Abstract
A group of new fluorescent dye materials for single-molecule imaging applications comprised of an amine donor, a π-system comprised of phenyl and thiophene rings and a 2-dicyanomethylene-3-cyano-2,5-dihydrofuran acceptor group have been synthesized. Relative to comparable single-ring compounds these doubly aromatic conjugated fluorophores have red-shifted absorption and emission usually accompanied by significantly increased quantum yields. Solvatochromism studies indicate that the photophysical properties of these dyes are sensitive to the solvent polarity and environmental rigidity. Photophysical studies demonstrate that these DCDHF dye materials are strong single-molecule emitters and the total number of detected photons per molecule is among the highest thus far for this family of fluorophores.
Keywords: Single-molecule Imaging, Fluorophore, Solvatochromism, Synthesis, Quantum Yield
Introduction
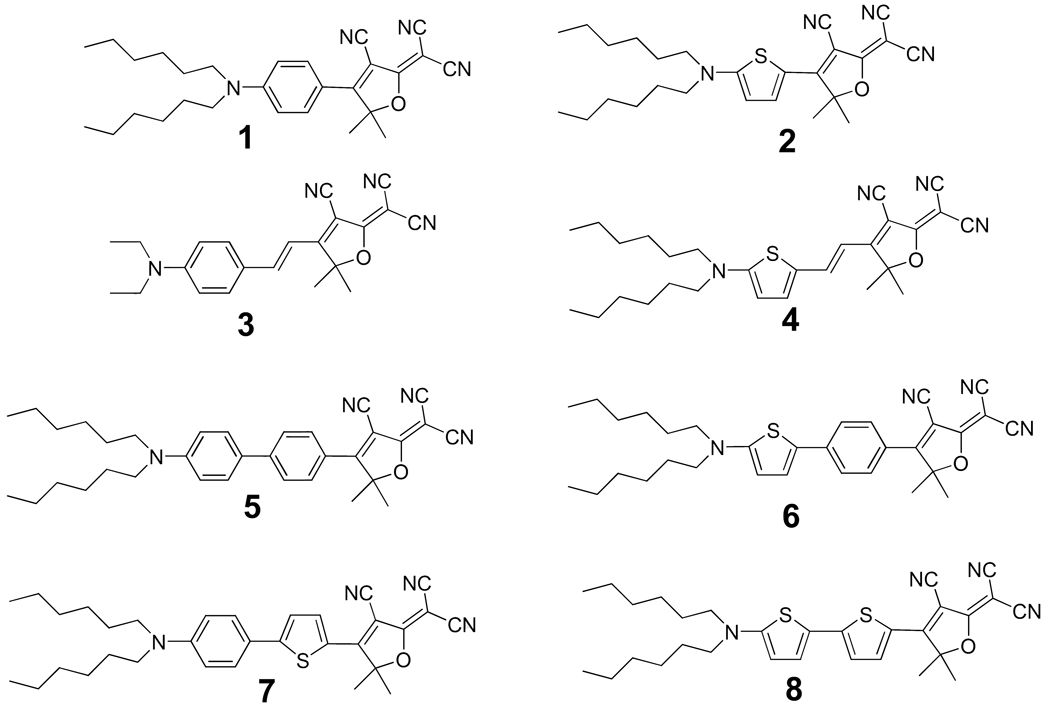
Fluorescence imaging of single molecules is a powerful technique in which characteristics of the structure and dynamics of complex condensed materials are revealed that are otherwise hidden in ensemble measurements.1 The successful imaging of individual molecules requires strong and stable fluorescent emitters that can be detected at the single-copy level. We have demonstrated that the family of molecules comprised of a dicyanodihydrofuran (DCDHF) acceptor and a dialkylamino donor linked by a π-conjugated unit are well suited for this purpose.2 The DCDHF acceptor group was first described by Melikian and coworkers,3 and soon found extensive applications in electro-optic materials4 and photorefractive materials.5 The original DCDHF fluorophores which evolved from the photorefractive materials had a single benzene or thiophene ring (Fig. 1, molecules 1 and 2) as the conjugated bridge. These compounds are pumped at 488 nm, but at this wavelength autofluorescence occurs in typical biological systems and emission from single fluorophores is not easily observed. As a result, fluorophore materials with red-shifted absorption and emission are highly desired; specifically, autofluorescence is significantly reduced at pumping wavelengths longer than 514 or 532 nm, beyond where flavins absorb.6 Introduction of an olefin unit, as is usually employed in electro-optic chromophores, red shifts the absorption very effectively but the fluorescence quantum yield is significantly reduced, probably due to the introduction of nonradiative processes involving rotations about the olefins (Fig. 1, molecules 3 and 4). This loss of quantum yield prompted us to explore an alternative extension of conjugation by addition of aromatic rings such as phenyl and thiophene. This approach complements our earlier successful push to longer wavelengths using fused rings.7 We proposed that DCDHFs with only oligoaromatic conjugation would have the desired red shift but with a smaller reduction of quantum yields. The more extensively conjugated bisaromatic systems 5, 6, 7 and 8 in Fig. 1, are expected to show a bathochromic shift of the absorption and emission band. In addition, a trisaromatic dye and two bisaromatic dyes with an olefin unit were also synthesized for comparison.
Figure 1.
Structures of DCDHF chromophores. Compounds 1–4 are typical of those examined earlier while the set of new bisaromatic compounds 5–8 are described here in detail.
The conjugated donor-π-bridge-acceptor push-pull chromophores with π-bridges of phenyl and thiophene rings and their combinations are widely recognized for applications in materials science because of their interesting and useful optoelectronic properties.8 Extension of the conjugation unit from one aromatic ring to two aromatic rings leads to a bathochromic shift for their charge-transfer electronic absorption. For example, a biphenyl unit has been broadly applied to optoelectronic material synthesis.9 Chromophores bearing a benzo-1,3-dithiol-2-ylidene donor and dicyanomethylene acceptor with a 4,4'-biphenyl instead of a 1,4-benzene push the absorption from the visible to the near-infrared.10 Since the thiophene ring has less resonance energy than benzene it permits more effective π-electron delocalization.11 Bithiophene provides a pronounced red shift compared with biphenyl indicative of enhanced electron transmission from the donor to the acceptor.12
Here we describe the synthetic aspects as well as spectroscopic properties—including absorption, fluorescence, solvatochromism, and single-molecule imaging properties—of DCDHF dyes with an extended π-system comprised of combinations of benzene and thiophene rings. Most of these compounds have the desired combination of enhanced quantum yields with absorption and fluorescence at longer wavelengths that very effectively overcome the background autofluorescence seen in many biological samples. On the single-molecule level, this new group of dyes includes some of the brightest and most long-lived amongst the DCDHF class of single-molecule emitters. For many of these dyes in PMMA, single copies emit brightly for tens of seconds to minutes under typical epifluorescence imaging conditions (even without oxygen scavengers), emitting several millions of photons before photobleaching. Moreover, as with other DCDHFs studied,2(b) these fluorophores seldom exhibit blinking on the time scale of our experiments (100-ms integration time). These DCDHF fluorophore compare favorably with other excellent single-molecule emitters, such as rhodamines and perylene diimides, which emit millions of photons;13 Terrylene diimides can emit tens or even hundreds of millions of photons, but face significant solubility hurdles.14 Overall, these new DCDHF dyes show excellent promise as bright, long-lived red emitters—necessary criteria for single-molecule experiments in the cellular environment.
Experimental Section
Bulk Spectroscopy
Solutions were prepared in pure solvents and bulk absorption and emission spectra were obtained on a Perkin-Elmer Lambda 19 UV-Vis spectrometer and a SPEX Fluromax-2 fluorimeter, respectively. Quantum yields were measured against standards with known quantum yields, and corrected for differences in optical density and solvent refractive index.15 Bulk samples in poly(methyl methacrylate) (PMMA, Tg = 105 °C, MW = 75,000 g/mol, atactic, polydispersity ~2.8, PolySciences Inc.) were prepared by spin-casting 20% (m:m) solutions of PMMA in toluene, doped with a small volume of highly concentrated dye solution, onto clean glass slides. These colored films were placed in the spectrometer and fluorimeter to obtain quantitative absorption and emission spectra, respectively.
Single-Molecule Imaging
Single-molecule samples were prepared by spin-casting 1% (m:m) solutions of PMMA in toluene, doped with nanomolar fluorophore concentrations, onto clean glass coverslips. After drying, these samples were studied using a Nikon Diaphot 200 inverted microscope in an epifluorescence configuration.16 Samples were illuminated using a continuous-wave 532-nm Spectra-Physics Millenia laser; the intensity at the sample was between 0.5 and 3.5 kW/cm2. The emission was collected through a 100×, 1.4 N.A. Nikon microscope objective, filtered to remove scattered excitation light, and imaged onto a back-illuminated frame-transfer Si CCD camera (Roper Scientific MicroMax) with an integration time of 100 ms.
Crystal Structures and Calculations
To perform x-ray crystallography, crystals of 5, 6 and 21 were mounted onto a thin glass fiber from a pool of Fluorolube™ and immediately placed under a low-temperature N2 stream on a Bruker AXS diffractometer. The radiation used was graphite monochromatized Mo Kα radiation (λ = 0.7107 Å). The lattice parameters were optimized from a least-squares calculation on carefully centered reflections. Lattice determination, data collection, structure refinement, scaling, and data reduction were carried out using APEX2 version 1.0-27 software package.
Each structure was solved using direct methods. This procedure yielded a number of the C, N, and O atoms. Subsequent Fourier synthesis yielded the remaining atom positions. The hydrogen atoms were fixed in positions of ideal geometry and refined within the XSHELL software. These idealized hydrogen atoms had their isotropic temperature factors fixed at 1.2 or 1.5 times the equivalent isotropic U of the C atoms to which they were bonded. The final refinement of each compound included anisotropic thermal parameters on all non-hydrogen atoms. Data collection parameters are listed in Table S1.
Gaussian 0317 was used to calculate the optimized ground-state geometries for molecules 1–8 and 21. In order to determine the best level of theory and optimal basis set for these molecules, we performed a series of systematic tests. We used our measured crystal structure of molecule 6 as a starting structure and ran ground-state geometry optimizations using HF, BLYP, and B3LYP; for each level of theory, we used 3-21G and 6-31G(d) basis sets. We also ran calculations using more elaborate basis sets (e.g. 6-31+G(d)) and/or higher levels of theory (e.g. MP2), but found only modest increases in accuracy at very high costs. HF theory, using either basis set, produces a structure that is qualitatively incorrect: the dihedral angles between the phenyl and thiophene rings are off by approximately 40 degrees. DFT performed much better, and BLYP and B3LYP structures were all qualitatively comparable to each other and the known crystal structure. To quantify the errors, we compared each of the bond lengths, bond angles, and dihedral angles for each of optimized structures directly with the corresponding values in the known crystal structure using a home-written program. When ignoring errors in hydrogen dihedral angles—which should have minimal effect on the photophysics of the molecules—we determined that BLYP/6-31G outperformed the other viable combinations of basis set and level of theory; therefore, we used this combination to calculate the optimized geometries of molecules 1–8 and 21. In cases where there were conformers, we report the structures with the lowest calculated energy.
Synthetic Procedures
The preparative details for some of the compounds are found in the supplemental information. This is especially the case where the method is described here as “by a similar procedure”.
4-Bromo-4′-(N,N-dihexylamino)biphenyl (11a)
In a 200 mL round bottom flask with stirbar was added 4-amino-4′-bromobiphenyl (4.55 g, 0.018 mol), 1-bromohexane (10.59 g, 9.1 mL, 0.064 mol, 3.5 eq.), potassium carbonate (7.60 g, 0.055 mol, 3 eq.) and DMF (40 mL). The mixture was stirred under nitrogen at 110 °C for 10 h. The reaction was cooled to room temperature and water (100 mL) was added to the reaction mixture. The product was extracted with hexane (200 mL) twice. The organic layers were combined and washed twice with water (100 mL), and then the combined organic layers were dried over magnesium sulfate. The organic layer was adsorbed on silica gel, placed at the top of a silica gel column and eluted with hexane. The desired product was obtained as a white solid (4.82 g, 63% yield). Mp 67 °C. IR (neat): 2955, 2925, 2854, 1604, 1525, 1482, 804 cm−1. 1H NMR (400 MHz, CDCl3): δ 7.55-7.40 (m, 6H), 6.73 (d, J = 8.8 Hz, 2H), 3.34 (t, J = 7.6 Hz, 4H), 1.67-1.62 (m, 4H), 1.40-1.30 (m, 12H), 0.96 (t, J = 6.8 Hz, 6H); 13C NMR (100 MHz, CDCl3): δ 147.8, 140.3, 131.7, 127.7, 127.6, 126.3, 119.6, 111.8, 51.1, 31.8, 27.3, 26.9, 22.8, 14.1. Anal. Calcd for C24H34BrN: C, 69.22; H, 8.23; N, 3.36. Found: C, 69.41; H, 8.24; N, 3.48.
1-(4′-Dihexylamino-biphenyl-4-yl)-2-hydroxy-2-methyl-propan-1-one (12a)
In a dry 100 mL two-neck round bottom flask with stirbar was added magnesium turnings (0.17 g, 7.0 mmol) and 4-bromo-4′-(N,N-dihexylamino)biphenyl (2.08 g, 5.0 mmol) under nitrogen. Anhydrous THF (10 mL) was added with stirring to the mixture and then 1,2-dibromoethane (4 drops) was added. Once the reaction was initiated, another portion of anhydrous THF (20 mL) was added to the mixture by syringe. The reaction mixture was heated to reflux for 3 h. Then the mixture was cooled to room temperature and 2-methyl-2-trimethylsilanyloxy-propionitrile (1.60 g, 10.0 mmol) was added. The reaction was reheated to reflux overnight and then cooled to room temperature. Hydrochloric acid (6 N, 5 mL) was added to the reaction mixture and it was stirred at room temperature for 3 h. Solid sodium bicarbonate was added to the reaction mixture until it was neutral. The reaction mixture was extracted with EtOAc (100 mL) and the crude product was adsorbed on silica gel, placed at the top of a silica column and eluted (pure hexane to hexane/ EtOAc = 10/1) to give the desired product as a green-yellow liquid (1.70 g, 80% yield). IR (neat): 3460, 2954, 2927, 2856, 1659, 1611, 1530, 1497, 1165 cm−1; 1H NMR (400 MHz, CDCl3): δ 8.08 (d, J = 8.4 Hz, 2H), 7.66 (d, J = 8.4 Hz, 2H), 7.56 (d, J = 8.8 Hz, 2H), 6.73 (d, J = 9.2 Hz, 2H), 4.35 (br s, 1H), 3.34 (t, J = 8.0 Hz, 4H), 1.70 (s, 6H), 1.64-1.62 (m, 4H), 1.39-1.36 (m, 12H), 0.95-0.92 (m, 6H); 13C NMR (100 MHz, CDCl3): δ 203.7, 148.4, 146.0, 130.6, 128.1, 127.4, 125.4, 115.5, 111.9, 111.8, 51.1, 31.7, 28.7, 27.3, 26.9, 22.7, 14.1. HRMS m/z Calcd. for C28H41NO2 (M+H): 424.3215. Found: 424.3206.
3-Cyano-2-dicyanomethylen-5,5-dimethyl-4-[(4'-dihexylamino-biphenyl)-4-yl]-2,5-dihydrofuran (5)
In a 200 mL round bottom flask with stirbar was added 1-(4′-dihexylamino-biphenyl-4-yl)-2-hydroxy-2-methyl-propan-1-one (1.40 g, 0.0033 mol), malononitrile (1.59 g, 0.024 mol), pyridine (20 mL) and acetic acid (4 drops). The reaction mixture was stirred at room temperature for 48 h. The reaction mixture was poured into 400 mL ice water and vigorously stirred. The precipitate was filtered out and recrystallized from dichloromethane and methanol to give purple crystals (0.60 g, 35% yield). Mp 189 °C. IR (neat): 2952, 2925, 2856, 2225 cm−1; 1H NMR (400 MHz, CDCl3): δ 7.97 (ddd, J = 8.0, 2.4, 2.0 Hz, 2H), 7.78 (ddd, J = 8.0, 2.4, 2.0 Hz, 2H), 7.60 (ddd, J = 9.0, 3.1, 2.0 Hz, 2H), 6.74 (ddd, J = 9.0, 3.1, 2.0 Hz, 2H), 3.36 (t, J = 7.6 Hz, 4H), 1.90 (s, 6H), 1.66-1.63 (m, 4H), 1.39-1.36 (m, 12H), 0.94 (t, J = 6.8 Hz, 6H); 13C NMR (100 MHz, CDCl3): δ 176.0, 175.8, 149.2, 147.4, 129.7, 128.2, 126.2, 124.0, 123.4, 111.9, 111.5, 110.9, 98.7, 98.3, 57.6, 51.1, 31.7, 27.3, 27.0, 26.8, 22.7, 14.1 UV-Vis(CH2Cl2): λmax = 535 nm, εmax = 2.84×104 L·mol−1·cm−1. Anal. Calcd for C34H40N4O: C, 78.42; H, 7.74; N, 10.76. Found: C, 78.33; H, 7.47; N, 10.93.
4-(4-Bromophenyl)-4-oxo-butyric acid dihexylamide (9)
In a 500 mL round bottom flask was placed 4-(4-bromophenyl)-4-oxobutanoic acid (12.9 g, 50 mmol), triethylamine (24 mL, 170 mmol) and THF (200 mL). The flask was cooled to between −40 and −50 °C. To the stirred solution was added ethyl chloroformate (6.1 g, 56 mmol) via syringe over 10 min. The reaction mixture was stirred for 40 min and the temperature was allowed to rise to −20 °C. Dihexylamine (10.2 g, 55 mmol) was added and the reaction was stirred overnight at room temperature. Hexane (400 mL) was added to the reaction flask and stirred for additional 1 h. The precipitate was filtered off and the solution was washed with 5% hydrochloric acid, brine and dried over anhydrous MgSO4. Solvent was removed by rotary evaporation and the residue was purified by silica gel flash chromatography (hexane/EtOAc = 4/1) to give the amide (17.2 g, 81% yield). IR (neat): 2955, 2927, 2857, 1687, 1640 cm−1; 1HNMR (400 MHz, CDCl3): δ 7.89 (ddd, J = 8.6, 2.4, 1.9 Hz, 2H), 7.60 (ddd, J = 8.6, 2.4, 1.9 Hz, 2H), 3.33-3.26 (m, 6H), 2.77 (t, J = 6.5 Hz, 2H), 1.68-1.58 (m, 2H), 1.57-1.46 (m, 2H), 1.38-1.22 (m, 12H), 0.95-0.85 (m, 6H). 13C NMR (100 MHz, CDCl3): δ 198.4, 170.8, 135.7, 131.8, 129.7, 128.1, 48.0, 46.2, 33.8, 31.62, 31.56, 28.9, 27.8, 27.2, 26.7, 26.6, 22.62, 22.60, 14.04, 14.02. HRMS m/z Calcd. for C22H34BrNO2 (M+Na): 446.1671. Found: 446.1693.
2-Dihexylamino-5-(4-bromophenyl)-thiophene (11b)
A mixture of 4-(4-bromophenyl)-4-oxobutyric acid dihexylamide (7.4 g, 17.4 mmol) and Lawesson’s reagent (12.0 g, 29.7 mmol) in 200 mL toluene was refluxed for 4 h. The mixture was cooled and 2 M Na2CO3 (30 mL) was added to the flask and stirred overnight. The organic layer was separated and the aqueous layer was extracted with hexane. The combined organic layers were washed with water, brine and dried over anhydrous MgSO4. Solvent was removed with a rotary evaporator and the residue was purified by flash chromatography on silica gel with hexane as eluent. The product was obtained as yellow oil (6.34 g, 86% yield). IR (neat): 2953, 2926, 2855, 1500 cm−1; 1HNMR (400 MHz, CDCl3): δ 7.41 (ddd, J = 8.6, 2.5, 1.0 Hz, 2H), 7.32 (ddd, J = 8.6, 2.5, 1.0 Hz, 2H), 7.03 (d, J = 4.0 Hz, 1H), 5.79 (d, J = 4.0 Hz, 1H), 3.25 (t, J = 7.6 Hz, 4H), 1.70-1.61 (m, 4H), 1.40-1.32 (m, 12H), 0.94 (t, J = 6.9 Hz, 6H).13C NMR (100 MHz, CDCl3): δ 157.9, 134.5, 131.6, 125.3, 124.5, 123.5, 118.3, 101.6, 53.7, 31.7, 27.0, 26.8, 22.7, 14.1. HRMS m/z Calcd. For C22H32BrNS (M+H): 422.1517. Found: 422.1519.
[4-(5-Dihexylaminothien-2-yl)phenyl)]-2-hydroxy-2-methylpropan-1-one (12b)
In a 200 mL round bottom flask was placed 2-dihexylamino-5-(4-bromophenyl)-thiophene (2.1 g, 5.0 mmol) and 15 mL anhydrous THF. The mixture was cooled to −78 °C and 2.4 mL (6.0 mmol) of n-BuLi in hexane (2.5 M) was added to this stirred mixture over 10 min. The resulting mixture was stirred for an additional 1 h at −78 °C and then 2-methyl-2-trimethylsilyloxypropionitrile (1.57 g, 10.0 mmol) was added to the resulting mixture via a syringe over 10 min. After completion of addition the reaction was allowed to warm to room temperature and then stirred for an additional 12 h at room temperature. The reaction was quenched with 50 mL water and 10 mL 6 M hydrochloric acid, stirred overnight and then extracted with ethyl acetate. The organic layer was washed with water, brine and dried with anhydrous MgSO4. Solvent was removed with a rotary evaporator and the residue was purified by silica gel chromatography (hexane/EtOAc = 20/1) to give an orange oil (1.79 g, 83% yield). IR (neat): 3451, 2954, 2927, 2856, 1655, 1595, 1505, 1483, 1445, 1162, 1066 cm−1; 1H NMR (400 MHz, CDCl3): δ 8.00 (ddd, J = 8.6, 2.2, 1.9 Hz, 2H), 7.48 (ddd, J = 8.6, 2.2, 1.9 Hz, 2H), 7.33 (d, J = 4.1 Hz, 1H), 5.82 (d, J = 4.1 Hz, 1H), 4.20 (br, s, 1H), 3.29 (t, J = 7.6 Hz, 4H), 1.67 (s, 6H), 1.71-1.62 (m, 4H), 1.38-1.31 (m, 12H), 0.93 (t, J = 7.0 Hz, 6H). 13C NMR (100 MHz, CDCl3): δ 202.9, 159.6, 140.2, 130.7, 125.8, 124.7, 124.0, 122.8, 102.3, 75.8, 53.7, 31.6, 28.6, 27.1, 26.7, 22.5, 13.8. HRMS m/z Calcd. for C26H39NO2S (M+H): 430.2780. Found: 430.2768.
3-Cyano-2-dicyanomethylen-5,5-dimethyl-4-[4-(5-dihexylaminothien-2-yl)phenyl]-2,5-dihydrofuran (6)
The title compound was synthesized by a similar procedure as compound 5. It was given as green-gold crystals (1.66 g, 73% yield). Mp 129.7-131.0 °C. IR (neat): 3011, 3079, 2955, 2924, 2854, 2224 cm−1; 1H NMR (400 MHz, CDCl3): δ 7.90 (ddd, J = 9.0, 2.4, 1.9 Hz, 2H), 7.50 (ddd, J = 9.0, 2.4, 1.9 Hz, 2H), 7.39 (d, J = 4.3 Hz, 1H), 5.91 (d, J = 4.3 Hz, 1H), 3.34 (t, J = 7.7 Hz, 4H), 1.86 (s, 6H), 1.74-1.64 (m, 4H), 1.41-1.31 (m, 12H), 0.93 (t, J = 6.8 Hz, 6H). 13C NMR (100 MHz, CDCl3): δ 176.2, 174.1, 161.8, 142.1, 130.2, 129.3, 123.1, 122.4, 121.4, 112.4, 112.2, 111.4, 103.2, 98.1, 95.4, 56.2, 53.8, 31.6, 27.3, 27.1, 26.7, 22.6, 14.0. APCI-MS m/z 527.0. UV-Vis (CH2Cl2): λmax = 625 nm, εmax = 5.54×104 Lcm−1mol−1. Anal. Calcd for C32H38N4OS: C, 72.97; H, 7.27; N, 10.64; S, 6.09. Found: C, 72.85; H, 6.94; N, 10.94; S, 6.15.
Dihexyl-(4-thiophen-2-yl-phenyl)-amine (11c)
In a dry 500 mL two-neck round bottom flask with stirbar was added Mg turnings (2.92 g, 0.12 mol), anhydrous THF (10 mL) and 1,2-dibromoethane (2 drops). Next, 2-bromothiophene (16.3 g, 0.10 mol) in THF (60 mL) was added via an addition funnel during 30 min under nitrogen. After refluxing for 1 h, the reaction was cooled to 0 °C, and dry ZnCl2 (20.44 g, 0.15 mol) in anhydrous THF (200 mL) was added to the reaction mixture through a cannula. After all of the ZnCl2 had been added, the reaction was stirred at room temperature for another 10 min, and then Pd(PPh3)4 (2.85 g, 0.0024 mol, 2mol%) and 4-bromo-N,N-dihexylaniline (27.23 g, 0.08 mol) were added to the reaction mixture and the reaction was heated to reflux for 14 h. The reaction was cooled to room temperature and all of the salt was removed from the THF solution through suction filtration. The solid was washed several times with hexane and ether. The filtrate and washing solution were combined and adsorbed on silica gel, placed at the top of a silica column and eluted (hexane/ether = 20/1) to give the product as a light yellow liquid (19.0 g, 69% yield). IR (neat): 2954, 2926, 2853, 1609, 1538, 1505, 1146, 805 cm−1; 1H NMR (400 MHz, CDCl3): δ 7.48 (ddd, J = 8.77, 3.20, 2.05 Hz, 2H), 7.16-7.14 (m, 2H), 7.06-7.03 (m, 1H), 6.65 (ddd, J = 8.77, 3.20, 2.05 Hz, 2H), 3.30 (t, J = 7.76 Hz, 4H), 1.64-1.56 (m, 4H), 1.45-1.31 (m, 12H), 0.94 (t, J = 6.70 Hz, 6H); 13C NMR (100 MHz, CDCl3): δ 147.6, 145.5, 127.8, 127.1, 122.4, 121.6, 120.5, 111.7, 51.1, 31.8, 27.3, 26.9, 22.7, 14.1; HRMS m/z Calcd. for C22H33NS (M+H): 344.2412. Found: 344.2425.
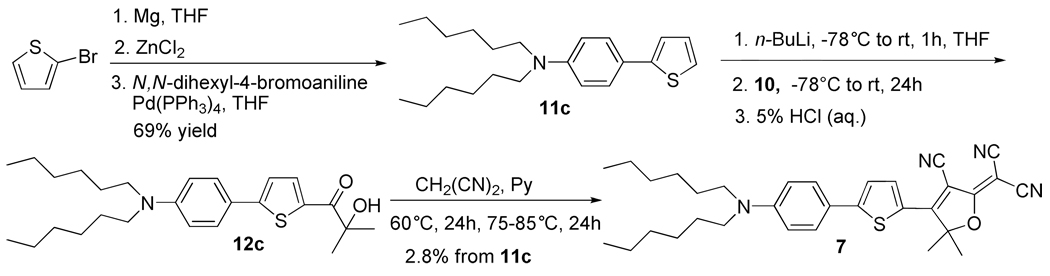
3-Cyano-2-dicyanomethylen-5,5-dimethyl-4-[5-(4-dihexylaminophenyl)thien-2-yl]-2,5-dihydrofuran (7) [from nitrile 10]
The title compound was synthesized in two steps similar to the synthesis of compounds 12b and 5 respectively. It was given as a blue solid (46 mg, 2.8% yield for two steps). Mp 131.9-133.6 °C. IR (neat): 2953, 2923, 2853, 2224, 1603, 1582, 1550 cm−1; 1H NMR (400 MHz, CDCl3): δ 8.13 (d, J = 4.5 Hz, 1H), 7.60 (d, J = 9.1 Hz, 2H), 7.38 (d, J = 4.5 Hz, 1H), 6.67 (d, J = 9.1 Hz, 2H), 3.36 (t, J = 7.7 Hz, 4H), 1.90 (s, 6H), 1.66-1.58 (m, 4H), 1.40-1.32 (m, 12H), 0.93 (t, J = 6.7 Hz, 6H). 13C NMR (100 MHz, CDCl3): δ 176.4, 166.9, 160.5, 150.3, 138.3, 128.5, 124.7, 122.9, 118.3, 112.6, 112.3, 111.7, 97.1, 91.5, 55.0, 51.2, 31.7, 27.5, 27.4, 26.7, 22.7, 14.0. APCI-MS m/z 526.9. UV-Vis (CH2Cl2): λmax = 599 nm, εmax = 6.11×104 Lcm−1mol−1. Anal. Calcd for C32H38N4OS: C, 72.97; H, 7.27; N, 10.64; S, 6.09. Found: C, 72.56; H, 7.09; N, 10.86; S, 6.12.
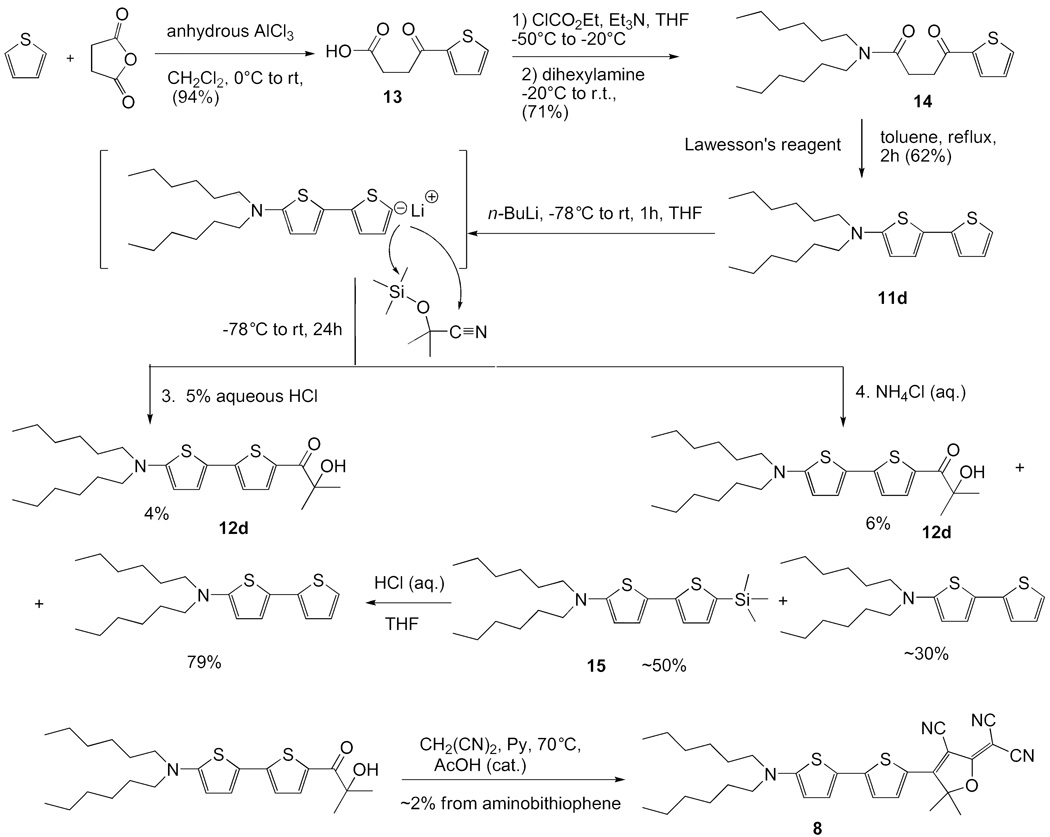
N,N-Dihexyl-4-oxo-4-thiophen-2-yl-butylamide (14)
The title compound was synthesized by a similar procedure to compound 9. The amide was given in 34.0 g with 90% yield. IR (neat): 2955, 2927, 2857, 1639, 1519, 1459, 1416 cm−1. 1H NMR (400 MHz, CDCl3): δ 7.81 (dd, J = 3.8, 1.2 Hz, 1H), 7.62 (dd, J = 5.0, 1.2 Hz, 1H), 7.14 (dd, J = 5.0, 3.8 Hz, 1H), 3.34-3.27 (m, 6H), 2.77 (t, J = 6.7 Hz, 2H), 1.66-1.58 (m, 2H), 1.55-1.47 (m, 2H), 1.37-1.24 (m, 12H), 0.94-85 (m, 6H). 13C NMR (100 MHz, CDCl3): δ 192.3, 170.8, 144.1, 133.4, 132.0, 128.1, 48.0, 46.2, 34.5, 31.6, 31.55, 29.0, 27.8, 27.2, 26.7, 26.6, 22.6, (2C) 14.04, 14.01. HRMS m/z Calcd. for C20H33NO2S (M+Na): 374.2130. Found: 374.2129.
5-N,N-Dihexylamino-2,2′-bithiophene (11d)
The title compound was synthesized by a similar procedure as compound 11b. It was given as yellow oil (26.0 g, 77% yield). IR (neat): 2954, 2926, 2855, 1556, 1514, 1493, 1451 cm−1. 1H NMR (300 MHz, CDCl3): δ 7.07 (dd, J = 4.8, 1.5 Hz, 1H), 6.99-6.94 (m, 2H), 6.90 (d, J = 3.9 Hz, 1H), 5.75 (d, J = 3.9 Hz, 2H), 3.25 (t, J = 7.5 Hz, 4H), 1.70-1.62 (m, 4H), 1.44-1.28 (m, 12H), 0.97 (t, J = 6.8 Hz, 6H). 13C NMR (75 MHz, CDCl3): δ 157.3, 139.3, 127.7, 124.0, 121.8, 120.7, 120.0, 101.5, 53.9, 31.9, 27.2, 27.0, 22.9, 14.3. HRMS m/z Calcd. for C20H31NS2 (M+H): 350.1976. Found: 350.1983.
3-Cyano-2-dicyanomethylen-5,5-dimethyl-4-[5'-(dihexylamino)-2,2'-bithien-5-yl]-2,5-dihydrofuran (8) [from nitrile 10]
The title compound was synthesized in two steps by similar procedures as in the synthesis of compounds 12b and 5 respectively. It was given as green crystals (0.12 g, 2.5% yield for two steps). Mp 191.2-192.8 °C. IR (neat): 2952, 2928, 2854, 2222, 1574, 1544 cm−1. 1H NMR (400 MHz, CDCl3): δ 8.00 (d, J = 4.6 Hz, 1H), 7.36 (d, J = 4.5 Hz, 1H), 7.05 (d, J = 4.6 Hz, 1H), 5.93 (d, J = 4.5 Hz, 1H), 3.35 (t, J = 7.7 Hz, 4H), 1.86 (s, 6H), 1.74-1.65 (m, 4H), 1.40-1.32 (m, 12H), 0.93 (t, J = 6.8 Hz, 6H). 13C NMR (100 MHz, CDCl3): δ 176.6, 165.1, 163.3, 154.2, 138.4, 132.1, 122.4, 121.8, 116.4, 113.1, 112.9, 112.2, 104.1, 96.5, 54.1, 31.5, 27.4, 27.0, 26.6, 22.6, 14.0. APCI-MS m/z 532.2. UV-Vis (CH2Cl2): λmax = 671 nm, εmax = 10.5×104 Lcm−1mol−1. Anal. Calcd for C30H36N4OS2: C, 67.63; H, 6.81; N, 10.52; S, 12.04. Found: C, 67.46; H, 6.60; N, 10.64; S, 12.31.
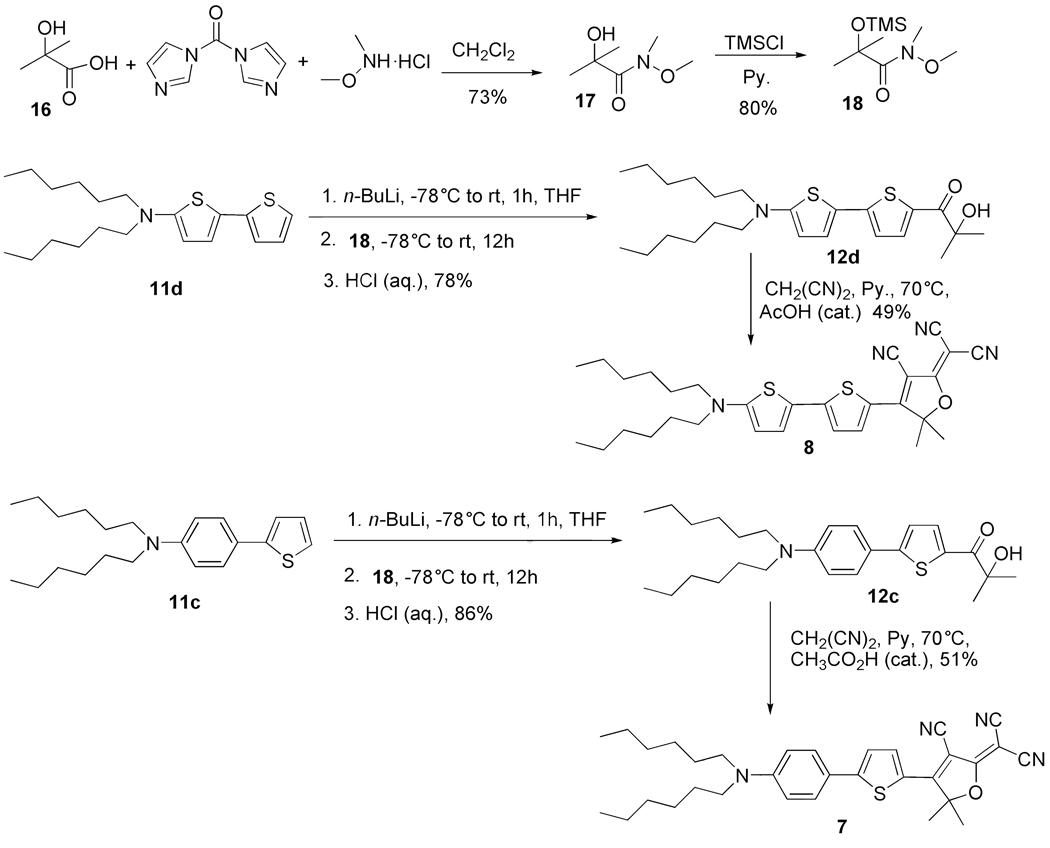
2-Hydroxy-N-methoxy-2,N-dimethyl-propioamide (17)
To a solution of 2-hydroxyisobutyric acid (16) (2.08 g, 20 mmol) in 40 mL dry dichloromethane was added 1,1′-carbonyldiimidazole (4.21 g, 26 mmol) in portions over 10 min. After the final addition, the solution was stirred for 15 min, then N,O-dimethylhydroxylamine hydrochloride (2.54 g, 26 mmol) was added in one portion. The reaction was allowed to stir at room temperature overnight. Diethyl ether (50 mL) was added and after stirring for 5 min the clear solution was decanted. The residue was stirred again with diethyl ether (40 mL), which was decanted off. The combined organic solution was washed with saturated sodium bicarbonate, brine and dried over anhydrous MgSO4. The crude product was purified by flash chromatography (hexane/EtOAc = 5/1) to give 2-hydroxy-N-methoxy-2,N-dimethyl-propionamide as a colorless liquid (1.83 g, 73% yield). IR (neat): 3468, 2989, 2943, 1744 cm−1; 1H NMR (400 MHz, CDCl3): δ 3.70 (s, 3H), 3.25 (s, 3H), 1.44 (s, 6H); 13C NMR (100 MHz, CDCl3): δ 177.2, 72.1, 61.0, 33.6, 26.5. HRMS m/z Calcd. for C6H13NO3 (M+Na): 170.0793. Found: 170.0793.
N-Methoxy-2,N-dimethyl-2-trimethylsilanyloxy-propionamide (18)
To a solution of 2-hydroxy-Nmethoxy-2,N-dimethyl-propionamide (1.5 g, 10.2 mmol) in pyridine (0.98 g, 12.4 mmol) was added chlorotrimethylsilane (1.6 mL, 12.5 mmol) dropwise while chilling in an ice-water bath. The resulting mixture was stirred at room temperature for 8 h. After that, the mixture was poured into saturated sodium bicarbonate solution (100 mL) and ethyl ether (100 mL). After stirring for 1 h, the mixture was separated, washed with brine and dried over magnesium sulfate. After evaporation of the solvent, the residue was distilled under vacuum to give product as a clear liquid (1.8 g, 80% yield). IR (neat): 2957, 1658, 1462, 1032 cm−1. 1H NMR (400 MHz, CDCl3): δ 3.73 (s, 3H, OCH3), 3.35 (s, 3H, NCH3), 1.52 (s, 6H, C(CH3)2), 0.19 (s, 9H, Si(CH3)3); 13C NMR (100 MHz, CDCl3): δ 174.2, 76.6, 60.4, 28.2, 26.5, 2.2. HRMS m/z Calcd. for C9H21NO3Si (M+Na): 242.1188. Found: 242.1185.
1-(5'-Dihexylamino-[2,2']bithiophenyl-5-yl)-2-hydroxy-2-methyl-propan-1-one (12d) [from the Weinreb amide 18]
In a 100 mL round bottom flask was placed (5-N,N-dihexylamino)-2,2′-thiophene (1.47 g, 4.2 mmol) and 15 mL anhydrous THF. The mixture was cooled to −78 °C and 1.93 mL (4.84 mmol) of n-BuLi in hexane (2.5 M) was added to this stirred mixture over 5 min. The resulting mixture was stirred for an additional 1 h at −78 °C and then at room temperature for 10 min. Subsequently the reaction mixture was cooled to −78 °C and N-methoxy-2,N-dimethyl-2-trimethylsilanyloxy-propionamide (18) (1.11 g, 5.04 mmol) was added via a syringe over 5 min. After completion of addition the temperature was allowed to increase to room temperature over 3 h and the reaction mixture was stirred for an additional 6 h at room temperature. The reaction was quenched with 5% hydrochloric acid (10 mL) and the mixture was stirred for 5 h and extracted with dichloromethane. The organic layer was washed with water, brine and dried with anhydrous MgSO4. Solvent was removed with a rotary evaporator and the residue was purified by flash column chromatography (hexane/EtOAc = 5/1), and a 1-(5′-dihexylamino-[2,2′]bithiophenyl-5-yl)-2-hydroxy-2-methyl-propan-1-one was obtained as yellow oil (1.73 g, 94% yield). IR (neat): 3442, 2954, 2927, 2856, 1619, 1551, 1513, 1485, 1419, 1048 cm−1. 1H NMR (400 MHz, CDCl3): δ 7.73 (d, J = 4.1 Hz, 1H), 7.12 (d, J = 4.1 Hz, 1H), 6.89 (d, J = 4.1 Hz, 1H), 5.76 (d, J = 4.1 Hz, 1H), 4.11 (br, s, 1H), 3.27 (t, J = 7.6 Hz, 4H), 1.70-1.60 (m, 10H), 1.38-1.31 (m, 12H), 0.91 (t, J = 6.7 Hz, 6H). 13C NMR (100Hz, CDCl3): δ 195.6, 159.6, 149.4, 135.8, 132.7, 127.5, 120.1, 117.5, 101.6, 75.6, 53.7, 31.6, 29.0, 27.0, 26.7, 22.6, 14.0. HRMS m/z Calcd. for C24H37NO2S2 (M+Na): 458.2163. Found: 458.2172.
3-Cyano-2-dicyanomethylen-5,5-dimethyl-4-[5'-(dihexylamino)-2,2'-bithien-5-yl]-2,5-dihydrofuran(8)
The title compound was synthesized in a similar procedure as compound 5. It was obtained as a green solid (0.90 g, 47% yield).
1-[5-(4-Dihexylamino-phenyl)-thiophen-2-yl]-2-hydroxy-2-methyl-propan-1-one (12c). [from the Weinreb amide 18]
The title compound was synthesized by a similar procedure as compound 12d, from the Weinreb amide 18. It was obtained as oil (1.90 g, 86%). IR(neat): 3446, 2955, 2927, 2857, 1739, 1603, 1366 cm−1. 1H NMR (400 MHz, CDCl3): δ 7.82 (d, J = 4.1 Hz, 1H), 7.54 (ddd, J = 9.0, 3.1, 2.1 Hz, 2H), 7.18 (d, J = 4.1 Hz, 1H), 6.65 (ddd, J = 9.0, 3.1, 2.1 Hz, 2H), 4.14(br, s, 1H), 3.22 (t, J = 7.7 Hz, 4H), 1.67 (s, 6H), 1.67-1.60 (m, 4H), 1.39-1.32 (m, 12H), 0.95 (t, J = 6.7 Hz, 6H). 13C NMR (100Hz, CDCl3): δ 196.2, 155.6, 148.9, 135.8, 134.5, 127.6, 121.1, 119.8, 111.5, 75.7, 51.1, 31.7, 29.0, 27.2, 26.8, 22.7, 14.1. APCI m/z 430.5. HRMS m/z Calcd. for C26H39NO2S (M+Na): 452.2599. Found: 452.2603.
3-Cyano-2-dicyanomethylen-5,5-dimethyl-4-[5-(4-dihexylaminophenyl)thien-2-yl]-2,5-dihydrofuran (7)
The title compound was synthesized by a similar procedure as compound 5. It was obtained as a purple solid (1.0 g, 51% yield).
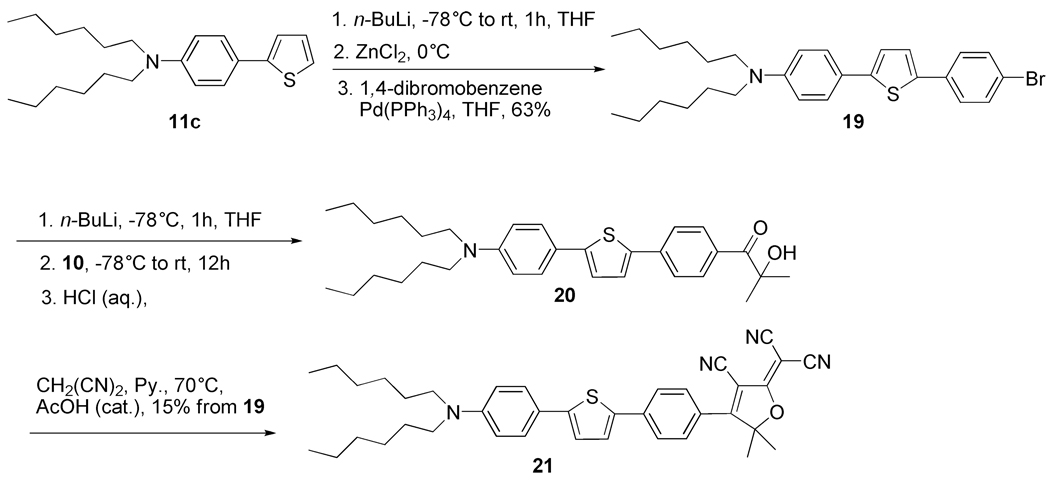
{4-[5-(4-Bromophenyl)-thiophen-2-yl]-phenyl}-dihexyl-amine (19)
In a dry 200 mL two-neck round bottom flask with stirbar was added dihexyl-(4-thiophen-2-yl-phenyl)-amine (3.44 g, 10 mmol) and anhydrous THF (35 mL). Then the mixture was cooled to −78 °C. After stirring for about 5 min, n-BuLi (2.5 M in hexane, 0.77 g, 4.8 mL, 12 mmol) was added. The mixture was stirred at −78 °C under nitrogen for 20 min, then the temperature was increased slowly over 30 min to room temperature. The reaction mixture was cooled to −78 °C again and dry ZnCl2 (2.05 g, 15 mmol) in THF (15 mL) was added to the reaction mixture. After about 5 min the reaction mixture was slowly increased to room temperature. After another 10 min, 1,4-dibromobenzene (11.8 g, 50 mmol, 5 eq.) and Pd(PPh3)4 (0.23 g, 0.2 mmol, 2 mol%) were added. The reaction mixture was stirred under nitrogen overnight. The reaction mixture was diluted with hexane, washed with water and dried with anhydrous MgSO4. After removal of solvent, the residue was adsorbed on silica gel, placed at the top of a silica column and eluted (hexane) to give a yellow solid crude product which was recrystallized from 1-propanol to give the desired product as a pure yellow solid (3.10 g, 63% yield). Mp 90 °C. IR (neat): 2955, 2927, 2857, 2361, 1607 cm−1. 1H NMR (400 MHz, CDCl3): δ 7.51-7.46 (m, 6H), 7.25 (d, J = 3.8 Hz, 1H), 7.10 (d, J = 3.8 Hz, 1H), 6.66 (ddd, J = 8.9, 3.1, 2.2 Hz, 2H), 3.31 (t, J = 7.7 Hz, 4H), 1.64-1.61 (m, 4H), 1.41-1.32 (m, 12H), 0.94 (t, J = 6.8 Hz, 6H); 13C NMR (100 MHz, CDCl3): δ 147.8, 145.6, 139.4, 133.7, 131.9, 126.9, 126.8, 124.3, 121.4, 121.2, 120.5, 111.6, 51.1, 31.8, 27.3, 26.9, 22.7, 14.1; Anal. Calcd for C28H36BrNS: C, 67.45; H, 7.28; N, 2.81; S, 6.43. Found: C, 67.58; H, 6.99; N, 2.96; S, 6.16.
2-(3-Cyano-4-{4-[5-(4-dihexylamino-phenyl)-thiophen-2-yl]-phenyl}-5,5-dimethyl-5H-furan-2-ylidene)-malononitrile (21)
The title compound was synthesized by two steps by methods similar to the synthesis of compounds 12b and 5 respectively. It was obtained as purple crystals (0.25 g, 9.2% yield for two steps). Mp 178 °C. IR (neat): 2954, 2922, 2854, 2224 cm−1. 1H NMR (400 MHz, CDCl3): δ 7.94 (ddd, J = 8.8, 2.3, 2.0 Hz, 2H), 7.79 (ddd, J = 8.8, 2.3, 2.0 Hz, 2H), 7.52 (ddd, J = 9.0, 3.0, 2.0 Hz, 2H), 7.50 (d, J = 4.0 Hz, 1H), 7.20 (d, J = 4.0 Hz, 1H), 6.67 (ddd, J = 9.0, 3.0, 2.0 Hz, 2H,), 3.33 (t, 4H), 1.89 (s, 6H), 1.69-1.57 (m, 4H), 1.41-1.31 (m, 12H), 0.88 (t, 6H); 13C NMR (100 MHz, CDCl3): δ 175.5, 175.4, 149.2, 148.4, 140.8, 137.7, 129.7, 127.4, 127.1, 125.6, 124.3, 122.0, 120.4, 111.7, 111.6, 111.2, 110.7, 99.1, 98.7, 58.1, 51.1, 31.7, 27.3, 26.9, 26.8, 22.7, 14.1; UV-Vis (CH2Cl2): λmax = 555 nm, εmax=2.6×104 L·mol−1·cm−1. Anal. Calcd for C38H42N4OS: C, 75.71; H, 7.02; N, 9.29; S, 5.32. Found: C, 75.82; H, 6.89; N, 9.44; S, 5.40.
5-(4-Dihexylamino-phenyl)-thiophene-2-carbaldehyde (22)
In a 200 mL round bottom flask was placed 2-(4-N,N-dihexylaminophenyl)thiophene (1.03 g, 3.0 mmol) and 15 mL anhydrous THF. The mixture was cooled to −78 °C and 1.44 mL (3.6 mmol) of n-BuLi in hexane (2.5 M) was added to this stirred mixture over 10 min. The resulting mixture was stirred for an additional 1 h at −78 °C and then at room temperature for 10 min. Subsequently, the reaction mixture was cooled to −78 °C and anhydrous DMF (459 mg, 6.0 mmol) was added to the resulting mixture via a syringe as quick as possible. After completion of addition the temperature was allowed to increase to room temperature over 3 h and the reaction mixture was stirred for an additional 24 h at room temperature. The reaction was quenched with 30 mL water and 1 mL 6 M hydrochloric acid and extracted with dichloromethane. The organic layer was washed with water and brine and dried with anhydrous MgSO4. Solvent was removed with a rotary evaporator and the residue was purified by silica gel chromatography (dichloromethane) to give the desired product as oil (0.97 g, 87% yield). IR (neat): 2954, 2926, 2855, 2791, 2740, 1656, 1601, 1440 cm−1. 1H NMR (400 MHz, CDCl3): δ 9.83 (s, 1H), 7.68 (d, J = 4.0 Hz, 1H), 7.55 (ddd, J = 9.1, 3.1, 2.1 Hz, 2H), 7.23 (d, J = 4.0 Hz, 1H), 6.65 (ddd, J = 9.1, 3.1, 2.1 Hz, 2H), 3.32 (t, J = 7.7 Hz, 4H), 1.66-1.57 (m, 4H), 1.41-1.25 (m, 12H), 0.94 (t, J = 6.8 Hz, 6H). 13C NMR (100 MHz, CDCl3): δ 182.4, 156.4, 149.0, 139.7, 138.2, 127.7, 121.1, 119.7, 111.5, 51.1, 31.7, 27.2, 26.8, 22.7, 14.1. HRMS m/z Calcd. for C23H33NOS(M+H): 372.2361. Found: 372.2369.
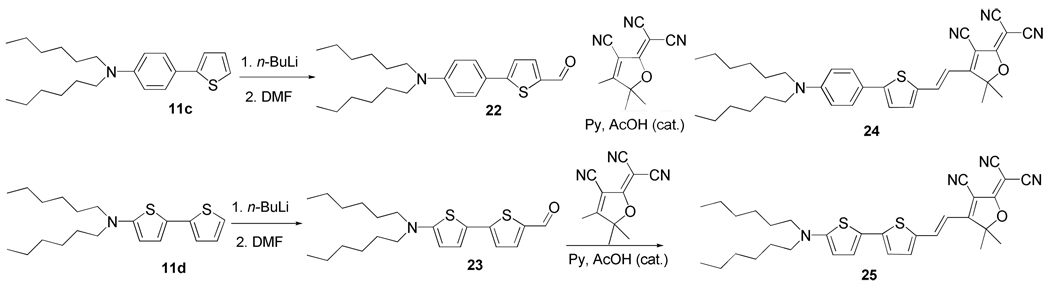
1-(3-Cyano-2-dicyanomethylen-5,5-dimethyl-2,5-dihydrofuran-4-yl)-2-{5-[4-(N,N-dihexylaminophenyl)]thien-2-yl}ethene (24)
A mixture of 5-(4-dihexylaminophenyl)-thiophene-2-carbaldehyde (557 mg, 1.5 mmol) and 2-dicyanomethylen-3-cyano-4,5,5-trimethyl-2,5-dihydrofuran (301 mg, 1.4 mmol) was dissolved in 15 mL dry pyridine. After addition of 3 drops of acetic acid the mixture was stirred at room temperature for 48 h. The reaction mixture was transferred to a separatory funnel and 150 mL of ethyl acetate was added. The solution was washed with 5% hydrochloric acid, water, brine and dried over anhydrous MgSO4. Solvent was removed with a rotary evaporator and the residue was purified by silica gel chromatography with hexane and hexane/ethyl acetate mixture as eluent to give a green solid, which was recrystallized from dichloromethane/methanol to give the desired product as green crystals (610 mg, 77% yield). Mp. 185.7–186.7 °C. IR (neat): 3093, 2957, 2925, 2857, 2225, 1592 cm−1. 1H NMR (400 MHz, CDCl3): δ 7.81 (d, J = 15.5 Hz, 1H), 7.55 (d, J = 9.1 Hz, 2H), 7.45 (d, J = 4.1 Hz, 1H), 7.26 (d, J = 4.1 Hz, 1H), 6.66 (d, J = 9.1 Hz, 2H), 6.56 (d, J = 15.5 Hz, 1H), 3.35 (t, J = 7.8 Hz, 4H), 1.77 (s, 6H), 1.65-1.59 (m, 4H), 1.40-1.33 (m, 12H), 0.93 (t, J = 6.8 Hz, 6H). 13C NMR (100 MHz, CDCl3): δ 176.0, 173.2, 156.6, 149.6, 139.9, 138.5, 136.5, 127.9, 122.6, 119.4, 112.5, 111.72, 111.67, 111.3, 110.8, 96.9, 95.1, 55.4, 51.1, 31.7, 27.3, 26.8, 26.6, 22.7, 14.1. UV-Vis (CH2Cl2): λmax = 658 nm, εmax = 5.37×104 Lcm−1mol−1. Anal. Calcd for C34H40N4OS: C, 73.88; H, 7.29; N, 10.14; S, 5.80. Found: C, 73.62, H, 7.42; N, 9.99; S, 5.93.
5'-Dihexylamino-[2,2']-bithiophenyl-5-carbaldehyde (23)
The title compound was synthesized in a similar procedure as compound 22. It was given as oil (1.21 g, 93% yield). IR (neat): 2953, 2926, 2856, 1651, 1518, 1424, 1042 cm−1. 1H NMR (400 MHz, CDCl3): δ 9.75 (s, 1H), 7.57 (d, J = 4.1 Hz, 1H), 7.12 (d, J = 4.1, 1H), 6.92 (d, J = 4.1 Hz, 1H), 5.76 (d, J = 4.1 Hz, 1H), 3.27 (t, J = 7.6 Hz, 4H), 1.71-1.62 (m, 4H), 1.40-1.29 (m, 12H), 0.92 (t, J = 6.9 Hz, 6H); 13C NMR (100 MHz, CDCl3): δ 181.7, 159.9, 150.0, 138.2, 138.0, 127.9, 120.0, 117.4, 101.6, 53.7, 31.6, 27.0, 26.7, 22.6, 14.0. HRMS m/z Calcd. For C21H31NOS2 (M+H): 378.1925. Found: 378.1935.
1-(3-Cyano-2-dicyanomethylen-5,5-dimethyl-2,5-dihydrofuran-4-yl)-2-[5'-(N,N-dihexylamino)-2,2'-bithien-5-yl]ethene (25)
The title compound was synthesized in a similar procedure as compound 24. It was given as green crystals (0.62 g, 63% yield). Mp 194.0-196.1°C. IR (neat): 2927, 2856, 2222, 1593 cm−1. 1H NMR (400 MHz, CDCl3): δ 7.77 (d, J = 15.2 Hz, 1H), 7.36 (d, J = 4.3 Hz, 1H), 7.24 (d, J = 4.3 Hz, 1H), 6.95 (d, J = 4.3 Hz, 1H), 6.34 (d, J = 15.2 Hz, 1H), 5.89 (d, J = 4.3 Hz, 1H), 3.33 (t, J = 7.7 Hz, 4H), 1.77 (s, 6H), 1.71-1.62 (m, 4H), 1.40-1.33 (m, 12H), 0.93 (t, J = 6.8 Hz, 6H). 13C NMR (100 MHz, CDCl3): δ 176.3, 172.4, 162.1, 150.9, 139.5, 139.0, 135.1, 130.5, 121.9, 117.7, 113.0, 112.3, 111.9, 109.2, 103.6, 96.4, 92.6, 54.0, 53.7, 31.6, 27.1, 26.7, 26.66, 22.6, 14.0. UV-Vis (CH2Cl2): λmax = 761 nm, εmax = 7.74×104 L·cm−1·mol−1. Anal. Calcd for C32H38N4OS2: C, 68.78; H, 6.85; N, 10.03; S, 11.48. Found: C, 68.73; H, 6.71; N, 10.02; S, 11.62.
Results and Discussion
Synthesis
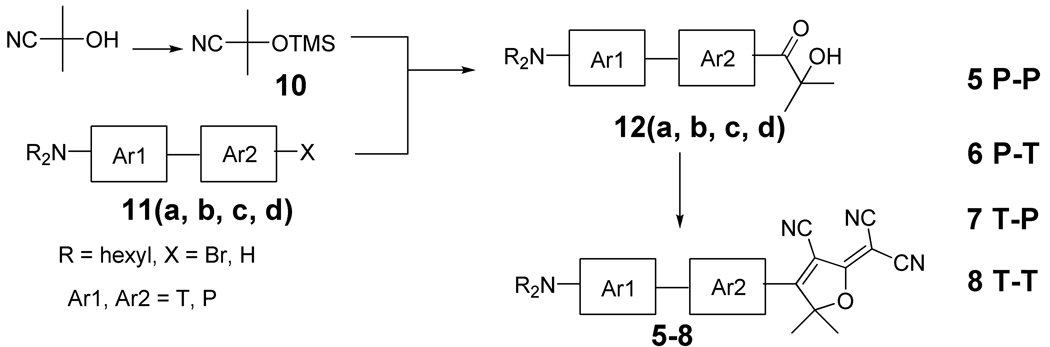
Much of the general synthetic strategy for the DCDHF chromophores with multiple rings and without a vinyl link seen in Scheme 1 follows from our previous procedures.18,2(g) The first step is to make the dialkylaminobisaromatic compounds 11a–11d, which are converted to organometallic derivatives and then reacted with nitrile 10 (the TMS protected acetone cyanohydrin) leading to the α-ketols 12a–12d after hydrolysis of the imine intermediate. Knoevenagel condensation between the α-ketols 12a–12d and malononitrile directly provides the target DCDHF fluorophores 5–8. Subsequently, we describe separately the preparation of these individual bisaromatic DCDHF compounds with Ar1-Ar2 = P-P, P-T, T-P and T-T. (The naming scheme specifies the π system: Ar1 is the π unit closest to DCDHF acceptor, Ar2 is the π unit one away from the acceptor, and so on. The π units are denoted P = phenylene, V = vinyl, T = thiophene.)19 In particular, we discuss a problem involving thienyllithium reaction with 10 at the trimethylsilyl group predominating over the cyano group and the development of a solution using a Weinreb amide 18 to make the thienyl α-ketols much more efficiently.
Scheme 1.
A general strategy for synthesis of DCDHF chromophores with two aromatic rings between the donor and acceptor units.
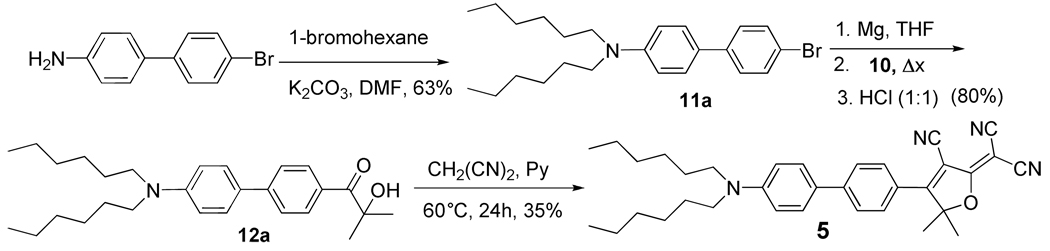
Compound 5 (Scheme 2), (Ar1-Ar2 = P-P) was prepared starting with nitration of 4-bromobiphenyl with fuming nitric acid to give 4-bromo-4′-nitrobiphenyl which was then reduced to 4-amino-4′-bromobiphenyl.20 The 4-amino-4′-bromobiphenyl was N,N-dialkylated, treated with magnesium in anhydrous THF, and the resulting Grignard reagent was quenched with nitrile 10 to afford, after hydrolysis of the imine, the α-ketol which was then converted to DCDHF dye 5 by treatment with malononitrile in pyridine.
Scheme 2.
Synthesis of DCDHF dye 5 with Ar1-Ar2 = P-P.
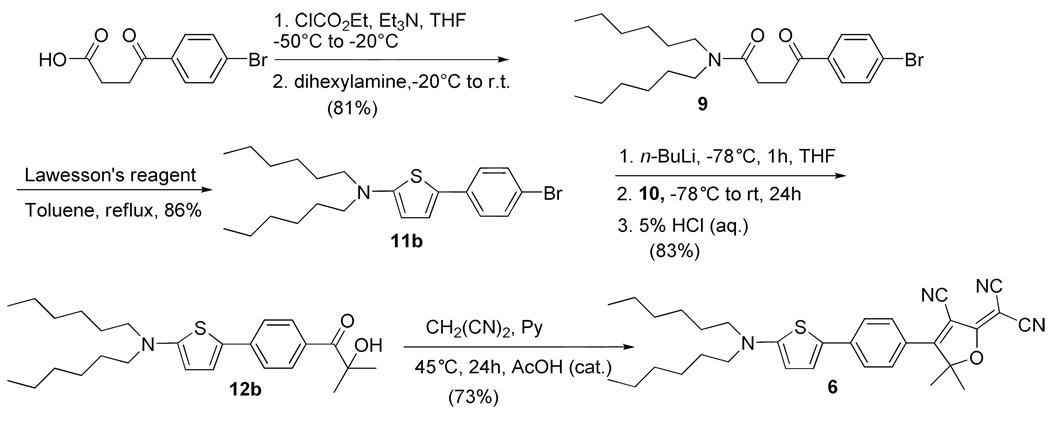
Compound 6 (Scheme 3), (Ar1-Ar2 = P-T) features a combination of a thiophene and a phenyl ring with the thiophene ring on the donor side and the phenyl ring linked to the acceptor group (Scheme 3). The synthesis starts with the Friedel-Crafts acylation of bromobenzene with succinic anhydride using a modified procedure of Seed and coworkers to give 4-(bromothien-2-yl)-4-oxobutanoic acid.21 Conversion of the carboxylic acid to the amide 9 used the method of Kozikowski et al and the resulting γ-ketoamide was cyclized with Lawesson's reagent in toluene.22,23 We found that 1.0 equivalent of Lawesson’s reagent gave only a low yield of the thiophene while more than 1.5 equivalents of Lawesson’s reagent was required for the cyclization reaction to proceed in good yield. Halogen exchange of dihexylamino-(4-thiophen-2-yl-phenyl)-amine (11b) was accomplished with n-BuLi and the resulting lithium reagent was then trapped with nitrile 10 to afford the α-ketol (12b) which was converted to DCDHF dye 6 with Ar1-Ar2 = P-T by treatment with malononitrile in pyridine.
Scheme 3.
Synthesis of DCDHF dye 6 with Ar1-Ar2 = P-T.
Molecule 7 (Scheme 4), (Ar1-Ar2 = T-P) is again a combination of phenyl and thiophene rings but in this case with reversed order having phenyl on the donor side and thiophene directly linked to the acceptor. To synthesize 7, 2-bromothiophene was reacted with magnesium in THF to give a Grignard reagent which was treated with anhydrous zinc chloride and then subjected to a Pd catalyzed Negishi coupling with N,N-dihexyl-4-bromoaniline to give 11c.24 Next, 11c was lithiated with n-BuLi at −78 °C and then warmed to 10 °C over 1 h, quenched with nitrile 10 and hydrolyzed with aqueous hydrochloric acid to afford a mixture containing α-ketol 12c, which was not immediately purified after hydrolysis due to low yield. The crude α-ketol was directly used to run the DCDHF ring formation reaction to give a low yield of 7. This problem of low yield is common to the syntheses of all α-ketols with the acceptor on the thiophene side and a solution for this problem is discussed in detail later.
Scheme 4.
Synthesis of DCDHF dye 7 with Ar1-Ar2 = T-P.
Synthesis of chromophore 8 (Ar1-Ar2 = T-T) with a thiophene-thiophene π-system is shown in Scheme 5. It began with a Friedel-Crafts acylation reaction of thiophene with succinic anhydride to afford keto-acid 13.25 Keto-acid 13 was treated with ethyl chloroformate at ~ −45 °C to give a mixed anhydride and dihexylamine was added to the same flask to give amide 14 which was subsequently reacted with Lawesson’s reagent to give bithiophene 11d using similar procedures as in the synthesis of 6. Dialkylaminobithiophene 11d was then deprotonated with n-BuLi and the organolithium derivative was subsequently trapped with nitrile 10 and hydrolyzed with 5% hydrochloric acid to afford α-ketol 12d. The crude α-ketol 12d was not further purified but directly reacted with malononitrile to give DCDHF dye 8 but in only 2.5% yield from 11d.
Scheme 5.
Synthesis of DCDHF dye 8 with Ar1-Ar2 = T-T. The side reactions of metallated 11d leading to byproducts and/or return of starting material are also shown.
The low yields of both 7 and 8, which share the common feature of a thiophene linked directly to the acceptor, prompted us to further explore the problems involved in their preparation in which the lithiated thiophene is trapped with the TMS protected acetone cyanohydrin. We carefully examined the conversion of 11d to 12d, and found that after our usual work-up with hydrochloric acid, most of the starting bithiophene was recovered and that only a low yield of α-ketol 12d was obtained. We initially assumed that this outcome was due to a failure to form the lithiated thiophene, or the failure of the lithiated thiophene to react with the TMS protected cyanohydrin or possibly due to instability of the α-ketol 12d if formed.26 However, and much to our surprise, when the reaction mixture was instead worked up with ammonium chloride solution, about 50% of dihexyl-(5′-trimethylsilanyl-[2,2]bithiophenyl-5-yl)-amine (15) was isolated. This product results from thienyllithium attack on 10 at the trimethylsilyl group strongly predominating over attack at the cyano group. Such attack of an organolithium reagent on a trimethylsilyl group is uncommon but has been documented in the literature. For example, phenyl lithium reacts with ethoxytrimethylsilane in ethereal solution to give phenyltrimethylsilane in 72% yield.27 So, in the case at hand, the formation of 15 was obscured since the reaction was worked up with the ordinary hydrochloric acid under which conditions 15 would be protiodesilylated to 11d. In order to confirm this assumption, a THF solution of 15 (itself isolated after a mild ammonium chloride workup) was treated with 5% hydrochloric acid at room temperature and the trimethylsilyl group was indeed removed in a protiodesilylation reaction leading cleanly to aminobithiophene 11d.
Because of the unfavorable result for the reaction requiring thienyllithium attack on the cyano functionality of 10, an alternative electrophile for the formation of the α-hydroxyketone is desired. The Weinreb amide is widely used for the acylation reaction of organometallics since it will stabilize the organometallic intermediate formed by chelation to prevent tertiary alcohol formation.28 Thus, Weinreb amide 18 was proposed to replace the TMS protected acetone cyanohydrin 10 (Scheme 6). The synthesis of 18 began with reaction of 2-hydroxyisobutyric acid with 1,1′-carbonyldiimidazole followed by addition of HN(OMe)Me·HCl to afford the alcohol-containing Weinreb amide 17.29 The hydroxyl functionality in 17 was protected with TMS by reacting 17 with trimethylsilylchloride in pyridine to produce the final Weinreb amide 18. Now, 11d was deprotonated with n-BuLi and the organolithium derivative was subsequently trapped with Weinreb amide 18 and hydrolyzed with 5% hydrochloric acid to afford α-ketol 12d, which was now isolated in a much improved 78% yield. The ring formation of α-ketol 12d with malononitrile in pyridine at 70 °C now gave DCDHF dye 8 in 49% yield and the overall yield from 11d to dye 8 was 38%. A comparable good result was also obtained when the Weinreb amide 18 was used for the synthesis of DCDHF dye 7 with the thiophene directly linked to the DCDHF and the α-ketol 12c was isolated in 86% yield. The corresponding ring formation reaction gave 51% yield and the overall yield from 11c to dye 7 was 44%. So, the Weinreb amide derivative 18 is much more reactive and useful than the TMS cyanohydrin 10 originally used for creation of the α-hydroxyketone needed as a DCDHF intermediate, particularly in the case of less reactive nucleophiles such as thiophene derived anions.
Scheme 6.
Synthesis of the T-P dye 7 and T-T dye 8 using the Weinreb intermediate 18.
In addition to the set of four new DCDHF dye materials just discussed we have also synthesized a three-ring system and a pair of two ring compounds extended with an alkene. DCDHF dye 21 with a PT- P conjugation between the donor and the acceptor unit was prepared in order to examine the influence of the additional aromatic unit on the photophysical properties (Scheme 7). To synthesize 21, biaromatic 11c was deprotonated with n-BuLi in THF and subsequently treated with anhydrous zinc chloride, the organozinc reagent was then subjected to a Pd-catalyzed Negishi coupling with large excess of 1,4-dibromobenzene to give the bromine-terminated triaromatic 19.24 Bromide 19 was lithiated with n-BuLi, quenched with nitrile 10 and hydrolyzed with 6N hydrochloric acid to afford a mixture containing α-ketol 20, which was not further purified after hydrolysis. The crude α-hydroxyketone was used directly in the DCDHF ring formation reactions to give 21 in 15% yield from 19.
Scheme 7.
The synthesis of DCDHF fluorophore 21 with a three-ring P-T-P linkage.
The synthesis of the additional two vinylogous dyes with V-T-P structure 24 and V-T-T structure 25 is presented in Scheme 8. Both of them are synthesized by converting the requisite dialkylamino bisaromatic compound 11c and 11d to their corresponding aldehydes 22 and 23 which were subsequently converted to 24 and 25 by condensing the aldehydes 22 and 23 with 2-dicyanomethylen-3-cyano-4,5,5-trimethyl-2,5-dihydrofuran respectively.2(g)
Scheme 8.
The synthesis of two DCDHF fluorophores 24 and 25 with V-Ar1-Ar2 linkages.
Absorption Spectra
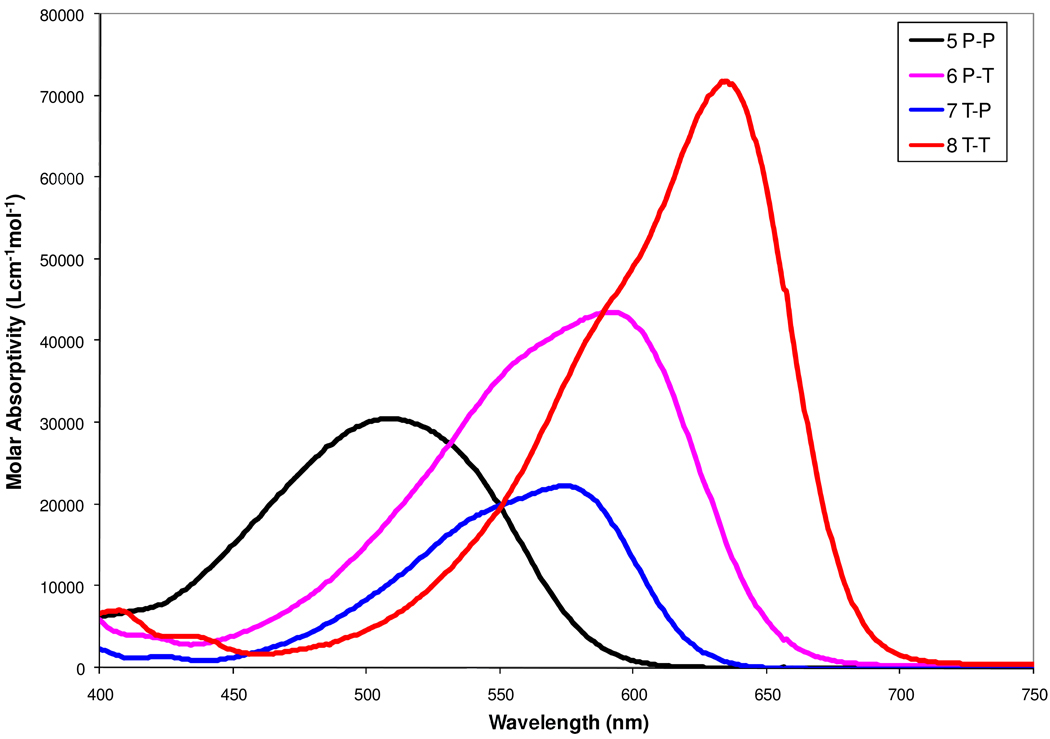
The absorption and other photophysical properties of DCDHFs 1–8, 21, 24 and 25 are all summarized in Table 1. All these dyes have the same dialkylamine donor and the same DCDHF acceptor and differ only in the π-conjugation linkage present. Thus, differences in the longest wavelength absorption band (the charge-transfer band) reflect only the composition of the long axis π–conjugation bridge of the molecules. A bathochromic shift results from addition of a second ring, either a thiophene or benzene, to the parent DCDHF 1 or 2. The absorption spectra of the set of four Ar1-Ar2 DCDHF chromophores 5–8 in toluene are shown in Fig. 2. Addition of a benzene ring to the single phenyl linkage in 1 led to a bathochromic shift of 20 nm (5), while a thiophene ring addition induced a shift of 105 nm (6) or 89 nm (7) depending on the position of the new ring. Similarly, an addition of a second phenyl ring to the thiophene linkage dye 2 led to a bathochromic shift of 77 nm (6) or 61 nm (7) and an additional thiophene ring for dye 2 gave a 120 nm (8) bathochromic shift. Overall, the most effective conjugation was observed with two thiophene rings. Comparison of the absorption maxima of the different DCDHF derivatives indicates that the charge-transfer properties can be effectively tuned by iterations of the π-bridge.
Table 1.
Summary of the Photophysical properties of DCDHF chrornophores 1-8, 21, 24 and 25 photostability value Ntot,emitted will be discussed in the following single molecule section.
| DCDHF- (π)-NR2 |
εmax (M−1cm−1)a |
λabs (nm)a |
λem (nm)a |
Stokes Shift (cm−1)a |
λex (nm)a |
ΦF Toluene [PMMA] |
Ntot,emittedb | |
|---|---|---|---|---|---|---|---|---|
| 1 | P | 71,000 | 486 | 505 | 774 | 470 | 0.044c [0.92] | 2.4×106 |
| 2 | T | 100,000 | 514 | 528 | 516 | 488 | 0.11 | ~9.1×105 |
| 3 | V-P | 45,500 | 562 | 603 | 1210 | 532 | 0.02 [0.39] | ~1.9×106 |
| 4 | V-T | 114,000 | 614 | 646 | 807 | 594 | 0.02 | ~2.3×105 |
| 5 | P-P | 31,000 | 506 | 623 | 3711 | 488 | 0.82 | 4.5×106 |
| 6 | P-T | 44,000 | 591 | 663 | 1838 | 532 | 0.21 | 6.4×106 |
| 7 | T-P | 22,000 | 575 | 631 | 1543 | 532 | 0.74 | 2.9×106 |
| 8 | T-T | 71,800 | 634 | 679 | 1045 | 600 | 0.50 | >2.3×105 d |
| 21 | P-T-P | 28,000 | 541 | 709 | 4380 | 488 | 0.34 | 9.1×105 |
| 24 | V-T-P | 47,300 | 611 | 723 | 2535 | 570 | 0.07 | >5.4×105 d |
| 25 | V-T-T | 49,800 | 708 | 779 | 1287 | 600 | 0.13 | >3.8×104 d |
All the absorption and emissions were measured in toluene
For comparison, Ntot,emitted for R6G = 1.4×106 photons per molecule (bulk measurement in PMMA).
The quantum yield value reported before (10%) is less accurate and has been corrected in this paper.
These values were determined from bulk measurements of the dyes doped in PMMA films, excited at 633 nm. They are lower limits because the calculation uses the quantum yields in toluene, which are lower than those in PMMA; if the (correct) higher PMMA quantum yields were easily measurable, the Ntot,emitted values would be higher (in a linear fashion).
Figure 2.
Visible range absorption spectra of DCDHF bisaromatic dyes 5–8 in toluene.
The P-P linkage (5) shows diminished π-overlap efficiency due to a confinement of π electrons associated with the high aromatic stabilization energy of the benzene ring and the potential barriers arising from the steric hindrance of the ortho hydrogens that inhibit the coplanarity between the two phenyl rings. In its X-ray structure, the dihedral angle (dr2 in Table 2) between the two aromatic (phenyl) rings is −19.8°, which is larger than that between thiophene and phenyl ring in compound 6 (10.0°). It is well known that the absorption wavelength increases as the number of phenyl units in the push-pull system increases, but this effect saturates (usually with the bi- or terphenyls); after saturation, adding more phenyl units eventually causes a hypsochromic shift.30 Adding a third phenyl ring does indeed cause a hypsochromic shift: the trisaromatic P-T-P dye 21 has an additional phenyl ring compared to the T-P dye 7, and the absorption maximum of 21 is 541 nm, 34 nm to the blue of 7. This blue shift occurs because the additional phenyl ring disturbs the charge transfer since the whole molecule deviates from a planar structure, as shown from the dihedral angle (dr3) between thiophene and phenyl ring of 11.8° in Table 2. In contrast, the introduction of thiophene in the π–conjugated system tolerates more thiophene units before the absorption maxima saturates. This has been explained by the reduced aromaticity of the thiophene ring and relative ease of co-planarity for adjacent thiophene rings.9(a) As such, it is no surprise to observe that the absorption maxima of the P-T 6 and T-P 7 dyes fall in the range of the absorption maxima between the P-P 5 and the T-T 8 dyes. To some extent, P-T 6 has better electronic push-pull character than T-P 7 (the former’s Stoke shift is 16 nm longer), which can be explained from the calculated dihedral angles (dr2) between phenyl and thiophene ring of these two molecules (Table 2). In the T-T dye 8, the trans conformation between two thiophene rings produces little steric interaction of hydrogens at the β,β′ position, thus a preferred coplanar structure is possible.31 This is also consistent with dihedral angles calculation of this molecule in Table 2. Finally, and in contrast to the addition of a third aromatic ring, dyes 24 and 25 (with an additional vinyl unit on the T-P dye 7 and the T-T dye 8 between the aromatic core and the acceptor) continue to shift the absorption to the red significantly.
Table 2.
Calculated torsion angles between the carbon off the amine and the average plane of the ring adjacent to the amine (da), between the DCDHF and the adjacent ring (dr1), between the first ring and its adjacent ring (dr2), and between the second and third rings (dr3), where applicable. Values in brackets are from available X-ray structures.
| da | dr1 | dr2 | dr3 | |
|---|---|---|---|---|
| 1 | 0.5 | 9.7 | ||
| 2 | 8.5 | 0.9 | ||
| 3 | −0.1 | 0.0 | 0.0 | |
| 4 | −170.5 | −0.3 | −0.2 | |
| 5 | 1.3 [−7.8] | 13.9 [−2.2] | −27.2 [−23.0] | |
| 6 | 10.0 [−3.2] | 7.5 [13.2] | −0.1 [8.1] | |
| 7 | 0.7 | 1.5 | −11.6 | |
| 8 | −167.9 | −0.4 | −179.4 | |
| 21 | 3.6 [1.1] | −3.1 [1.0] | −172.2 [−162.3] | 162.9 [−168.5] |
Dihedral angles from both of the available x-ray results and model calculations indicate that the rings are twisted, although the calculations may not always capture the correct direction or exact magnitude. Errors in calculating values for da may be due to the use of a dimethylamino donor group in the calculation model rather than the dihexyl amino group found in the actual molecules. This analysis shows that the dihedral angle between aromatic rings (dr2) is a better indicator of the degree of charge-transfer character than that between DCDHF ring and the adjacent ring (dr1). Although these dr2 values for each compound from x-ray results and model calculations are different, they have the same trends, with P-P 5 having the largest dihedral angle and P-T 6 having the smallest value.
Emission Spectra
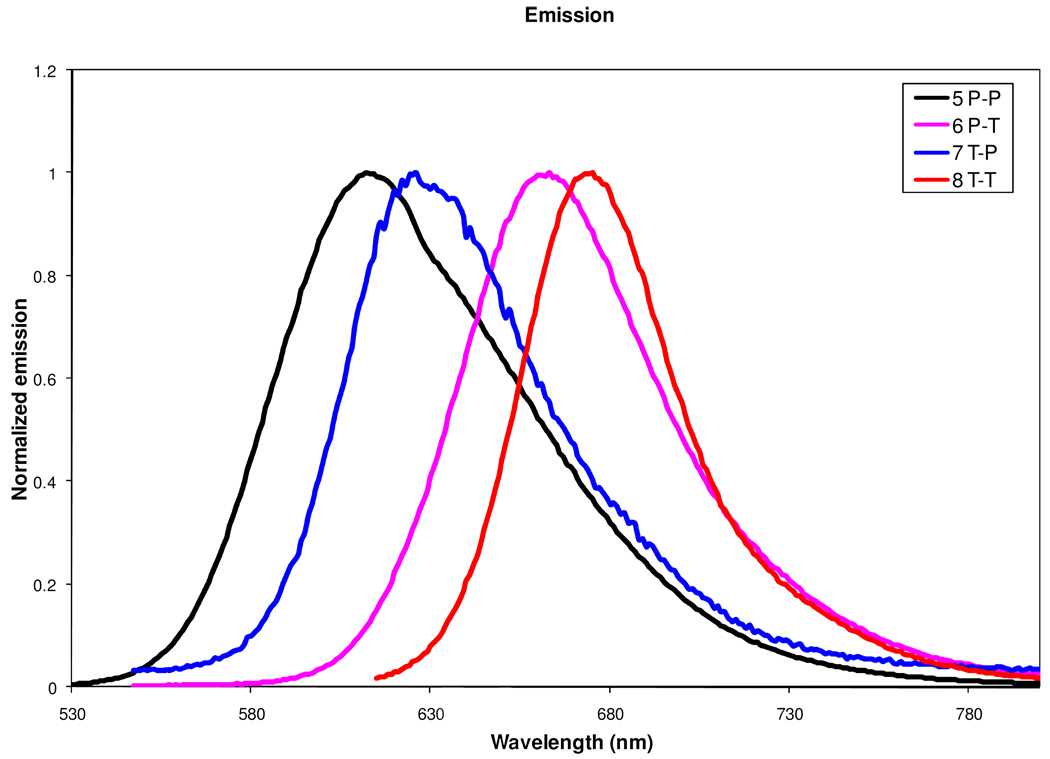
The emission maxima, Stokes shift and quantum yield of the DCDHF dyes measured in toluene are summarized in Table 1 while Fig. 3 shows the normalized emission spectra of the four Ar1-Ar2 DCDHF fluorophores 5–8 in toluene. It is clear that the addition of an olefin to the single aromatic ring dye provides a significant bathochromic shift, which is accomplished with a sacrifice of quantum yield (in solution) (Table 1). On the contrary, introduction of the second aromatic ring, particularly a thiophene ring, affords not only a red shift but also increased quantum yield (ΦF) in toluene compared with the original dyes with a single benzene or thiophene π-bridge. For example, addition of a benzene ring to the single phenyl linkage provides a small bathochromic shift of 22 nm from 486 nm for 1 to 506 nm for 5, but this is accompanied by an order-of-magnitude increase in quantum yield (4.4% to 82%). If the second ring is a thiophene, both the absorption wavelength and quantum yield are further enhanced. The T-P dye 7 has the highest quantum yield (74%) and the T-T dye 8 has the longest absorption wavelength and offers five times the quantum yield (50%) compared to the single thiophene dye 2 (11%). The trisaromatic 21 has a longer wavelength emission maximum than any of the bisaromatic dyes 5–8 while its absorption maximum wavelength is less than any of the thiophene-containing dyes 6–8; this triaromatic possesses the highest Stokes shift (4380 cm−1) amongst all the DCDHF dyes studied here. The bisaromatic-vinyl compounds 24 and 25 have longer wavelength emission maxima and higher quantum yields 7% (24), 13% (25) compared with V-P 3 (2%) and V-T 4 (2%), but the quantum yields are still much lower than their corresponding bisaromatic counterparts 7 and 8.
Figure 3.
Normalized emission spectra of DCDHF fluorophores 5–8 in toluene
It is interesting to note that the P-P dye 5 has the highest Stokes shift among all the bisaromatic dyes. The Stokes shift provides information about the excited state and it is quite possible that flattening (reduction of torsion angles) of the excited state structure plays a role in this phenomenon. In the ground state, there exists a significant torsion angle between the two phenyl rings, which prevents the effective conjugation and results in a short-wavelength absorption. The very large Stokes shift is indicative of a large excited-state dipole moment and the change in electronic structure due to molecular flattening.32 The high Stokes shift for the trisaromatic 21 may also due to flattening in the excited state.
Solvatochromism
These Ar-Ar dye materials exhibit strong solvatochromism. Because the DCDHF dyes are donor–acceptor fluorophores, the dipole moment of the molecule is expected to increase upon excitation and charge transfer. The influence of the solvent polarity on the Stokes shift was explored with the Lippert-Mataga equation (Eq. 1), in which the fluorophore is modeled as a dipole located in a cavity with radius of a in a continuous solvent-dipole environment. The polarity of the solvent is approximated using the orientation polarizability Δf, which represents how easily solvent molecules rearrange around a dipole in a continuous medium.15
Most light-induced charge transfers further increase the charge separation in a molecule. When a charge-transfer fluorophore is excited from the ground to excited state, the solvent dipole can reorient around the excited-state dipole moment. This solvent relaxation stabilizes the structure and lowers the energy of the excited state, an effect that becomes larger as the solvent polarity is increased. Thus the emission will be red shifted in more polar solvents. The Lippert-Mataga equation is:
| (eq. 1) |
and ν̅A and ν̅F are the wavenumbers of the absorption and emission, μG and μE are the ground- and excited-state dipole moments, n is the refractive index and εr is the relative low-frequency dielectric constant of the solvent, h is Planck’s constant, and c is the speed of light.
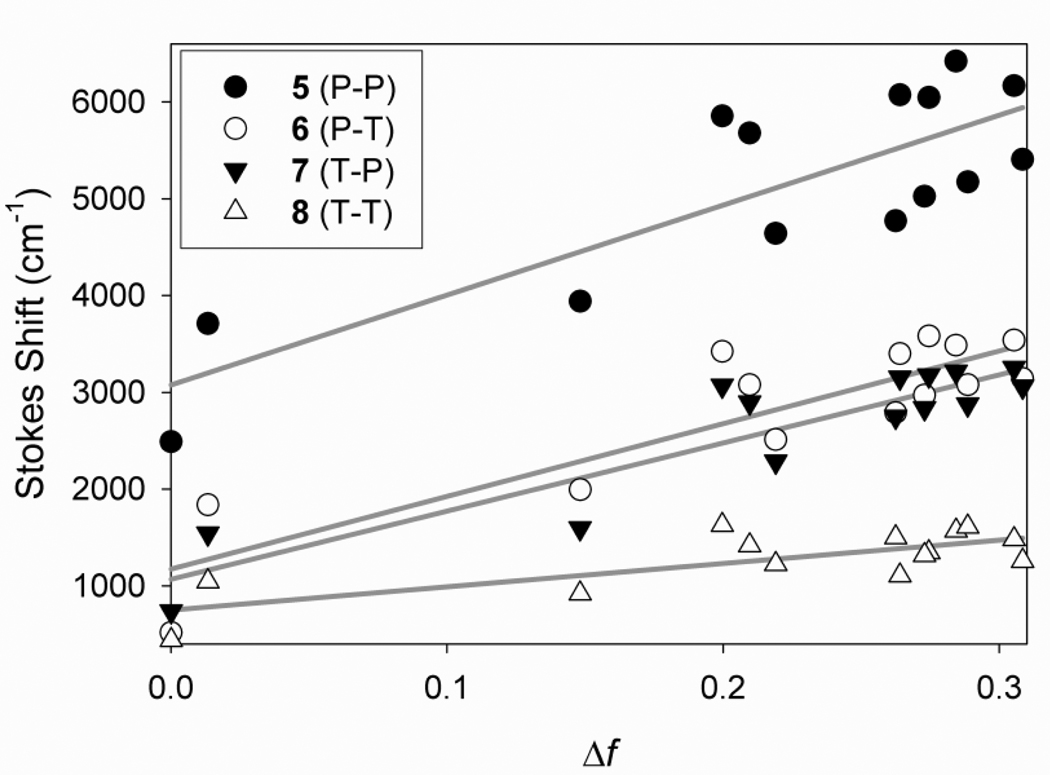
Plots of the Stokes shift as a function of the solvent Δf for 5–8 are shown in Figure 4. The absorption and emission wavelengths of Ar1-Ar2 dyes 5–8 in solvents with a range of Δf (orientation polarizability) values are found in a table in the supporting information. The slopes for the fits are 9290, 7517, 7047, and 2416 cm−1, respectively. The previously reported highest slope value for a DCDHF is 7757 cm−1 for DCDHF-A, a red-emitting fluorophore with an anthracene core.7(a) The Lippert-Mataga slope for the phenyl DCDHF (1) is only 1588 cm−1. The steeper the slope, the greater the change in dipole moment upon photoexcitation (i.e. μE – μG) and more sensitive solvatochromism is exhibited. The significant solvatochromism of the bisaromatic DCDHFs is not surprising because the extended conjugation allows for more charge separation upon charge transfer in the excited state. Such large Stokes shifts can be usefully applied in practice by using emission filters with longer wavelength cutoff, further reducing background. Moreover, it is possible to use the changes in emission wavelength to probe local (i.e. nanometer-scale) polarity.
Figure 4.
Lippert-Mataga plots of molecules 5–8. The slopes for the fits are 9290 (R2 = 0.70), 7517 (R2 = 0.77), 7047 (R2 = 0.83), and 2416 cm−1 (R2 = 0.56), respectively. The steeper the slope, the greater the excited-state charge transfer and solvatochromism is exhibited.
Single-Molecule Imaging
In the context of optical imaging, one rigorous test of the utility of a fluorophore is its ability to be successfully imaged at the single-molecule level, which requires strong fluorescence, weak coupling with dark states, and photostability. These bisaromatic DCDHF fluorophores are strong single-molecule emitters: single copies of 6 in PMMA are easily visible in an epifluorescence image of a typical sample (Figure 5). To better characterize the quality of these single-molecule emitters using one simple parameter, we recorded the distribution of the number of photons detected from single fluorophores before photobleaching, and converted this value to total photons emitted (Ntot,emitted) using the known losses in our collection system. The photon collection efficiency of our setup is D = ηQFcollFoptFfilter, the product of the camera quantum efficiency, the angular collection factor determined by the objective NA, the transmission factor through the microscope optics, and the transmission factor through the various filters, respectively. At the emission wavelengths, ηQ = 85% for our camera; the maximum possible Fcoll for our setup is 38% for a single dipole emitter aligned horizontally;33 we measured Fopt for our setup to be 50%; and we measured Ffilter to be between 50–65% for the different emission ranges. After histogramming a single-molecule emitted photon distribution, a fit to one or two exponential decays yielded the average number of photons.
Figure 5.
Epifluorescence image of single copies of DCDHF fluorophore 6 in PMMA.
Values for total number of photons detected and total number of photons emitted Ntot,emitted are reported in Table 1. (Some of the Ntot,emitted values in Table 1 are measured from bulk samples in PMMA. This measurement used bleaching curves and the rate of photon absorption to estimate the total photons emitted. The equation for this calculation can be found in reference 7a.) The Ntot,emitted values for these derivatives are all high; in fact, some are higher than those for Rhodamine 6G (1.9×106 photons emitted per molecule),13(a) which is a demonstrably good single-molecule fluorophore. These red bisaromatic DCDHF emit millions of photons, without requiring rigorous removal of oxygen from the sample,34 and thus offer a potentially useful tool for high-resolution measurements of location or dynamics within living cells.
Conclusions
A group of new fluorescent dye materials for single-molecule imaging applications containing a 2-dicyanomethylene-3-cyano-2,5-dihydrofuran acceptor group and a combination of phenyl and thiophene π-conjugation have been synthesized. The synthesis of some thiophene containing fluorophores required development of some improved synthetic methodology to obtain useful yields. These fluorophores with two aromatic rings providing conjugation linkage have absorption shifted to the red, generally exhibit significantly increased quantum yields, and show increased resistance to photobleaching in many cases. The thiophene-thiophene combination provided the longest absorption wavelength while the phenylthiophene gave the best quantum yield. Single-molecule analysis reveals that bisaromatic DCDHFs include some of the brightest and longest-lived emitters in this class of fluorophores studied so far. These red emitters are easily imaged and observed for minutes before photobleaching, making them attractive for applications in single-molecule biological and cellular studies.
Supplementary Material
X-ray data table, thermal ellipsoid plots for compounds 5, 6 and 21, Detailed synthesis procedures for compounds 6, 7, 8, 11d, 12c, 14, 21, 23, 25 and a standard 1D 1H NMR and 13C NMR spectrum for all new compounds mentioned in this paper are available free of charge via the internet at http://pubs.acs.org.
Acknowledgment
Support from DOE (DG-FG02-04ER63777), NIH (1P20HG003638-01) and the Ohio Board of Regents are acknowledged.
REFERENCES
- 1.(a) Moerner WE, Orrit M. Science. 1999;283:1670–1676. doi: 10.1126/science.283.5408.1670. [DOI] [PubMed] [Google Scholar]; (b) Weiss S. Science. 1999;283:1676–1683. doi: 10.1126/science.283.5408.1676. [DOI] [PubMed] [Google Scholar]
- 2.(a) Willets KA, Nishimura SY, Schuck PJ, Twieg RJ, Moerner WE. Accounts of Chemical Research. 2005;38:549–556. doi: 10.1021/ar0401294. [DOI] [PubMed] [Google Scholar]; (b) Willets KA, Ostroverkhova O, He M, Twieg RJ, Moerner WE. J. Am. Chem. Soc. 2003;125:1174–1175. doi: 10.1021/ja029100q. [DOI] [PubMed] [Google Scholar]; (c) Twieg R, Wang H, Lu Z, Kim SY, Lord S, Nishimura S, Schuck PJ, Willets KA, Moerner WE. Nonlinear Optics, Quantum Optics. 2005;34:241–246. [Google Scholar]; (d) Schuck PJ, Willets KA, Fromm DP, Twieg RJ, Moerner WE. Chem. Phys. 2005;318:7–11. [Google Scholar]; (e) Willets KA, Callis PR, Moerner WE. J. Phys. Chem. B. 2004;108:10465–10473. [Google Scholar]; (f) Nishimura S, Lord S, Willets K, Moerner WE, He M, Lu Z, Twieg RJ. J. Phys. Chem. B. 2006;110:8151–8157. doi: 10.1021/jp0574145. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Wang H, Lu Z, Lord SJ, Willets KA, Bertke JA, Bunge SD, Moerner WE, Twieg RJ. Tetrahedron. 2007;63:103–114. [Google Scholar]; (h) Wang H, Lu Z, Lord SJ, Moerner WE, Twieg RJ. Tetrahedron Lett. 2007;48(19):3471–3474. doi: 10.1016/j.tetlet.2007.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Melikian G, Rouessac FP, Alexandre C. Synth. Commun. 1995;25:3045–3051. [Google Scholar]
- 4.For example: Zhang C, Ren AS, Wang F, Zhu JS, Dalton LR, Woodford JN, Wang CH. Chem. Mat. 1999;11:1966–1968. Zhang C, Dalton LR, Oh MC, Zhang H, Steier WH. Chem. Mat. 2001;13:3043–3050. Gopalan P, Katz HE, McGee DJ, Erben C, Zielinski T, Bousquet D, Muller D, Grazul J, Olsson Y. J. Am. Chem. Soc. 2004;126:1741–1747. doi: 10.1021/ja039768k. He MQ, Leslie T, Garner S, DeRosa M, Cites J. J. Phys. Chem. B. 2004;108:8731–8736. Liu S, Haller MA, Ma H, Dalton LR, Jang SH, Jen AKY. Adv. Mater. 2003;15:603–607. Qiu L, Shen YQ, Hao JM, Zhai JF, Zu FH, Zhang T, Zhao YX, Clays K, Persoons A. J. Mater. Sci. 2004;39:2335–2340. Sullivan PA, Rommel H, Liao Y, Olbricht BC, Akelaitis AJP, Firestone KA, Kang JW, Luo JD, Davies JA, Choi DH, Eichinger BE, Reid PJ, Chen AT, Jen AKY, Robinson BH, Dalton LR. J. Am. Chem. Soc. 2007;129:7523–7530. doi: 10.1021/ja068322b. Beverina L, Fu J, Leclercq A, Zojer E, Pacher P, Barlow S, Van Stryland EW, Hagan DJ, Bredas JL, Marder SR. J. Am. Chem. Soc. 2005;127:7282–7283. doi: 10.1021/ja050688l. Bouit PA, Wetzel G, Berginc G, Loiseaux B, Toupet L, Feneyrou P, Bretonniere Y, Kamada K, Maury O, Andraud C. Chem. Mat. 2007;19:5325–5335.
- 5.(a) Wright D, Gubler U, Roh Y, Moerner WE, He M, Twieg RJ. Appl. Phys. Lett. 2001;79:4274–4276. [Google Scholar]; (b) Ostroverkhova O, Wright D, Gubler U, Moerner WE, He M, Sastre-Santos A, Twieg RJ. Adv. Funct. Mater. 2002;12:621–629. [Google Scholar]; (c) He M, Twieg RJ, Gubler U, Wright D, Moerner WE. Chem. Mat. 2003;15:1156–1164. [Google Scholar]; (d) You W, Cao SK, Hou ZJ, Yu LP. Macromolecules. 2003;36:7014–7019. [Google Scholar]; (e) Hayden LM, Sinyukov AM, Leahy MR, French J, Lindahl P, Herman WN, Twieg RJ, He M. J. Polym. Sci. Pt. B-Polym. Phys. 2003;41:2492–2500. [Google Scholar]; (f) Ostroverkhova O, He M, Twieg RJ, Moerner WE. Chem. Phys. Chem. 2003;4:732–744. doi: 10.1002/cphc.200200633. [DOI] [PubMed] [Google Scholar]; (g) Ostroverkhova O, Moerner WE, He M, Twieg RJ. Appl. Phys. Lett. 2003;82:3602–3604. [Google Scholar]; (h) You W, Hou ZJ, Yu LP. Adv. Mater. 2004;16:356–360. [Google Scholar]; (i) Hou ZJ, You W, Yu LP. Appl. Phys. Lett. 2004;85:5221–5223. [Google Scholar]
- 6.Harms GS, Cognet L, Lommerse PHM, Blab GA, Schmidt T. Biophys J. 2001;80:2396–2408. doi: 10.1016/S0006-3495(01)76209-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.(a) Lord SJ, Lu Z, Wang H, Willets KA, Schuck PJ, Lee HD, Nishimura SY, Twieg RJ, Moerner WE. J. Phys. Chem. 2007;111(37):8934–8941. doi: 10.1021/jp0712598. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Lu Z, Lord SJ, Wang H, Moerner WE, Twieg RJ. J. Org. Chem. 2006;71:9651–9657. doi: 10.1021/jo0616660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.For example: Nesterov EE, Skoch J, Hyman BT, Klunk WE, Bacskai BJ, Swager TM. Angew. Chem.-Int. Edit. 2005;44:5452–5456. doi: 10.1002/anie.200500845. Aoki S, Kagata D, Shiro M, Takeda K, Kimura E. J. Am. Chem. Soc. 2004;126:13377–13390. doi: 10.1021/ja040095v.
- 9.(a) Cheng LT, Tam W, Marder SR, Stiegman AE, Rikken G, Spangler CW. J. Phys. Chem. 1991;95:10643–10652. [Google Scholar]; (b) Goodbrand HB, Hu NX. J. Org. Chem. 1999;64:670–674. [Google Scholar]; (c) Lim SF, Friend RH, Rees ID, Li J, Ma YG, Robinson K, Holmes AB, Hennebicq E, Beljonne D, Cacialli F. Adv. Funct. Mater. 2005;15:981–988. [Google Scholar]; (d) Goodall W, Wild K, Arm KJ, Williams JAG. J. Chem. Soc.-Perkin Trans. 2002;2:1669–1681. [Google Scholar]
- 10.Inoue S, Aso Y, Otsubo T. Chem. Commun. 1997:1105–1106. [Google Scholar]
- 11.(a) Dirk CW, Katz HE, Schilling ML, King LA. Chem. Mat. 1990;2:700–705. [Google Scholar]; (b) Jen AKY, Rao VP, Wong KY, Drost KJ. J. Chem. Soc.-Chem. Commun. 1993:90–92. [Google Scholar]; (c) Raimundo JM, Blanchard P, Gallego-Planas N, Mercier N, Ledoux-Rak I, Hierle R, Roncali J. J. Org. Chem. 2002;67:205–218. doi: 10.1021/jo010713f. [DOI] [PubMed] [Google Scholar]; (d) Moylan CR, McNelis BJ, Nathan LC, Marques MA, Hermstad EL, Brichler BA. J. Org. Chem. 2004;69:8239–8243. doi: 10.1021/jo049230c. [DOI] [PubMed] [Google Scholar]; (e) Jia WL, Bai DR, McCormick T, Liu QD, Motala M, Wang RY, Seward C, Tao Y, Wang SM. Chem.-Eur. J. 2004;10:994–1006. doi: 10.1002/chem.200305579. [DOI] [PubMed] [Google Scholar]
- 12.(a) Effenberger F, Wurthner F, Steybe F. J. Org. Chem. 1995;60:2082–2091. [Google Scholar]; (b) Bedworth PV, Cai YM, Jen A, Marder SR. J. Org. Chem. 1996;61:2242–2246. [Google Scholar]; (c) Steybe F, Effenberger F, Gubler U, Bosshard C, Gunter P. Tetrahedron. 1998;54:8469–8480. [Google Scholar]; (d) Milian B, Orti E, Hernandez V, Navarrete JTL, Otsubo T. J. Phys. Chem. B. 2003;107:12175–12183. [Google Scholar]; (e) Raposo MMA, Fonseca AMC, Kirsch G. Tetrahedron. 2004;60:4071–4078. [Google Scholar]; (f) Tabet A, Hartmann H. Synthesis. 2005:610–616. [Google Scholar]; (g) Takahashi T, Takimiya K, Otsubo T, Aso Y. Org. Lett. 2005;7:4313–4316. doi: 10.1021/ol0513037. [DOI] [PubMed] [Google Scholar]
- 13.(a) Soper SA, Nutter HL, Keller RA, Davis LM, Shera EB. Photochem. Photobiol. 1993;57:972–977. [Google Scholar]; (b) Margineanu A, Hofkens J, Cotlet M, Habuchi S, Stefan A, Qu J, Kohl C, Mullen K, Vercammen J, Engelborghs Y, Gensch T, Deschryver FC. J. Phys. Chem. B. 2004;108:12242–12251. [Google Scholar]
- 14.Jung C, Muller BK, Lamb DC, Nolde F, Mullen K, Brauchle C. J. Am. Chem. Soc. 2006;128:5283–5291. doi: 10.1021/ja0588104. [DOI] [PubMed] [Google Scholar]
- 15.Lakowicz JR. Principles of Fluorescence Spectroscopy. New York: Springer; 2006. [Google Scholar]
- 16.Moerner WE, Fromm DP. Rev. Sci. Instrum. 2003;74:3597–3619. [Google Scholar]
- 17.Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA, Jr, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA. Gaussian 03, Revision C.01. Wallingford CT: Gaussian, Inc.; 2004. [Google Scholar]
- 18.He M, Twieg RJ, Ostroverkhova O, Gubler U, Wright D, Moerner WE. Proceedings of SPIE. 2002;4802:9–20. [Google Scholar]
- 19.Lord SJ, Conley NR, Lee H-LD, Nishimura SY, Pomerantz AK, Willets KA, Lu Z, Wang H, Liu N, Samuel R, Weber R, Semyonov A, He M, Twieg RJ, Moerner WE. ChemPhysChem. 2009 doi: 10.1002/cphc.200800581. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lemenovskii DA, Makarov MV, Dyadchenko VP, Bruce AE, Bruce MRM, Larkin SA, Averkiev BB, Starikova ZA, Antipin MY. Russ. Chem. Bull. 2003;52:607–615. [Google Scholar]
- 21.Sonpatki VM, Herbert MR, Sandvoss LM, Seed AJ. J. Org. Chem. 2001;66:7283–7286. doi: 10.1021/jo015644j. [DOI] [PubMed] [Google Scholar]
- 22.Kozikowski AP, Ma D, Brewer J, Sun S, Costa E, Romeo E, Guidotti A. J. Med. Chem. 1993;36:2908–2920. doi: 10.1021/jm00072a010. [DOI] [PubMed] [Google Scholar]
- 23.Raposo MMM, Kirsch G. Heterocycles. 2001;55:1487–1498. [Google Scholar]
- 24.(a) Negishi E, King AO, Okukado N. J. Org. Chem. 1977;42:1821–1823. [Google Scholar]; (b) Nicolaou KC, Bulger PG, Sarlah D. Angew. Chem.-Int. Edit. 2005;44:4442–4489. doi: 10.1002/anie.200500368. [DOI] [PubMed] [Google Scholar]
- 25.Cauquil-Caubere I, Kamenka JM. Eur. J. Med. Chem. 1998;33:867–877. [Google Scholar]
- 26.Liska R. Heterocycles. 2001;55:1475–1486. [Google Scholar]
- 27.Gilman H, Ingham RK, Smith AG. J. Org. Chem. 1953;18:1743–1749. [Google Scholar]
- 28.Nahm S, Weinreb SM. Tetrahedron Lett. 1981;22:3815–3818. [Google Scholar]
- 29.Garg NK, Caspi DD, Stoltz BM. J. Am. Chem. Soc. 2005;127:5970–5978. doi: 10.1021/ja050586v. [DOI] [PubMed] [Google Scholar]
- 30.Meier H. Angew. Chem.-Int. Edit. 2005;44:2482–2506. doi: 10.1002/anie.200461146. [DOI] [PubMed] [Google Scholar]
- 31.(a) Effenberger F, Würthner F. Angew. Chem.-Int. Edit. 1993;32:719–721. [Google Scholar]; (b) Garcia P, Pernaut JM, Hapiot P, Wintgens V, Valat P, Garnier F, Delabouglise D. J. Phys. Chem. 1993;97:513–516. [Google Scholar]
- 32.(a) Klock AM, Rettig W, Hofkens J, Vandamme M, Deschryver FC. J. Photochem. Photobiol. A-Chem. 1995;85:11–21. [Google Scholar]; (b) Swiatkowski G, Menzel R, Rapp W. J. Lumines. 1987;37:183–189. [Google Scholar]; (c) Berlman IB. J. Phys. Chem. 1970;74:3085–3093. [Google Scholar]
- 33.Zander C, Enderlein J, Keller RA, editors. Single-Molecule Detection in Solution: Methods and Applications. Wiley-VCH: Berlin; 2002. [Google Scholar]
- 34.Aitken CE, Marshall AR, Puglisi J. Biophys. J. 2008;94:1826–1835. doi: 10.1529/biophysj.107.117689. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
X-ray data table, thermal ellipsoid plots for compounds 5, 6 and 21, Detailed synthesis procedures for compounds 6, 7, 8, 11d, 12c, 14, 21, 23, 25 and a standard 1D 1H NMR and 13C NMR spectrum for all new compounds mentioned in this paper are available free of charge via the internet at http://pubs.acs.org.