Abstract
Objective
Light to moderate alcohol consumption has been widely established to be protective against coronary heart disease (CHD), whereas heavy alcohol consumption has been shown to have a potential detrimental effect. The reduction in risk of CHD associated with light and moderate alcohol intake is generally attributed to the beneficial effects of alcohol on high-density lipoprotein (HDL) cholesterol levels. Previous research in the Atherosclerosis Risk in Communities (ARIC) study showed all levels of alcohol consumption to be protective against CHD in whites but to be associated with an increased risk of CHD in black men. We investigated the ARIC cohort to determine whether risk of incident CHD is influenced by an interaction effect between alcohol intake and genetic variation involved in the regulation of HDL cholesterol. Genes of interest included cholesterol ester transfer protein (CETP), lipoprotein lipase (LPL), hepatic lipase (HL), and paraoxonase-1 (PON1).
Method
Participants were selected from the ARIC study, a prospective investigation of atherosclerosis and its clinical sequelae, involving 15,792 individuals, ages 45–64 years at recruitment (1987–1989). Incident CHD was identified through annual telephone calls and hospital and death certificate surveillance. Because of ethnic differences in the alcohol-CHD relationship observed in the ARIC cohort and pattern of alcohol consumption differences, statistical analyses were evaluated separately for each race-gender stratum (white men/women, black men/women).
Results
Genotype modified the relationship between heavy drinking and CHD risk but did not modify this relationship for light or moderate drinking. Interaction analyses were significant for heavy alcohol intake and PON1 genotype (p = .02) in black men, with a suggested interaction for heavy alcohol intake and CETP genotype (p = .06) in black men. Heavy drinking was associated with an increased risk for CHD in black men with the PON1 QQ and CETP GG genotypes (PON1 hazard rate ratio [HRR] = 17.3, 95% confidence interval [CI]: 1.76–170.2; CETP HRR = 2.23, 95% CI: 1.01–4.91).
Conclusions
Results from the current study suggest that interaction effects between alcohol consumption and HDL cholesterol metabolism gene variation influence the risk of incident CHD in black men. Additional studies are warranted to confirm these findings.
Most studies to date have established light to moderate alcohol consumption to be associated with decreased incidence of coronary heart disease (CHD), but there are exceptions (Fraser and Upsdell, 1981; Gronbaek et al., 2000; Klatsky, 1999; Marmot and Brunner, 1991; Rehm et al., 1997; Rimm et al., 1996; Stampfer et al., 1988; Thun et al., 1997). Although the exact mechanisms for this association are unknown, alcohol consumption has been shown to affect lipoprotein metabolism, hemostasis, and vascular wall functioning, with the protective effects of light and moderate alcohol consumption partially explained by increased plasma high-density lipoprotein (HDL) cholesterol (Hannuksela et al., 2002; Lucas et al., 2005; Savolainen and Kesaniemi, 1995). Previous research in the Atherosclerosis Risk in Communities (ARIC) study examined the association between different strata of alcohol consumption and risk of incident CHD (Fuchs et al., 2004). According to this previous study, a trend toward a more protective effect against CHD as the amount of alcohol increased was observed only in white men and women, compared with nondrinkers. In black men, CHD risk was increased for all alcohol-intake groups compared with nondrinkers, but no specific trend was observed (Fuchs et al., 2004). The infrequent drinking among black women precluded any conclusions about the alcohol-CHD relationship in this group (Fuchs et al., 2004).
Genes involved in the regulation of HDL cholesterol may mediate some of the cardioprotective effects of alcohol, with alcohol affecting lipoprotein levels only in the presence of certain genotypes. The aim of the present study was to determine whether risk of incident CHD is influenced by an interaction effect between alcohol consumption and genetic variation involved in the regulation of HDL. Genes coding for proteins involved in HDL cholesterol metabolism included in the present study were cholesterol ester transfer protein (CETP), lipoprotein lipase (LPL), hepatic lipase (HL), and paraoxonase-1 (PON1).
Many studies have demonstrated a consistent relationship between the CETP Taq1B polymorphism and increased HDL levels (Boekholdt et al., 2005; de Grooth et al., 2004; Ordovas et al., 2000; Stein and Stein, 2005). However, associations between this polymorphism and risk of cardiovascular disease have been contradictory, showing either no effect or a protective effect dependent on metabolic, genetic, and/or environmental contexts (Boekholdt et al., 2005; Carlquist et al., 2003; Freeman et al., 2003; Fumeron et al., 1995; Liu et al., 2002; Ordovas et al., 2000). The LPL S447X polymorphism increases LPL activity and results in increased levels of HDL cholesterol, and the activity of LPL has been shown to be enhanced by both moderate and heavy alcohol intake (Contaldo et al., 1989; Kastelein et al., 1999; Nishiwaki et al., 1994; Taskinen et al., 1982; van Bockxmeer et al., 2001). Associations between LPL genetic variation and CHD have been contradictory, with one study finding a significant relation in women but not in men (Wittrup et al., 1997), one showing a negative association only in men (Gagne et al., 1999), and others detecting a positive association only in men (Kastelein et al., 1998; van Bockxmeer et al., 2001; Wittrup et al., 1999). HL activity has been shown to be either unaffected or reduced by moderate alcohol consumption, which may increase HDL cholesterol. Genetic variation within the HL gene has been associated with carotid wall thickness and CHD (Hokanson et al., 2003; Rundek et al., 2002). Decreased PON1 activity is suggested to be associated with an increased risk of cardiovascular disease (van der Gaag et al., 1999). Studies in both humans and rats have demonstrated low amounts of alcohol to increase PON1 levels and high amounts to decrease PON1 levels compared with no alcohol intake (Rao et al., 2003; Sierksma et al., 2002; van der Gaag et al., 1999). Although many studies have examined the separate association of alcohol consumption and gene variation on CHD, few studies have examined their potential interactions, and even fewer have done so in a longitudinal population-based cohort study.
Method
The ARIC study
Participants were selected from the ARIC study, a prospective investigation of atherosclerosis and its clinical sequelae involving 15,792 individuals, ages 45–64 years at recruitment (1987–1989). Participants were re-examined every 3 years, with the first screen (baseline) occurring in 1987–1989, the second in 1990–1992, the third in 1993–1995, and the fourth and final examination in 1996–1998. Follow-up occurs yearly by telephone to maintain contact with participants and to assess health status of the cohort. Institutional review boards approved the ARIC study, and all participants included in the present analysis provided their written informed consent. A detailed description of the ARIC study design and methods, as well as details on quality assurance for ascertainment and classification of CHD events, have been published elsewhere (ARIC Investigators, 1989; Rosamond et al., 1999; White et al., 1996). Briefly, subjects were selected by probability sampling from four communities: Forsyth County, NC; Jackson, MS; northwestern suburbs of Minneapolis, MN; and Washington County, MD. Incidence of CHD was determined by contacting participants annually to identify hospitalizations during the previous year and by surveying discharge lists from local hospitals and death certificates from state vital statistics offices for potential cardiovascular events (ARIC Investigators, 1989; Rosamond et al., 1999; White et al., 1996). Participants were excluded from analyses (n = 7,004) if they (1) had a positive or unknown history of prevalent CHD or stroke or a history of transient ischemic attack/stroke symptoms at the initial clinic visit; (2) requested nonuse of their DNA for research purposes; (3) had an ethnic background other than white or black; (4) were classified as a former drinker; (5) were classified as a current drinker but reported 0 g per week of alcohol consumed; (6) were missing information for HDL cholesterol levels, alcohol intake, or other covariates included in the analyses; or (7) had insufficient genotype information for all four selected HDL cholesterol metabolism gene polymorphisms. Incident CHD cases were defined as a definite or probable myocardial infarction, a silent myocardial infarction between examinations by electrocardiogram, a definite CHD death, or a coronary revascularization.
Baseline examination and laboratory measures
Seated blood pressure was measured three times with a random-zero sphygmomanometer, and the last two measurements were averaged. Hypertension was defined as systolic blood pressure 140 mm Hg or higher, diastolic blood pressure 90 mm Hg or higher, or current use of antihypertensive medications. Questionnaires and in-person interviews were used to assess use of medications. Diabetes was defined by a fasting glucose level 126 mg/dl or higher, a nonfasting glucose level 200 mg/dl or higher, and/or history of or treatment for diabetes. Body mass index (measured in kilograms per square meter) was calculated from height and weight measurements. Plasma total cholesterol was measured by an enzymatic method (Siedel et al., 1983). HDL cholesterol was measured after dextran-magnesium precipitation of non-HDL lipoproteins (Warnick et al., 1982). Alcohol consumption was ascertained at baseline by means of an interviewer-administered dietary questionnaire. Subjects were asked whether they currently drank alcoholic beverages, and, if not, whether they had done so in the past. The amount of ethanol consumed (in grams per week) was calculated assuming the following alcohol content: 4 oz of wine = 10.8 g; 12 oz of beer = 13.2 g; and 1.5 oz of distilled spirits = 15.1 g. For a drinker who reported less than one drink per week, the alcohol consumption was recorded as 0 g per week. Polymorphisms studied included CETP Taq1B (G > A) (rs708272), HL C-514T (rs1800588), PON1 Q192R (rs662), and LPL S447X (rs328). For each of the polymorphisms studied, genotypes were designated by the two alleles observed (i.e., the three PON1 Q192R genotypes observed were QQ [homozygous wildtype], QR [heterozygous], and RR [homozygous variant]). Genotyping was carried out using standard polymerase chain reaction-based methods. Primer and probe sequences are available from the authors on request. The ARIC study has a rigorous blind duplicate program. Percentage of agreement for blind duplicate data for these polymorphisms was 95% for CETP Taq1B, 96% for HL C-514T and PON1 Q192R, and 98% for LPL S447X.
Statistical analysis
All statistical analyses were conducted using STATA version 9.2 (StataCorp LP, College Station, TX). Because allele frequencies were different between the races and patterns of alcohol consumption were different between genders, all analyses were done separately for each race-gender stratum (black men, black women, white men, and white women). Allele frequencies were estimated by gene counting. Hardy-Weinberg equilibrium expectations (mathematical equations used to describe the relationship between allele and genotype frequencies) were tested using a chi-square goodness-of-fit test. Cox proportional hazards models were used to estimate the hazard rate ratios (HRRs) of CHD. An HRR is a measure of the effect obtained when the outcome variable of interest is time until an event occurs (i.e., CHD). All analyses were performed assuming a dominant mode of inheritance because of the low frequency of participants with homozygous variant genotypes. For each polymorphism, the more frequent allele in whites was designated as wildtype. Subjects with two wildtype alleles were coded as “0” (wildtype genotype) and those with one or more variant alleles were coded as “1” (variant genotypes). All race-gender strata (except black women) were classified into four alcohol intake groups: nondrinkers (0 g per week), light drinkers (1–105 g per week or ~1 drink per day), moderate drinkers (105–210 g per week or ~2 drinks per day), and heavy drinkers (> 210 g per week or > 2 drinks/day). Black women were classified into three groups: nondrinkers, light drinkers, and moderate drinkers, with the few classified as heavy drinkers (n = 16) excluded from further analyses. The nondrinker reference group included only those who never drank (former drinkers were excluded), thus avoiding the potential problem of including former drinkers, which may include persons who have abstained from alcohol because of poor health (the “sick-quitter effect”) (Fuchs et al., 2004; Shaper, 1990). For analyses of CHD cases, follow-up time intervals were defined as the time between the initial clinical visit and the date of the first CHD event. For noncases, follow-up continued until December 31, 2003, the date of death, or the date of last contact if lost to follow-up, whichever came first. Covariates were assessed for statistical significance in the models by the Wald chi-square statistic and included age, center (referring to ARIC community), body mass index, HDL and total cholesterol, and diabetes and hypertension status. Evidence for an alcohol-specific effect of variation was assessed by including Genotype × Alcohol Intake Group interaction terms in the model, with statistical significance assessed in each gene model by the Wald chi-square statistic.
Results
Race and gender-specific characteristics of the study population are presented in Table 1. CETP, LPL, HL, and PON1 genotype distributions for each race-gender stratum were in accordance with Hardy-Weinberg equilibrium expectations. Variant genotypes for CETP and LPL were more common in whites, and variant genotypes for HL and PON1 were more common in blacks. The majority of white men, white women, and black men were classified as light drinkers (48%, 45%, and 36%, respectively), whereas the majority of black women were classified as nondrinkers (80%). More black men were classified as nondrinkers compared with white men (33% vs 16%, respectively), whereas more white women were classified as light and moderate drinkers (45% and 9%, respectively) compared with black women (17% and 3%, respectively).
TABLE 1.
Baseline characteristics of study population, by race and gender
| White | Black | |||||
|---|---|---|---|---|---|---|
| Characteristics | Men | Women | Men | Women | ||
| Age, in years, mean (SEM) | 54.5 (0.1) | 54.0 (0.1) | 53.2 (0.2) | 53.2 (0.1) | ||
| Body mass index, kg/m2, mean (SEM) | 27.3 (0.07) | 26.3 (0.09) | 27.3 (0.2) | 30.7 (0.2) | ||
| HDL cholesterol, mg/dl, mean (SEM) | 44.7 (0.2) | 59.5 (0.3) | 52.8 (0.6) | 58.3 (0.4) | ||
| Total cholesterol, mmol/l, mean (SEM) | 5.47 (0.02) | 5.64 (0.02) | 5.47 (0.04) | 5.60 (0.03) | ||
| Diabetic, n (%) | 234 (8%) | 240 (7%) | 133 (15%) | 304 (18%) | ||
| Hypertensive, n (%) | 783 (26%) | 830 (26%) | 472 (52%) | 891 (54%) | ||
| Alcohol intake, n (%) | ||||||
| None | 475 (16%) | 1,375 (43%) | 298 (33%) | 1,306 (80%) | ||
| Light (~1 drink/day) | 1,431 (48%) | 1,470 (45%) | 322 (36%) | 272 (17%) | ||
| Moderate (~2 drinks/day) | 616 (20%) | 299 (9%) | 151 (17%) | 47 (3%) | ||
| Heavy (>2 drinks/day) | 476 (16%) | 103 (3%) | 131 (14%) | – | ||
| CETP Taq1B (G > A), n (%) | ||||||
| GG | 922 (32%) | 1,030 (33%) | 452 (52%) | 875 (55%) | ||
| AG | 1,427 (49%) | 1,480 (48%) | 338 (39%) | 610 (39%) | ||
| AA | 551 (19%) | 606 (19%) | 73 (9%) | 92 (6%) | ||
| HL C-514T, n (%) | ||||||
| CC | 1,883 (63%) | 2,000 (62%) | 208 (23%) | 370 (23%) | ||
| TC | 947 (32%) | 1,076 (34%) | 454 (51%) | 816 (50%) | ||
| TT | 144 (5%) | 142 (4%) | 236 (26%) | 450 (27%) | ||
| LPL S447X, n (%) | ||||||
| SS | 2,375 (81%) | 2,585 (81%) | 758 (90%) | 1,313 (86%) | ||
| SX | 541 (18%) | 592 (18%) | 82 (9.8%) | 199 (13%) | ||
| XX | 28 (1%) | 22 (1%) | 2 (0.2%) | 10 (1%) | ||
| PON1 Q192R, n (%) | ||||||
| 1,476 (50%) | 1,662 (52%) | 101 (11%) | 195 (12%) | |||
| QR | 1,240 (42%) | 1,295 (40%) | 389 (44%) | 766 (47%) | ||
| RR | 256 (8%) | 261 (8%) | 402 (45%) | 671 (41%) | ||
Notes: CETP = cholesterol ester transfer protein; HL = hepatic lipase; LPL = lipoprotein lipase; PON1 = paraoxonase-1.
Cox proportional hazards models for incident CHD in each race-gender stratum showed significant effect modification of the genetic polymorphisms on the relationship between heavy drinking and incident CHD in black men only. There was no effect modification by the genetic polymorphisms and the relationship between light or moderate drinking on incident CHD. Specifically, there was a significant PON1 Genotype × Heavy Alcohol Intake interaction (p = .02) in black men only (Table 2). There was a suggested interaction effect for CETP Genotype × Heavy Alcohol Intake in black men (p = .06). Noting that the two interaction effects were detected in one race-gender stratum, additional analyses were performed only in black men for the CETP and PON1 genes.
TABLE 2.
Genotype × Alcohol Intake Group interaction p values for each gene and race-gender stratum
| Interaction p values | ||||
|---|---|---|---|---|
| Gene | Race-gender stratum | Genotype × Light Alcohol Intake |
Genotype × Moderate Alcohol Intake |
Genotype × Heavy Alcohol Intake |
| CETP | White men | .4 | .3 | .2 |
| White women | .3 | .7 | .3 | |
| Black men | .9 | .9 | .06 | |
| Black women | .4 | 1.0 | – | |
| HL | White men | .5 | .3 | .2 |
| White women | .9 | .1 | 1.0 | |
| Black men | .6 | .6 | .1 | |
| Black women | .6 | 1.0 | – | |
| LPL | White men | .6 | 1.0 | .2 |
| White women | .4 | .3 | .7 | |
| Black men | .2 | .2 | .1 | |
| Black women | .5 | 1.0 | – | |
| PON1 | White men | .3 | .8 | .6 |
| White women | 1.0 | .1 | .7 | |
| Black men | .2 | .7 | .02 | |
| Black women | 1.0 | 1.0 | – | |
Notes: CETP = cholesterol ester transfer protein; HL = hepatic lipase; LPL = lipoprotein lipase; PON1 = paraoxonase-1.
Table 3 provides the HRRs for each alcohol intake group (relative to the no alcohol intake group) for black men stratified by genotype. Note that the significant interaction or effect modification of genotype was seen only for the heavy drinkers; the influence of light or moderate drinking on incident CHD was not modified by genotype (Table 2). After adjustment for age and center (Model 1), heavy drinking (compared with no drinking) was a significant predictor of incident CHD in black men with the PON1 QQ genotype (HRR = 6.64, 95% confidence interval [CI]: 1.04–42.4), with significance remaining after adjustment for established CHD risk factors (Model 2: HRR = 17.3, 95% CI: 1.76–170.2). Heavy drinking was not significantly associated with incident CHD in black men with the PON1 variant genotypes (QR and RR). Heavy drinking (compared with no drinking) was a significant predictor of incident CHD in black men with the CETP GG genotype after adjustment for established CHD risk factors (Model 2: HRR = 2.23, 95% CI: 1.01–4.91). Heavy drinking was not significantly associated with incident CHD in black men with the CETP variant genotypes (AG and AA).
TABLE 3.
Relationship between alcohol intake and incident CHD case status in black men, by genotype
| Genotype | Genotype counts |
Alcohol intake group |
Model 1a HRR (95% CI), p |
Model 2b HRR (95% CI), p |
|---|---|---|---|---|
| CETP | ||||
| GG | 152 | None | 1 | 1 |
| 164 | Light | 1.36 (0.73–2.54), .3 | 1.41 (0.75–2.65), .3 | |
| 73 | Moderate | 1.69 (0.81–3.55), .2 | 1.67 (0.78–3.56), .2 | |
| 63 | Heavy | 2.08 (0.96–4.47), .06 | 2.23 (1.01–4.91), .05 | |
| AG + AA | 133 | None | 1 | 1 |
| 145 | Light | 1.25 (0.66–2.36), .5 | 1.40 (0.74–2.65), .3 | |
| 68 | Moderate | 1.72 (0.84–3.53), .1 | 2.03 (0.92–4.02), .1 | |
| 65 | Heavy | 0.77 (0.28–2.10), .6 | 0.81 (0.30–2.23), .7 | |
| PON1 | ||||
| 33 | None | 1 | 1 | |
| 43 | Light | 3.08 (0.63–14.9), .2 | 3.91 (0.74–20.7), .1 | |
| 14 | Moderate | 2.80 (0.39–20.0), .3 | 3.47 (0.43–28.2), .2 | |
| 11 | Heavy | 6.64 (1.04–42.4), .04 | 17.3 (1.76–170.2), .01 | |
| QR + RR | 263 | None | 1 | 1 |
| 275 | Light | 1.26 (0.79–2.01), .3 | 1.35 (0.85–2.16), .2 | |
| 135 | Moderate | 1.87 (1.11–3.16), .02 | 2.03 (1.19–3.45), .01 | |
| 118 | Heavy | 1.17 (0.61–2.25), .6 | 1.29 (0.67–2.47), .4 |
Notes: HRR = hazard rate ratio; 95% CI = 95% confidence interval; CHD = coronary heart disease; CETP = cholesterol ester transfer protein; PON1 = paraoxonase-1.
Adjusted for age and center;
adjusted for age, center, body mass index, high density lipoprotein and total cholesterol, diabetes, and hypertension status.
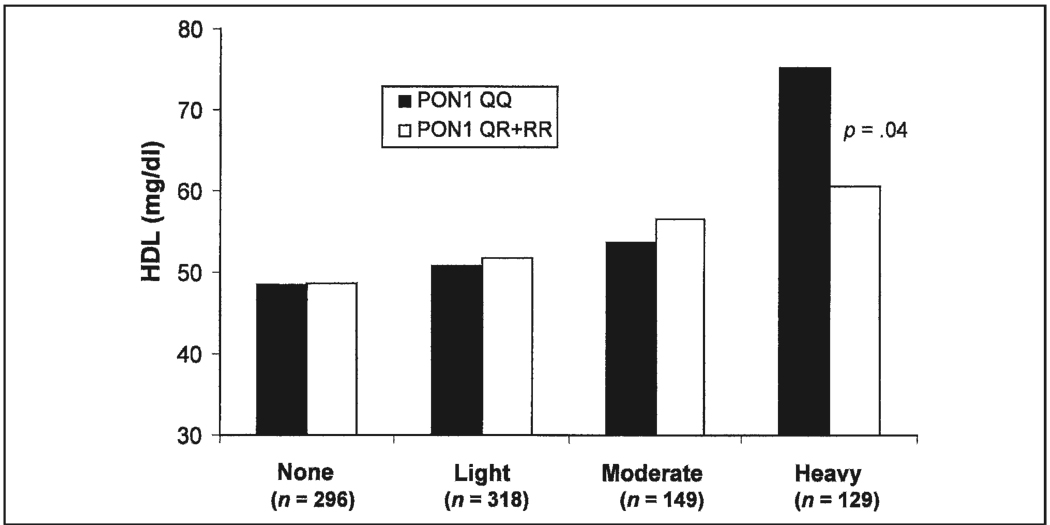
We next investigated whether the observed effect on incident CHD risk could be accounted for by the effects of these genes and alcohol intake on HDL cholesterol levels. With regard to HDL cholesterol levels, results from interaction analyses in regression models revealed a significant interaction between PON1 genotype and heavy alcohol intake in black men (p = .02). Figure 1 shows HDL cholesterol levels in black men by alcohol intake group and PON1 genotype. HDL cholesterol levels are positively associated with alcohol intake for both wildtype and variant genotypes. Within the heavy alcohol intake group, HDL cholesterol levels were significantly higher in black men with the QQ genotype compared with the variant (QR and RR) genotypes (p = .04). Therefore, the increased CHD risk associated with heavy alcohol intake for those black men with the PON1 QQ genotype is not the result of decreased levels of HDL cholesterol, because HDL cholesterol levels are highest in those with the QQ genotype. Using a model for incident CHD risk that included PON1 genotype by alcohol intake interaction terms, as well as HDL by alcohol intake interaction terms, an interaction effect was still observed for heavy-drinking black men. Thus, the positive association between heavy alcohol intake and CHD risk in black men with the PON1 QQ genotype is not the result of the gene’s effect on HDL cholesterol levels.
FIGURE 1.
Mean high-density lipoprotein (HDL) cholesterol levels in black men, by paraoxonase-1 (PON1) genotype and alcohol intake group. Results from interaction analyses in regression models revealed a significant association for PON1 with HDL cholesterol levels only in black men who were heavy drinkers (p = .02). HDL cholesterol levels were positively associated with alcohol intake for both wildtype and variant genotypes. Within the heavy alcohol intake group, HDL cholesterol levels were significantly higher in black men with the wildtype genotype compared with the variant genotypes (p = .04). Interaction p value for PON1 × Heavy Alcohol Intake = .02.
Discussion
Although most studies have shown low to moderate alcohol consumption to be associated with a decreased incidence of CHD, there are exceptions (Camacho et al., 1987; Fraser and Upsdell, 1981; Fuchs et al., 2004; Gronbaek et al., 2000; Klatsky, 1999; Marmot and Brunner, 1991; Rehm et al., 1997; Rimm et al., 1996; Stampfer et al., 1988; Thun et al., 1997). Previous research in the ARIC study has shown all levels of alcohol consumption, compared with nonconsumption, to be associated with a decreased risk of incident CHD in whites but associated with an increased risk of incident CHD in black men (Fuchs et al., 2004). The present ARIC study demonstrates that (1) CETP, HL, LPL, and PON1 genotype frequencies differ markedly between blacks and whites; (2) the effect of alcohol intake on incident CHD risk was significant only for heavy-drinking black men with PON1 QQ and CETP GG genotypes; and (3) the observed effect of heavy alcohol intake on incident CHD risk in black men cannot be attributed to changes in HDL cholesterol levels.
Although most studies have shown increased PON1 levels to be associated with decreased risk of cardiovascular disease, results regarding associations of PON1 polymorphisms with disease risk have been contradictory (Mackness et al., 2004; Mackness and Mackness, 2004; Robertson et al., 2003). These inconsistencies are believed to be the result of differences in environmental factors (e.g., diet, smoking, alcohol use) that influence PON1 activity and possibly override any underlying genetic effect (Costa et al., 2005; Deakin and James, 2004; Mackness et al., 2004; Robertson et al., 2003). PON1 genotype status is not believed to be a reliable predictor of PON1 levels, with differences in levels varying up to 13-fold within a single PON1 genotype (Costa et al., 2005). Compared with no alcohol intake, studies have demonstrated low amounts of alcohol to increase PON1 levels and high amounts of alcohol to decrease PON1 levels (Rao et al., 2003; Sierksma et al., 2002; van der Gaag et al., 1999). Our finding that heavy drinkers with the PON1 QQ genotype have an increased risk of CHD suggests that genetic factors, in addition to alcohol effects, influence CHD risk in black men.
Previous studies have demonstrated a consistent relationship between the CETP Taq1B variant AA genotype, decreased CETP activity, and increased HDL cholesterol levels (Boekholdt et al., 2005; de Grooth et al., 2004; Ordovas et al., 2000; Stein and Stein 2005). Alcohol has been shown to increase HDL cholesterol levels, but not affect CETP activity, in persons with the CETP AA genotype (Fumeron et al., 1995). However, associations between this polymorphism and risk of cardiovascular disease have been contradictory, with protective effects dependent on the environmental context considered (Boekholdt et al., 2005; Carlquist et al., 2003; Freeman et al., 2003; Fumeron et al., 1995; Liu et al., 2002; Ordovas et al., 2000). The current study suggests that the association of heavy drinking with increased risk of CHD in black men is modulated by the CETP Taq1B GG genotype. However, a mechanistic interpretation of these findings will require experimental analyses beyond the scope of this study.
Limitations of the current study include the inability to identify an underlying mechanistic link between heavy drinking and incident CHD that is determined by the genes studied. Although the observed associations are consistent with biological plausibility, enzyme activity levels were not available for investigation in the current study to strengthen and corroborate the observed associations. Another concern is that current findings among heavy-drinking black men in the ARIC cohort might be secondary to the inability to test for associations with various patterns of alcohol use, such as heavy episodic drinking, but, as previously stated by Fuchs and colleagues (2004), there is evidence that the questionnaire administered to ARIC participants captured drinking patterns reasonably well, and there was overall agreement between classification of participants’ alcohol use on their first questionnaire and the average of use reported from their first and second questionnaires administered 3 years apart (Fuchs et al.). We must also acknowledge that our results may be the result of chance findings when one considers multiple testing and that the significant interaction effects were observed only in heavy-drinking black men (a small sample size group). The effects of smoking status on the current results have also been examined, but the conclusions were not altered by additional analyses including smoking status as a covariate (data not shown).
In summary, the current study suggests that interaction effects between heavy alcohol consumption and genetic variation within HDL cholesterol metabolism genes influence the risk of incident CHD in black men. The incidence of disease varies between racial groups, as does the impact of risk factors and genetic factors, with neither one working independently in the pathogenesis of disease. Results from the present study provide evidence that the differential effect of alcohol use on CHD risk in blacks may be modulated in part by variations in genes coding for proteins involved in HDL cholesterol metabolism. Additional studies in blacks are needed to confirm the findings of the present study and should investigate the impact of alcohol consumption on different genotypic profiles associated with cardiovascular disease risk.
Acknowledgment
The authors thank the staff and participants of the ARIC study for their important ontributions.
Footnotes
The Atherosclerosis Risk in Communities (ARIC) study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts N01-C-55015, N01-HC-55016, N01-HC-55018, N01-HC-55019, N01-HC-55020, N01-HC-55021, and N01-HC-55022.
References
- ARIC Investigators. The Atherosclerosis Risk in Communities (ARIC) Study: Design and objectives. Amer. J. Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- Boekholdt SM, Sacks FM, Jukema JW, Shepherd J, Freeman DJ, McMahon AD, Cambien F, Nicaud V, De Grooth GJ, Talmud PJ, Humphries SE, Miller GJ, Eiriksdottir G, Gudnason V, Kauma H, Kakko S, Savolainen MJ, Arca M, Montali A, Liu S, Lanz HJ, Zwinderman AH, Kuivenhoven JA, Kastelein JJ. Cholesteryl ester transfer protein TaqIB variant, high-density lipoprotein cholesterol levels, cardiovascular risk, and efficacy of pravastatin treatment: Individual patient meta-analysis of 13,677 subjects. Circulation. 2005;111:278–287. doi: 10.1161/01.CIR.0000153341.46271.40. [DOI] [PubMed] [Google Scholar]
- Camacho TC, Kaplan GA, Cohen RD. Alcohol consumption and mortality in Alameda County. J. Chron. Dis. 1987;40:229–236. doi: 10.1016/0021-9681(87)90158-5. [DOI] [PubMed] [Google Scholar]
- Carlquist JF, Muhlestein JB, Home BD, Hart NI, Bair TL, Molhuizen HO, Anderson JL. The cholesteryl ester transfer protein Taq1B gene polymorphism predicts clinical benefit of statin therapy in patients with significant coronary artery disease. Amer. Heart J. 2003;146:1007–1014. doi: 10.1016/S0002-8703(03)00501-5. [DOI] [PubMed] [Google Scholar]
- Contaldo F, D’Arrigo E, Carandente V, Cortese C, Coltorti A, Mancini M, Taskinen MR, Nikkila EA. Short-term effects of moderate alcohol consumption on lipid metabolism and energy balance in normal men. Metabolism. 1989;38:166–171. doi: 10.1016/0026-0495(89)90257-6. [DOI] [PubMed] [Google Scholar]
- Costa LG, Vitalone A, Cole TB, Furlong CE. Modulation of paraoxonase (PON1) activity. Biochem. Pharmacol. 2005;69:541–550. doi: 10.1016/j.bcp.2004.08.027. [DOI] [PubMed] [Google Scholar]
- Deakin SP, James RW. Genetic and environmental factors modulating serum concentrations and activities of the antioxidant enzyme paraoxonase-1. Clin. Sci. 2004;107:435–447. doi: 10.1042/CS20040187. [DOI] [PubMed] [Google Scholar]
- de Grooth GJ, Klerkx AHEM, Stroes ESG, Stalenhoef AFH, Kastelein JJP, Kuivenhoven JA. A review of CETP and its relation to atherosclerosis. J. Lipid Res. 2004;45:1967–1974. doi: 10.1194/jlr.R400007-JLR200. [DOI] [PubMed] [Google Scholar]
- Fraser GE, Upsdell M. Alcohol and other discriminants between cases of sudden death and myocardial infarction. Amer. J. Epidemiol. 1981;114:462–476. doi: 10.1093/oxfordjournals.aje.a113212. [DOI] [PubMed] [Google Scholar]
- Freeman DJ, Samani NJ, Wilson V, McMahon AD, Braund PS, Cheng S, Caslake MJ, Packard CJ, Gaffney D. A polymorphism of the cholesteryl ester transfer protein gene predicts cardiovascular events in non-smokers in the West of Scotland Coronary Prevention Study. Europ. Heart J. 2003;24:1833–1842. doi: 10.1016/j.ehj.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Fuchs FD, Chambless LE, Folsom AR, Eigenbrodt ML, Duncan BB, Gilbert A, Szklo M. Association between alcoholic beverage consumption and incidence of coronary heart disease in whites and blacks. Amer. J. Epidemiol. 2004;160:466–474. doi: 10.1093/aje/kwh229. [DOI] [PubMed] [Google Scholar]
- Fumeron F, Betoulle D, Luc G, Behague I, Ricard S, Poirier O, Jemaa R, Evans A, Arveiler D, Marques-Vidal P, Bard J-M, Fruchart J-C, Ducimetière P, Apfelbaum M, Cambien F. Alcohol intake modulates the effect of a polymorphism of the cholesteryl ester transfer protein gene on plasma high density lipoprotein and the risk of myocardial infarction. J. Clin. Invest. 1995;96:1664–1671. doi: 10.1172/JCI118207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagne SE, Larson MG, Pimstone SN, Schaefer EJ, Kastelein JJ, Wilson PW, Ordovas JM, Hayden MR. A common truncation variant of lipoprotein lipase (Ser447X) confers protection against coronary heart disease: The Framingham Offspring Study. Clin. Genet. 1999;55:450–454. doi: 10.1034/j.1399-0004.1999.550609.x. [DOI] [PubMed] [Google Scholar]
- Gronbaek M, Becker U, Johansen D, Gottschau A, Schnohr P, Hein HO, Jensen G, Sorensen TI. Type of alcohol consumed and mortality from all causes, coronary heart disease, and cancer. Ann. Intern. Med. 2000;133:411–419. doi: 10.7326/0003-4819-133-6-200009190-00008. [DOI] [PubMed] [Google Scholar]
- Hannuksela ML, Liisanantti MK, Savolainen MJ. Effect of alcohol on lipids and lipoproteins in relation to atherosclerosis. Crit. Rev. Clin. Lab. Sci. 2002;39:225–283. doi: 10.1080/10408360290795529. [DOI] [PubMed] [Google Scholar]
- Hokanson JE, Kamboh MI, Scarboro S, Eckel RH, Hamman RF. Effects of hepatic lipase gene and physical activity on coronary heart disease risk. Amer. J. Epidemiol. 2003;158:836–843. doi: 10.1093/aje/kwg230. [DOI] [PubMed] [Google Scholar]
- Kastelein JJ, Groenemeyer BE, Hallman DM, Henderson H, Reymer PW, Gagne SE, Jansen H, Seidell JC, Kromhout D, Jukema JW, Bruschke AV, Boerwinkle E, Hayden MR. The ASN9 variant of lipoprotein lipase is associated with the -93G promoter mutation and an increased risk of coronary heart disease. Clin. Genet. 1998;53:27–33. doi: 10.1034/j.1399-0004.1998.531530106.x. [DOI] [PubMed] [Google Scholar]
- Kastelein JJ, Ordovas JM, Wittekoek ME, Pimstone SN, Wilson PWF, Gagne SE, Larson MG, Schaefer EJ, Boer JM, Gerdes C, Hayden MR. Two common mutations (D9N, N291S) in lipoprotein lipase: A cumulative analysis of their influence on plasma lipids and lipoproteins in men and women. Clin. Genet. 1999;56:297–305. doi: 10.1034/j.1399-0004.1999.560407.x. [DOI] [PubMed] [Google Scholar]
- Klatsky AL. Moderate drinking and reduced risk of heart disease. Alcohol Res. Hlth. 1999;23:15–23. [PMC free article] [PubMed] [Google Scholar]
- Liu S, Schmitz C, Stampfer MJ, Sacks F, Hennekens CH, Lindpaintner K, Ridker PM. A prospective study of Taq1B polymorphism in the gene coding for cholesteryl ester transfer protein and risk of myocardial infarction in middle-aged men. Atherosclerosis. 2002;161:469–474. doi: 10.1016/s0021-9150(01)00673-6. [DOI] [PubMed] [Google Scholar]
- Lucas DL, Brown RA, Wassef M, Giles TD. Alcohol and the cardiovascular system: Research challenges and opportunities. J. Amer. Coll. Cardiol. 2005;45:1916–1924. doi: 10.1016/j.jacc.2005.02.075. [DOI] [PubMed] [Google Scholar]
- Mackness M, Durrington P, Mackness B. Paraoxonase 1 activity, concentration and genotype in cardiovascular disease. Curr. Opin. Lipidol. 2004;15:399–404. doi: 10.1097/01.mol.0000137227.54278.29. [DOI] [PubMed] [Google Scholar]
- Mackness M, Mackness B. Paraoxonase 1 and atherosclerosis: Is the gene or the protein more important? Free Rad. Biol. Med. 2004;37:1317–1323. doi: 10.1016/j.freeradbiomed.2004.07.034. [DOI] [PubMed] [Google Scholar]
- Marmot M, Brunner E. Alcohol and cardiovascular disease: The status of the U shaped curve. Brit. Med. J. 1991;303:565–568. doi: 10.1136/bmj.303.6802.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiwaki M, Ishikawa T, Ito T, Shige H, Tomiyasu K, Nakajima K, Kondo K, Hashimoto H, Saitoh K, Manabe M, Miyajima E, Nakamura H. Effects of alcohol on lipoprotein lipase, hepatic lipase, cholesteryl ester transfer protein, and lecithin: Cholesterol acyltransferase in high-density lipoprotein cholesterol elevation. Atherosclerosis. 1994;111:99–109. doi: 10.1016/0021-9150(94)90195-3. [DOI] [PubMed] [Google Scholar]
- Ordovas JM, Cupples LA, Corella D, Otvos JD, Osgood D, Martinez A, Lahoz C, Coltell O, Wilson PW, Schaefer EJ. Association of cholesteryl ester transfer protein-TaqIB polymorphism with variations in lipoprotein subclasses and coronary heart disease risk: The Framingham study. Arterioscler. Thromb. Vasc. Biol. 2000;20:1323–1329. doi: 10.1161/01.atv.20.5.1323. [DOI] [PubMed] [Google Scholar]
- Rao MN, Marmillot P, Gong M, Palmer DA, Seeff LB, Strader DB, Lakshman MR. Light, but not heavy alcohol drinking, stimulates paraoxonase by upregulating liver mRNA in rats and humans. Metabolism Clin. Exp. 2003;52:1287–1294. doi: 10.1016/s0026-0495(03)00191-4. [DOI] [PubMed] [Google Scholar]
- Rehm JT, Bondy SJ, Sempos CT, Vuong CV. Alcohol consumption and coronary heart disease morbidity and mortality. Amer. J. Epidemiol. 1997;146:495–501. doi: 10.1093/oxfordjournals.aje.a009303. [DOI] [PubMed] [Google Scholar]
- Rimm EB, Klatsky A, Grobbee DG, Stampfer MJ. Review of moderate alcohol consumption and reduced risk of coronary heart disease: Is the effect due to beer, wine, or spirits? Brit. Med. J. 1996;312:731–736. doi: 10.1136/bmj.312.7033.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson KS, Hawe E, Miller GJ, Talmud PJ, Humphries SE. Human paraoxonase gene cluster polymorphisms as predictors of coronary heart disease risk in the prospective Northwick Park Heart Study II. Biochim. Biophys. Acta. 2003;1639:203–212. doi: 10.1016/j.bbadis.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Rosamond WD, Folsom AR, Chambless LE, Wang C-H, McGovern PG, Howard G, Copper LS, Shahar E. Stroke incidence and survival among middle-aged adults: 9-year follow-up of the Atherosclerosis Risk in Communities (ARIC) cohort. Stroke. 1999;30:736–743. doi: 10.1161/01.str.30.4.736. [DOI] [PubMed] [Google Scholar]
- Rundek T, Elkind MS, Pittman J, Boden-Albala B, Martin S, Humphries SE, Juo SH, Sacco RL. Carotid intima-media thickness is associated with allelic variants of stromelysin-1, interleukin-6, and hepatic lipase genes: The Northern Manhattan Prospective Cohort Study. Stroke. 2002;33:1420–1423. doi: 10.1161/01.STR.0000015558.63492.B6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savolainen MJ, Kesaniemi YA. Effects of alcohol on lipoproteins in relation to coronary heart disease. Curr. Opin. Lipidol. 1995;6:243–250. doi: 10.1097/00041433-199508000-00009. [DOI] [PubMed] [Google Scholar]
- Shaper AG. Alcohol and mortality: A review of prospective studies. Brit. J. Addict. 1990;85:837–847. doi: 10.1111/j.1360-0443.1990.tb03710.x. [DOI] [PubMed] [Google Scholar]
- Siedel J, Hagele EO, Ziegenhorn J, Wahlefeld AW. Reagent for the enzymatic determination of serum total cholesterol with improved lipolytic efficiency. Clin. Chem. 1983;29:1075–1080. [PubMed] [Google Scholar]
- Sierksma A, van der Gaag MS, van Tol A, James RW, Hendriks HFJ. Kinetics of HDL cholesterol and paraoxonase activity in moderate alcohol consumers. Alcsm Clin. Exp. Res. 2002;26:1430–1435. doi: 10.1097/01.ALC.0000030639.57507.60. [DOI] [PubMed] [Google Scholar]
- Stampfer MJ, Colditz GA, Willett WC, Speizer FE, Hennekens CH. A prospective study of moderate alcohol consumption and the risk of coronary disease and stroke in women. New Eng. J. Med. 1988;319:267–273. doi: 10.1056/NEJM198808043190503. [DOI] [PubMed] [Google Scholar]
- Stein O, Stein Y. Lipid transfer proteins (LTP) and atherosclerosis. Atherosclerosis. 2005;178:217–230. doi: 10.1016/j.atherosclerosis.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Taskinen MR, Valimaki M, Nikkila EA, Kuusi T, Ehnholm C, Ylikahri R. High density lipoprotein subfractions and postheparin plasma lipases in alcoholic men before and after ethanol withdrawal. Metabolism Clin. Exp. 1982;31:1168–1174. doi: 10.1016/0026-0495(82)90169-x. [DOI] [PubMed] [Google Scholar]
- Thun MJ, Peto R, Lopez AD, Monaco JH, Henley SJ, Heath CW, Jr, Doll R. Alcohol consumption and mortality among middle-aged and elderly U.S. adults. New Eng. J. Med. 1997;337:1705–1714. doi: 10.1056/NEJM199712113372401. [DOI] [PubMed] [Google Scholar]
- van Bockxmeer FM, Liu Q, Mamotte C, Burke V, Taylor R. Lipoprotein lipase D9N, N291S and S447X polymorphisms: Their influence on premature coronary heart disease and plasma lipids. Atherosclerosis. 2001;157:123–129. doi: 10.1016/s0021-9150(00)00717-6. [DOI] [PubMed] [Google Scholar]
- van der Gaag MS, van Tol A, Scheek LM, James RW, Urgert R, Schaafsma G, Hendriks HF. Daily moderate alcohol consumption increases serum paraoxonase activity: A diet-controlled, randomized intervention study in middle-aged men. Atherosclerosis. 1999;147:405–410. doi: 10.1016/s0021-9150(99)00243-9. [DOI] [PubMed] [Google Scholar]
- Warnick GR, Benderson JM, Albers JJ. Quantitation of high-density-lipoprotein subclasses after separation by dextran sulfate and Mg2+ precipitation (abstract) Clin. Chem. 1982;28:1574. [PubMed] [Google Scholar]
- White AD, Folsom AR, Chambless LE, Sharret AR, Yang K, Conwill D, Higgins M, Williams OD, Tyler HA. Community surveillance of coronary heart disease in the Atherosclerosis Risk in Communities (ARIC) Study: Methods and initial 2 years’ experience. J. Clin. Epidemiol. 1996;49:223–233. doi: 10.1016/0895-4356(95)00041-0. [DOI] [PubMed] [Google Scholar]
- Wittrup HH, Tybjaerg-Hansen A, Abildgaard S, Steffensen R, Schnohr P, Nordestgaard BG. A common substitution (Asn291Ser) in lipoprotein lipase is associated with increased risk of ischemic heart disease. J. Clin. Invest. 1997;99:1606–1613. doi: 10.1172/JCI119323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittrup HH, Tybjaerg-Hansen A, Steffensen R, Deeb SS, Brunzell JD, Jensen G, Nordesgaard BG. Mutations in the lipoprotein lipase gene associated with ischemic heart disease in men: The Copenhagen City Heart Study. Arterioscler. Thromb. Vasc. Biol. 1999;19:1535–1540. doi: 10.1161/01.atv.19.6.1535. [DOI] [PubMed] [Google Scholar]