. 2001a;25:118–30. doi: 10.1016/S0893-133X(00)00248-7. [DOI] [PubMed] [Google Scholar]
- Walker QD, Francis R, Cabassa J, Kuhn CM. Effect of ovarian hormones and estrous cycle on stimulation of the hyopothalamo-pituitary-adrenal axis by cocaine. Journal of Pharmacology and Experimental Therapeutics. 2001b;297:291–8. [PubMed] [Google Scholar]
- Walker QD, Ray R, Kuhn CM. Sex differences in neurochemical effects of dopaminergic drugs in rat striatum. Neuropsychopharmacology. 2006;31:1193–202. doi: 10.1038/sj.npp.1300915. [DOI] [PubMed] [Google Scholar]
- Walker QD, Rooney MB, Wrightman RM, Kuhn CM. Dopamine release and uptake are greater in female than male rat striatum as measured by fast cyclic voltammetry. Neuroscience. 2000;95:1061–70. doi: 10.1016/s0306-4522(99)00500-x. [DOI] [PubMed] [Google Scholar]