Abstract
Treg from mice bearing a breast tumor were elevated (tumor Treg). In vitro, whereas tumor Treg ability to inhibit tumor-primed CD4+ T cell activity is comparable to Treg from naïve mice (naïve Treg), only tumor Treg suppress naïve CD8+ T cell activation and DC function. Neither tumor Treg nor naïve Treg can suppress antitumor immunity at the effector phase of the immune response induced by adoptively-transferred tumor-primed CD4+ T cells. This is consistent with the observation that, in this model, neither tumor Treg nor naïve Treg can inhibit effectors in vitro or in vivo. However, tumor Treg abrogate tumor-specific CD8+ T cell responses in TDLN and antitumor immunity at the early stage of the immune response induced by adoptively-transferred tumor-primed CD4+ T cells. These data indicate that, in this model, tumor Treg potently abrogate tumor-specific CD8+ T cell responses in TDLN, thereby suppressing antitumor immunity at the early stage of the immune response induced by adoptively-transferred tumor-primed CD4+ T cells.
Keywords: Treg, Antitumor immunity
Introduction
CD4+CD25+ regulatory T cells (Treg)3 constitute 5–10% of peripheral CD4+ T cells in naïve mice (1). Tumors may differentiate, expand, recruit and activate Treg (tumor Treg) via multiple mechanisms (2–5). The frequency of tumor Treg is commonly elevated in tumors, peripheral blood or lymphoid organs of many tumor-bearing hosts (2). When compared with naïve Treg, suppressor functions of tumor Treg have been reported to be ineffective, comparable or enhanced in various tumor models (5–11). Tumor Treg may promote local tumor growth at tumor sites, and may be relevant to the progression of systemic disease in the peripheral blood or lymphoid organs (12). Thus, tumor Treg may be a major obstacle for immunotherapy of cancer (2, 4–5). It has become clear that understanding the mechanisms of action by Treg in tumor immunity is critical in developing an effective tumor vaccine or immunotherapy (2, 5).
Treg suppress multiple cells such as CD4+ and CD8+ T cells, B cells, NK cells, NKT cells, dendritic cells (DC) and macrophages (13–17). Cell targets and stages of the immune response that are critical for Treg-mediated suppression are still unclear (13–17). Several biological events of Treg-mediated suppression including inhibition of proliferation, cytokine production, differentiation, and migration by effectors have been reported (13–17). Also, down-regulation of expression of costimulatory molecules on DC, disruption of stable naïve T-DC contact, induction of tolerogenic antigen-presenting cells (APC), reduction of DC ability to present Ag and stimulate T cell proliferation, and control of cytotoxicity of tumor-infiltrating DC have been suggested (13–18). Several mechanisms of Treg-mediated suppression have been proposed: 1) a yet unknown direct cell-cell contact, 2) killing B cells, NK or CD8+ T cells via molecules such as perforin or granzyme B, 3) metabolic disruption via molecules such as CD39 or CD73, 4) modulating DC via molecules such as CTLA-4, LFA-1, LAG-3 or neuropilin-1, 5) indirect suppression via cytokines such as IL-10, TGF-β or IL-35, and 6) in vivo consumption of survival and growth-promoting cytokines (13–17, 19–23). Recent reports show that Treg suppress mast cells via cell-cell contact involving OX40-OX40L signaling in mice and kill autologous CD8+ T cells by Fas-mediated apoptosis in humans (24–25).
Multiple mechanisms underlying Treg-mediated immune suppression may be not mutually exclusive, and may vary depending on the nature of the immune response being regulated in various diseases and/or models (13–17). One complication, in particular, is how tumor Treg, which are usually elevated and likely activated during tumor progression, execute suppressor function (2–5).
Adoptive tumor-primed CD4+ T cell transfer induced an effective host CD8+ T cell-dependent tumor protection in an aggressive spontaneous metastatic murine breast tumor model (26). Using this tumor model, we tested the suppressor function of both tumor and naïve Treg in vitro and in vivo.
Materials and Methods
Mice and tumor cell lines
BALB/c mice were purchased from Taconic and housed in specific pathogen-free conditions in the University of Pittsburgh animal facility. All animal procedures were performed according to approved protocols and in accordance with recommendations for the proper use and care of laboratory animals. Murine breast tumor cell 4T1.2-Neu (27) was maintained in DMEM (IRVINE Scientific) supplemented with 10% fetal bovine serum (FBS) (Hyclone), 2mM glutamine (Invitrogen), antibiotic antimycotic solution (Sigma), G-418 (500 μg/ml) (Invitrogen). Murine colon carcinoma cell CT26 (ATCC) was cultured in RPMI 1640 (IRVINE Scientific) supplemented with 10% FBS, 2mM glutamine, antibiotic antimycotic solution.
Treg
6–8 wk old female BALB/c mice were inoculated s.c. with 4T1.2-Neu (1×105) in 20μl endotoxin-free 1xPBS (Sigma) at the 4th mammary fat pad. 3–4 wk post tumor inoculation, CD4+CD25+ T cells were purified from splenocytes of tumor-bearing mice or age-matched naïve BALB/c mice using mouse CD4+CD25+ regulatory T cell isolation kit (Miltenyi Biotec) (Purity was confirmed by flow cytometry and consistently resulted in greater than 95%.). For surface staining, splenocytes or purified CD4+CD25+ T cells were stained by anti-CD4-PETXRED (or -FITC) (GK1.5) and anti-CD25-PE (PC61 or 7D4) or anti-CTLA-4-biotin mAb (UC10-4B9) (streptavidin-FITC as a second Ab) (isotype control of each antibody was used in control staining) (BD Biosciences or eBioscience), and analyzed by flow cytometry. For intracellular staining of CTLA-4, purified tumor CD4+CD25+ or naïve CD4+CD25+ T cells were cultured in RPMI1640 10%FBS in 12-well plate at 37°C, 5%CO2 for 4 d. Monensin (BD Biosciences) (4μl/ml) was added for additional 4 h of culture. Cells were washed and resuspended in Cytoperm/Cytofix solution (BD Biosciences) for 20 min on ice, and subsequently stained using anti-CTLA-4-biotin mAb as described above. The expression of Foxp3 protein in purified tumor CD4+CD25+, tumor CD4+CD25− or naïve CD4+CD25+ T cells was detected using the specific Foxp3 (FJK-16s) intracellular staining kit (eBioscience).
Suppression of tumor-primed CD4+ T cell activity in vitro
Preparation of tumor-primed CD4+ T cells was described previously (26). Briefly, BABL/c mice were injected i.p. with 600μg of anti-CD25 mAb (purified from hybridoma cell PC61 culture supernatants using MAb purification kit). 3 d later, these mice were inoculated s.c with 4T1.2-Neu (1×105) as described above. Tumor-primed CD4+ T cells were purified from splenocytes of anti-CD25 mAb-pretreated mice that rejected the tumor using anti-mouse CD4 micro-beads (Miltenyi Biotec). Purified tumor-primed CD4+ T cells (2×106/ml) were cultured in the presence or absence of tumor Treg, tumor CD4+CD25− T cells or naïve Treg (2–8×106/ml) in RPMI 1640 10%FBS at 37°C, 5% CO2 for 2 d. The concentration of IL-2 in the culture supernatants was determined by ELISA (BD Biosciences).
Suppression of naïve CD8+ T cell activation in vitro
Activation of naïve CD8+ T cells in vitro was described previously (26). Briefly, tumor-primed CD4+ T cells were obtained as described above. CD11c+ DC or CD8+ T cells were purified from splenocytes of naïve BALB/c mice using anti-mouse CD11c or CD8 micro-beads (Miltenyi Biotec). Purified CD11c+ DC were loaded with 4T1.2-Neu tumor Ag. Tumor-primed CD4+ T cells (2×105), tumor Ag-loaded DC (2×105) and naïve CD8+ T cells (2×105) were cultured in the presence or absence of purified tumor CD4+CD25− T cells, tumor Treg or naïve Treg (2–8×105) in 200μl RPMI 1640 10%FBS at 37°C, 5% CO2 for 2 d. The concentration of IFN-γ in the culture supernatants was determined by ELISA (BD Biosciences).
Suppression of DC function in vitro
Naïve splenic CD11c+ DC and tumor-primed CD4+ T cells were prepared as described above. CD11c+ DC (2×105) and tumor-primed CD4+ T cells (2×105) were cultured alone, with tumor Treg or naïve Treg (2–8×105) in the presence of LPS (1μg/ml, Sigma) in 200μl RPMI 1640 10%FBS at 37°C, 5% CO2 for 18 or 48 h. The concentration of IL-12(p40) in the culture supernatant was measured by ELISA (BD Biosciences). These cells were harvested and treated with 5 mM EDTA. Fc receptor binding of Abs was minimized by incubation with rat anti-mouse CD16/CD32 mAb (BD Biosciences) prior to staining with anti-CD11c-APC (HL3) and anti-CD80-PE (16-10A1) or anti-CD86-PE (GL1) (isotype control of each antibody was used in control staining) (BD Biosciences or eBioscience), and analyzed by flow cytometry. Propidium iodide (BD Biosciences) was used to check cell viability. Forward scatter and side scatter was used to exclude cell debris. The flow cytometric data was analyzed using Flowjo software (Tree star).
Suppression of effectors in vitro and in vivo
BALB/c mice were adoptively transferred i.v with tumor-primed CD4+ T cells (1×107). 1 d later, these mice were inoculated s.c. with 4T1.2-Neu (1×105). Primary tumor was observed in all tumor-inoculated mice. Tumor-rejection usually started on d 9 and completed on d 21 post tumor inoculation. To examine Treg-mediated suppression of effectors in vitro, d 21 post tumor inoculation, mice that rejected the tumor were sacrificed and splenic cells (1×105) were restimulated in vitro with mitomycin C-treated 4T1.2-Neu (1×104) in the presence of tumor Treg or naïve Treg (1×105) in 200μl RPMI 1640 10%FBS at 37°C, 5%CO2 for 2–3 d. To examine Treg-mediated suppression of effectors in vivo, d 21 post tumor inoculation, effectors in mice that rejected the tumor were restimulated in vivo by immunization s.c. with mitomycin C-treated 4T1.2-Neu (2×106) and these mice were adoptively transferred i.v. with tumor Treg or naïve Treg (1×107) at the same time. 6 d later, these mice were sacrificed and splenic cells (4×105) were cultured without in vitro stimulation in 200μl RPMI 1640 10%FBS at 37°C, 5%CO2 for 3 d. The concentration of IFN-γ in the culture supernatant was measured by ELISA.
Suppression of tumor-specific CD8+ T cell responses in vivo
Tumor-primed CD4+ T cells (1×107) and tumor Treg or naïve Treg (1×107) were adoptively cotransferred i.v. into naïve BALB/c mice on d −1. These mice were inoculated s.c. with 4T1.2-Neu (1×105) on d 0. On d 5, CD8+ T cells were purified from pooled tumor-draining lymph nodes (TDLN) using anti-mouse CD8 micro-beads. CD11c+ DC were purified from splenocytes of naïve BALB/c mice as descried above, and loaded with 4T1.2-Neu or CT26 tumor Ag (26). Tumor Ag-loaded DC (4×104) and purified CD8+ T cells (1×105) were cultured in 200μl RPMI 1640 10%FBS at 37°C, 5% CO2 for 5 d. The concentration of IFN-γ in the culture supernatants was determined by ELISA.
Suppression of antitumor immunity in vivo
To examine the influence of Treg on antitumor immunity at the early stage of the immune response induced by adoptively-transferred tumor-primed CD4+ T cells, tumor Treg or naïve Treg (1×107) and tumor-primed CD4+ T cells (1×107) were adoptively cotransferred i.v. into naïve BALB/c mice on d −1. These mice were inoculated s.c. with 4T1.2-Neu (1×105) on d 0. To examine the influence of Treg on antitumor immunity at the effector phase of the immune response induced by adoptively-transferred tumor-primed CD4+ T cells, tumor-primed CD4+ T cells (1×107) were adoptively transferred i.v. into naïve BALB/c mice on d −1. These mice were inoculated s.c. with 4T1.2-Neu (1×105) on d 0. Tumor-rejection usually started on d 9 post tumor inoculation, adoptive tumor Treg or naïve Treg (1×107) transfer thus was performed on d 9. Primary tumor was observed in all tumor-inoculated mice and measured by electric caliper in two perpendicular diameters every other day. Mice were sacrificed for humane reasons when a primary tumor reached 10mm in mean diameter, when ulceration, bleeding or both developed, or when mice became ill due to metastatic diseases.
Statistics
We analyzed significance between groups with Student’s t test. For evaluation of data from animal survival studies, we used the Log Rank test. All analyses were performed using Prism software (GraphPad Software, Inc.). P < 0.05 is considered to be statistically significant.
Results
Splenic CD4+CD25+ T cells from tumor-bearing mice are elevated, express CTLA-4 and Foxp3, and exhibit suppressor function
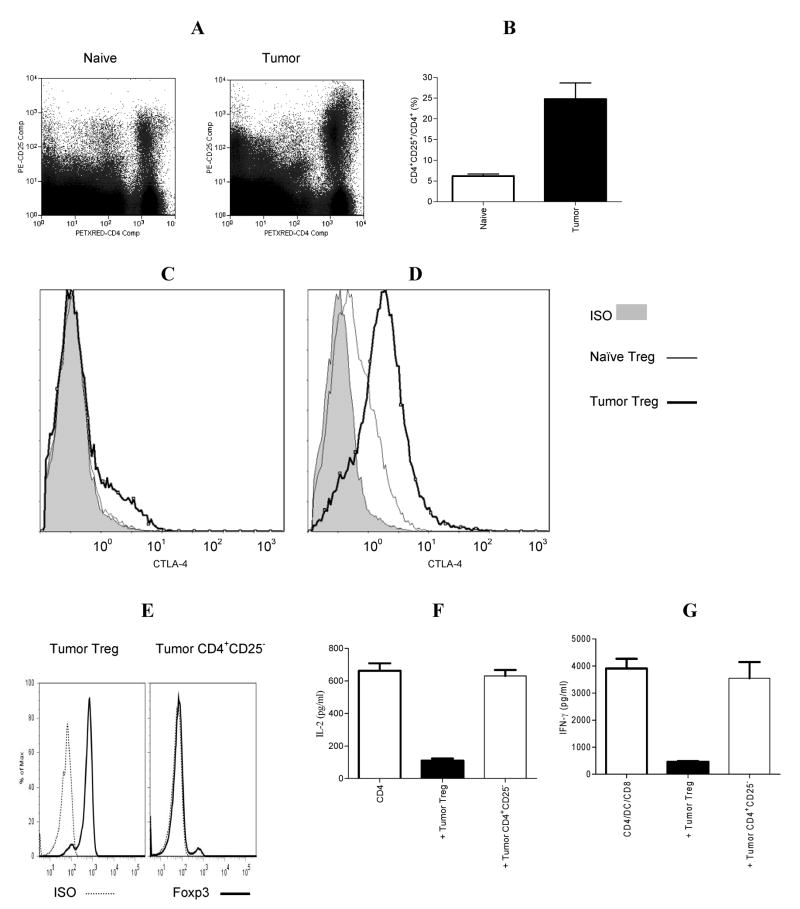
The increased frequency of CD4+CD25+ T cells in TDLN or tumors has been reported in a 4T1 tumor model (11, 28). This population was also elevated in splenocytes of 4T1.2-Neu tumor (a 4T1 related model)-bearing mice when compared with naïve mice (Fig. 1A–B). Tumor progression induced splenomegaly with a marked increase in absolute numbers of CD4+CD25+ T cells after 2-wk tumor inoculation (data not shown). When compared with CD4+ T cells from splenocytes of naïve mice, CD4+ T cells from splenocytes of tumor-bearing mice expressed higher levels of CD25 and CTLA-4 (Fig. 1A, C–D). In line with naïve Treg (data not shown), tumor Treg but not tumor CD4+CD25− T cells expressed Foxp3 (Fig. 1E). Importantly, tumor Treg but not tumor CD4+CD25− T cells suppressed the production of IL-2 by tumor-primed CD4+ T cells and naïve CD8+ T cell activation in vitro (Fig. 1F–G). These data show that, in the breast tumor model, CD4+CD25+ T cells from splenocytes of tumor-bearing mice are actually functional Treg.
FIGURE 1.
CD4+CD25+ T cells from splenocytes of tumor-bearing mice are elevated, express CTLA-4 and Foxp3, and exhibit suppressor function in vitro. A. Splenocytes from tumor-bearing or age-matched naïve mice were stained by anti-CD4-PETXRED and anti-CD25-PE, and analyzed by flow cytometry. One of two independent experiments with similar results is shown. B. The frequency of CD4+CD25+ T cells in CD4+ T cells in naïve (n=4) or tumor-bearing (n=7) mice is shown. Tumor vs. naïve: p<0.005. The surface (C) or intracellular (D) expression of CTLA-4 in purified tumor Treg or naïve Treg is shown in one of two independent experiments with similar results. E. The expression of Foxp3 in tumor Treg or tumor CD4+CD25− T cells is shown in one of three independent experiments with similar results. F. Tumor-primed CD4+ T cells were cultured alone, with tumor Treg or tumor CD4+CD25− T cells. IL-2 in the culture supernatant was determined by ELISA. CD4 vs. CD4 + tumor Treg: p<0.0005. CD4 vs. CD4 + tumor CD4+CD25−: NS. G. Tumor-primed CD4+ T cells, tumor Ag-loaded DC and naïve CD8+ T cells were cultured alone, with tumor Treg or tumor CD4+CD25− T cells. IFN-γ in the culture supernatants was determined by ELISA. CD4/DC/CD8 vs. CD4/DC/CD8 + tumor Treg: p<0.0005; CD4/DC/CD8 vs. CD4/DC/CD8 + tumor CD4+CD25−: NS. The data represents three independent experiments.
Tumor Treg are functionally indistinguishable from naïve Treg in inhibiting tumor-primed CD4+ T cell activity in vitro
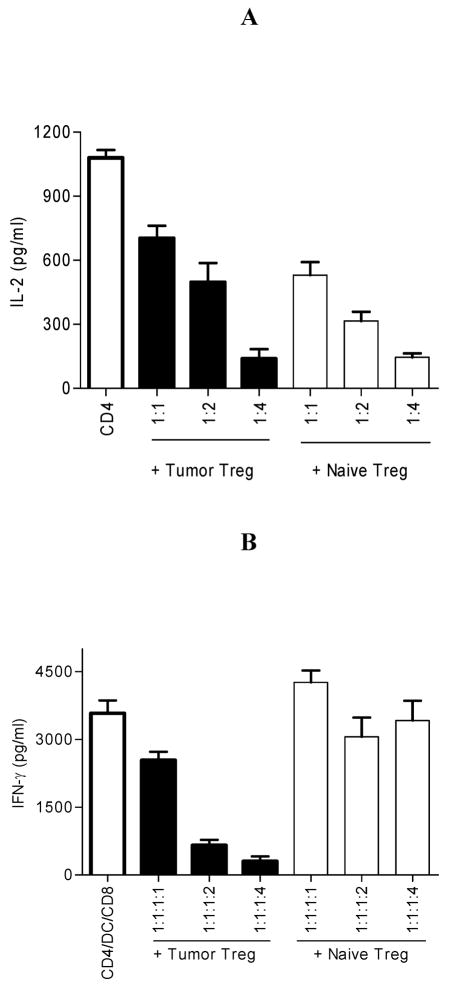
Tumor-primed CD4+ T cells spontaneously produced IL-2 in culture (26). To test whether there was any special property of tumor Treg, tumor-primed CD4+ T cells were cultured alone, with tumor Treg or naïve Treg at ratios of 1:1, 1:2 or 1:4 (CD4:Treg). The production of IL-2 by tumor-primed CD4+ T cells was monitored. As shown in Fig. 2A, both tumor Treg and naïve Treg inhibited the production of IL-2 by tumor-primed CD4+ T cells in a dose-dependent manner. These data suggest that tumor Treg ability to inhibit tumor-primed CD4+ T cell activity is comparable to naïve Treg in vitro.
FIGURE 2.
Suppressor functions of tumor Treg and naïve Treg in vitro. A. Tumor-primed CD4+ T cells were cultured alone, with tumor Treg or naïve Treg at ratios of 1:1, 1:2 or 1:4 (CD4:Treg). IL-2 in the culture supernatants was determined by ELISA. CD4 vs. CD4 + tumor Treg or naïve Treg: p<0.0005. B. Tumor-primed CD4+ T cells, tumor Ag-loaded DC and naïve CD8+ T cells were cultured alone, with tumor Treg or naïve Treg at ratios of 1:1:1:1; 1:1:1:2 or 1:1:1:4 (CD4:DC:CD8:Treg). IFN-γ in the culture supernatant was determined by ELISA. CD4/DC/CD8 vs. CD4/DC/CD8 + tumor Treg: p<0.005. CD4/DC/CD8 vs. CD4/DC/CD8 + naïve Treg: NS. The data represents three independent experiments.
Tumor Treg are functionally distinguishable from naïve Treg in suppressing naïve CD8+ T cell activation in vitro
Naïve CD8+ T cells were activated by tumor Ag-loaded DC in the presence of tumor-primed CD4+ T cells in vitro (26). To test the special property of tumor Treg, we examined tumor Treg or naïve Treg ability to suppress naïve CD8+ T cell activation in vitro. Tumor-primed CD4+ T cells, tumor Ag-loaded DC and naïve CD8+ T cells were cultured alone, with tumor Treg or naïve Treg at ratios of 1:1:1:1, 1:1:1:2 or 1:1:1:4 (CD4:DC:CD8:Treg). IFN-γ in the culture supernatants was monitored. As shown in Fig. 2B, tumor Treg inhibited naïve CD8+ T cell activation in vitro in a dose-dependent manner. However, naïve Treg did not do so. These data show that tumor Treg ability to suppress naïve CD8+ T cell activation is distinguishable from naïve Treg in vitro.
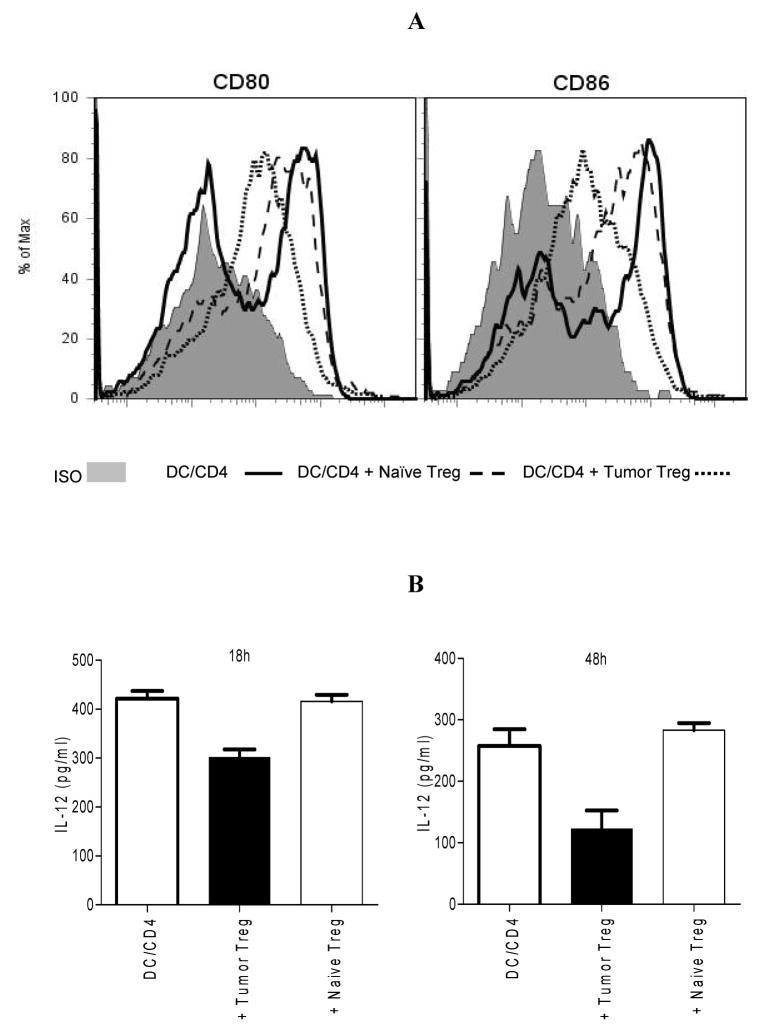
Tumor Treg suppress DC function in vitro
It has been shown that both naïve Treg and tumor Treg inhibited DC function in vitro and in vivo (22, 29–39). To examine the influence of tumor Treg or naïve Treg on DC, we compared the expression of CD80 and CD86 and the production of IL-12 by splenic DC in vitro. Splenic DC and tumor-primed CD4+ T cells were cultured alone, with tumor Treg or naïve Treg in the presence of LPS at ratios of 1:1:1, 1:1:2 or 1:1:4 (DC:CD4:Treg). When compared with naïve Treg, tumor Treg down-regulated CD80 and CD86 expression, and inhibited the production of IL-12 by DC in a dose-independent manner in vitro (Fig. 3A–B, data not shown). These data indicate that tumor Treg potently suppress DC function in vitro.
FIGURE 3.
Tumor Treg suppress DC function in vitro. Splenic DC and tumor-primed CD4+ T cells were cultured alone, with tumor Treg or naïve Treg at a ratio of 1:1:1 (DC:CD4:Treg) in the presence of LPS. A. The data represents one of three independent experiments with similar results showing CD80 or CD86 expression on gated CD11c+ DC. B. IL-12 in the culture supernatant was determined by ELISA. DC/CD4 vs. DC/CD4 + tumor Treg: p<0.0005 (18h), p<0.005 (48h); DC/CD4 vs. DC/CD4 + naïve Treg: NS. The data represents three independent experiments.
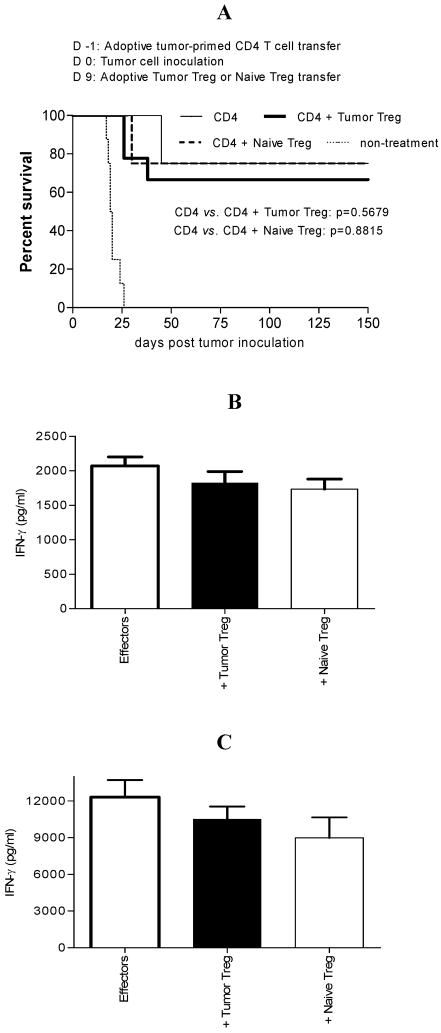
Neither tumor Treg nor naïve Treg suppress antitumor immunity at the effector phase of the immune response induced by adoptively-transferred tumor-primed CD4+ T cells
Adoptive tumor-primed CD4+ T cell transfer ignited potent protective antitumor immunity (26). To determine whether tumor Treg or naïve Treg inhibit antitumor immunity at the effector phase of the immune response, mice were adoptively transferred with tumor-primed CD4+ T cells (1×107) on d −1, and inoculated with tumor cells on d 0. Tumor Treg or naïve Treg (1×107) were adoptively transferred on d 9. As shown in Fig. 4A, neither tumor Treg nor naïve Treg suppressed antitumor immunity at the effector phase of the immune response. To understand the mechanism behind this observation, we examined tumor Treg or naïve Treg ability to inhibit effectors generated by adoptively-transferred tumor-primed CD4+ T cells. Effectors from tumor-rejection mice were cultured with tumor Treg or naïve Treg in vitro. As shown in Fig. 4B, neither tumor Treg nor naïve Treg suppressed effectors in vitro. To confirm this in vivo, tumor Treg or naïve Treg were adoptively transferred into tumor-rejection mice. As shown in Fig. 4C, neither tumor Treg nor naïve Treg suppressed effectors in vivo. These data suggest that, in this model, effectors are resistant to suppression by tumor Treg or naïve Treg. This may result in the failure of Treg-mediated suppression of antitumor immunity at the effector phase of the immune response.
FIGURE 4.
Neither tumor Treg nor naïve Treg suppress antitumor immunity at the effector phase of the immune response induced by adoptively-transferred CD4+ T cells. A. Purified tumor-primed CD4+ T cells were adoptively transferred into naïve BALB/c mice on d −1. These mice were inoculated with tumor cells on d 0. Tumor Treg or naïve Treg were adoptively transferred on d 9. Mice adoptively transferred with tumor-primed CD4+ T cells alone served as a positive control. Mice without treatment served as a negative control. Animal survival is presented using Kaplan-Meier Survival Curves. The data represents two independent experiments with 4–5 mice per group. CD4 (n=8), CD4 + tumor Treg (n=9), CD4 + naïve Treg (n=8), non-treatment (n=8). B. Effectors from tumor-rejection mice were cultured with tumor Treg or naïve Treg in vitro. IFN-γ in the culture supernatant was determined by ELISA. Effectors vs. Effectors + tumor Treg or naïve Treg: NS. The data represents two independent experiments. C. Tumor Treg or naïve Treg were adoptively transferred into tumor-rejection mice, and effectors from these mice were cultured in vitro. IFN-γ in the culture supernatant was determined by ELISA. Effectors vs. Effectors + tumor Treg or naïve Treg: NS. The data represents two independent experiments.
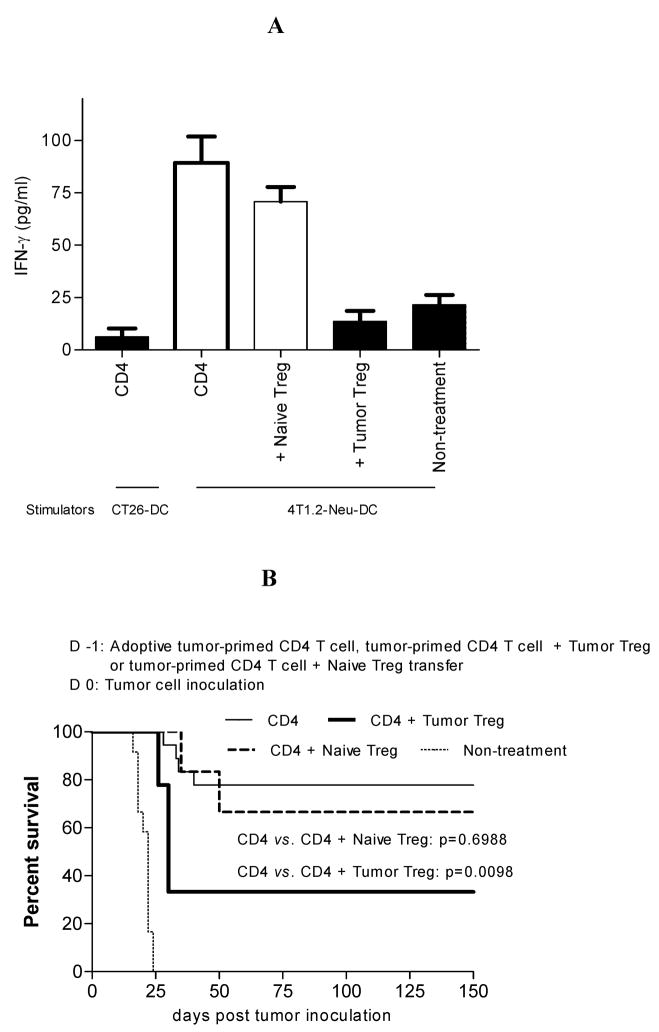
Tumor Treg abrogate tumor-specific CD8+ T cell responses in TDLN and antitumor immunity at the early stage of the immune response induced by adoptively-transferred tumor-primed CD4+ T cells
Next we explored the influence of tumor Treg or naïve Treg on the induction of antitumor immunity. We first tested whether tumor Treg abrogate tumor-specific CD8+ T cell responses induced by adoptively-transferred tumor-primed CD4+ T cells in TDLN. Mice were adoptively cotransferred with tumor-primed CD4+ T cells (1×107) and tumor Treg or naïve Treg (1×107) on d −1, and inoculated with tumor cells on d 0. On d 5, CD8+ T cells were purified from pooled TDLN. The in vivo tumor-specific CD8+ T cell responses were evaluated ex vivo by stimulation of purified CD8+ T cells with tumor Ag-loaded DC. As shown in Fig. 5A, CD8+ T cells purified from TDLN of mice that were adoptively transferred by tumor-primed CD4+ T cells alone or cotransferred by tumor-primed CD4+ T cells and naïve Treg responded to a tumor-specific restimulation. However, CD8+ T cells purified from TDLN of mice that were adoptively cotransferred by tumor-primed CD4+ T cells and tumor Treg did not do so (Fig. 5A). We next examined whether tumor Treg suppress antitumor immunity at the early stage of the immune response. Mice were adoptively cotransferred with tumor-primed CD4+ T cells (1×107) and tumor Treg or naïve Treg (1×107) on d −1, and inoculated with tumor cells on d 0. As shown in Fig. 5B, only tumor Treg inhibited antitumor immunity at the early stage of the immune response. These data indicate that, in this model, tumor Treg potently abrogate tumor-specific CD8+ T cell responses in TDLN and antitumor immunity at the early stage of the immune response induced by adoptively-transferred tumor-primed CD4+ T cells.
FIGURE 5.
Tumor Treg abrogate tumor-specific CD8+ T cell responses in TDLN and antitumor immunity at the early stage of the immune response induced by adoptively-transferred tumor-primed CD4+ T cells. A. Mice were adoptively cotransferred with tumor-primed CD4+ T cells and tumor Treg or naïve Treg on d −1, and inoculated with tumor cells on d 0. Mice adoptively transferred with tumor-primed CD4+ T cells alone served as a positive control. Mice without treatment served as a negative control. On d 5, CD8+ T cells purified from TDLN were restimulated by 4T1.2-Neu- or CT26-loaded DC in vitro. IFN-γ in the culture supernatants was determined by ELISA. CD4 vs. non-treatment: p<0.0005; CD4 vs. CD4 + tumor Treg: p<0.0005; CD4 vs. CD4 + naïve Treg: NS. The data represents two independent experiments with 3–4 mice per group. B. Mice were adoptively cotransferred with tumor-primed CD4+ T cells and tumor Treg or naïve Treg on d −1, and inoculated with tumor cells on d 0. Mice adoptively transferred with tumor-primed CD4+ T cells alone served as a positive control. Mice without treatment served as a negative control. Animal survival is presented using Kaplan-Meier Survival Curves. The data represents two independent experiments with 3–5 (with Treg) to 6–9 (without Treg) mice per group. CD4 (n=18), CD4 + tumor Treg (n=9), CD4 + naïve Treg (n=6), non-treatment (n=12).
Discussion
Both Treg and effectors could be primed during tumor progression (40–41). Thus, Foxp3 expression together with suppressor function (a cardinal feature of Treg) may be the best way to judge whether tumor CD4+CD25+ T cells are actually Treg (14). In this report, CD4+CD25+ but not CD4+CD25− T cells from splenocytes of tumor-bearing mice expressed Foxp3, inhibited tumor-primed CD4+ T cell activity and CD8+ T cell activation (Fig. 1), suggesting they are functional Treg.
During tumor progression, tumors may trigger Treg activation in vivo (3, 5). The function of Treg from tumor-bearing mice was different from naïve Treg activated by anti-CD3/IL-2 in vitro (42). Suppressive activity of Treg from cancer patients was enhanced when compared with Treg from healthy donors (9–10). In a related 4T1 model, suppressive activity of tumor-infiltrating Treg was increased (11). It is therefore interesting to know whether tumor Treg are functionally distinguishable from naïve Treg (5). Treg are usually expanded and activated in vitro, and adoptively transferred into mice to examine their function in vivo. In this breast tumor model, sufficient Treg were obtained from splenocytes (but not lymph nodes or tumors) of tumor-bearing mice at a later stage of tumor progression (3–4 wk post tumor inoculation) which made it possible to evaluate suppressor function of tumor Treg expanded and activated under a physiological (tumor progression) condition.
With regard to the inhibition of the production of IL-2 by tumor-primed CD4+ T cells in vitro, tumor Treg was comparable to naïve Treg (Fig. 2A). However, only tumor Treg suppressed naïve CD8+ T cell activation in vitro (Fig. 2B). Naïve Treg must be activated to exhibit suppressor function in vitro (43). In this model, naïve Treg were cultured with tumor-primed CD4+ T cells or tumor-primed CD4+ T cells and tumor Ag-loaded DC, and exhibited suppressor function (Fig. 2A), indicating that naïve Treg were activated during ex vivo culture since tumor-primed CD4+ T cells spontaneously produced IL-2 (Figs. 1D and 2A, 26), which is required to activate Treg (43–44).
DC have been suggested to be the most relevant targets of Treg in vivo (33–34). Consistently with others (35), we found that tumor Treg hindered DC ability to stimulate T cell proliferation in vitro and DC incubated with tumor Treg in vitro induced less robust Ag-specific T cell responses in vivo after vaccination (data not shown). A recent report indicates that naïve Treg in vitro down-regulate CD80 or CD86 expression on splenic DC at a ratio of 1:10 (DC:Treg) (39). In this study, tumor Treg in vitro down-regulated CD80 or CD86 expression and reduced the production of IL-12 by splenic DC at a ratio of 1:1 (DC:Treg) (Fig. 3), indicating a potent suppressor function of tumor Treg. These data suggest that tumor Treg might be important in disarming DC function, although whether their effects on DC account for immunopathology is still an open question to be determined.
It has been speculated that the mechanisms of Treg-mediated suppression observed in vitro may be different from the mechanisms operative in vivo (23, 45–46). Therefore, it is important to examine the function of tumor Treg and naïve Treg in vivo. Treg depletion in tumors resulted in an advanced tumor regression and adoptive transfer of purified Treg inhibited adoptively-transferred tumor-specific CD8+ T cell- or NK cell-mediated antitumor immunity in vivo (19, 47–49). These data suggest that tumor Treg or naïve Treg suppress antitumor effectors. In this model, neither tumor Treg nor naïve Treg impaired antitumor immunity at the effector phase of the immune response induced by adoptively-transferred tumor-primed CD4+ T cells (Fig. 4A). This correlated with the observation that effectors from tumor-rejection mice were resistant to suppression by tumor Treg or naïve Treg in vitro or in vivo (Fig. 4B–C). Thus, strategies aimed at generating effectors that are resistant to Treg suppression may be promising for an effective tumor vaccine or immunotherapy.
Treg have been indicated to mediate suppression of T cell immunity at the early stage of the immune response (2, 50). Tumor Treg suppressed naïve CD8+ T cell activation in vitro (Fig. 2B). Furthermore, tumor Treg in vivo suppressed antitumor immunity at the early stage of the immune response induced by adoptively-transferred tumor-primed CD4+ T cells (Fig. 5B). Tumor-primed CD4+ T cells, tumor Treg and naïve Treg were detected in TDLN after adoptive transfer (data not shown). Importantly, tumor Treg (but not naïve Treg) potently abrogated tumor-specific CD8+ T cell responses in TDLN (Fig. 5A). This observation is in line with a report that activated CD8+ T cells were not detectable in TDLN in 4T1 tumor-bearing mice (11). These data indicate that in vivo tumor Treg suppress tumor-specific CD8+ T cell responses at the early stage of the immune response induced by adoptively-transferred tumor-primed CD4+ T cells. This can be interpreted that 1) tumor Treg abrogate naïve CD8+ T cell priming, 2) CD8+ T cells primed in the presence of tumor Treg are anergic or 3) tumor Treg suppress function and/or differentiation of primed CD8+ T cells. The precise mechanisms of action by tumor Treg in TDLN will be investigated in the future studies. It is now recognized that Treg function may be compartmentalized (15, 51–52). How the function of tumor Treg from other compartments compares to that of the spleen needs further investigation.
In summary, in this study, in vitro data show the comparable ability between tumor Treg and naïve Treg to inhibit tumor-primed CD4+ T cell activity, and the potent ability of tumor Treg to suppress CD8+ T cell activation and hinder DC function. In vivo results demonstrate that tumor Treg are potent to abrogate tumor-specific CD8+ T cell responses in TDLN and suppress antitumor immunity at the early stage of the immune response induced by adoptively-transferred tumor-primed CD4+ T cells. Collectively, these data suggest that tumor Treg effectively abrogate antitumor immunity.
Acknowledgments
We are indebted to H. Shen, D. Falkner, J. Chen, H. Noh and C. Donahue (University of Pittsburgh) for their help, and the University of Pittsburgh Experimental Animal Facility technicians for animal care.
Footnotes
This work was supported by a start-up fund from Department of Dermatology of The University of Pittsburgh, and by NIH grant R01CA108813 (to Z.Y.), P01CA73743, R01AI060008, and R01CA106662 (to L.D.F.).
Abbreviation used in this paper: Treg, CD4+CD25+ regulatory T cells; DC, dendritic cells; TDLN, tumor-draining lymph nodes; APC, antigen-presenting cells
Disclosures
The authors have no financial conflict of interest.
References
- 1.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor -chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 2.Zou W. Regulatory T cells, tumor immunity and immunotherapy. Nat Rev Immunol. 2006;6:295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]
- 3.Munn DH, Mellor AL. The tumor-draining lymph node as an immune-privileged site. Immunol Rev. 2006;213:146–158. doi: 10.1111/j.1600-065X.2006.00444.x. [DOI] [PubMed] [Google Scholar]
- 4.Knutson KL, Disis ML, Salazar LG. CD4 regulatory T cells in human cancer pathogenesis. Cancer Immunol Immunother. 2007;56:271–285. doi: 10.1007/s00262-006-0194-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Curiel TJ. Regulatory T cells and treatment of cancer. Curr Opin Immunol. 2008;20:241–246. doi: 10.1016/j.coi.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turk MJ, Guevara-Patiño JA, Rizzuto GA, Engelhorn ME, Sakaguchi S, Houghton N. Concomitant tumor immunity to a poorly immunogenic melanoma is prevented by regulatory T cells. J Exp Med. 2004;200:771–782. doi: 10.1084/jem.20041130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lutsiak CME, Tagaya Y, Adams AJ, Schlom J, Sabzevari H. Tumor-Induced Impairment of TCR Signaling Results in Compromised Functionality of Tumor-Infiltrating Regulatory T Cells. J Immunol. 2008;180:5871–5881. doi: 10.4049/jimmunol.180.9.5871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Webster WS, Thompson RH, Harris KJ, Frigola X, Kuntz S, Inman BA, Dong H. Targeting Molecular and Cellular Inhibitory Mechanisms for Improvement of Antitumor Memory Responses Reactivated by Tumor Cell Vaccine. J Immunol. 2007;179:2860–2869. doi: 10.4049/jimmunol.179.5.2860. [DOI] [PubMed] [Google Scholar]
- 9.Hilchey SP, De A, Rimsza LM, Bankert RB, Bernstein SH. Follicular Lymphoma Intratumoral CD4+CD25+GITR+ Regulatory T Cells Potently Suppress CD3/CD28-Costimulated Autologous and Allogeneic CD8+CD25 and CD4+CD25 T Cells. J Immunol. 2007;178:4051–4061. doi: 10.4049/jimmunol.178.7.4051. [DOI] [PubMed] [Google Scholar]
- 10.Yokokawa J, Cereda V, Remondo C, Gulley JL, Arlen PM, Schlom J, Tsang KY. Enhanced functionality of CD4+CD25highFoxP3+ regulatory T cells in the peripheral blood of patients with prostate cancer. Clin Cancer Res. 2008;14:1032–1040. doi: 10.1158/1078-0432.CCR-07-2056. [DOI] [PubMed] [Google Scholar]
- 11.Chaput N, Darrasse-Jéze G, Bergot AS, Cordier C, Ngo-Abdalla S, Klatzmann D, Azogui O. Regulatory T Cells Prevent CD8 T Cell Maturation by Inhibiting CD4 Th Cells at Tumor Sites. J Immunol. 2007;179:4969–4978. doi: 10.4049/jimmunol.179.8.4969. [DOI] [PubMed] [Google Scholar]
- 12.Liyanage UK, Moore TT, Joo HG, Tanaka Y, Herrmann V, Doherty G, Drebin JA, Strasberg SM, Eberlein TJ, Goedegebuure PS, Linehan DC. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol. 2002;169:2756–2761. doi: 10.4049/jimmunol.169.5.2756. [DOI] [PubMed] [Google Scholar]
- 13.Shevach EM. CD4+CD25+ suppressor T cells: more questions than answers. Nat Rev Immunol. 2002;2:389–400. doi: 10.1038/nri821. [DOI] [PubMed] [Google Scholar]
- 14.Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6:345–352. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 15.von Boehmer H. Mechanisms of suppression by suppressor T cells. Nat Immunol. 2005;6:338–344. doi: 10.1038/ni1180. [DOI] [PubMed] [Google Scholar]
- 16.Tang Q, Bluestone JA. The Foxp3+ regulatory T cell: a jack of all trades, master of regulation. Nat Immunol. 2008;9:239–244. doi: 10.1038/ni1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vignali DAA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8:523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roux S, Apetoh L, Chalmin F, Ladoire S, Mignot G, Puig PE, Lauvau G, Zitvogel L, Martin F, Chauffert B, Yagita H, Solary E, Ghiringhelli F. CD4+CD25+ Tregs control the TRAIL-dependent cytotoxicity of tumor-infiltrating DCs in rodent models of colon cancer. J Clin Invest. 2008;118:3751–3761. doi: 10.1172/JCI35890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cao X, Cai SF, Fehniger TA, Song J, Collins LI, Piwnica-Worms DR, Ley TJ. Granzyme B and Perforin Are Important for Regulatory T Cell-Mediated Suppression of Tumor Clearance. Immunity. 2007;27:635–646. doi: 10.1016/j.immuni.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 20.Sarris M, Andersen KG, Randow F, Mayr L, Betz AG. Neuropilin-1 Expression on Regulatory T Cells Enhances Their Interactions with Dendritic Cells during Antigen Recognition. Immunity. 2008;28:402–413. doi: 10.1016/j.immuni.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liang B, Workman C, Lee J, Chew C, Dale BM, Colonna L, Flores M, Li N, Schweighoffer E, Greenberg S, Tybulewicz V, Vignali D, Clynes R. Regulatory T Cells Inhibit Dendritic Cells by Lymphocyte Activation Gene-3 Engagement of MHC Class II. J Immunol. 2008;180:5916–5926. doi: 10.4049/jimmunol.180.9.5916. [DOI] [PubMed] [Google Scholar]
- 22.Onishi Y, Fehervari Z, Yamaguchi T, Sakaguchi S. Foxp3+ natural regulatory T cells preferentially form aggregates on dendritic cells in vitro and actively inhibit their maturation. Proc Natl Acad Sci USA. 2008;105:10113–10118. doi: 10.1073/pnas.0711106105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pandiyan P, Zheng L, Ishihara S, Reed J, Lenardo MJ. CD4+CD25+Foxp3+ regulatory T cells induce cytokine deprivation-mediated apoptosis of effector CD4+ T cells. Nat Immunol. 2007;8:1353–1362. doi: 10.1038/ni1536. [DOI] [PubMed] [Google Scholar]
- 24.Gri G, Piconese S, Frossi B, Manfroi V, Merluzzi S, Tripodo C, Viola A, Odom S, Rivera J, Colombo MP, Pucillo CE. CD4+CD25+ Regulatory T Cells Suppress Mast Cell Degranulation and Allergic Responses through OX40-OX40L Interaction. Immunity. 2008;29:771–781. doi: 10.1016/j.immuni.2008.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Strauss L, Bergmann C, Whiteside TL. Human Circulating CD4+CD25highFoxp3+ Regulatory T Cells Kill Autologous CD8+ but Not CD4+ Responder Cells by Fas-Mediated Apoptosis. J Immunol. 2009;182:1469–1480. doi: 10.4049/jimmunol.182.3.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Z, Noh HS, Chen J, Kim JH, Falo LD, Jr, You Z. Potent Tumor-Specific Protection Ignited by Adoptively Transferred CD4+ T Cells. J Immunol. 2008;181:4363–4370. doi: 10.4049/jimmunol.181.6.4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim JH, Majumder N, Lin H, Chen J, Falo LD, Jr, You Z. Enhanced immunity by NeuEDhsp70 DNA vaccine is needed to combat an aggressive spontaneous metastatic breast cancer. Mol Ther. 2005;11:941–949. doi: 10.1016/j.ymthe.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 28.Valzasina B, Piconese S, Guiducci C, Colombo MP. Tumor-induced expansion of regulatory T cells by conversion of CD4+CD25− lymphocytes is thymus and proliferation independent. Cancer Res. 2006;66:4488–4495. doi: 10.1158/0008-5472.CAN-05-4217. [DOI] [PubMed] [Google Scholar]
- 29.Cederbom L, Hall H, Ivars F. CD4+CD25+ regulatory T cells down-regulate co-stimulatory molecules on antigen presenting cells. Eur J Immunol. 2000;30:1538–1543. doi: 10.1002/1521-4141(200006)30:6<1538::AID-IMMU1538>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 30.Misra N, Bayry J, Lacroix-Desmazes S, Kazatchkine MD, Kaveri SV. Cutting edge: human CD4+CD25+ T cells restrain the maturation and antigen-presenting function of dendritic cells. J Immunol. 2004;172:4676–4680. doi: 10.4049/jimmunol.172.8.4676. [DOI] [PubMed] [Google Scholar]
- 31.Houot R, Perrot I, Garcia E, Durand I, Lebecque S. Human CD4+CD25high regulatory T cells modulate myeloid but not plasmacytoid dendritic cells activation. J Immunol. 2006;176:5293–5298. doi: 10.4049/jimmunol.176.9.5293. [DOI] [PubMed] [Google Scholar]
- 32.Veldhoen M, Moncrieffe H, Hocking RJ, Atkins CJ, Stockinger B. Modulation of dendritic cell function by naïve and regulatory CD4+ T cells. J Immunol. 2006;176:6202–6210. doi: 10.4049/jimmunol.176.10.6202. [DOI] [PubMed] [Google Scholar]
- 33.Tang Q, Adams JY, Tooley AJ, Bi M, Fife BT, Serra P, Santamaria P, Locksley RM, Krummel MF, Bluestone JA. Visualizing regulatory T cell control of autoimmune responses in nonobese diabetic mice. Nat Immunol. 2006;7:83–92. doi: 10.1038/ni1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tadokoro CE, Shakhar G, Shen S, Ding Y, Lino AC, Maraver A, Lafaille JJ, Dustin ML. Regulatory T cells inhibit stable contacts between CD4+ T cells and dendritic cells in vivo. J Exp Med. 2006;203:505–511. doi: 10.1084/jem.20050783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Larmonier N, Marron M, Zeng Y, Cantrell J, Romanoski A, Sepassi M, Thompson S, Chen X, Andreansky S, Katsanis E. Tumor-derived CD4(+)CD25(+) regulatory T cell suppression of dendritic cell function involves TGF-beta and IL-10. Cancer Immunol Immunother. 2007;56:48–59. doi: 10.1007/s00262-006-0160-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bayry J, Triebel F, Kaveri SV, Tough DF. Human dendritic cells acquire a semimature phenotype and lymph node homing potential through interaction with CD4+CD25+ regulatory T cells. J Immunol. 2007;178:4184–4193. doi: 10.4049/jimmunol.178.7.4184. [DOI] [PubMed] [Google Scholar]
- 37.Mahnke K, Ring S, Johnson TS, Schallenberg S, Schönfeld K, Storn V, Bedke T, Enk AH. Induction of immunosuppressive functions of dendritic cells in vivo by CD4+CD25+ regulatory T cells: Role of B7-H3 expression and antigen presentation. Eur J Immunol. 2007;37:2117–2126. doi: 10.1002/eji.200636841. [DOI] [PubMed] [Google Scholar]
- 38.Hänig J, Lutz MB. Suppression of Mature Dendritic Cell Function by Regulatory T Cells In Vivo Is Abrogated by CD40 Licensing. J Immunol. 2008;180:1405–1413. doi: 10.4049/jimmunol.180.3.1405. [DOI] [PubMed] [Google Scholar]
- 39.Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, Nomura T, Sakaguchi S. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322:271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 40.Hiura T, Kagamu H, Miura S, Ishida A, Tanaka H, Tanaka J, Gejyo F, Yoshizawa H. Both regulatory T cells and antitumor effector T cells are primed in the same draining lymph nodes during tumor progression. J Immunol. 2005;175:5058–5066. doi: 10.4049/jimmunol.175.8.5058. [DOI] [PubMed] [Google Scholar]
- 41.Bui JD, Uppaluri R, Hsieh CS, Schreiber RD. Comparative Analysis of Regulatory and Effector T Cells in Progressively Growing versus Rejecting Tumors of Similar Origins. Cancer Res. 2006;66:7301–7309. doi: 10.1158/0008-5472.CAN-06-0556. [DOI] [PubMed] [Google Scholar]
- 42.Sharma MD, Baban B, Chandler P, Hou DY, Singh N, Yagita H, Azuma M, Blazar BR, Mellor AL, Munn DH. Plasmacytoid dendritic cells from mouse tumor-draining lymph nodes directly activate mature Tregs via indoleamine 2, 3-dioxygenase. J Clin Invest. 2007;117:2570–2582. doi: 10.1172/JCI31911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thornton AM, Donovan EE, Piccirillo CA, Shevach EM. IL-2 is critically required for the in vitro activation of CD4+CD25+ T cell suppressor function. J Immunol. 2004;172:6519–6523. doi: 10.4049/jimmunol.172.11.6519. [DOI] [PubMed] [Google Scholar]
- 44.Brandenburg S, Takahashi T, de la Rosa M, Janke M, Karsten G, Muzzulini T, Orinska Z, Bulfone-Paus S, Scheffold A. IL-2 induces in vivo suppression by CD4+CD25+Foxp3+ regulatory T cells. Eur J Immunol. 2008;38:1643–1653. doi: 10.1002/eji.200737791. [DOI] [PubMed] [Google Scholar]
- 45.Klein L, Khazaie K, von Boehmer H. In vivo dynamics of antigen-specific regulatory T cells not predicted from behavior in vitro. Proc Natl Acad Sci USA. 2003;100:8886–889. doi: 10.1073/pnas.1533365100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oliveira V, Sawitzki B, Chapman S, Appelt C, Gebuhr I, Wieckiewicz J, Long E, Wood KJ. Anti-CD4-mediated selection of Treg in vitro - in vitro suppression does not predict in vivo capacity to prevent graft . Eur J Immunol. 2008;38:1677–1688. doi: 10.1002/eji.200737562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ghiringhelli F, Ménard C, Terme M, Flament C, Taieb J, Chaput N, Puig PE, Novault S, Escudier B, Vivier E, Lecesne A, Robert C, Blay JY, Bernard J, Caillat-Zucman S, Freitas A, Tursz T, Wagner-Ballon O, Capron C, Vainchencker W, Martin F, Zitvogel L. CD4+CD25+ regulatory T cells inhibit natural killer cell functions in a transforming growth factor–β–dependent manner. J Exp Med. 2005;202:1075–1085. doi: 10.1084/jem.20051511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Antony PA, Piccirillo CA, Akpinarli A, Finkelstein SE, Speiss PJ, Surman DR, Palmer DC, Chan CC, Klebanoff CA, Overwijk WW, Rosenberg SA, Restifo NP. CD8+ T Cell Immunity Against a Tumor/Self-Antigen Is Augmented by CD4+ T Helper Cells and Hindered by Naturally Occurring T Regulatory Cells. J Immunol. 2005;174:2591–2601. doi: 10.4049/jimmunol.174.5.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yu P, Lee Y, Liu W, Krausz T, Chong A, Schreiber H, Fu YX. Intratumor depletion of CD4+ cells unmasks tumor immunogenicity leading to the rejection of late-stage tumors. J Exp Med. 2005;201:779–791. doi: 10.1084/jem.20041684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Elpek KG, Lacelle C, Singh NP, Yolcu ES, Shirwan H. CD4+CD25+ T Regulatory Cells Dominate Multiple Immune Evasion Mechanisms in Early but Not Late Phases of Tumor Development in a B Cell Lymphoma Model. J Immunol. 2007;178:6840–6848. doi: 10.4049/jimmunol.178.11.6840. [DOI] [PubMed] [Google Scholar]
- 51.Siegmund K, Feuerer M, Siewert C, Ghani S, Haubold U, Dankof A, Krenn V, Schön MP, Scheffold A, Lowe JB, Hamann A, Syrbe U, Huehn J. Migration matters: regulatory T-cell compartmentalization determines suppressive activity in vivo. Blood. 2005;106:3097–3104. doi: 10.1182/blood-2005-05-1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Strauss L, Bergmann C, Szczepanski M, Gooding W, Johnson JT, Whiteside TL. A unique subset of CD4+CD25highFoxp3+ T cells secreting interleukin-10 and transforming growth factor-beta1 mediates suppression in the tumor microenvironment. Clin Cancer Res. 2007;13:4345–4354. doi: 10.1158/1078-0432.CCR-07-0472. [DOI] [PubMed] [Google Scholar]