Abstract
A homologue of the mammalian translationally controlled tumor protein (TCTP) was cloned from the human parasite Schistosoma mansoni (SmTCTP). Sequence analysis showed that SmTCTP differed from other reported TCTPs in having only one signature sequence. Subsequently, SmTCTP was cloned in a T7 expression system and expressed as a histidine-tagged fusion protein. Recombinant SmTCTP (rSmTCTP) has a molecular mass of ~23 kDa with the histidine tag. Further analysis showed that SmTCTP transcripts and protein are expressed in all life cycle stages of the parasite within the vertebrate hosts. Interestingly, antibodies to SmTCTP were present in the sera of mice 9 weeks after infection with S. mansoni. Characterization studies showed that rSmTCTP is a calcium-binding protein that can cause histamine release from basophil/mast cells and induce eosinophil infiltration. These findings suggest that SmTCTP may have an important role in the development of allergic inflammatory responses associated with schistosomiasis and may be a target for new drug development.
A family of translationally controlled tumor proteins (TCTP)1 was initially demonstrated in the growth phase of tumor cells (1). Subsequently TCTP was found to be present in many cell types (2). Homologues of TCTP have been reported from several organisms including plants, earthworm, parasites, and hydras (3–6). Previous functional studies have shown that TCTP are calcium-binding proteins (7) that are induced in response to various stimuli within the cells (4). TCTP can bind to heme (5) and tubulin (8) and can induce histamine release (7) and secretion of interleukin-4 (9) from basophils. Despite their varied functions, ubiquitous distribution, and high level of conservation, the primary physiological function of TCTP still remains unclear (8).
Previously we reported the cloning of TCTP homologues from the filarial parasites Brugia malayi and Wuchereria bancrofti (10). In the present study we describe the cloning of a TCTP homologue from the human parasite Schistosoma mansoni. Results from the present study show that similar to the filarial TCTPs, the SmTCTP also exhibit a calcium-binding property and mediate histamine release from rat basophilic leukemia (RBL-2H3) cells.
EXPERIMENTAL PROCEDURES
Identification of SmTCTP
EST databases of S. mansoni were searched with Schistosoma japonicum TCTP homologue (GenBank™ on number U85483) at the parasite genome BLAST server (www.ebi.ac.uk/blast2/parasites.html). The search identified two ESTs, one from S. mansoni cercarial stage (EMBL accession No. AA559731) and second one from S. mansoni adult (EMBL accession No. N20681) with significant homology. By aligning these two sequences a forward primer specific to S. mansoni, TCTP was designed with a sequence of 5′-ATGCGAGTGTTCAAGGATG-3′. As the ESTs were partial, the 3′-end of the TCTP could not be identified. Using the 5′-end SmTCTP sequence as the forward primer and the T7 promoter primer located downstream to the multiple cloning sites as the reverse primer, a PCR reaction was performed on the S. mansoni schistosomula cDNA library. PCR parameters were as follows: 95 °C of denaturation for 30 s, 58 °C of primer annealing for 30 s, 72 °C of primer extension for 3 min, and cycled for 30 cycles; a final extension of 5 min was performed at 72 °C before storing the samples at 4 °C. The PCR product was cloned in pST-Blue vector (Novagen, Madison, WI), and the DNA insert was sequenced. The sequence was characterized by sequence analysis programs and named as SmTCTP.
Construction of SmTCTP Expression Vector
The open reading frame of SmTCTP was cloned in T7 expression vector. The forward PCR primer corresponded to the beginning of the open reading frame of SmTCTP with the addition of an upstream in-frame BamHI restriction site, (5′-CGCGGATCCATGATCGTGTATAAGGATATG-3′). The reverse primer corresponded to the 3′-end of SmTCTP open reading frame flanked by HindIII restriction site (5′-CCCAAGCTTTCAATATTTTTC-CTGAGTTAATCC-3′). PCR parameters were 95 °C of denaturation for 30 s, 52 °C of primer annealing for 30 s, 72 °C of primer extension for 3 min, and cycled for 30 cycles. A final extension of 5 min was performed at 72 °C before storing the samples at 4 °C. The PCR products obtained were digested with BamHI and HindIII enzymes and ligated to similarly digested T7 expression vector pRSET A (Invitrogen, La Jolla, CA). Insert DNA was sequenced to ensure the authenticity of the cloned nucleotide sequence.
Expression and Purification of SmTCTP
A recombinant construct of SmTCTP in T7 expression vector was maintained in XL-1 Blue (Stratagene, La Jolla, CA). For expression, the recombinant plasmid was transformed into BL21(DE3) containing pLysS (Invitrogen) to minimize toxicity due to the protein. WhenA600 of the cultures reached 0.6, 1 mM isopropyl-1-thio-β-D-galactopyranoside was added to the cultures to induce gene expression, and the cultures were incubated for an additional 3 h. Total proteins were separated in a 12% SDS-PAGE, and the presence of histidine-tagged protein was confirmed using an anti-Xpress antibody (Invitrogen). Subsequently, the histidine-tagged recombinant proteins were purified using a TALON metal affinity resin (CLONTECH, Palo Alto, CA) as per the manufacturer’s recommendations. The purified SmTCTP was passed through a polymyxin B-agarose column (Detoxi-gel, Pierce) to eliminate endotoxins if any in the preparation before using in functional assays.
Production of Polyclonal Antibodies to rSmTCTP
Polyclonal antibodies to purified rSmTCTP were generated in C57/BL6 mice. Mice were treated in accordance with an approved institutional protocol. For generating antibodies, mice were immunized with 2 μg of rSmTCTP in Gerbu adjuvant (CCBiotech Corp., Poway, CA) followed by three booster doses at 2-week interval. After the final booster dose, mice were bled, and the sera were separated and stored at −20 °C. Reactivity of the sera was tested on Western blots.
Infection Sera
Seven C57BL/6 mice were infected with 100 cercariae of S. mansoni as described previously (11). Following infection, blood samples were collected from each mouse on weeks 2, 3, 8, and 9 by ocular puncture, and sera were separated. Serum samples collected before infection (day 0) served as controls.
Analysis of Stage-specific Expression of SmTCTP Transcripts
Expression of SmTCTP transcript in various life cycle stages (sporocyst, cercaria, schistosomula, adult male, and adult female) of the parasite was determined by RT-PCR. Total RNA was extracted from each of the life cycle stages of the parasite using TRIzol reagent (Invitrogen) and was reverse-transcribed using RETROscript (Ambion, Austin, TX). Hepatopancreas from normal snails was used as a control for the sporocyst stage. First, the cDNA of actin was PCR-amplified from each sample (PerkinElmer Life Sciences) using S. mansoni actin-specific primers (forward primer, 5′-ACTAAGTGAACATGGCCGACG-3′; reverse primer, 5′-AGCATGTGGTAGAGCATAAC-3′). After the band densities were determined using NIH Image software, the concentrations of individual samples were then adjusted so that each sample contained approximately the same level of actin. Individual samples were then PCR-amplified using SmTCTP-specific primers (forward primer, 5′-ATGTTCACAGACTCGCACTGTCC-3′; reverse primer, 5′-TATGGTGTCATACCGTCCTC-3′). Primers for actin and SmTCTP amplify 536- and 447-bp target fragments, respectively. PCRs were performed as follows: for actin, 3 min at 94 °C, 30 s at 55 °C, and 30 s at 72 °C for 30 cycles; For SmTCTP, 3 min at 94 °C, 30 s at 56 °C, and 30 s at 72 °C for 28 cycles. The final extension was followed by 5 min at 72 °C for all target cDNA amplifications. The products were resolved on a 1.5% agarose gel and stained with ethidium bromide. Photographs of gels were scanned, and band densities were analyzed using NIH Image software. Results are shown as scanned photographs and are expressed as target band intensity divided by β-actin band density.
Analysis of Stage-specific Expression of SmTCTP Protein
Expression of SmTCTP protein in various life cycle stages (sporocyst, cercaria, schistosomula, adult male, and adult females) of the parasite was determined by an immunoblot analysis. Excretory secretions (ES) of schistosomula were prepared as described previously (12). Soluble antigens of the parasites were prepared by sonication (Sonic Dismembrator, Fisher Scientific) in the presence of a protease inhibitor mixture (Sigma). Cross-reactive antigens from these preparations were then removed by incubating them for 1 h at room temperature with nitrocellulose membrane (Bio-Rad) strips adsorbed with mouse pre-immune serum (1:10). These antigens were then resolved on a 12% SDS-PAGE, transferred onto nitrocellulose membranes, and probed with mouse anti-SmTCTP (1:100 dilution) or mouse pre-immune serum for 1 h at room temperature. After washing the membrane three times with phosphate-buffered saline containing 0.05% Tween 20, horseradish peroxidase-conjugated goat anti-mouse antibody (Pierce Chemicals) was added at a 1:5000 dilution, and color was developed using a chemiluminescence substrate (Amersham Biosciences, Inc.). Purified rSmTCTP was used as a positive control.
45CaCl2 Overlay Assay
The ability of SmTCTP to bind calcium was studied in vitro as described previously (10). Briefly, purified rSmTCTP transblotted onto nitrocellulose membranes was incubated with 20 μCi/ml 45CaCl2 (ICN Biomedicals, Costa Mesa, CA) for 10 min, washed with distilled water for 5 min, air-dried, and exposed to x-ray film for 10 min.
Histamine Release Assay
The ability of SmTCTP to induce the release of histamine from a rat basophilic cell line, RBL-2H3 (ATCC, Manassas, VA) was studied as described previously (10). Briefly, rSmTCTP at final concentrations of 10, 1.25, and 0.3 μg/ml was added to cultured RBL-2H3 cells and incubated at 37 °C for 30 min. Histamine released into the culture supernatant was determined using a kit purchased from Beckman-Coulter (Immunotech, Miami, FL) as per the protocol provided by the manufacturer. Substance 48/80 (Sigma), an ionophore added at 0.3 μg/ml final concentration, served as a positive control for histamine release assay. Another recombinant protein of S. mansoni, Sm-G-binding factor (SmGBF), expressed in our laboratory and purified under similar conditions, was used as negative control.
Measuring Cellular Responses to rSmTCTP in the Peritoneum
C57BL/6 mice were sensitized first by injecting 20 μg of ovalbumin (Sigma) intraperitoneally. One week after sensitization, 5 μg of rSmTCTP suspended in 100 μl of saline was injected into the peritoneum. rSmGBF or sterile phosphate-buffered saline was used as the negative control. At 24 h after injection, peritoneal cells were collected, and a differential count was made on a cytospin (Cytopro, Wescor Inc. Logan, UT) smear preparation of the cells stained with Giemsa.
Statistical Analysis
Statistical analyses to test the significance of variance between control and experimental groups were determined by a Mann-Whitney U test using a Sigmastat program (Jandel Scientific, San Rafael, CA).
RESULTS
Isolation and Sequence Analysis of SmTCTP
A cDNA fragment of 513 bp was isolated from S. mansoni cercarial cDNA using primers derived from S. japonicum TCTP sequence and EST sequences of S. mansoni as described under “Experimental Procedures.” Translation of the nucleotide sequence revealed a putative open reading frame of 170 amino acids with a molecular mass of 19.6 kDa and pI of 4.68. BLAST analysis of encoded polypeptide sequence confirmed that the isolated cDNA clone is a homologue of TCTP protein. Multiple sequence alignment of the SmTCTP-encoded polypeptide with the TCTP family of proteins is shown in Fig. 1A. SmTCTP displayed 58% identity and 77% similarity with S. japonicum TCTP. Human and mouse TCTP proteins share 35–38% identity and 60% similarity with smTCTP, whereas filarial TCTP proteins from B. malayi and W. bancrofti had only about 27% identity and 52% similarity with SmTCTP. The phylogenetic tree, depicted in Fig. 1B, shows that S. mansoni and S. japonicum TCTP are closely related, while they are distinctly separate from filarial and mammalian TCTP proteins.
Fig. 1.
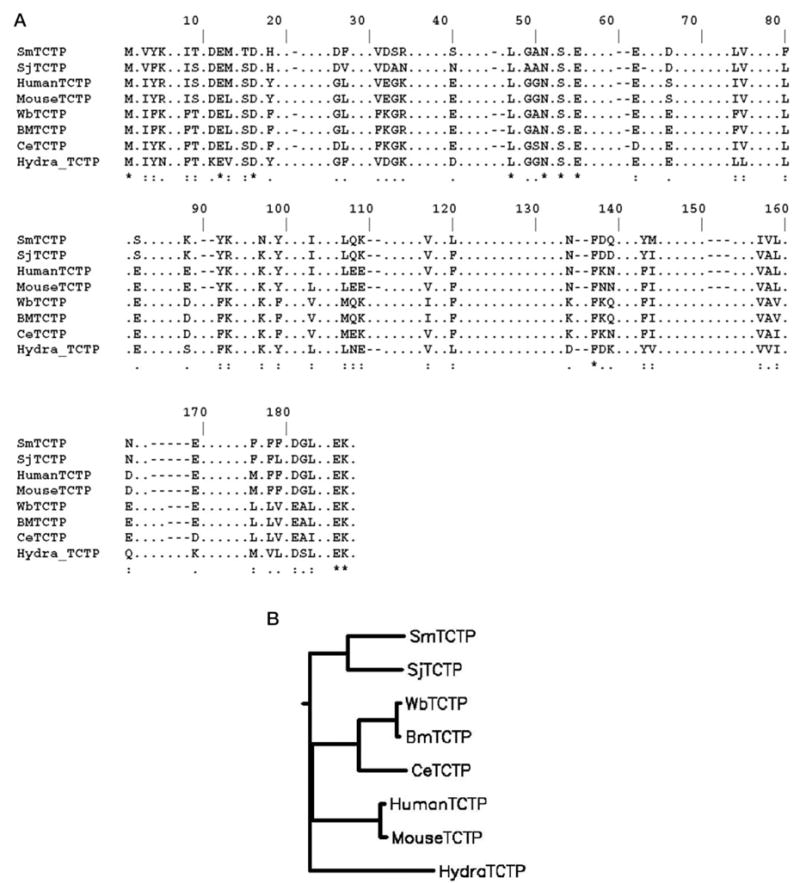
A, multiple alignment (ClustalW) of deduced amino acid sequences of SmTCTP with S. japonicum (SjTCTP, GenBank™ accession No. U85483) B. malayi (BmTCTP, H97276), W. bancrofti (WbTCTP, AY039808), C. elegans (CeTCTP, Q93573), Hydra vulgaris (Hydra_TCTP, U76187), human (Human TCTP, NP_003286), and mouse (MouseTCTP, NP_033455). B, phylogenetic tree analysis of TCTP family of proteins. The tree distances were generated according to the ClustalW algorithm, and the tree was constructed using DRAWGRAM (Phylip program).
A search of amino acid sequence of SmTCTP with the pattern data base of Prosite (13) revealed that SmTCTP contained 100% identical TCTP1 signature sequences, (I/A)G(G/A/S)N(P/A)SAE(G/D/E)(P/A/G/E)X(0/1)(D/E/G)X(D/E/N)X2(D/E), which corresponded to amino acid positions 46–56. However, it lacked a conspicuous signature sequence 2, which is present in all of the known TCTPs except the TCTP of hydra (GenBank™ accession No. 6094440). SmTCTP contained several potential phosphorylation sites and a myristoylation site (data not shown). In addition, SmTCTP also contained the typical Lupas coiled-coil structure as described previously with the filarial TCTPs (10).
Expression, Purification, and Immunoreactivity of rSm-TCTP
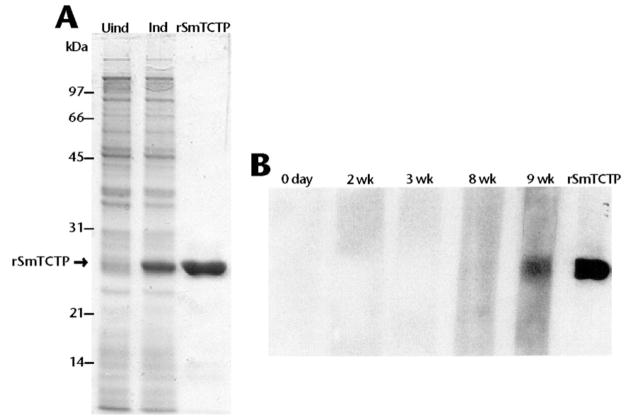
SmTCTP cloned in T7 expression vector pRSET A was expressed as a histidine-tagged fusion protein. The resultant recombinant fusion protein showed the expected molecular size of 23 kDa (Fig. 2), which corresponded to 19.6 kDa of SmTCTP open reading frame and ~3 kDa encoded by cloning vector from the translational start codon to the cloning site BamH1, that included a hexa-histidine tag. The recombinant protein was then purified using metal affinity column chromatography to >98% purity (Fig. 2).
Fig. 2. Expression, purification, and immunoreactivity of rSmTCTP.
A, ~20 μg of protein extracted from uninduced (Uind) and induced (Ind) cultures of Escherichia coli containing SmTCTP expression construct were separated in a 12% SDS-PAGE. rSmTCTP was then purified from the cultures using a nickel-nitrilotriacetic acid chromatography column, and ~1 μg of the protein was separated in a 12% SDS-PAGE (rSmTCTP). B, affinity-purified rSmTCTP probed with sera collected from C57BL/6 mice on day 0 or week 2, 3, 8, or 9 after infection with S. mansoni. Purified rSmTCTP probed with a mouse anti-SmTCTP serum served as a positive control.
Purified rSmTCTP was probed with sera collected from mice at different time points after infection with S. mansoni cercariae. Recombinant SmTCTP was reactive with 9-week post-infection sera but not with 0-, 2-, 3-, or 8-week sera (Fig. 2). Anti-rSmTCTP sera generated in mice were a positive control. Interestingly, rSmTCTP did not react with antisera raised against filarial TCTPs and vice-versa (data not shown).
Stage-specific Expression of SmTCTP
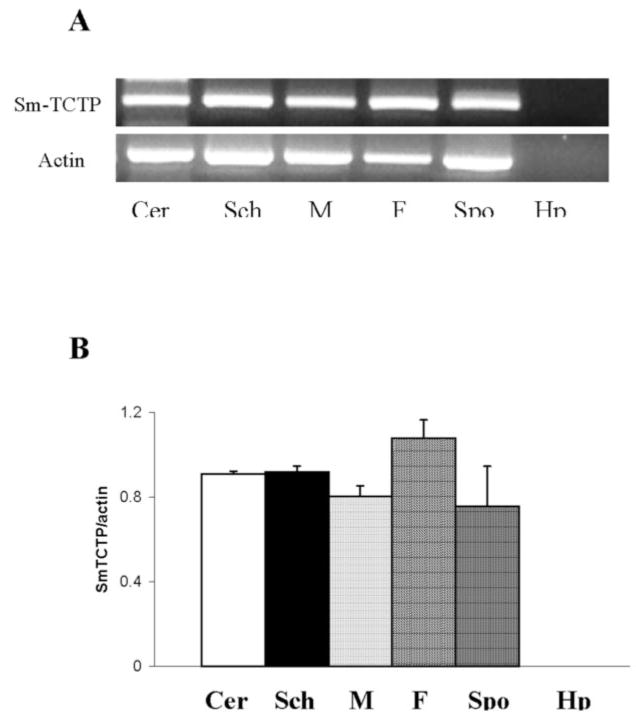
RT-PCR amplification of the SmTCTP gene from different life cycle stages of S. mansoni showed that the gene is transcribed in all the stages evaluated (Fig. 3A). Semiquantitative analysis done by comparing the ratio of target to actin transcript suggested that the SmTCTP expression level is slightly higher in female adult worms than at other life cycle stages (Fig. 3B).
Fig. 3. Expression of SmTCTP transcripts in various life cycle stages of S. mansoni.
TCTP transcript was PCR-amplified from cDNA collected from various life cycle stages (sporocyst (Spo), cercariae (Cer), schistosomula (Sch), adult male (M), and adult female (F) worms) of S. mansoni using primers specific for SmTCTP. A, PCR products were resolved in a 1% agarose gel, and the band intensity was normalized to actin amplified from the same samples using actin-specific primers. cDNA from normal snail hepatopancreas (Hp) remained as a negative control for the sporocystic stage. B, the ratio of SmTCTP to actin was calculated after scanning the images using NIH Image software. The data represent results obtained from one of three similar experiments.
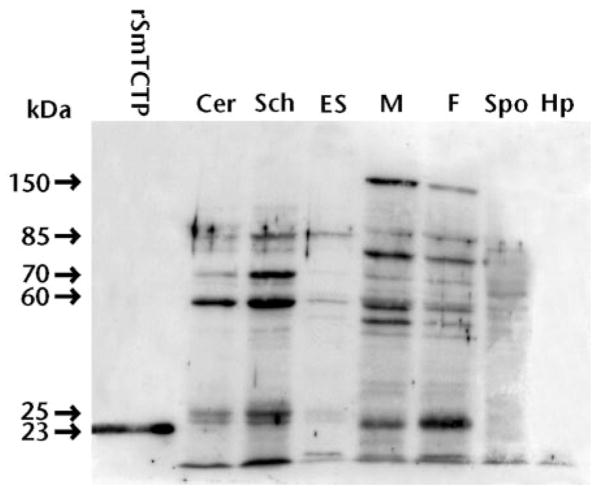
Immunoblot analyses done on the soluble protein extracts of the different life cycle stages of the parasite also showed that the expression of SmTCTP protein was present in sporocyst, cercaria, schistosomula, adult male, and adult female stages (Fig. 4). However, SmTCTP expression was comparatively lower in sporocystic stages. Interestingly SmTCTP was also present in the ES products of schistosomula. A protein at 85 kDa in the ES products reacted strongly with the anti-SmTCTP antibodies. Based on densitometric scanning of this band, it was estimated that the schistosomula of S. mansoni secrete approximately 1 μg of SmTCTP/1000 schistosomula. As has been reported previously with other TCTP proteins (10, 14, 15), the SmTCTP antibody also recognized several proteins most prominently around 25, 60, 85, and 150 kDa in molecular size in the worm homogenates of schistosomula, adult male worms, and adult female worms (Fig. 4).
Fig. 4. Expression of SmTCTP in various life cycle stages of S. mansoni.
Soluble protein extracts (10 μg/lane) of different life cycle stages such as sporocyst (Sp), cercariae (Cer), schistosomula (Sch), ES products of schistosomula (ES), adult male (M), or adult female (F) worms of the parasite were resolved on a 12% SDS-PAGE, transferred to nitrocellulose membrane, and probed with a mouse anti-SmTCTP polyclonal antibody (1:1000 dilution). Peroxidase-labeled goat anti-mouse IgG was used as the secondary antibody, and the reactive bands were detected using a chemiluminescent substrate. Recombinant SmTCTP was used as a positive control, and soluble proteins from normal snail hepatopancreas (Hp) were used as a negative control for the sporocystic stages. Arrows indicate bands of strong immunoreactivity. Data are from one of three experiments with similar results.
Calcium-binding Property of rSmTCTP
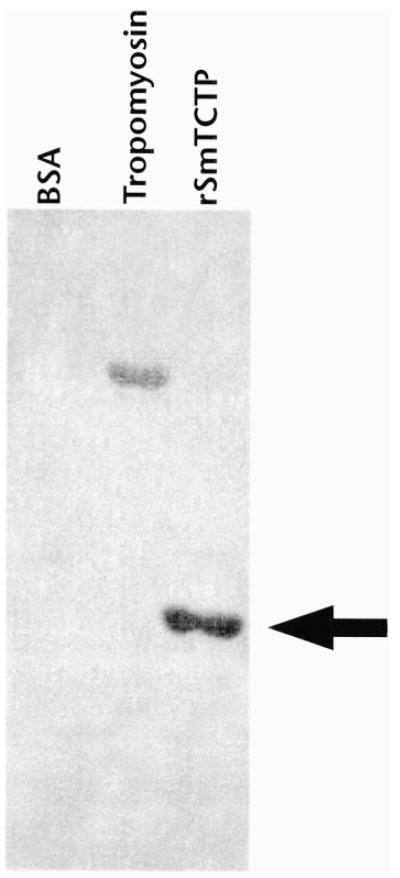
Mammalian and filarial TCTP are shown to bind calcium (7, 10). The calcium-binding domain of SmTCTP showed significant similarity with the calcium-binding domain of mammalian and filarial TCTPs. Hence, we wanted to test for whether rSmTCTP could also bind calcium. Overlay studies with radioactive Ca45 demonstrated that rSmTCTP could indeed bind calcium (Fig. 5). This suggested that SmTCTP is a calcium-binding protein.
Fig. 5. SmTCTP is a calcium-binding protein.
About 1 μg of purified rSmTCTP was transferred to nitrocellulose membrane and probed with radioactive 45CaCl2 as described under “Experimental Procedures.” Bovine muscle tropomyosin was a positive control, and bovine serum albumin (BSA) was used as a negative control. The data shown are representative of two similar experiments.
rSmTCTP-induced Histamine Release from a Basophil/Mast Cell Line
Because mammalian and parasite TCTPs can induce the release of histamine from basophils (16), we wanted to evaluate whether SmTCTP also possess similar property. Histamine releasing ability of rSmTCTP was evaluated using a rat basophilic cell line, RBL-2H3. Results showed that rSmTCTP induced histamine release in a dose-dependent manner (Fig. 6). This release was significantly (p < 0.01) higher than spontaneous release. The addition of another S. mansoni recombinant protein, rSmGBF, which was expressed and purified under conditions similar to rSmTCTP, did not release histamine from RBL-2H3 above base line.
Fig. 6. SmTCTP mediates histamine release from RBL-2H3 cells.
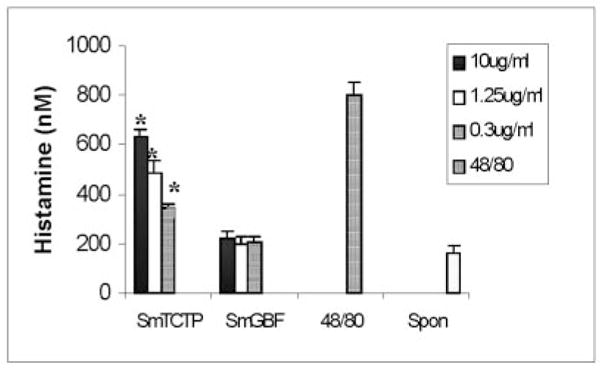
About 1 × 105 RBL-2H3 cells were incubated with 10, 1.25, or 0.3 μg/ml rSmTCTP for 30 min at 37 °C; the amount of histamine released into the culture supernatant was measured using a kit. As a positive control, 48/80 was added to the wells at a concentration of 0.3 μg/ml. Recombinant SmGBF was used as a negative control. The data shown are the mean ± S.D. of five different experiments. All three concentrations of rSmTCTP released significantly higher amounts of histamine than found in negative controls or upon spontaneous release (p < 0.01).
rSmTCTP-induced Infiltration of Eosinophil into the Peritoneal Cavity
Injection of rSmTCTP into the peritoneal cavity of mice sensitized 1 week previously with ovalbumin resulted in significant cellular infiltration within 24 h after the injection. A differential count showed that eosinophils are the predominant infiltrating cells in the peritoneal cavity (Fig. 7). Injection of rSmGBF, a recombinant protein from the schistosomular stages of S. mansoni expressed and purified under similar conditions as rSmTCTP, did not stimulate eosinophil accumulation in the peritoneal cavity.
Fig. 7. Effect of rSmTCTP on eosinophil infiltration into the peritoneal cavity of mice.
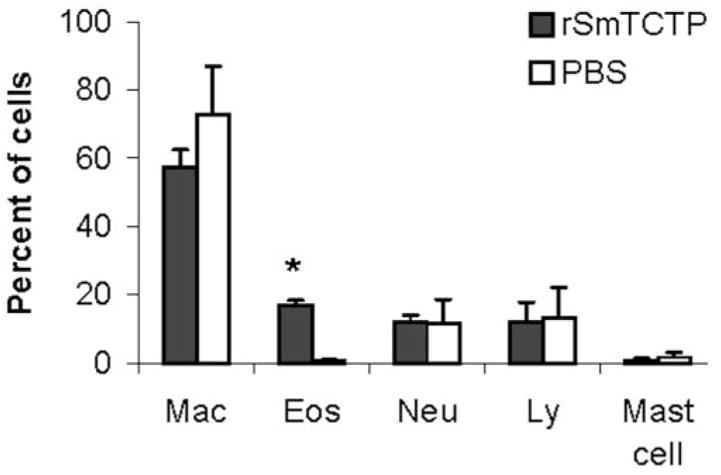
C57BL/6 mice were sensitized 1 week prior to the injection of rSmTCTP with ovalbumin. Peritoneal cells were collected 24 h after injection with 5 μg of endotoxin-free rSmTCTP, and a differential count was made on a cytospin smear preparation stained with Giemsa. Control animals received 100 μl of sterile saline. The data presented are from one of three identical experiments with similar results. Values shown are the mean (±S.E.) from five animals/group. *, significant at p < 0.01.
DISCUSSION
We have cloned a TCTP homologue from S. mansoni using S. japonicum TCTP sequences and schistosome EST data base sequences. Similar to other TCTP proteins, SmTCTP was found to be a calcium-binding protein with a histamine-releasing function. To our knowledge, SmTCTP is the first recombinant protein characterized from S. mansoni that has a histamine-releasing function.
A comparison of amino acid sequence data of SmTCTP with other TCTP proteins showed that S. mansoni and S. japonicum TCTP proteins share a high percentage of homology but are divergent from filarial TCTP proteins. In terms of percent similarity and percent identity, schistosome TCTP proteins share slightly more similarity with mammalian TCTP proteins (38% identity and 61% similarity) than filarial TCTPs (27% identity and 52% similarity). Immunoblot analysis showed that rSmTCTP antisera did not recognize filarial TCTP proteins (data not shown), indicating that there is no immunological cross-reactivity between filarial and schistosome TCTPs. This probably indicates that SmTCTP has evolved independently. The phylogenetic analysis (Fig. 1B) also suggests that the schistosome TCTP is well separated from filarial TCTPs. Another striking feature is that the signature sequence 2 is not conserved in SmTCTP and shows only 63% similarity with other TCTPs. All known TCTP proteins, (including S. japonicum TCTP) have a region with 100% similarity to signature sequence 2, except the TCTP homologue reported from the metazoan hydra (6). Hydra TCTP showed 86% similarity with signature sequence 2, with a substitution of threonine for glycine at position 9. In S. mansoni, amino acid positions 3, 5, 7, and 13 are substituted with methionine, proline, serine, and isoleucine, respectively, for isoleucine, glutamic acid, methionine, and valine. Interestingly, proline can introduce bends in the α-helix, potentially causing structural changes to the SmTCTP molecule. This may explain the lack of cross-reactivity between filarial TCTPs and SmTCTP. Nevertheless, the functional significance of signature sequences in any of the TCTP proteins has not been established yet.
Calcium-binding studies using radioactive calcium showed that SmTCTP binds calcium in a manner similar to filarial and other TCTP proteins. Previously Ram et al. (17) described several calcium-binding proteins in S. mansoni having different molecular sizes (20, 19, 16, and 8 kDa). These proteins, especially the 20-kDa antigen, possess the four EF-hand motifs for calcium binding and show significant homology with other members of the calcium-binding family of proteins such as calmodulin, troponin C, and light-chain myosin. The 20-kDa antigen appears to be expressed in schistosomula and adult worms but not in the egg stages (17). Another 58-kDa calcium-binding protein of S. mansoni, the calreticulin, is expressed in all life cycle stages of the parasite including cercariae, adult worm, and eggs (18). Interestingly, SmTCTP appears to be significantly different from all of these calcium-binding proteins of S. mansoni in the absence of any sequence homology either with them or with other members of the calcium-binding family of proteins and in the absence of any EF-hand calcium-binding motifs. These findings therefore suggest that SmTCTP is a novel calcium-binding protein.
Analysis of various life-cycle stages of S. mansoni for the expression of SmTCTP showed that although transcripts for SmTCTP are present in all life-cycle stages, only those stages that are present in the vertebrate host (schistosomula and adults) express high levels of SmTCTP (Fig. 4). A recent study by Niak et al. (19) showed that heat induction increases TCTP expression in the infective stage of Trichenella spiralis by 5.7-fold more than in the uninduced parasite. Therefore, it is possible that entry of the schistosomula stages from a cold-blooded snail vector to a warm-blooded host might have triggered the higher expression of SmTCTP. Other parasites are also known to express TCTP differentially (5). Longitudinal screening of sera for antibodies against SmTCTP in infected mice showed that antibodies to rSmTCTP appear in the sera of infected mice only around 9 weeks after infection. This time period coincides with the initiation of egg-induced granulomatous pathology in this infection. Therefore, it is possible that SmTCTP expressed in the adult/egg stages of the parasite may have potential significance in the pathology associated with this infection.
Sequence analysis of SmTCTP showed that it lacks leader sequences. Yet, significant amounts of SmTCTP were present in the ES of schistosomula (Fig. 4). MacDonald et al. (20) have also showed that human TCTP are secretory proteins despite the lack of signal sequence. Similar observations were made previously with filarial TCTPs (10), P. falciparum TCTP (21), and mouse TCTP (22). Neither the mechanism of SmTCTP secretion nor its functional significance in host-parasite interaction is fully understood at this time. Given its partial homology with host TCTP it is possible that SmTCTP may play an important role in host modulation similar to the malarial TCTP (21).
Another interesting finding was the ability of SmTCTP to induce histamine release from a rat basophilic cell line in a dose-dependent manner. TCTPs from other parasites are also known to possess histamine-releasing function (10, 21). Numerous earlier studies, in experimental animals and in patients with acute or chronic schistosomiasis, have reported the release of histamine during infections with S. mansoni (23–27). It is possible that these histamine-releasing effects during infection may be due to the release of SmTCTP by the parasites in infected individuals. Recently, using a glass microfiber histamine release assay, it has been shown that whole worm antigen homogenate and soluble egg antigens of S. mansoni can induce histamine release from basophils that are passively sensitized with sera from individuals with schistosomiasis (28). Similarly, soluble egg antigens of S. mansoni have been shown to induce release of histamine and interleukin-4 from human basophils in a dose-dependent manner (29). Despite all of these observations, there has been no report to date on the identification of a histamine-releasing antigen from S. mansoni. SmTCTP is probably the first fully characterized histamine-releasing recombinant antigen of S. mansoni.
Histamine release has also been demonstrated routinely in cell cultures within 1 h after incubation with antigens from adult, egg, or cercarial stages of S. mansoni. This histamine release, which occurs in an antigen-specific fashion, is thought to account for the specific suppression of lymphocyte responsiveness to parasite antigens (30). Because SmTCTP is one of the major antigens of S. mansoni that can induce release of histamine into the microenvironment around the parasite, it is possible that SmTCTP has the potential of regulating host immune responses as well. This conclusion is supported by the previous findings that immunoregulation of egg-induced granulomas in S. mansoni infection is mediated by histamine (H2) receptor bearing granuloma lymphocytes (31, 32).
SmTCTP has significant sequence homology with human TCTP, also designated as human histamine-releasing factor (9). Studies from Susan Macdonald’s laboratory (16) suggested that histamine-releasing factor might exert its histamine-releasing activity independently of IgE and FcεR1 suggesting that it may have a unique receptor on the surface of basophils. Our studies show that rSmTCTP also induces its histamine-releasing function independent of IgE. Previous studies by Catto et al. (26) suggested that cercariae of S. mansoni produce a mast cell-triggering factor that can release histamine from rat peritoneal mast cells in vitro. They also note that this activation occurs in the absence of serum and does not require adherence of cercariae to mast cells. It is possible that the factor described by Catto et al. (26) could likely be SmTCTP. It is believed that histamine and possibly other vasoactive amines released in the skin during infection can cause vasodilation, which facilitates easy migration of the parasite into the blood vessels (33). Therefore, secretion of SmTCTP by skin-migrating schistosomula may be a survival strategy of the parasite.
The present study also shows that rSmTCTP can induce eosinophil infiltration in a manner similar to the filarial TCTPs (10). Furthermore, this findings also support recent report that histamine-releasing factors are chemotactic for eosinophils (34). Eosinophil infiltration around schistosomula and egg stages of the parasite has long been demonstrated (35–37). Several mechanisms have been proposed for the accumulation of eosinophils around the parasite (38, 39). The present study demonstrates another possible mechanism by which eosinophils are recruited around the parasite. Because SmTCTP can induce eosinophilia and release histamine from mast cells/basophils, it is highly probable that SmTCTP play an important role in the development of allergic inflammation associated with schistosomiasis mansoni.
Exogenously added histamines are known to increase the neuromuscular activity of schistosomes (40) possibly by binding to a 65-kDa G protein-coupled histamine receptor expressed on the surface of S. mansoni (41). Because SmTCTP can induce release of histamine, the above findings might suggest an important physiological function for SmTCTP in the regulation of motor activity of the worm. Similarly, Bhisutthibhan et al. (5) recently demonstrated that malarial TCTPs are targets for the anti-malarial drug artimisinin. Because, artimisinin derivatives are also highly effective against schistosomiasis (42), it is conceivable that the target for artimisinin derivatives in schistosomes could be SmTCTP. Thus, SmTCTP is an interesting molecule that may play a crucial role in the development of pathology and/or survival of the parasite in the host. Developing drugs or intervention that specifically target SmTCTP may potentially help in the control of pathology and/or infection due to S. mansoni.
Acknowledgments
We thank Dr. Fred Lewis, Biomedical Research Institute, Rockville, MD, for supplying schistosome life cycle stages through National Institutes of Health-NIAID Contract N01-A1-55270 and Dr. He Yi-Xun for collecting different stages of the parasite for antigen preparation. S. mansoni cDNA libraries were the kind gift of Dr. Philip T. Loverde, State University of New York, Buffalo.
Footnotes
This work was supported by National Institutes of Health Grant AI 39066 (to K. R.).
The nucleotide sequence(s) reported in this paper has been submitted to the GenBank™/EBI Data Bank with accession number(s)AF358139.
The abbreviations used are: TCTP, translationally controlled tumor protein; SmTCTP, Schistosoma mansoni TCTP; rSmTCTP, recombinant SmTCTP; SmGBF, S. mansoni G-binding factor; RBL, rat basophilic leukemia cells; ES, excretory secretions; EST, expressed sequence tag; BLAST, basic local alignment search tool.
References
- 1.Bohm H, Bendorf R, Gaestel M, Gross B, Nurnberg P, Kraft RAO, Bielka H. Biochem Int. 1989;19:277–286. [PubMed] [Google Scholar]
- 2.Sanchez J, Schaller D, Ravier F, Golaz O, Jaccoud S, Belet M, Wilkins M, James R, Deshusses J, Hochstrasser D. Electrophoresis. 1997;18:150–155. doi: 10.1002/elps.1150180127. [DOI] [PubMed] [Google Scholar]
- 3.Pay A, Heberle-Bors E, Hirt H. Plant Mol Biol. 1992;19:501–503. doi: 10.1007/BF00023399. [DOI] [PubMed] [Google Scholar]
- 4.Sturzenbaum S, Kille P, Morgan A. Biochim Biophys Acta. 1998;1398:294–304. doi: 10.1016/s0167-4781(98)00077-3. [DOI] [PubMed] [Google Scholar]
- 5.Bhisutthibhan J, Pan X, Hossler P, Walker D, Yowell C, Carlton J, Dame J, Meshnick S. J Biol Chem. 1998;273:16192–16198. doi: 10.1074/jbc.273.26.16192. [DOI] [PubMed] [Google Scholar]
- 6.Yan L, Fei K, Bridge D, Sarras M. Dev Genes Evol. 2000;210:507–511. doi: 10.1007/s004270000088. [DOI] [PubMed] [Google Scholar]
- 7.Kim M, Jung Y, Lee K, Kim C. Arch Pharm Res. 2000;23:633–636. doi: 10.1007/BF02975253. [DOI] [PubMed] [Google Scholar]
- 8.Gachet Y, Tournier S, Lee M, Lazaris-Karatzas A, Poulton T, Bommer U. J Cell Sci. 1999;112:1257–1271. doi: 10.1242/jcs.112.8.1257. [DOI] [PubMed] [Google Scholar]
- 9.Schroeder JT, Lichtenstein LM, MacDonald SM. J Exp Med. 1996;183:1265–1270. doi: 10.1084/jem.183.3.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gnanasekar M, Rao K, Chen L, Narayanan R, Geetha M, Scott A, Ramaswamy K, Kaliraj P. Mol Biochem Parasitol. 2002;121:107–118. doi: 10.1016/s0166-6851(02)00027-0. [DOI] [PubMed] [Google Scholar]
- 11.Ramaswamy K, He YX, Salafsky B. Exp Parasitol. 1997;86:118 –132. doi: 10.1006/expr.1997.4178. [DOI] [PubMed] [Google Scholar]
- 12.Ramaswamy K, Salafsky B, Potluri S, He YX, Li JW, Shibuya T. J Inflamm. 1995;46:13–22. [PubMed] [Google Scholar]
- 13.Bairoch A, Bucher P, Hofman K. Nucleic Acids Res. 1997;25:217–221. doi: 10.1093/nar/25.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhisutthibhan J, Meshnick SR. Antimicrob Agents Chemother. 2001;45:2397–2399. doi: 10.1128/AAC.45.8.2397-2399.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoon T, Jung J, Kim M, Lee KM, Choi EC, Lee K. Arch Biochem Biophys. 2000;384:379–382. doi: 10.1006/abbi.2000.2108. [DOI] [PubMed] [Google Scholar]
- 16.MacDonald S. Int Arch Allergy Immunol. 1997;113:187–189. doi: 10.1159/000237542. [DOI] [PubMed] [Google Scholar]
- 17.Ram D, Romano B, Schechter I. Parasitology. 1994;108:289–300. doi: 10.1017/s0031182000076137. [DOI] [PubMed] [Google Scholar]
- 18.Khalife J, Liu JL, Pierce R, Porchet E, Godin C, Capron A. Parasitology. 1994;108:527–532. doi: 10.1017/s0031182000077398. [DOI] [PubMed] [Google Scholar]
- 19.Niak CH, Su KW, Ko RC. Parasitology. 2001;123:293–300. doi: 10.1017/s0031182001008320. [DOI] [PubMed] [Google Scholar]
- 20.MacDonald S, Rafnar T, Langdon J, Lichtenstein L. Science. 1995;269:688 –690. doi: 10.1126/science.7542803. [DOI] [PubMed] [Google Scholar]
- 21.MacDonald SM, Bhisutthibhan J, Shapiro TA, Rogerson SJ, Taylor TE, Tembo M, Langdon JM, Meshnick SR. Proc Natl Acad Sci U S A. 2001;98:10829–10832. doi: 10.1073/pnas.201191498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Teshima S, Rokutan K, Nikawa T, Kishi K. J Immunol. 1998;161:6356–6366. [PubMed] [Google Scholar]
- 23.Schoenbechler MJ, Sadun EH. Proc Soc Exp Biol Med. 1968;127:601–606. doi: 10.3181/00379727-127-32752. [DOI] [PubMed] [Google Scholar]
- 24.El-Din AH, Peters SM, Attia WM, Liu WJ, Ali MK, Bellanti JA. Ann Allergy. 1983;50:190 –194. [PubMed] [Google Scholar]
- 25.Harris WG. Immunology. 1973;24:567–577. [PMC free article] [PubMed] [Google Scholar]
- 26.Catto BA, Lewis FA, Ottesen EA. Am J Trop Med Hyg. 1980;29:886 –889. doi: 10.4269/ajtmh.1980.29.886. [DOI] [PubMed] [Google Scholar]
- 27.el-Hawey AM, Selim AS, Mousa AH. J Egypt Med Assoc. 1970;53:530–537. [PubMed] [Google Scholar]
- 28.Satti MZ, Ebbesen F, Vennervald B, Lind P, Ghalib H, Sulaiman S, Daffalla A, Skov PS. Trop Med Int Health. 1996;1:655–666. doi: 10.1111/j.1365-3156.1996.tb00091.x. [DOI] [PubMed] [Google Scholar]
- 29.Falcone FH, Dahinden CA, Gibbs BF, Noll T, Amon U, Hebestreit H, Abrahamsen O, Klaucke J, Schlaak M, Haas H. Eur J Immunol. 1996;26:1147–1155. doi: 10.1002/eji.1830260528. [DOI] [PubMed] [Google Scholar]
- 30.Hofstetter M, Fasano MB, Ottesen EA. J Immunol. 1983;130:1376–1380. [PubMed] [Google Scholar]
- 31.Weinstock JV, Chensue SW, Boros DL. J Immunol. 1983;130:423–427. [PubMed] [Google Scholar]
- 32.Barsoum IS, Dahawi HS, Gamil FM, Habib M, El Alamy MA, Colley DG. J Immunol. 1984;133:1576–1580. [PubMed] [Google Scholar]
- 33.Gerken SE, Mota-Santos TA, Vaz NM, Correa-Oliveira R, Dias-da-Silva W, Gazzinelli G. Braz J Med Biol Res. 1984;17:301–307. [PubMed] [Google Scholar]
- 34.Bheekha-Escura R, MacGlashan D, Langdon J, MacDonald S. Blood. 2000;96:2191–2198. [PubMed] [Google Scholar]
- 35.Sabin EA, Kopf MA, Pearce EJ. J Exp Med. 1996;184:1871–1878. doi: 10.1084/jem.184.5.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Capron M, Capron A. Mem Inst Oswaldo Cruz. 1992;87:167–170. doi: 10.1590/s0074-02761992000800025. [DOI] [PubMed] [Google Scholar]
- 37.Lichtenberg F, Sher A, Gibbons N, Doughty BL. Am J Pathol. 1976;84:479–500. [PMC free article] [PubMed] [Google Scholar]
- 38.Owhashi M, Maruyama H, Nawa Y. Int J Parasitol. 1996;26:705–711. doi: 10.1016/0020-7519(96)00054-9. [DOI] [PubMed] [Google Scholar]
- 39.Horii Y, Owhashi M, Ishii A. Parasitol Res. 1990;76(Suppl 4):602–605. doi: 10.1007/BF00932570. [DOI] [PubMed] [Google Scholar]
- 40.Ercoli N, Payares G, Nunez D. Exp Parasitol. 1985;59:204–216. doi: 10.1016/0014-4894(85)90074-8. [DOI] [PubMed] [Google Scholar]
- 41.Hamdan FF, Abramovitz M, Mousa A, Xie J, Durocher Y, Ribeiro P. Mol Biochem Parasitol. 2002;119:75–86. doi: 10.1016/s0166-6851(01)00400-5. [DOI] [PubMed] [Google Scholar]
- 42.Shuhua X, Tanner M, N’Goran EK, Utzinger J, Chollet J, Bergquist R, Minggang C, Jiang Z. Acta Trop. 2002;82:175–181. doi: 10.1016/s0001-706x(02)00009-8. [DOI] [PubMed] [Google Scholar]