ABSTRACT
Objective: To evaluate the benefits and risks of performing an optic nerve sheath incision to supplement standard osseous optic canal decompression for traumatic optic neuropathy. Methods: Before-after analysis of 57 cases undergoing optic nerve decompression at a tertiary referral centre from 1988–2006. Fifty-five cases had adequate post surgical follow-up for evaluation for improvement. Group A (n = 35) had decompression of the osseous optic canal and surgical slitting of the optic nerve sheath; Group B (n = 22) had osseous decompression alone. The groups were comparable for age, injury severity, and injury-surgery interval. Main outcome measure: Percentage visual improvement, which was calculated by conversion of the pre- and post-intervention visual acuity measurements to the logarithm of the minimum angle of resolution (logMAR) scale. Results: No significant recovery was noted in subjects with persistent complete blindness (PL-ve vision). In subjects with residual pre-op vision, the quantum of recovery was greater in Group A than in Group B (46% and 33% respectively, p = 0.10). The difference was especially evident in subjects with no optic canal/posterior orbit fracture (p = 0.07). Three cases with the sheath incision developed transient CSF rhinorrhea in the initial experience, but this was subsequently alleviated with modification of surgical technique. Conclusion: The addition of optic nerve sheath incision to osseous decompression may improve recovery in optic nerve injury, especially in subjects without optic canal fracture.
Keywords: Optic nerve injury, optic nerve decompression, optic nerve sheath, endoscopic skull base
Surgical decompression of the optic nerve is one of the advocated treatments for visual loss secondary to optic nerve injury.1,2,3 Of the three segments of the optic nerve—intraorbital, canalicular, and intracranial—surgical decompression is directed to the canalicular segment, where the nerve passes through a bony canal and is prone to compression. Compression may occur either because of a fracture of the bony canal or because of intraneural contusion and hemorrhage, secondary vasospasm and veno-occlusion, edema, and necrosis.4,5 These pathophysiological developments cause intraneural swelling leading to a compartmental syndrome within the constricting confines of the bony optic canal with progressively increasing veno-occlusion, consequent tissue edema, and consequent further veno-occlusion. The visual loss caused by veno-occlusion and compression may be initially reversible, but progressive compression may lead to arterial obstruction and irreversible infarction.6
Surgical decompression is effective because it releases the compartmental syndrome. Opinion in the literature is, however, divided on whether this decompression should be restricted to surgical decompression of the bony canal alone, or should include decompression of the nerve sheath, too. The optic nerve sheath is an anterior extension of the middle cranial fossa dura, which envelopes the canalicular optic nerve and condenses at its anterior end to a concentric fibrous ring known as the annulus of Zinn. Proponents of decompression of the nerve sheath note that the post-traumatic compartmental syndrome is best relieved by releasing the constricting sheath enveloping the nerve.7,8 Detractors of nerve sheath slitting, however, doubt its efficacy and also note that this may cause an occasional cerebrospinal fluid (CSF) leak, may potentially disrupt the pial vessels contributing to nerve vascularity, may disrupt the nerve fascicles, and may also risk injury to the ophthalmic artery coursing in the optic canal.7,8,9 Most surgeons have fixed positions on the advisability of whether to slit the nerve sheath; the potential benefits of nerve sheath decompression combined with bony canal decompression versus bony canal decompression alone have never been tested.
The authors' policy on optic nerve sheath decompression has changed over time due to the varying perceptions regarding its efficacy and risks. The dataset includes patients with bony decompression alone and those with nerve sheath decompression in addition to bony decompression. The quantum of improvement in the two groups and the complications resulting from nerve sheath decompression are presented.
MATERIALS AND METHODS
Participants
The study group comprises 57 patients in the period from 1988 to 2006 who underwent optic nerve decompression for optic neuropathy secondary to blunt head injury. All pre- and post-treatment data pertaining to these patients were prospectively collected at the time of treatment as per our study design.
Optic nerve injury was diagnosed on the basis of significant visual loss with an afferent pupillary defect, and no significant pathology found in the anterior or posterior segment ocular evaluation. All patients had computed tomography (CT) scanning with specific cuts for the optic nerve and canal (axial cuts at an angle of –5 degrees to the radiological 0-degree plane and 2-mm thickness with 1-mm overlap). In recent years, all patients with a fracture of the optic canal have also had a magnetic resonance imaging (MRI) scan to rule out vascular injuries in the cavernous sinus and the internal carotid artery. Optic nerve pathology was localized to the canalicular segment on the basis of radiology, the fundus examination, and visual field evaluation (if feasible).
Initial treatment was with corticosteroids, either in conventional doses or megadoses. Only patients not improving adequately with steroid treatment (i.e., residual visual deficit with visual acuity ≤ 6/60 or visual fields < 20 degrees with no further apparent progressive improvement) or having progressive deterioration despite steroid treatment were offered the option of a surgical decompression. All patients gave informed consent and were counseled of other treatment options as advocated in the literature (no treatment and steroid treatment).1,2 They were also cautioned that visual improvement following the surgical procedure was not consistent and usually not complete.
The preoperative visual acuity ranged from no perception of light (PL)−ve to 6/60. The interval from the onset of visual loss to the surgical decompression ranged from 1 to 374 days (median, 63 days). Many patients' surgeries were delayed after injury for various reasons, including treatment of other life-threatening injuries, referral from distant locations, and the time taken by patients to come to an informed decision regarding their treatment. Five patients had an interval > 180 days from injury to surgery. Such cases were informed of the possible poorer prognosis because of the delay, but surgery was not withheld on the basis of the literature, which demonstrated that the operation remains worthwhile even if undertaken after a delay of a few weeks or months.7,10,11,12,13,14,15,16,17
Surgical Procedure
Access to the sphenoid sinus was via external sphenoethmoidectomy (with an operating microscope for magnification) in the initial 35 patients (before 1999), and via transnasal endoscopy in 22 patients since 1999. The subsequent operation, following access to the sphenoid, was similar (whether initial access was by external sphenoethmoidectomy or by endoscopic sphenoethmoidectomy). The optic canal was identified either as the bulge on the lateral wall of the sphenoid or by following the orbital periosteum posteriorly until it converged on the opening of the optic canal. The bony canal was then decompressed by removing its medial wall. The anterior end of the canal was often thick and required a drill to ensure an atraumatic decompression. The posterior transition of the intracanalicular segment of the nerve to its intracranial segment cannot be easily discerned when the nerve is viewed from its medial aspect from the sphenoid. The posterior limit of bone removal was, therefore, arbitrarily set to the point where the nerve curved from an oblique to a transverse direction in its posterior course, indicating that it was nearing the chiasmal region and well posterior to the encasing bony canal. In four patients, however, decompression had to be restricted to the anterior portion because the posterior bone was extremely thick and vascular—possibly secondary to callus formation.
Following osseous optic canal decompression, further slitting of the optic nerve sheath was undertaken in 35 of 57 patients. The initial policy in the early years (1988–1990) was to incise the nerve sheath. Subsequently, because of complications, slitting of the nerve sheath was discontinued. An analysis in 1999, however, suggested the quantum of visual recovery to be possibly better in patients that had had incision of the nerve sheath;7 therefore, the nerve sheath is again being incised. The sheath incision was undertaken by a Bard-Parker knife with a disposable sickle shaped blade (no. 12) in the superomedial quadrant of the circumference of the nerve. It extended posteriorly to anteriorly to include the anterior fibrous annulus of Zinn and to continue for 3 to 5 mm into the posterior orbital periosteum. In the overall dataset, nerve sheath incision was undertaken in 35 patients, 14 in the initial years (1988–1990) and 21 in the years since 1999 (1999–2006).
In our current practice (after 1999), after slitting the nerve sheath, a specific attempt is made to precipitate occult CSF leaks by undertaking an “anesthetic Valsalva maneuver” to transiently increase the CSF pressure. In cases where this maneuver precipitates any suspicion of a leak, a mucosal graft from the ethmoid sinuses is then placed over the slit sheath. Three of 21 cases have required such a mucosal graft.
Patients were discharged from the hospital at a mean of 3.75 days (median, 3 days) after surgery.
Assessment and Analysis
Following discharge, patients were assessed at 1 week, 1 month, and 3 months for improvements in visual acuity and visual fields and for complications. Visual acuity recorded at the 3-month postoperative visit was taken as the final maximal improvement. In three patients for whom 3-month follow-up information was not available, the observations of the 1-month follow-up were used. Two other patients with follow-ups of less than 1 month were excluded from the analysis.
The severity of injury was graded (Table 1) based on the system proposed by Cook et al.1 Grade of injury is based on fractures of the optic canal or posterior orbit and the worst recorded vision (which usually corresponds to the immediate post-traumatic vision). For purposes of grading, fractures of the posterior orbit and the optic canal were treated similarly, and no differentiation was made between displaced and undisplaced fractures.
Table 1.
Comparison between Patients with and without Sheath Incision
| All Patients (n = 55)* |
Preoperative Vision > PL−ve (n = 42)* |
|||
|---|---|---|---|---|
| Group A (Sheath Incised) (n = 33) | Group B (Sheath not Incised) (n = 22) | Group A (Sheath Incised) (n = 24) | Group B (Sheath not Incised) (n = 18) | |
| PL−ve, no perception of light; PL+ve, perception of light. | ||||
| Age (y): mean (range) | 27.3 (6–52) | 25 (6–45) | 26.5 (6–52) | 25.6 (6–45) |
| Preoperative visual acuity | ||||
| • PL−ve | 9 | 4 | − | − |
| • PL+ve | 4 | 2 | 4 | 2 |
| • Hand movements | 6 | 7 | 6 | 7 |
| • 1/60 | 6 | 3 | 6 | 3 |
| • 2/60 | 2 | 1 | 2 | 1 |
| • 3/60 | 1 | 3 | 1 | 3 |
| • 6/60 | 5 | 2 | 5 | 2 |
| Fracture (optic canal/postorbit) | ||||
| • Fracture present | 12 | 10 | 6 | 6 |
| • No Fracture | 21 | 12 | 18 | 12 |
| Grade of Injury† | ||||
| • Grade 2 (no fracture, vision > PL−ve & ≤ 6/60) | 14 | 8 | 14 | 8 |
| • Grade 3 (fracture or PL−ve) | 11 | 7 | 8 | 7 |
| • Grade 4 (fracture & PL−ve) | 8 | 7 | 2 | 3 |
| Injury-operation interval (d) | ||||
| Median | 63 | 61 | 67 | 66.5 |
Two cases excluded from initial data set of 57 because of inadequate length of follow up.
Grade of injury is based on fractures of the optic canal or posterior orbit and the worst recorded vision.
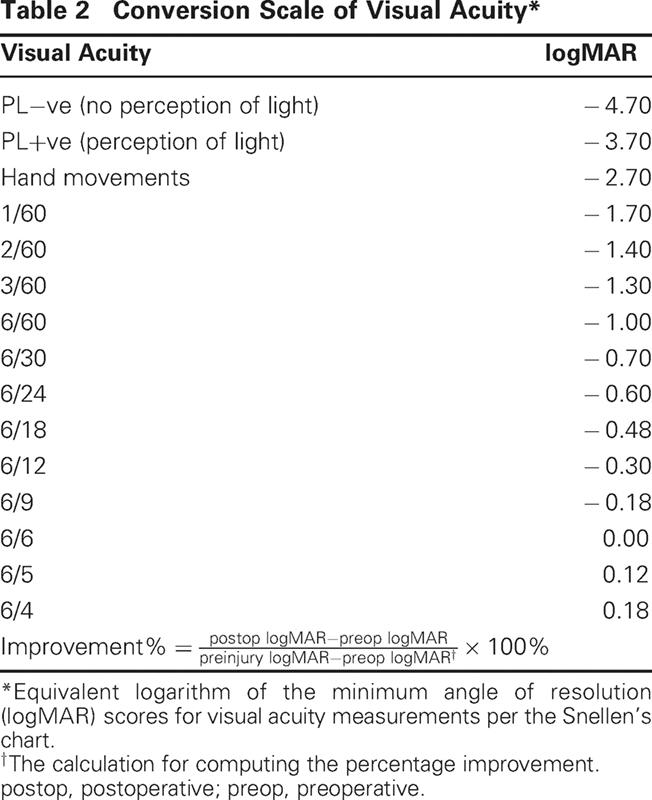
For a quantitative assessment of the degree of visual improvement following surgery, the visual acuity was converted to the logarithm of the minimum angle of resolution (logMAR) scale per the given conversion chart (Table 2), and the percentage improvement was calculated.1,7 The percentage improvement was calculated as the quantum of improvement following surgery (postoperative vision versus preoperative vision), as a proportion of the preoperative visual deficit (preinjury vision versus preoperative vision; Table 2). Because preinjury vision was never directly recorded by us, this was based on previous records, if available. In situations where no such records were available and the patient reported preinjury equivalent vision in both eyes, the contralateral visual acuity was recorded and presumed equivalent to the preinjury vision.
Recording and analysis of the dataset were done with Epi Info, version 6.04 (Centers for Disease Control and Prevention, Atlanta, GA). Statistical comparisons were done by the Mann-Whitney test and the Fisher exact test.
RESULTS
The 57 patients in the study group included 53 males and 4 females. The age ranged from 6 years to 52 years (mean, 26.7 years). Forty-three had sustained the injury in a traffic accident. Thirty-one had had some period of unconsciousness following the injury, and in 18 of these, this period was > 6 hours.
Fifty-five of the 57 patients had adequate postoperative follow-up examinations for evaluation of quantum of improvement. Thirty-three of these had a nerve sheath incision in addition to osseous canal decompression (group A), and the other 22 had a decompression of the osseous optic canal alone (group B). A comparison of the two groups with regard to patient age, fractures of the optic canal and posterior orbit, preoperative vision, grade of injury, and time since injury indicates that the two groups are comparable (Table 1). Because visual outcome analysis was mainly relevant in the patients with partial visual loss, these patients are illustrated separately (Table 1).
A marked difference in outcome was observed among patients with complete visual loss (PL−ve) and patients with partial visual loss (vision > PL−ve). Only 1 of 13 patients with complete visual loss showed some minimal improvement following optic nerve decompression (PL−ve to PL+ve). This one patient had decompression on the fourth day postinjury. No other patient with complete visual loss responded to surgery. Thirty-four of 42 patients with partial visual loss improved following surgery, with a mean improvement of 40.6% (standard error, 4.2%; range, 0 to 89.1%). Ten patients developed visual acuity of 6/18 or better; five had visual acuity of 6/12 or better.
The quantum of recovery in the two groups is illustrated in Table 3. In patients with partial visual loss, the mean quantum of recovery in group A was 46.6% compared with a mean recovery of 32.8% in group B (p = 0.10). Because patients with complete visual loss have a uniformly poor prognosis, subgroup analysis was restricted to patients with partial visual loss. The analysis indicates the difference between the two groups to be particularly manifest in patients with no fractures of the posterior orbit or optic canal (p = 0.07) and in patients with grade 2 injury (p = 0.10).
Table 3.
Percentage Improvement among Patients in Groups A and B*
| Group A (n = 33) |
Group B (n = 22) |
Statistical Significance (Mann-Whitney Test) | |||
|---|---|---|---|---|---|
| Proportion Improving | Mean Improvement (%) | Proportion Improving | Mean Improvement (%) | ||
| vn, vision; PL−ve, no perception of light; PL+ve, perception of light. | |||||
| Preop vn = PL−ve† (n = 13) | 1/9 | 1.6 | 0/4 | − | − |
| Preop vn > PL−ve† (n = 42) | 20/24 | 46.4 | 14/18 | 32.8 | 0.10 |
| Subsequent Analysis Restricted to Patients with Residual Vision before Surgery (vn > PL−ve†) | |||||
| n = 24 | n = 18 | ||||
| Fracture (optic canal/postorbit) | |||||
| • Fracture present (n = 12) | 4/6 | 30.3 | 4/6 | 29.0 | 0.87 |
| • No fracture (n = 30) | 16/18 | 51.8 | 10/12 | 34.6 | 0.07 |
| Grade of injury‡ | |||||
| • Grade 2 (n = 22)(no fracture, vn > PL−ve & ≤ 6/60) | 13/14 | 57.5 | 8/8 | 43.0 | 0.10 |
| • Grade 3 (n = 15) (fracture or PL−ve) | 6/8 | 35.2 | 5/7 | 28.2 | 0.60 |
| • Grade 4 (n = 5) (fracture & PL−ve) | 1/2 | 13.5 | 1/3 | 16.1 | − |
Group A patients had optic canal decompression supplemented with incision of the nerve sheath; group B patients had canal decompression alone and no incision of the nerve sheath.
Categorization of patients into vn = PL−ve and Vn > PL−ve is based on the preoperative vision after medical treatment and spontaneous improvement. Patients with persistent PL−ve despite medical treatment and spontaneous therapy are considered an extremely poor prognostic group.
Grade of injury is based on fractures of the optic canal or posterior orbit and the worst recorded vision.
A final visual acuity of 6/18 or better was achieved by a greater proportion of patients in group A (8 of 24 patients) than in group B (2 of 18 patients; Table 4). A final visual acuity of 6/12 or better was achieved by 5 of 24 in group A compared with none in group B (p = 0.06).
Table 4.
Proportion of Patients Developing Useful Vision*
| Group A (Sheath Incised) n = 24 | Group B (Sheath not Incised) n = 18 | Odds Ratio | P Value (Fisher Exact Test) | |
|---|---|---|---|---|
| CI, confidence interval; PL−ve, no perception of light. | ||||
| Final vision ≥ 6/18 | 8/24 | 2/18 | 4.0 | 0.14 |
| (95% CI 0.6–32.5) | ||||
| Final vision ≥ 6/12 | 5/24 | 0/18 | Undefined | 0.06 |
Useful vision here is considered to be ≥ 6/18. The analysis is restricted to patients with residual vision before surgery (i.e., preoperative vision > PL−ve), which in this study was 42 patients.
Complications encountered in the two groups are listed in Table 5. The complications include the those encountered in all patients and, separately, those in the subgroup with partial visual loss. Two patients, one each in group A and group B, had a transient decrease in vision following decompression that improved to preoperative levels or better after treatment (with steroids in one patient, and with the evacuation of a sphenoid hematoma in the other patient). Three of the 14 initial patients in group A (nerve sheath incision group) had transient and self-limiting CSF rhinorrhea. No further CSF leaks occurred in the subsequent 21 patients with a nerve sheath incision, in whom care was taken to detect even minor intraoperative CSF leaks by transiently raising the CSF pressure following the sheath incision, and placing a mucosal graft on the site if even the slightest leak was suspected.
Table 5.
Complications Encountered in Patients with and without Slitting of Nerve Sheath
| All Patients (n = 57) | Preoperative Vision > PL−ve (n = 44) | |
|---|---|---|
| PL−ve, no perception of light; CSF, cerebrospinal fluid; ICA, internal carotid artery. | ||
| Group A (sheath incised) | n = 35 | n = 26 |
| Initial 14 patients (no mucosal graft) | ||
| • Intraoperative transient CSF leak | 1 | 1 |
| • Immediate postoperative CSF leak | 1 | 1 |
| • Delayed CSF leak (complicated by meningitis) | 1 | 1 |
| Subsequent 21 patients (Valsalva maneuver & prophylactic mucosal graft if required) | ||
| • CSF leak | — | — |
| • Transient decrease in vision | 1 | 1 |
| • Secondary hemorrhage/epistaxis | 1 | — |
| • Incomplete posterior decompression | 1 | 1 |
| Group B (sheath not incised) | n = 22 | n = 18 |
| • Transient decrease in vision | 1 | 1 |
| • Intraoperative bleeding (not related to ICA) | 1 | 1 |
| • Incomplete posterior decompression | 3 | 1 |
DISCUSSION
Surgical decompression of the optic nerve seeks to relieve the compression on the nerve fibers. Such compression may be either “external” (e.g., displaced fracture or extraneural hematoma) or “internal” (e.g., secondary to intraneural edema or hematoma and consequential compartmental syndrome). Internal compression may not be completely relieved by bony decompression alone and would be better relieved by a further slitting of the optic nerve sheath and the fibrous annulus of Zinn. This study indicates that the quantum of improvement following surgical decompression is better in patients in whom the decompression involves both decompression of the osseous canal and the nerve sheath rather than the osseous canal alone (46.4% versus 32.8%, p = 0.10). This is especially so in patients with no evidence of fractures, in whom the compression may be largely internal (51.8% versus 34.6%, p = 0.07).
Significant visual improvement to a final visual acuity of 6/18 or better was achieved in 10 of 42 patients with partial visual loss. Such improvement was more likely in patients in whom the nerve sheath was slit than in patients in whom it was not (odds ratio, 4.0; 95% confidence interval [CI] 0.6 to 32.5) (Table 4).
Statistical support for these findings is borderline (0.10 < p < 0.05) (Tables 3 and 4), but they provide the current best evidence to guide surgical practice. In the context of the limited number of such patients, it remains unlikely that further and more statistically robust answers would be available to this particular question in the near future.
Previous literature has documented that prognosis following optic nerve decompression may be influenced by patient age,18,19 presence or absence of fractures,1,7,20 preoperative vision,1,17,18,20 grade of injury,1,7 and delay from injury to surgery.21 Table 1 demonstrates that patients in both groups were comparable for these parameters. A slight discordance was noted in the proportion of fractures and the grade of injury in the two groups, and a stratified analysis was undertaken for these factors (Table 3).
The layers of the optic nerve sheath as viewed from a medial trans-sphenoidal approach include the periosteal and dural layers of the dura mater, the arachnoid sheath, the subarachnoid space, and the pial sheath, which is adherent to the nerve.22 Nerve sheath incisions may potentially be limited to the superficial dura mater alone and may not violate the subarachnoid CSF space. An obvious intraoperative CSF leak was noted by us in only 1 of 35 sheath incisions (Table 5). Other surgeons have also noted CSF egress being unusual following sheath incision for traumatic neuropathy,12,23 and it has been speculated that canalicular trauma may lead to obstruction of communication between the chiasmal cistern and the subarachnoid spaces around the optic nerve.12
Two other patients in this series, however, demonstrated CSF leakage in the immediate postoperative period. No intraoperative CSF leaks had been noted in these patients. Based on this experience, we have since become extremely cognizant of silent and delayed CSF leaks resulting from the nerve sheath incision. Our current practice is to specifically precipitate any incipient CSF leaks by transiently raising the CSF pressure and to repair the incision site with a mucosal graft at the slightest suspicion of a leak. With such a policy, no further morbidity has occurred with CSF leaks in the last 21 patients with a nerve sheath incision. Other authors have similarly suggested that fibrin glue be applied to the nerve sheath following a surgical incision.8
The ophthalmic artery also traverses with the optic nerve in its sheath, but it is anatomically lateral to the nerve and is not normally encountered in the medial trans-sphenoid approach. The artery may, however, occasionally be in an inferior or inferomedial location to the nerve,8,22 and it is therefore standard practice to place the nerve sheath incision in the superomedial quadrant of the nerve circumference.8
Controversy continues over the best timing for nerve decompression. It would be logical to assume that early surgical decompression would be more efficacious in reversing pathology than decompression that has been delayed by a few weeks. Early surgical decompression, however, has not been proved to be more efficacious than early treatment with steroids in conventional doses or megadoses.1,2 Moreover, optic nerve injury may be associated with other life-threatening and progressive injuries and also injury to the internal carotid artery;21 in these scenarios medical management is perceived as the safer initial treatment option. Our policy, therefore, is initial treatment with megadoses of steroids, with surgical decompression recommended only for patients who, despite such medical treatment, continue to have poor vision or whose vision progressively deteriorates. The efficacy of this treatment policy has been previously documented in the literature,7,15 and many other studies have noted the potential of the nerve to recover even if surgery is delayed by a few months.10,11,12,13,14,16,17 In the context of the analysis of this study, both groups are similarly matched with regard to the injury-to-surgery interval.
Good improvements in vision were achieved in this study even though the time from injury to decompression was significantly delayed. This may be attributed to case selection, where patients with poor prognostic factors such as PL−ve vision and fractures are limited in number. Many workers have restricted optic nerve decompression mainly to patients with very poor vision (finger counting or worse)8,17,18,21,24 because of fears of further worsening vision as a result of surgery.24 Such patients, however, have a poor prognosis,7,13 and the results of nerve decompression in such subgroups have, therefore, proved disappointing. This study indicates that surgical decompression of patients with persistently complete loss of vision (PL−ve) despite steroid treatment is almost always disappointing, and we no longer recommend surgical decompression for such patients. Our experience further indicates that the operation is safe, and, with appropriate expertise, there is little risk of worsening vision because of surgery. We advise surgical decompression for all patients who, following steroid treatment, have residual vision of 6/60 or worse, but to exclude all patients with complete visual loss.
CONCLUSION
This article demonstrates the efficacy of a nerve sheath incision in enhancing the visual outcome in patients with traumatic optic neuropathy who undergo decompression of the osseous optic canal. The potential of the nerve sheath incision to provoke an immediate or delayed CSF leak is also noted, and the efficacy of current surgical techniques to reliably detect and seal such leaks is demonstrated.
A better quantum of improvement and a greater probability of achieving useful vision (≥ 6/18) was noted in patients who underwent slitting of the nerve sheath in addition to osseous optic canal decompression. This was especially so in patients with no optic canal and/or posterior orbital fracture, and is consistent with the pathophysiological mechanisms that suggest nerve fiber compression secondary to intraneural compartmental syndrome.
REFERENCES
- Cook M W, Levin L A, Joseph M P, Pinczower E F. Traumatic optic neuropathy. A meta-analysis. Arch Otolaryngol Head Neck Surg. 1996;122:389–392. doi: 10.1001/archotol.1996.01890160031006. [DOI] [PubMed] [Google Scholar]
- Levin L A, Beck R W, Joseph M P, Seiff S, Kraker R. The treatment of traumatic optic neuropathy: the International Optic Nerve Trauma Study. Ophthalmology. 1999;106:1268–1277. doi: 10.1016/s0161-6420(99)00707-1. [DOI] [PubMed] [Google Scholar]
- Tandon D A, Thakar A, Mahapatra A K, Ghosh P. Trans-ethmoidal optic nerve decompression. Clin Otolaryngol. 1994;19:98–104. doi: 10.1111/j.1365-2273.1994.tb01190.x. [DOI] [PubMed] [Google Scholar]
- Walsh F B. Pathological–clinical correlations. I. Indirect trauma to the optic nerves and chiasm. II. Certain cerebral involvements associated with defective blood supply. Invest Ophthalmol. 1966;5:433–449. [PubMed] [Google Scholar]
- Steinsapir K D, Goldberg R A. Traumatic optic neuropathy. Surv Ophthalmol. 1994;38:487–518. doi: 10.1016/0039-6257(94)90145-7. [DOI] [PubMed] [Google Scholar]
- Chen Y R, Breidahl A, Chang C N. Optic nerve decompression in fibrous dysplasia: indications, efficacy and safety. Plast Reconstr Surg. 1997;99:22–30. doi: 10.1097/00006534-199701000-00004. [DOI] [PubMed] [Google Scholar]
- Thakar A, Mahapatra A K, Tandon D A. Delayed optic nerve decompression for indirect optic nerve injury. Laryngoscope. 2003;113:112–119. doi: 10.1097/00005537-200301000-00021. [DOI] [PubMed] [Google Scholar]
- Luxenberger W, Stammberger H, Jebeles J A, Walch C. Endoscopic optic nerve decompression: the Graz experience. Laryngoscope. 1998;108:873–882. doi: 10.1097/00005537-199806000-00016. [DOI] [PubMed] [Google Scholar]
- Onofrey C B, Tse D T, Johnson T E, et al. Optic canal decompression: a cadaveric study of the effects of surgery. Ophthal Plast Reconstr Surg. 2007;23:261–266. doi: 10.1097/IOP.0b013e3180cac220. [DOI] [PubMed] [Google Scholar]
- Niho S, Niho M, Niho K. Decompression of the optic canal by the transethmoidal route and decompression of the superior orbital fissure. Can J Ophthalmol. 1970;5:22–40. [PubMed] [Google Scholar]
- Fukado Y. Results in 400 cases of surgical decompression of the optic nerve. Mod Probl Ophthalmol. 1975;14:474–481. [PubMed] [Google Scholar]
- Sofferman R A. The recovery potential of the optic nerve. Laryngoscope. 1995;105(supplement 72):1–38. [PubMed] [Google Scholar]
- Matsuzaki H, Kunita M, Kawai K. Optic nerve damage in head trauma: clinical and experimental studies. Jpn J Ophthalmol. 1982;26:447–461. [PubMed] [Google Scholar]
- Kountakis S E, Maillard A A, Urso R, Stiernberg C M. Endoscopic approach to traumatic visual loss. Otolaryngol Head Neck Surg. 1997;116:652–656. doi: 10.1016/S0194-59989770243-2. [DOI] [PubMed] [Google Scholar]
- Fujitani T, Inoue K, Takahashi T, Ikushima K, Asai T. Indirect traumatic optic nerve neuropathy- visual outcome of operative and nonoperative cases. Jpn J Ophthalmol. 1986;30:125–134. [PubMed] [Google Scholar]
- Girard B C, Bouzas E A, Lamas G, Soudant J. Visual improvement after transethmoid-sphenoid decompression in optic nerve injuries. J Clin Neuroophthalmol. 1992;12:142–148. [PubMed] [Google Scholar]
- Mine S, Yamakami I, Yamaura A, et al. Outcome of traumatic optic neuropathy. Comparison between surgical and nonsurgical treatment. Acta Neurochir (Wien) 1999;141:27–30. doi: 10.1007/s007010050262. [DOI] [PubMed] [Google Scholar]
- Chen C T, Huang F, Tsay P K, et al. Endoscopically assisted transconjunctival decompression of traumatic optic neuropathy. J Craniofac Surg. 2007;18:19–26. doi: 10.1097/01.scs.0000248654.15287.89. [DOI] [PubMed] [Google Scholar]
- Levin L A, Joseph M P, Rizzo J F, III, Lessell S. Optic canal decompression in indirect optic nerve trauma. Ophthalmology. 1994;101:566–569. doi: 10.1016/s0161-6420(94)31299-1. [DOI] [PubMed] [Google Scholar]
- Wang B H, Robertson B C, Girotto J A, et al. Traumatic optic neuropathy: a review of 61 patients. Plast Reconstr Surg. 2001;107:1655–1664. doi: 10.1097/00006534-200106000-00003. [DOI] [PubMed] [Google Scholar]
- Rajiniganth M G, Gupta A K, Gupta A, Bapuraj J R. Traumatic optic neuropathy: visual outcome following combined therapy protocol. Arch Otolaryngol Head Neck Surg. 2003;129:1203–1206. doi: 10.1001/archotol.129.11.1203. [DOI] [PubMed] [Google Scholar]
- Lang J. In: Samii M, Draf W, editor. Surgery of the Skull Base, an Interdisciplinary Approach. Berlin: Springer; 1989. Anatomy of optic nerve decompression, in surgical anatomy of the skull base. pp. 16–18.
- Joseph M P, Lessel S, Rizzo J, Momose J. Extracranial optic nerve decompression for traumatic optic neuropathy. Arch Ophthalmol. 1990;108:1091–1093. doi: 10.1001/archopht.1990.01070100047032. [DOI] [PubMed] [Google Scholar]
- Wohlrab T M, Maas S, de Carpentier J P. Surgical decompression in traumatic optic neuropathy. Acta Ophthalmol Scand. 2002;80:287–293. doi: 10.1034/j.1600-0420.2002.800311.x. [DOI] [PubMed] [Google Scholar]


