ABSTRACT
Objective: Report the experience of the Karmanos Cancer Institute with sinonasal mucosal melanoma (SNMM) in patients diagnosed between 1995 and 2007. Results: Eighteen patients, ages 31 to 85 (mean, 67), whose most common presenting symptoms included epistaxis and facial pressure. Most common anatomic locations were the maxillary sinus and nasal cavity. Seventy-two percent presented with tumors extending to the skull base, frontal sinus, orbit, or cranium. Tumor size ranged from 0.3 cm to 5.3 cm. Most common surgical procedure was medial maxillectomy (12 patients). Eight patients received chemotherapy, ten received radiotherapy and six received both. One third of patients received interferon-α. Median recurrence-free survival (RFS) was 14.4 months, with a 1-year RFS rate of 55%. Median overall survival (OS) was 19.3 months with a 1-year OS rate of 60% and a 2-year OS rate of 42%. The 5-year OS rate was 34%. Conclusion: SNMM remains a disease that has eluded breakthroughs in treatment. Patients are typically treated with wide local resection; however unique to our institution was the frequent use of interferon and chemoradiation. Further research in adjuvant therapies will be necessary to improve outcomes.
Keywords: Melanoma, sinus, nasal cavity, sinonasal mucosal melanoma
In North America, primary sinonasal mucosal melanoma (SNMM) accounts for 0.3% to 2% of all malignant melanomas, roughly 4% of head and neck melanoma cases,1 and comprise 3.5% of all malignancies in the sinonasal region.2 Microscopically, SNMM has a highly variable appearance, and similarities to lymphoma, rhabdomyosarcoma, plasmacytoma, olfactory neuroblastoma, and poorly differentiated carcinoma make diagnosis difficult. In fact, reported cases in the past may have been misdiagnosed because proper histochemical and immunohistochemical staining methods were not available to discern melanoma from these other cancers.3
Although literature on the cutaneous forms of melanoma is abundant, reports on primary SNMM remain scant. Many of the past studies are case reports, and others focus solely on radiological features, pathological features, or therapeutic regimens, without analyzing a series of patients and their experiences with SNMM. Countries such as China have published the most complete studies due to the higher incidence of SNMM in the Asian population.4
Here we report the experience with this disease in a major head and neck cancer program of a National Cancer Institute (NCI)-designated comprehensive cancer center. We reviewed and assessed our management of SNMM over a period of 13 years.
PATIENTS AND METHODS
Patient Population
Wayne State University Human Investigation Committee (HIC) and Institutional Review Board (IRB) approval was obtained to review patients' medical records. Sinonasal mucosal melanoma was defined as a pathological diagnosis of malignant melanoma arising on the mucosa of the nasal cavity, nasopharynx, or sinuses, according to the final pathological report. Patients with cutaneous melanomas, including cutaneous melanomas encroaching on the sinonasal area, were excluded from the study. Patients were selected using the CoPath pathology database, in which the natural language search function was performed using keywords “melanoma” and “sinus,” or “sinonasal,” or “nasal cavity.” In this way, every patient with a pathological diagnosis of paranasal sinus or nasal melanoma (excluding skin) between 1995 and 2007 at the Karmanos Cancer Institute at Wayne State University was selected for review.
Methods
We performed a thorough review of both outpatient clinic-based and hospital-based medical records of the Karmanos Cancer Institute. In addition, surgical pathology reports were reviewed to characterize the histological features of our patients' tumors.
Data obtained from clinical records included patient characteristics, tumor characteristics, anatomic site and size of the tumor, clinical and pathological staging and metastatic locations of the disease, histological features, types of surgeries performed, and types of postoperative adjuvant treatments. In addition, patient follow-up was performed for disease recurrence and survival.
Statistical Methods
Patient characteristics were described with summary statistics. Recurrence-free survival (RFS) was measured from surgery to the date of clinically documented disease recurrence or death from any cause, whichever came first. Surviving patients still recurrence-free as of the date of their last tumor assessment were censored on that date. Overall survival (OS) was measured from surgery to the date of death from any cause. Surviving patients were censored as of the last date on which they were known to be still alive. Standard Kaplan-Meier estimates of the censored RFS and OS distributions were computed. Due to the small sample sizes, survival statistics (e.g., median, 1-year rate, etc.) were estimated more conservatively using linear interpolation among successive event times on the Kaplan-Meier curves.5
RESULTS
Demographic and clinical characteristics of patients are listed in Table 1. Disease was almost equally common in men and women; our patient population included 10 women and 8 men. This differs from cutaneous melanoma, where men generally have higher incidence of disease. Similar to other studies on this topic,3 our patients were generally elderly at diagnosis, although we did have two patients diagnosed in their 30s. Our patients' ages ranged from 31 to 85 years, with median age of 67.5 years. Patients' most common complaints included epistaxis (44%), facial pressure (22%), mass (22%), and obstruction (22%). The median duration of symptoms before presenting to an otolaryngologist was 1 month (range 15 days to 8 months).
Table 1.
Patient Characteristics
| No. of Patients (%) | |
|---|---|
| Gender | |
| M | 8 (44) |
| F | 10 (56) |
| Age at Diagnosis (y) | |
| Median, 67.5 (range, 31 to 85) | |
| Symptoms | |
| Epistaxis | 8 (44) |
| Facial pressure | 4 (22) |
| Mass | 4 (22) |
| Obstruction | 4 (22) |
| Numbness | 3 (17) |
| Pain | 1 (6) |
| Rhinorrhea | 1 (6) |
| Neck mass | 1 (6) |
| Symptom Duration (mo) | |
| Median, 1 (range, 0.5 to 8.0) |
Anatomic location, stage, and tumor size are given in Table 2. Most frequently, tumors were in the maxillary sinus (67%), nasal cavity (33%), and nasal septum (17%). Only four patients (22%) had disease localized to maxillary sinus alone. Thirteen patients (72%) exhibited tumors in prognostically poor locations such as the skull base (5), orbit (4), cribriform plate (2), brain (1), and nasopharynx (1). This might account for such a high percentage (56%) of patients with stage IV disease. Staging was based on the American Joint Committee on Cancer (AJCC) classification for sinus cancer staging. According to AJCC criteria, involvement of the frontal sinus, nasopharynx, orbit, or intracranial extension confers a higher T stage and a worse prognosis. Thirteen patients had involvement of multiple anatomic areas. Tumor size ranged from 0.3 cm to 5.3 cm (median, 3.8 cm) based on pathological sectioning.
Table 2.
Anatomic Characteristics
| No. of Patients (%) | |
|---|---|
| Anatomic Site | |
| Maxillary sinus | 12 (67) |
| Nasal cavity | 6 (33) |
| Septum | 3 (17) |
| Prognostically Poor Locations | |
| Total | 13 (72) |
| Skull base invasion | 5 (28) |
| Orbital invasion | 4 (22) |
| Cribriform plate invasion | 2 (11) |
| Brain | 1 (6) |
| Frontal Sinus | 1 (6) |
| Nasopharynx | 1 (6) |
| Overall Stage (%) | |
| I | 2 (11) |
| II | 2 (11) |
| III | 4 (22) |
| IV | 10 (56) |
| Tumor Maximal Diameter (cm) | |
| Median, 3.8 (range, 0.8 to 5.3) |
Lymph node and distant metastasis data are listed in Table 3. Eleven patients (61%) had disease in the cervical lymph nodes. Five (28%) had level I involvement, three (17%) had level II involvement, and only one each had level IV, level V, and periparotid involvement. Six patients had distant metastatic disease, with the liver (four patients [22%]) and the lung (three patients [17%]) being the most common locations. Three patients had distant metastatic disease in more than one location.
Table 3.
Metastasis
| No. of Patients (%) | |
|---|---|
| Cervical Lymph Nodes | |
| Total | 11 (61) |
| Level I | 5 (28) |
| Level II | 3 (17) |
| Level III | 0 |
| Level IV | 1 (6) |
| Level V | 1 (6) |
| Periparotid | 1 (6) |
| Retropharyngeal | 0 |
| Distant Metastases | |
| Total | 6 (33) |
| Liver | 4 (22) |
| Lung | 3 (17) |
| Brain | 1 (6) |
| Bone | 1 (6) |
Each tumor specimen exhibited a characteristic appearance based on one of the major melanoma histologies—pleomorphic, spindle cell, plasmacytoid, or epithelioid. Based on their first biopsy at presentation, seven patients (39%) exhibited spindle cell histology, four (22%) exhibited pleomorphic and epithelioid variants, two (11%) had undifferentiated melanoma, and one (6%) had the plasmacytoid form.
The type of surgery varied based on anatomic location, ranging from simple maxillectomies and septectomies to advanced craniofacial resections. The majority of patients (12 [67%]) had medial maxillectomy performed, ethmoidectomy was the second most common (8 [44%]), followed by septectomy (5 [28%]). One patient had a series of 10 procedures ranging from minimally invasive CO2 laser, to parotidectomy and partial rhinectomy. Three patients had neck dissection performed, one had frontal sinus cranialization, one had orbital exenteration, one had rhinectomy, and one had an infraorbital nerve resection for pain control. Except for this last patient, all patients had attempted curative resections.
Postoperative adjuvant treatments are listed in Table 4. Eight patients (61%) received chemotherapy. The most common immunotherapies were interferon (INF) in six patients (33%) and interleukin (IL)-2 in two patients (11%). Patients receiving adjuvant INF typically got low-dose treatment (i.e., three million units subcutaneously three times a week for 1 year). One patient received high-dose IFN at 10 million units five times a week for 4 weeks, then 5 million units three times week for 48 weeks. Six patients received cisplatin or carboplatin together with radiation. Radiotherapy (RT) was administered to 10 patients (56%). Dosage information was known for eight of these patients, and ranged from 30 to 70 Gy. These patients typically received a total of 10 fractions over 2 to 4 weeks.
Table 4.
Adjuvant Treatments
| No. of Patients (%) | |
|---|---|
| Chemotherapy | |
| Total | 10 (56) |
| Cisplatin | 5 (28) |
| Veltane | 1 (6) |
| Tamoxifen | 1 (6) |
| Temodar | 1 (6) |
| Immunotherapy* | |
| INF-α | 6 (33) |
| Interleukin-2 | 2 (11) |
| Radiation Therapy† | |
| Total | 10 (56) |
Median dose INF-α, 5 million units (range, 3 to 15 million units); derived from the 5 patients for whom INF-α dose was known.
Median total dose, 54.7 Gy (range, 30 to 70 Gy); derived from the 8 patients for whom total radiation dose was known.
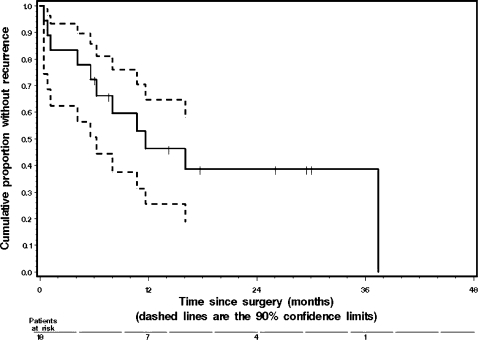
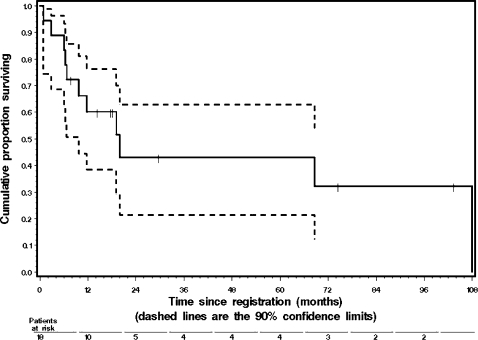
Figures 1 and 2 illustrate the RFS and OS curves, respectively. Median RFS was 14.4 months, with a 1-year RFS rate of 55%. Median OS was 19.3 months, with a 1-year OS rate of 60% and a 2-year OS rate of 42%. The 5-year OS rate was 34%.
Figure 1.
Kaplan-Meier graph of recurrence-free survival (RFS) with 90% confidence limits shown as dashed lines. Median RFS was 14.4 months, with 90% confidence interval (CI) of 9.1 to 24.4 months. The 1-year RFS rate was 55%, with 90% CI of 0.32 to 0.78. The 2-year RFS rate was 30%, with 90% CI of 0.08 to 0.52. Three patients were still alive and recurrence-free at 26.1, 29.5, and 30.0 months after surgery.
Figure 2.
Kaplan-Meier graph of overall survival (OS) with 90% confidence limits shown as dashed lines. Median OS was 19.3 months, with 90% confidence interval (CI) 9.1 to 107.3 months. The 2-year OS rate was 42%, with 90% CI 0.20 to 0.64. The 5-year OS rate was 34%, with 90% CI 0.12 to 0.57. Two patients are currently still alive at 74.4 and 103.4 months after surgery.
DISCUSSION
Typically, SNMM presents as an expansive mass encroaching on several subsites of the paranasal sinuses, orbit, or cranial fossa. Interestingly, almost three quarters of our patients had involvement of the skull base, intracranial extension, nasopharyngeal involvement, or orbital invasion. Our patient population seems to present at a higher AJCC stage, although, based on past literature, only disease in the nasopharynx confers a worse clinical outcome.4,6
Lymph node involvement was somewhat different than expected. Generally, the major lymphatic drainage route of the anterior nose, maxillary sinus, and anterior and middle ethmoid cells is into the lateral and inferior collecting trunks to the submandibular, parotid, and jugulodigastric nodes, whereas the nasal cavity and posterior ethmoid cells drain through the superoposterior trunk to retropharyngeal and deep cervical lymph nodes. Six patients had nodal involvement, and most did not exhibit the classic drainage pattern. Most had involvement of submandibular and upper jugular disease, regardless of primary site. One patient with nasopharyngeal extension had involvement of periparotid lymph nodes.
The actual purpose and function and the melanocytes present in the respiratory epithelium are unknown. The most common histological subtypes of SNMM include spindle cell, epithelioid, pleomorphic, and cytologically in small blue or plasmacytoid forms. Spindle cell melanomas mimic sarcomas; they have variable nuclei and interlacing fascicles of cells. The differential diagnoses include leiomyosarcoma, neurogenic sarcoma, and malignant fibrous histiocytoma. Pleomorphic-type melanomas have multinucleated tumor cells and differential diagnoses including undifferentiated carcinoma and rhabdomyosarcoma. The plasmacytoid variants mimic “small blue cell” tumors, and the differential diagnoses include olfactory neuroblastoma, plasmacytoma, and lymphoma.7,8,9
Immunohistochemical staining with anti–S-100, HMB-45, and antivimentin may confirm the diagnosis of melanoma. For example, plasmacytoma typically has leukocyte antigen on staining, whereas neuroblastomas have a unique S-100 staining pattern.9 Although the spindle cell melanoma confers the worst prognosis in the cutaneous form, in SNMM only the undifferentiated form confers a poorer prognosis.4
Past literature has not yielded much information on improvements in the management of SNMM. The rarity of this condition obviously renders studies and trials limited. Wide local excision has been the standard form of primary treatment at most institutions.
In addition, we administered several adjuvant therapies for our patients, including cisplatin/carboplatin, IL-2, INF, and tamoxifen. There is clearly no standard systemic treatment regimen for SNMM, presumably based on lack of evidence supporting adjuvant treatment for this disease. Thus, a variety of systemic therapies have been employed.
Current studies show that adjuvant chemotherapy does not appear to improve survival. A multicenter phase III randomized trial of patients with high-risk primary limb melanoma did not show a benefit from isolated limb perfusion with melphalan in regard to disease-free survival or OS when compared with surgery alone.10
Interferon was used in a third of our patients as adjuvant treatment. Several studies have analyzed its efficacy in cutaneous melanomas. In one prospective randomized controlled trial, adjuvant high-dose INF was shown to increase RFS and OS when compared with observation.11 Another randomized trial conducted by the same group of researchers using the same high-dose INF regimen confirmed the RFS advantage but not the OS advantage.12 Adjuvant therapy with lower doses of INF has not consistently shown an impact on either RFS or OS.13 As previously mentioned, our patients on INF typically received low-dose treatment of three million units subcutaneously three times a week for 1 year.
More than half of our patients received some form of adjuvant radiation treatment. Kingdom and Kaplan noted that postoperative RT lengthened disease-free intervals and OS.14 Gilligan and Slevin's study15 concluded that definitive RT could be employed for melanoma of the nasal cavity. However, they reported a 5-year survival rate of only 18%. No patients in our study received primary RT as treatment. Although melanoma is thought to be radioresistant, other sources have pointed to its radiosensitivity to higher doses of RT per fraction.15
Our study's inability to evaluate the efficacy of specific treatments is a limitation. Our small sample size makes conclusions on the value of INF, radiation, and chemotherapy impossible. Nor could we assess the relationship between pathology/metastasis and prognosis. Because surgery has been the mainstay for treatment, further studies need to evaluate adjuvant therapies for this disease.
The 34% 5-year OS rate observed in our patients compares favorably with that reported in other studies of SNMM. Lund et al16 report a 28% 5-year OS rate and a similar median OS (21 months) to that from our study (19.3 months). Their study assessed the impact of chemotherapy (melphalan and Bacille Calmette-Guérin [BCG] vaccine) and RT treatments. They found no statistically significant difference in either local control or survival in patients with versus without adjuvant therapy. Kingdom and Kaplan14 reported a 20% 5-year survival rate in their series of 13 patients. Brandwein et al17 reported a 36% 5-year survival in their retrospective review of 25 patients. Interestingly, few of the patients in these last two studies received chemotherapy or RT. The Brandwein study also attempted to evaluate long-term trends given the advances in imaging and treatment in the past 30 years. They concluded that 5-year survival decreased from 40% before 1980 to 30% after 1980.
CONCLUSIONS
Sinonasal mucosal melanoma remains a disease that has eluded breakthroughs in treatment. Survival is low and recurrence rates are still high. Patients typically received primary surgical treatment consisting of wide local resection of their tumor, with INF and chemoradiation used in many instances. Further research in the use of adjuvant therapy (chemotherapy, immunotherapy, biological therapy) will be necessary to improve the outcome of patients with SNMM
ACKNOWLEDGMENTS
This study was partially supported by National Institutes of Health Cancer Center Support Grant, CA-22453.
Material in this article was presented at the 7th International Conference on Head and Neck Cancer, San Francisco, CA, July 19–23, 2008.
REFERENCES
- Thompson A C, Morgan D A, Bradley P J. Malignant melanoma of the nasal cavity and paranasal sinuses. Clin Otolaryngol. 1993;18:34–36. doi: 10.1111/j.1365-2273.1993.tb00806.x. [DOI] [PubMed] [Google Scholar]
- Conley J J, Ackerman A B. Melanoma of the Head and Neck. 1st ed. New York: Georg Thieme Verlag; 1990. pp. 154–178.
- Thompson L DR, Wienke J A, Miettinen M. Sinonasal tract and nasopharyngeal melanomas: a clinicopathologic study of 115 cases with a proposed staging system. Am J Surg Pathol. 2003;27(5):594–611. doi: 10.1097/00000478-200305000-00004. [DOI] [PubMed] [Google Scholar]
- Cheng Y F, Lai C C, Ho C Y, Shu C H, Lin C Z. Toward a better understanding of sinonasal mucosal melanoma: clinical review of 23 cases. J Chin Med Assoc. 2007;70(1):24–29. doi: 10.1016/S1726-4901(09)70296-5. [DOI] [PubMed] [Google Scholar]
- Lee E. Statistical Methods for Survival Data Analysis. 3rd ed. Hoboken, New Jersey: Wiley & Sons, Inc.; 2003. pp. 76–91.
- Manolidis S, Donald P J. Malignant mucosal melanoma of the head and neck: review of the literature and a report of 14 patients. Cancer. 1997;80(8):1373–1386. doi: 10.1002/(sici)1097-0142(19971015)80:8<1373::aid-cncr3>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Holmstrom M, Lund V J. Malignant melanomas of the nasal cavity after occupational exposure to formaldehyde. Br J Ind Med. 1991;48(1):9–11. doi: 10.1136/oem.48.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett A W, Scully C. Human oral mucosal melanocytes: a review. J Oral Pathol Med. 1994;23:97–103. doi: 10.1111/j.1600-0714.1994.tb01095.x. [DOI] [PubMed] [Google Scholar]
- Wick M R, Stanley S J, Swanson P E. Immunohistochemical diagnosis of sinonasal melanoma, carcinoma, and neuroblastoma with monoclonal antibodies HMB-45 and anti-synaptophysin. Arch Pathol Lab Med. 1988;112:616–620. [PubMed] [Google Scholar]
- Koops H S, Vaglini M, Suciu S, et al. Prophylactic isolated limb perfusion for localized, high-risk limb melanoma: results of a multicenter randomized phase III trial. European Organization for Research and Treatment of Cancer Malignant Melanoma Cooperative Group Protocol 18832, the World Health Organization Melanoma Program Trial 15, and the North American Perfusion Group Southwest Oncology Group-8593. J Clin Oncol. 1998;16(9):2906–2912. doi: 10.1200/JCO.1998.16.9.2906. [DOI] [PubMed] [Google Scholar]
- Kirkwood J M, Strawderman M H, Ernstoff M S, et al. Interferon alfa-2b adjuvant therapy of high-risk resected cutaneous melanoma: the Eastern Cooperative Oncology Group Trial EST 1684. J Clin Oncol. 1996;14(1):7–17. doi: 10.1200/JCO.1996.14.1.7. [DOI] [PubMed] [Google Scholar]
- Kirkwood J M, Ibrahim J, Lawson D H, et al. High-dose interferon alfa-2b does not diminish antibody response to GM2 vaccination in patients with resected melanoma: results of the Multicenter Eastern Cooperative Oncology Group Phase II Trial E2696. J Clin Oncol. 2001;19(5):1430–1436. doi: 10.1200/JCO.2001.19.5.1430. [DOI] [PubMed] [Google Scholar]
- Hancock B W, Wheatley K, Harris S, et al. Adjuvant interferon in high-risk melanoma: the AIM HIGH Study–United Kingdom Coordinating Committee on Cancer Research randomized study of adjuvant low-dose extended-duration interferon alfa-2a in high-risk resected malignant melanoma. J Clin Oncol. 2004;22(1):53–61. doi: 10.1200/JCO.2004.03.185. [DOI] [PubMed] [Google Scholar]
- Kingdom T T, Kaplan M. Mucosal melanoma of the nasal cavity and paranasal sinuses. Head Neck. 1995;17(3):184–189. doi: 10.1002/hed.2880170303. [DOI] [PubMed] [Google Scholar]
- Gilligan D, Slevin N J. Radical radiotherapy for 23 cases of mucosal melanoma in the nasal cavity and paranasal sinuses. Br J Radiol. 1991;64:1147–1150. doi: 10.1259/0007-1285-64-768-1147. [DOI] [PubMed] [Google Scholar]
- Lund V J, Howard D J, Harding L, Wei W I. Management options and survival in malignant melanoma of the sinonasal mucosa. Laryngoscope. 1999;109(2 Pt 1):208–211. doi: 10.1097/00005537-199902000-00007. [DOI] [PubMed] [Google Scholar]
- Brandwein M S, Rothstein A, Lawson W, Bodian C, Urken M L. Sinonasal melanoma: a clinicopathologic study of 25 cases and literature meta-analysis. Arch Otolaryngol Head Neck Surg. 1997;123(3):290–296. doi: 10.1001/archotol.1997.01900030064008. [DOI] [PubMed] [Google Scholar]