Abstract
BACKGROUND
Nutrient supply to the developing mammalian embryo is a fundamental requirement. Before completion of the chorioallantoic placenta, the visceral endoderm plays a crucial role in nurturing the embryo. We have found that visceral endoderm cells express folate receptor 1, a high-affinity receptor for the essential micronutrient folic acid, suggesting that the visceral endoderm has an important function for folate transport to the embryo. The mechanisms that direct expression of FOLR1 in the visceral endoderm are unknown.
METHODS
Sequences were tested for transcriptional activation capabilities in the visceral endoderm utilizing reporter gene assays in a cell model for extraembryonic endoderm in vitro, and in transgenic mice in vivo.
RESULTS
With F9 embryo carcinoma cells as a model for extraembryonic endoderm, we demonstrate that the P4 promoter of the human FOLR1 gene is active during differentiation of the cells towards visceral endoderm. However, transgenic mouse experiments show that promoter sequences alone are insufficient to elicit reporter gene transcription in vivo. Using sequence conservation as guide to choose genomic sequences from the human FOLR1 gene locus, we demonstrate that the sequence termed F1CE2 exhibits specific enhancer activity in F9 cells in vitro, in the visceral endoderm, and later the yolk sac in transgenic mouse embryos in vivo. We further show that the transcription factor HNF4-alpha can activate this enhancer sequence.
CONCLUSIONS
We have identified a transcriptional enhancer sequence from the FOLR1 locus with specific activity in vitro and in vivo, and suggest that FOLR1 is a target for regulation by HNF4-alpha.
Keywords: folate receptor, transcriptional regulation, visceral endoderm, enhancer, HNF4-alpha
INTRODUCTION
Folate, Birth Defects, and General Health
Folate deficiency has been linked to an increased incidence of congenital malformation (Molloy and Scott, 2001), heightened risk for certain types of cancer (Prinz-Langenohl et al., 2001; Ryan and Weir, 2001; Courtemanche et al., 2004b), reduced immune system performance (Courtemanche et al., 2004a), anemia, and reduced endurance (Lukaski, 2004). Suboptimal folate levels appear to be linked to impaired general health (Singh, 2004) and neurologic symptoms in aging (D'Anci and Rosenberg, 2004; Kim et al., 2008). Furthermore, our own experiments have demonstrated a beneficial effect of folate on the morphogenesis of the skeleton (Kappen et al., 2004). Historically, the finding that neural tube defect (NTD) frequencies may be associated with low folate levels in the mother (Smithells et al., 1976; Yates et al., 1987; Milunsky et al., 1989), and the resulting general hypothesis that nutritional deficiencies could be involved in the etiology of birth defects (Smithells et al., 1976, 1977; Shaw et al., 1995), represented a significant milestone in the understanding of congenital malformations. Importantly, this concept presented a highly feasible therapeutic approach to birth defect prevention simply by supplying vitamin preparations including folate to women who wished to become pregnant (Smithells et al., 1981). In fact, periconceptional supplementation with folate (Locksmith and Duff, 1998; Bailey, 2000; Ladipo, 2000) proved to be highly beneficial to the conceptus, resulting in significantly decreased occurrence of NTDs, craniofacial malformations, and cardiovascular abnormalities among newborns (Wald et al., 1991; Gelineau-van Waes and Finnell, 2001).
Folate Transport and Cellular Uptake
Mammalian cells have developed an elaborate mechanism to harvest extracellular folate (Trippett and Bertino, 1999), involving extracellular, glycolipid-anchored high-affinity folate receptors (Lacey et al., 1989; Wang et al., 1996; Wu et al., 1997), a low-affinity transmembrane carrier (Moscow et al., 1995; Wong et al., 1995), and a proton-coupled folate transporter (Qiu et al., 2006). Five folate receptor genes have been reported for the human genome (Elwood, 1989; Lacey et al., 1989; Ross et al., 1994; Spiegelstein et al., 2000), whereas in the mouse, three folate receptor genes are present. Expression studies on human folate receptors reveal that FOLR1 is mostly expressed in epithelial cells (Lacey et al., 1989; Page et al., 1993; Smith et al., 1999), FOLR3 is specific for the hematopoietic system (Shen et al., 1994), and FOLR2 (Reddy et al., 1999; Ross et al., 1999; Shen et al., 1994) and FOLR4 (Spiegelstein et al., 2000) are found at lower levels in diverse tissues. FOLR4 may play a role for the immune system because of its expression on regulatory T-cells (Walker, 2007; Yamaguchi et al., 2007). The carrier protein encoded by the RFC1 gene and the proton-coupled folate transporter PCFT seem to be widely distributed (Said et al., 1996; Wang et al., 2001; Maddox et al., 2003; Qiu et al., 2007). Mouse embryos lacking the Folr1 (folbp1) gene (Piedrahita et al., 1999) are arrested in their development shortly after gastrulation, fail to close the neural tube, and die in utero at mid-gestation, demonstrating that the mouse Folr1 gene is essential for embryonic development.
Expression of Folate Receptor Genes during Embryonic Development in the Mouse
The essential nature of folate intuitively would suggest that genes involved in folate transport and processing would be ‘housekeeping’ genes, with expression in every cell. In contrast, published observations (Saitsu et al., 2003) and our own in situ hybridization experiments (Kappen et al., 2004) demonstrate that this is clearly not the case: in the mouse embryo, genes for folate receptors are expressed in distinct and specific tissue distributions during development. The expression of Folr1 in the neural tube appears to represent a direct link to NTDs through a cell-autonomous function of the Folr1 gene (Saitsu et al., 2003), and folate supplementation is able to rescue embryos lacking the Folr1 gene (Spiegelstein et al., 2004). However, the literature is not clear about the causes for neurulation defects: both neural tube cells (Copp, 2005) and cells adjacent to the neural tube (Copp et al., 1988; van Straaten et al., 1993) are being implicated in a role for NTDs. Thus, a cell-autonomous model for Folr1 in NTDs may explain only part of the involvement of folate in neural tube closure.
In this context, it was interesting to note that the earliest and strongest expression signal for Folr1 in the developing embryo occurred in cells of the visceral endoderm (Saitsu et al., 2003), and our own data (Fig. 1). Expression was conspicuously absent from the embryo itself, raising the question as to how folate enters the majority of cells in the embryo during crucial periods of morphogenesis. Regarding the visceral endoderm, we found that Folr1 was the only gene of the folate receptor family expressed in this tissue. Consequently, it would appear that the FOLR1 protein represents the gateway for this important micronutrient through the visceral endoderm to the embryo itself. It is therefore possible that the lack of Folr1 in the gene knockout model may not only have a cell-autonomous effect on cells of the neural tube, but an additional indirect, pleiotropic effect on the whole embryo. Such pleiotropy may arise from the absence of Folr1 in the visceral endoderm, a resulting defect in folate transport in the visceral endoderm, failure to supply folate from the visceral endoderm to the embryo, and consequently, a condition of folate deficiency throughout the embryo, with negative consequences for cell proliferation and normal morphogenesis.
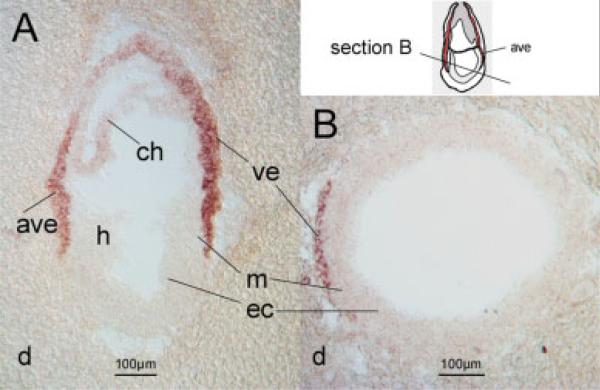
Figure 1.
Expression of the mouse Folr1 gene in the visceral endoderm. Sagittal (A) and transverse (B) sections from mouse decidua at 7.5 days’ gestation were hybridized with an antisense riboprobe specific for the murine Folr1 gene. Strong expression was observed in the visceral endoderm, with weaker signal in the chorion, and no expression detectable in the embryo itself. Abbreviations: ave, anterior visceral endoderm; ch, chorion; d, deciduum; ec, ectoderm; h, headfold region; m, mesoderm; ve, visceral endoderm.
Functions of the Visceral Endoderm
The visceral endoderm has important roles in patterning and in nurturing the developing embryo (Brent et al., 1990; Bielinska et al., 1999). Crucial patterning signals for determination of anterior identity arise from the anterior visceral endoderm (Thomas and Beddington, 1996; Tam and Behringer, 1997; Beddington and Robertson, 1998), whereas visceral endoderm adjacent to extraembryonic mesoderm is essential for the induction of blood vessel development (Boucher and Pedersen, 1996; Belaoussoff et al., 1998). In parallel, the visceral endoderm is responsible for nutrient uptake and transport to the embryo (Cross et al., 1994). This function for nutritional support is indispensable during establishment of the chorioallantoic placenta (Brent et al., 1990); once the placenta is functional, the embryo can switch from histiotrophic to hemotrophic nutrition (Burton et al., 2001). However, it is important to consider that crucial developmental processes, such as neural tube closure and patterning of the early heart, occur at a time when the burden of nurturing the embryo lies with the visceral endoderm (Brent et al., 1990). Thus, it stands to reason that birth defects involving the early embryonic patterning processes in relation to a lack of nutrients should be interpreted in the context of visceral endoderm function. Examples are spina bifida and folate deficiency (Smithells et al., 1976), or diabetic embryopathy and the detrimental nutritional milieu brought about by maternal diabetes (Reece et al., 1993; Reece and Eriksson, 1996). In fact, gene expression changes in the visceral yolk sac are thought to contribute to birth defects in diabetic pregnancies (Reece et al., 2006). Therefore, proper regulation of genes involved in nutrient uptake and transport in the visceral endoderm is a crucial prerequisite for successful development of the embryo itself. A targeted mutation of the transcription factor HNF4-alpha underscores that view, as many genes for nutrient transport or metabolism, such as apolipoproteins, glucose transporter 2, transferrin, and cytoplasmic retinoic acid binding proteins are downregulated in the visceral endoderm of HNF4-alpha−/− embryos (Stoffel and Duncan, 1997). In fact, the failure of HNF4-alpha−/− embryos to complete gastrulation has been ascribed to “death by starvation” (Copp, 1995; Duncan et al., 1997).
Regulation of Folate Receptor 1 Gene Expression
Gene transcription is typically dependent on regulatory DNA elements such as promoters and enhancers. Like all folate receptor genes, the human FOLR1 gene has multiple promoters that are well characterized. The FOLR1 P1 promoter (so designated as the transcript starts at exon 1) was studied in KB epidermal carcinoma cells and NIH/3T3 fibroblasts (Elwood et al., 1997; Galmozzi et al., 2001). Both the FOLR1 P1 and the FOLR1 P4 promoter (transcript starting at exon 4) contain initiator sequences and respond to the transcription factor SP1 (Sadasivan et al., 1994; Saikawa et al., 1995). The two promoters exhibit differential activity in KB cells, several adult tissues, and ovarian cancer cells (Elwood et al., 1997; Galmozzi et al., 2001). Furthermore, the transcription factor vHNF1 activates the P1 promoter in ovarian carcinoma cells (Tomassetti et al., 2003), and the FOLR1 P4 promoter is modulated by the estrogen receptor in cervical and ovarian carcinoma cells (Kelley et al., 2003). Interestingly, in several cell lines, the FOLR1 gene appears to regulated in response to cellular growth and not in response to folate levels (Doucette and Stevens, 2001), indicating that cellular requirements rather than a simple feedback mechanism control this gene. A recent study reported genetic variation in the FOLR1 promoter region (Nilsson and Borjel, 2004), with potential health implications presumed to be due to altered expression of the gene. As is evident from the literature on folate receptor gene promoters, most experiments have focused on carcinoma cell lines, with emphasis on FOLR1 gene regulation in cancer.
In contrast, regulatory mechanisms for FOLR1 gene expression during embryonic development have not been explored. The expression of FOLR1 in the visceral endoderm is consistent with a role of the visceral endoderm in folate uptake and subsequent release to cells of the embryo itself. Therefore, the mechanism that is responsible for the specific expression of Folr1 in the visceral endoderm is fundamental to ensure folate supply to the embryo. To identify this mechanism, we have undertaken reporter gene experiments to gain further insight into the regulatory events that control Folr1 expression during development, with our focus on the visceral endoderm.
Given the importance of folate for prevention of human birth defects, it is reasonable to assume that deficiencies in folate transport may be cause for susceptibility to congenital malformation in humans. Such deficiencies might arise from genetic variation in the structural part of the FOLR1 gene, but could also be based on mutations in regulatory regions that are required for proper expression of FOLR1. Identifying the human regulatory elements for FOLR1 expression would provide a means to characterize genetic variation in such elements, and investigate the relationship to birth defect susceptibility. To date, this approach was limited to promoter regions of FOLR1 (Barber et al., 2000), because the existence, identity, and location of enhancer sequences were unknown. Therefore, rather than using murine sequences, we chose to attempt identification of regulatory sequences from the human FOLR1 locus, using a transgenic mouse approach to provide an evolutionary conserved in vivo context. In this fashion, any human sequences with regulatory function in vivo may be readily checked for potential genetic variation in human populations in the future. In this study, we report the identification of a sequence from the human FOLR1 locus that can act as a transcriptional enhancer to direct gene expression specifically in the visceral endoderm, and we suggest that the FOLR1 gene, like many other genes for nutrient uptake or transport, is a target for the transcription factor HNF4-alpha.
MATERIALS AND METHODS
In Situ Hybridizations
Decidua with mouse embryos at 7.5 days’ gestation—with 12 PM on the day of the appearance of a vaginal plug designated as gestation day 0.5—were dissected from the uterus of FVB mice, embedded in O.C.T. compound (Sakura Finetek, Torrance, CA), frozen, and used to generate cryosections of 25 μm thickness. A digoxigenin-labeled antisense riboprobe was generated from a mouse Folr1 full-length cDNA clone, and sections were hybridized as described previously (Salbaum, 1998).
Plasmid Constructs
Reporter constructs for Luciferase assays were generated in pGL3 (Promega, Madison, WI). DNA fragments spanning the P1 and P4 promoter regions, as well as evolutionary conserved sequences flanking the human FOLR1 gene, were generated by PCR from commercially obtained human genomic DNA (Roche, Indianapolis, IN). Genomic coordinates (UCSC genome browser, human genome version hg18, March 2006 assembly) for the amplified fragments were as follows: promoter P1, chr11: 71,576,404-71,578,395; promoter P4, chr11:71,578,925-71,580,883. For conserved sequence elements from the FOLR1 gene, we use the abbreviation F1CE (for Folate receptor 1 Conserved Element) followed by a number; genomic coordinates were: F1CE1, chr11:71,560,901-71,561,809; F1CE2, chr11:71,565,324-71,566,907; F1CE3, chr11:71,591,596-71,592,126. The identity of each amplified DNA fragment was confirmed by DNA sequencing. Promoter fragments were generated with flanking MluI and XhoI restriction sites for cloning into pGL3 (Promega); fragments carrying conserved sequences were produced with flanking KpnI and MluI sites for cloning upstream of promoter sequences. Deletions in F1CE2-F1P4-GL3 were generated using existing restriction enzyme sites. A reporter plasmid carrying HcRed as reporter gene was generated by replacing the coding sequence for luciferase in pGL3 with the HcRed coding sequence from pHcRed-N1.1 (Clontech, Mountain View, CA). The plasmid F1CE2-F1P4-GhcR contains the same assembly of conserved sequence and promoter as F1CE2-F1P4-GL3 in the context of the fluorescent reporter, and was used for transgenic mouse experiments.
Transfection Experiments
Conditions to grow F9 mouse embryo carcinoma cells as well as differentiation to either visceral or parietal endoderm phenotype were as described elsewhere (Braunhut et al., 1992; Dong et al., 1990). Cells were seeded in 35-mm dishes at a density of 1 to 2 × 105 cells per dish; transfections using Effectene (Qiagen, Valencia, CA) and 400 to 600 ng of plasmid DNA were performed 24 hours after plating. Twenty-four hours after transfection, the transfection mixture was replaced with media containing differentiation agents; for visceral endoderm, cells were treated with 1 μM all-trans retinoic acid (Sigma, St. Louis, MO), whereas for parietal endoderm, cells were grown on dishes pretreated with 0.1% gelatin and received 1 μM all-trans retinoic acid and 250 μM dibutyryl-cAMP (Sigma). Sixty hours after induction of differentiation, a time at which any retinoic acid-mediated effects of FOLR1 have long ceased, cells were lysed in GloLysis buffer (Promega), and luciferase activity was determined using SteadyGlo substrate (Promega). Luciferase values were normalized to the protein content of the lysate as determined by BCA assay. Each transfection assay was performed in either five or ten replicates. For cotransfections, expression vectors encoding transcription factors (CMV-HNF4-alpha, CMV-TGIF) were obtained from the IMAGE Mammalian Gene Collection of full-length cDNAs (Open Biosystems, Huntsville, AL). The expression vector pMT7-HNF4-alpha (Jiang et al., 1995) was kindly provided by Dr. Francis Sladek (University of California, Irvine, CA). DNA for cotransfection experiments was a mixture of 500 ng of reporter construct DNA as well as 100 ng DNA of the plasmid encoding a transcription factor. An expression vector containing the HcRed fluorescent protein sequence was used as negative control (in place for transcription factor-expressing plasmids) for cotransfection experiments and served for normalization of reporter activity. For all experiments, fold changes were calculated by normalizing all observed values to the average of the respective control experiment. Statistical significance was determined by performing a double-sided t test on control and experimental values normalized to the average of the controls.
Transgenic Mouse Experiments
The construct F1CE2-F1P4-GhcR was used to generate transgenic mouse embryos that were then analyzed for reporter activity by confocal microscopy. Injection DNA free of plasmid backbone sequences was generated by digestion with Asp718 and SalI, followed by agarose gel electrophoresis and purification (Qiagen). DNA was injected into fertilized oocytes of FVB mice as published (Hogan et al., 1996). At gestation day 7.5 (E7.5) as well as 9.5 (E9.5), embryos were dissected from the uterus of CD-1 foster mice, and embryo as well as yolk sac (at E9.5) of each specimen were used for imaging HcRed-specific fluorescence on a Zeiss Confocal microscope (Carl Zeiss Inc., Thornwood, NY); all images were taken at identical intensity settings. Genotyping for transgene presence was performed on DNA extracted from embryos after imaging.
RESULTS
Expression of Folr1 in the Visceral Endoderm
To reveal sites of expression of the Folr1 gene, we performed in situ hybridization experiments on mouse embryos at stages prior to neural tube closure. While our results in general confirm previously published data (Saitsu et al., 2003), we were intrigued by the high level of expression of Folr1 in the visceral endoderm (Fig. 1) at embryonic day 7.5, and the yolk sac at later stages of development. The visceral endoderm is a cell layer thought to play an important role for nutrition of the embryo (Brent et al., 1990). We detected only Folr1 expression; neither Folr2 nor Folr4 expression was found (not shown). It is therefore likely that Folr1 represents the gateway for high-affinity folate transport in the visceral endoderm, and the regulatory mechanisms that direct expression of the Folr1 gene in the visceral endoderm are likely to be of high biologic significance for healthy development of the embryo.
Activity of FOLR1 Gene Promoters in F9 Cells Differentiated toward Visceral Endoderm
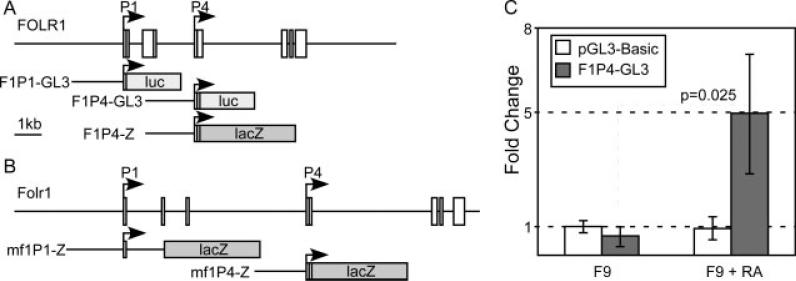
We generated reporter constructs comprising 2 kb of sequences of either the P1 or the P4 promoter of the human FOLR1 gene (Fig. 2A) to test their activity in F9 embryo carcinoma cells that were differentiated to visceral endoderm (Dong et al., 1990; Braunhut et al., 1992). Although we observed no activity from the P1 promoter in F9 cells under any circumstance, the construct carrying the P4 promoter showed activity in F9 cells, but only after they had undergone differentiation toward a visceral endoderm phenotype (Fig. 2B). In F9 cells grown without induction of differentiation, the P4 construct did not exhibit any activity higher than a promoterless luciferase control vector. We conclude that the P4 promoter of the human FOLR1 gene has the potential to contribute to expression of the gene in the visceral endoderm.
Figure 2.
Activity of the P4 promoter of the human FOLR1 gene. (A) Reporter constructs from the human FOLR1 gene. Promoter constructs included the publicly annotated transcription start site as well as 2 kb of upstream DNA for each respective construct. Firefly Luciferase as well as Escherichia coli β-galactosidase were used as reporter genes. (B) Reporter construct from the mouse Folr1 gene locus. (C) The human P4 promoter construct shows specific activity in F9 embryo carcinoma cells only after differentiation towards visceral endoderm.
Activity of FOLR1 Gene Promoters in Transgenic Mice
With the observation of cell type-specific promoter activity from the P4 promoter in the visceral endoderm model, we introduced reporter constructs with a β-galactosidase reporter gene (Fig. 2A) in transgenic mouse embryos to test whether promoter sequences of the human FOLR1, or the mouse Folr1 gene, were sufficient to drive expression of a reporter gene in a pattern resembling the expression of Folr1 in the mouse. Although we were able to generate transgenic specimen at expected frequencies (Table 1), none of those transgenic specimen showed reporter activity that matched expression of Folr1. A few embryos transgenic for the human FOLR1 P4 construct displayed some lacZ activity, but the spatial distributions of these activities were not consistent between individual transgenic samples, and did not match the known Folr1 expression pattern (Saitsu et al., 2003). We did not observe any reporter activity from transgenic samples carrying either the mouse Folr1 P1 or the mouse Folr1 P4 construct. We therefore conclude that the individual P1 or P4 promoter sequences of either the human or the mouse folate receptor 1 gene are not sufficient to drive gene expression in the correct pattern in vivo.
Table 1.
Reporter Constructs in Transgenic Mice
| Construct | Embryos | Transgenic | Expression |
|---|---|---|---|
| hF1P4-LacZ | 125 | 18 | 0 |
| mf1P4-LacZ | 106 | 16 | 0 |
| mf1P1-LacZ | 130 | 10 | 0 |
Conserved Sequence Elements at the FOLR1 Gene Locus
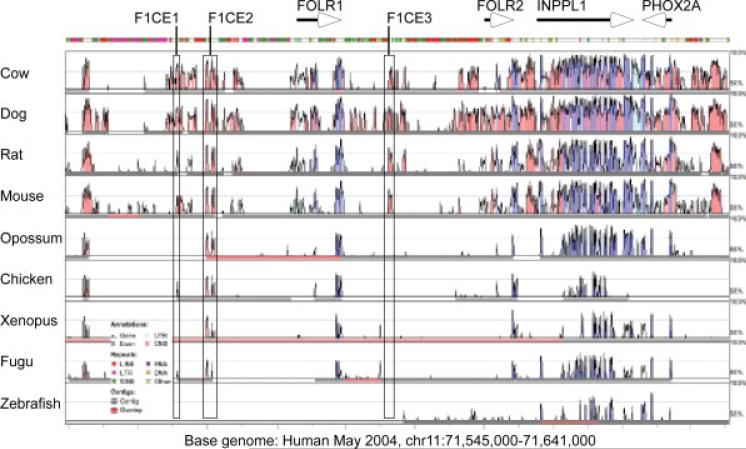
Because the gene for folate receptor 1 showed a high degree of sequence conservation between human and mouse, we hypothesized that the regulatory mechanisms controlling the expression of the gene might also be conserved. We used VISTA to generate a sequence conservation landscape around the human FOLR1 gene (Fig. 3). We initially selected the three conserved regions nearest to the FOLR1 gene and generated reporter constructs where each of the conserved sequences were placed in the context of the FOLR1 P4 promoter. An initial transfection survey experiment with constructs containing a fluorescent reporter (HcRed) suggested that the sequence termed F1CE2 conferred transcriptional activation activity upon the P4 promoter. We therefore decided to examine the F1CE2 sequence in further detail.
Figure 3.
Conservation profile at the human FOLR1 gene locus. Sequence conservation plot in the vicinity of the human FOLR1 gene locus. Genes are annotated by arrows, conserved sequence regions used in this study are outlined. Annotation of conservation peaks follows the VISTA convention, with conserved coding regions colored purple, transcribed non-coding regions in light blue, and conserved noncoding regions in pink. The colored bar above the conservation landscape indicates the presence of repetitive elements in the human sequence. Three sequences (termed F1CE, for FOLR1 Conserved Element) with conservation to multiple species were initially chosen to be included in reporter constructs and tested for transcriptional activation. Both F1CE1 and F1CE2 show deep conservation across vertebrates, whereas conservation in the F1CE3 sequence is limited to mammals.
A Conserved Sequence Upstream of the FOLR1 Gene Shows Enhancer Activity in vitro
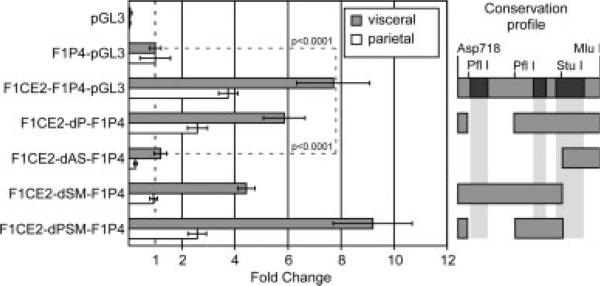
Using firefly luciferase as the reporter gene, we compared reporter activity for DNA constructs containing the FOLR1 P4 promoter in the presence or absence of the F1CE2 sequence, or with parts of the F1CE2 sequence deleted from the construct. We tested these constructs in F9 cells differentiated either towards the visceral or the parietal endoderm model. The results are summarized in Figure 4. Compared to the promoter-less vector pGL3, presence of the P4 promoter resulted in an increase in reporter activity at about the same magnitude as observed in the initial experiment; this was observed for both cell models. Activity of the F1P4-GL3 construct was then used to normalize reporter activities and calculate fold change. We found that addition of the conserved sequence F1CE2 to the P4 promoter resulted in an approximately eightfold increase of reporter activity in the visceral endoderm differentiation model, suggesting that the F1CE2 sequence can in fact act as an enhancer. Deletion of approximately two thirds of the F1CE2 sequence between the Asp718 and StuI restriction sites (F1CE2-dAS-F1P4) abolished that enhancement, suggesting that this enhancing activity may reside between these two coordinates. Deletion of the sequence between the two PflI restriction sites had little effect on enhancer activity, as seen for the F1CE2-dP-F1P4 and F1CE2-dPSM-F1P4 constructs. This suggests that the sequence between the second PflI site and the StuI site, which also contains a conserved sequence element, is a major contributor to the observed enhancer effect.
Figure 4.
Enhancer activity from a DNA fragment upstream of the human FOLR1 gene. F9 embryo carcinoma cells were transfected with various DNA constructs and differentiated either towards visceral or towards parietal endoderm. From top: pGL3, basic Luciferase vector without promoter sequences; F1P4-GL3, human FOLR1 P4 promoter construct as reference for the experiment; F1CE2-F1P4-GL3, conserved sequence F1CE2 tagged onto the human FOLR1 P4 promoter construct; F1CE2-dP-F1P4, deletion in the conserved F1CE2 sequence between the two PflI restriction sites; F1CE2-dAS-F1P4, deletion between Asp178 and StuI sites; F1CE2-dSM-F1P4, deletion between StuI and MluI sites; F1CE2-dPSM-F1P4, compound deletion with the sequence between the PflI sites as well as the sequence between the StuI and MluI sites absent from the F1CE2 sequence. All deletion constructs share the F1P4-Luciferase portion. Restriction sites and conservation regions (black) in the F1CE2 sequence are indicated to the right; gray bars represent the sequence present in the various deletion constructs. The presence of the F1CE2 sequence enhances the P4 promoter activity nearly eightfold in the visceral endoderm paradigm. F1P4 constructs also show activity in the parietal endoderm model, although the enhancing function of the F1CE2 sequence is diminished. Deletion of the sequence between the Asp718 and StuI sites from the F1CE2 sequence abolished the enhancement of transcription activity.
Tissue-Specific Enhancer Activity of a Conserved Sequence Upstream of the FOLR1 Gene
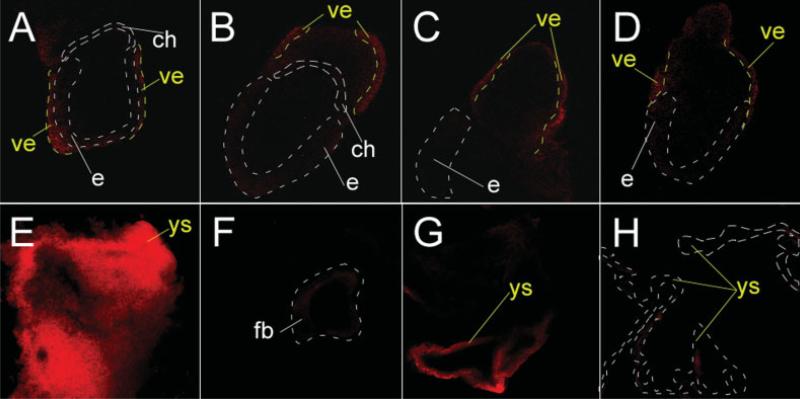
To determine whether the F1CE2 sequence would be able to confer enhancer activity in vivo, we generated transgenic mice with a construct carrying the same F1CE2-F1P4 configuration as described in the in vitro experiment, but using a gene for the red fluorescent protein HcRed as the reporter gene. We performed transient transgenic assays where the analysis for reporter activity was carried out directly on founder embryos. Analysis of embryos at 7.5 days of gestation (Fig. 5) revealed that all eight transgenic embryos exhibited red fluorescence restricted to the region of the visceral endoderm. A second experiment analyzed at 9.5 days of gestation yielded three transgenic specimen; all three showed consistent red fluorescence in the yolk sac. Fluorescence signals in embryos were spurious or not detectable at all, indicating that the reporter activity from this construct was specific for the visceral endoderm at E7.5, and for the yolk sac at E9.5, but not for the embryo proper. This was in excellent agreement with our earlier in situ hybridization results on the expression of the Folr1 gene itself, and suggests that the human F1CE2 region contains an enhancer sequence that is sufficient to drive the reporter gene expression in vivo in a pattern that resembles the expression of the mouse Folr1 gene. Based on the consistency between the in vitro and the in vivo results, we conclude that the F1CE2 sequence can function as an enhancer in the regulation of folate receptor gene expression in early development.
Figure 5.
Enhancer activity of the F1CE2 sequence in transgenic mice. (A, B, C, D) Images (confocal slices) of four different embryos at E7.5 that all carry the F1CE2-F1P4-HcRed transgene. Red fluorescence appeared to be restricted to the layer of visceral endoderm (ve) cells on the outside of the embryo. Stippled white lines show the location of the embryo (e) and the chorion (ch); stippled yellow lines mark the approximate boundary of the visceral endoderm (ve). (E) Yolk sac (ys) from a transgenic specimen at E9.5 showing bright red fluorescence (projection view of a stack of confocal images), which is indicative of high reporter activity. (F) Transgenic embryo (slice view) corresponding to the yolk sac shown in E with very little reporter fluorescence. Stippled area represents forebrain vesicle. No consistent pattern of red fluorescence was detected among independent transgenic embryos. (G) Yolk sac from a second, independent transgenic specimen (single confocal slice view), with a strong fluorescence signal. (H) Yolk sac from a nontransgenic specimen without fluorescent reporter activity.
HNF4-Alpha Can Activate the FOLR1 Enhancer
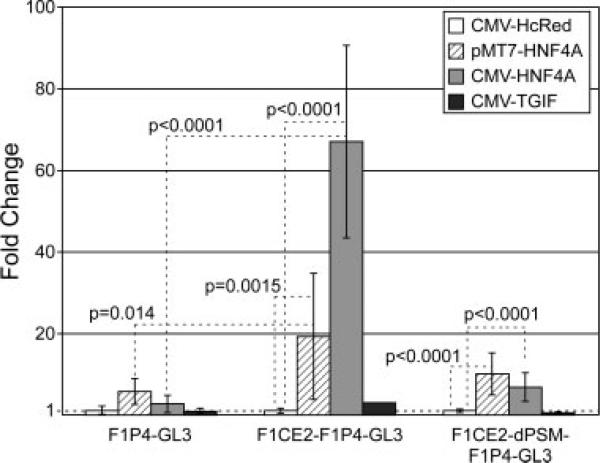
When we examined the PflI-StuI fragment of the F1CE2 region for conservation and for the presence of potential transcription factor binding sites, we noted a sequence 5′-TGGAATTGGACCT-3′ that was identified by rVISTA software (Loots et al., 2002; Loots and Ovcharenko, 2004) as a potential binding site for the transcription factor HNF4-alpha. This suggested the possibility that HNF4-alpha might be involved in the function of the F1CE2 enhancer function and thereby contribute to the regulation of the folate receptor 1 gene. We tested this possibility by performing cotransfection experiments in F9 cells differentiated towards the visceral endoderm. For these experiments, we compared the reporter activity of the F1P4 promoter alone, the F1P4 promoter carrying the full sequence of the F1CE2 region, and the F1P4 promoter with the F1CE2-dPSM deletion. Luciferase reporter plasmids were cotransfected with expression vectors that would express (1) HNF4-alpha from the MT7 promoter (Jiang et al., 1995), (2) HNF4-alpha from the CMV promoter, (3) the transcription factor TGIF from the CMV promoter, or (4) the red fluorescent protein HcRed from the CMV promoter. We used CMV/HcRed to control for the presence of a very strong enhancer/promoter sequence in the transfected cells, and any sequestering of general transcription factors that might occur because of the CMV sequence. To test whether any observed effect would be due to the function of a sequence-specific DNA binding protein and not just due to the increased presence of any DNA-binding protein, we used the transcription factor TGIF, which is not related to the biologic context of the experiment. All reporter activities were normalized to the CMV/HcRed co-transfection to calculate fold-change as a response to presence of HNF4-alpha. In these experiments, we observed that presence of HNF4-alpha lead to a robust and highly significant increase of reporter activity from the F1CE2-F1P4 construct compared to control (Fig. 6). The degree of increase was different for the two HNF4-alpha expression plasmids (60-fold for CMV vs 20-fold for MT7). One reason may be that the two plasmids express different splice variants of HNF4-alpha, which are thought to have slightly different transcriptional activity (Eeckhoute et al., 2003). More likely though, the difference is due to the higher degree of expression of HNF4-alpha from the CMV promoter plasmid compared with the MT7 promoter plasmid. As expected, cotransfection of TGIF as a control for increased DNA binding protein content in the cells did not affect the F1CE2-F1P4 construct in a significant way: as TGIF is a transcriptional co-repressor (Wotton et al., 1999a, 1999b), silencing of the construct might have been expected, but was not observed. Surprisingly, the F1CE2-F1P4 deletion construct carrying the presumed HNF4-alpha site, showed significantly lower activation in response to HNF4-alpha than the construct carrying the intact F1CE2 enhancer element. The construct carrying the F1P4 promoter alone also responded to the presence of HNF4-alpha, although not nearly as strong as the construct with the F1CE2 enhancer. Therefore, it is likely that transcriptional activation via HNF4-alpha involves more than a single binding site on F1CE2. Taken together, these results suggest HNF4-alpha as a part of the regulatory mechanism that controls folate receptor 1 gene expression specifically in the visceral endoderm and the yolk sac.
Figure 6.
HNF4alpha can activate the F1CE2 sequence from the FOLR1 gene. Cotransfection experiments indicate that the construct carrying the entire F1CE2 enhancer sequence responds very strongly to the presence of HNF4-alpha. F1P4-GL3, human FOLR1 P4 promoter fused to a luciferase reporter; F1CE2-F1P4-GL3, the F1P4-GL3 construct carrying the entire F1CE2 enhancer sequence; F1CE2-dPSM-F1P4-GL3, the F1P4-GL3 construct with a deletion version of the F1CE2 enhancer. A plasmid expression HcRed (instead of any transcription factor) from the CMV promoter was used as control. Values were normalized to the average of the control experiment to determine fold-changes. Cotransfection of HNF4-alpha leads to a strong activation of the reporter construct carrying the entire F1CE2 sequence, with only mild increases seen for the F1P4 promoter alone, or for the construct with the deletion version of the F1CE2 sequence.
DISCUSSION
In this study, we demonstrate that a DNA sequence located approximately 13 kb upstream of the P4 promoter of the human FOLR1 gene can act as a transcriptional enhancer for FOLR1 gene expression in the visceral endoderm and the yolk sac. Whereas the P4 promoter displays activity in an in vitro model of visceral endoderm, it appears that neither the P4 nor the P1 promoter of the human or the murine Folr1 genes alone contain the necessary regulatory elements to drive expression of this gene properly. Addition of the evolutionary conserved sequence F1CE2 to P4 reporter constructs confers increased transcriptional activity from the construct in vitro, and allows tissue-specific expression congruent with Folr1 gene expression in transgenic experiments in vivo. These results are consistent with our interpretation that the F1CE2 sequence functions as an enhancer.
In this context, it is important to note that in the absence of developmental expression data for the human FOLR1 gene, we are using the mouse Folr1 gene and its expression as a model and as guidance to evaluate the activity of sequences from the human FOLR1 gene locus. The fact that the human DNA sequences used in this study were able to generate specific transcriptional responses in our in vitro model of murine origin, as well as in transgenic mouse experiments in vivo, would argue that regulatory mechanisms are conserved between the human and mouse version of the folate receptor 1 gene. This would suggest that expression of the human gene may occur in a manner similar to the mouse gene during embryonic development.
We used a strategy of analyzing the transgenic specimen directly; in this fashion, every reporter expression signal arose from an independent transgenic event. Since transgene DNA introduced by pronuclear injection typically integrates in a random manner, it is highly unlikely that two independent transgenic events occur at the same genomic integration site. Consequently, the genomic neighborhood is unique for each transgenic event. The genomic neighborhood of a transgene can exert strong influences on a transgene expression (e.g., through methylation patterns or through the presence of strong regulatory elements). Therefore, if reporter gene expression matches between different transgenic founders, it is a strong indication that the observed reporter gene expression is due to a biologic function on the transgene sequence, and not due to the genomic integration site. The fact that we observed excellent congruency of reporter expression at both developmental time points is a compelling argument that the F1CE2 sequence harbors transcriptional enhancer function. The full characterization of the F1CE2 enhancer (e.g., the developmental time course) will have to await the establishment of transgenic mouse lines.
Our experiments show that the F1CE2 sequence has instructive properties and is sufficient to drive expression in the visceral endoderm. However, we used only the P4 promoter of FOLR1 in the pertinent experiments. We cannot rule out that the F1CE2 enhancer could also activate the P1 promoter of FOLR1. Preliminary experiments (not shown) using a heterologous promoter from the ICP4 gene of herpes simplex virus indicate that the F1CE2 sequence can activate such a heterologous promoter at least in F9 cells differentiated to visceral endoderm, and thereby fulfill the classic definition of an enhancer. It therefore stands to reason that the P1 promoter of the FOLR1 gene may also be activated by the F1CE2 sequence, although this remains to be proven experimentally.
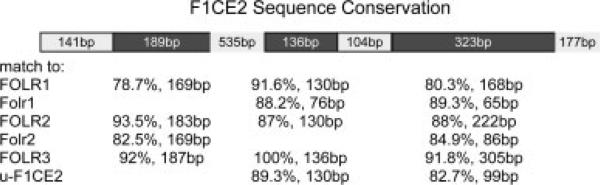
Although our data demonstrate the biologic activity of F1CE2, our studies cannot address whether the F1CE2 sequence is solely responsible, or even necessary for visceral endoderm expression of FOLR1. In fact, the DNA sequence conservation at the FOLR1 gene locus would suggest that there may be other sequences in the vicinity of the FOLR1 gene that may have similar properties as F1CE2. Closer examination of the F1CE2 sequence revealed that F1CE2 is in fact a remnant of a folate receptor gene, or a folate receptor pseudogene. No transcripts have been reported to arise from the human F1CE2 sequence, but a close sequence relationship for three small subregions on F1CE2 to the last three coding exons of other folate receptor genes is readily recognizable (Fig. 7). The F1CE2 sequence has a positional match in primate genomes, as well as in the genomes of dog and horse: in these genomes, a sequence matching F1CE2 exists in a location upstream of the cognate folate receptor 1 gene. Interestingly, no such positional match exists between the human and mouse genomes. In the mouse, the only sequences with relationship to F1CE2 are in fact the sequences for the two folate receptor genes Folr1 and Folr2. In line with the hypothesis of conservation of expression and regulation, we propose that sequences controlling the murine Folr1 gene may reside in either the Folr1 gene itself, or in the neighboring Folr2 gene. Based on sequence similarity and on the presence of another potential HNF4-alpha binding site, it appears that the Folr2 sequence is the more likely candidate for harboring enhancer function. It is therefore reasonable to assume that the human folate receptor gene locus on chromosome 11 with it's higher complexity of folate receptor genes – besides FOLR1 and FOLR2, there is also the FOLR3 gene, as well as two FOLR gene remnants (one of which is F1CE2) – might harbor more than one sequence capable of driving FOLR1 expression in the visceral endoderm.
Figure 7.
Conservation of the F1CE2 sequence. Comparison of the F1CE2 sequence to human and mouse genomes revealed the presence of three conserved regions (dark shading). The match to the F1CE2 sequence itself in the human genome is not shown. Capitalized gene names are human genes, u-F1CE2 denotes a sequence upstream of the F1CE2 sequence in the human genome that is also a remnant of a folate receptor gene. Percentage of sequence identity over a given nucleotide span is indicated. The regions of 525bp and 177bp are not drawn to scale.
It is well known that folate supplementation can reduce the incidence of NTDs (Smithells et al., 1981). In regard to the timing of neural tube closure, it is important to note that the process of neurulation occurs at a time when the chorioallantoic placenta has not been fully established. At that time, the visceral endoderm has the function of supplying nutrients to the embryo. In the quest of understanding how folate supplementation can exert its benefits on the embryo, it appears that the function of the visceral endoderm is of high biologic significance. In the mouse, the visceral endoderm mediates histiotrophic nutrition of the embryo, and it stands to reason that the presence of FOLR1 in cells of the visceral endoderm is connected to folate uptake and transport to the developing embryo just ahead and during the time of neurulation. Disturbance of this process may produce detrimental results for the embryo, and it appears that the regulatory mechanisms that ensure FOLR1 in the tissue that feeds the embryo during a crucial time play a very important role for proper development.
The role of the visceral endoderm in nutrition of the embryo has been addressed from the viewpoint of targeted gene mutations. In particular, mouse embryos lacking the transcription factor HNF4-alpha fail to complete gastrulation due to malfunction of the visceral endoderm (Chen et al., 1994; Duncan et al., 1994). In fact, it has been shown that HNF4-alpha controls the expression of several genes that are important for the transport of nutrients. In HNF4-alpha-mutant embryos, genes for nutrient transport or metabolism, such as apolipoproteins, glucose transporter 2, transferrin, and cytoplasmic retinoic acid binding proteins were downregulated in the visceral endoderm, and it is thought that these embryos die from starvation (Stoffel and Duncan, 1997). These findings suggest that HNF4-alpha could act as a master control gene for nutrition of the embryo. Therefore, it was interesting to find potential binding sites for HNF4-alpha on the F1CE2 enhancer sequence of the FOLR1 gene. As our experiments clearly demonstrate, the F1CE2 enhancer is highly responsive to the presence of HNF4-alpha, suggesting that FOLR1 expression in the visceral endoderm is driven by HNF4-alpha. The deletion analysis further suggests that more than one HNF4-alpha site may be involved in the function of the F1CE2 enhancer. At this time, it is not known whether FOLR1 expression is strictly dependent on HNF4-alpha; such an analysis would require either the study of HNF4-alpha-deficient mice, or the removal of potential HNF4-alpha binding sites from the F1CE2 enhancer sequence. Given that there are other conserved potential transcription factor binding sites on the F1CE2 sequence, it is likely that F1CE2 enhancer function requires other factors besides HNF4-alpha. Nevertheless, our results raise the possibility that folate transport is in fact a process controlled by HNF4-alpha, and support the concept that HNF4-alpha functions to integrate the general process of nurturing the embryo before the completion of the placenta and the onset of hemotrophic nutrition.
This study provides a first insight into the mechanisms that regulate FOLR1 gene expression during critical times of embryonic development. Understanding the signals that impinge on these mechanisms and their relationship to maternal folate status will provide further insight into the regulation of FOLR1 gene expression and folate transport in the embryo.
ACKNOWLEDGMENTS
Part of this work was carried out at and funded by the Munroe-Meyer Institute at the University of Nebraska Medical Center. We wish to acknowledge technical help by Andrew Wall, Ryan Taylor, and Don Harms, help with confocal microscopy by Dr. Bernd Fritzsch and Heather Thomas, as well support by grants NIH DE016315 (to RHF) and NIH DK063336 (to CK).
Footnotes
Presented at the 48th Annual Meeting of the Teratology Society, June 28 July 2, 2008, Monterey, CA.
REFERENCES
- Bailey LB. New standard for dietary folate intake in pregnant women. Am J Clin Nutr. 2000;71(5 Suppl):1304S–1307S. doi: 10.1093/ajcn/71.5.1304s. [DOI] [PubMed] [Google Scholar]
- Barber R, Shalat S, Hendricks K, et al. Investigation of folate pathway gene polymorphisms and the incidence of neural tube defects in a Texas hispanic population. Mol Genet Metab. 2000;70:45–52. doi: 10.1006/mgme.2000.2991. [DOI] [PubMed] [Google Scholar]
- Beddington RS, Robertson EJ. Anterior patterning in mouse. Trends Genet. 1998;14:277–284. doi: 10.1016/s0168-9525(98)01499-1. [DOI] [PubMed] [Google Scholar]
- Belaoussoff M, Farrington SM, Baron MH. Hematopoietic induction and respecification of A-P identity by visceral endoderm signaling in the mouse embryo. Development. 1998;125:5009–5018. doi: 10.1242/dev.125.24.5009. [DOI] [PubMed] [Google Scholar]
- Bielinska M, Narita N, Wilson DB. Distinct roles for visceral endoderm during embryonic mouse development. Int J Dev Biol. 1999;43:183–205. [PubMed] [Google Scholar]
- Boucher DM, Pedersen RA. Induction and differentiation of extraembryonic mesoderm in the mouse. Reprod Fertil Dev. 1996;8:765–777. doi: 10.1071/rd9960765. [DOI] [PubMed] [Google Scholar]
- Braunhut SJ, D'Amore PA, Gudas LJ. The location and expression of fibroblast growth factor (FGF) in F9 visceral and parietal embryonic cells after retinoic acid-induced differentiation. Differentiation. 1992;50:141–152. doi: 10.1111/j.1432-0436.1992.tb00669.x. [DOI] [PubMed] [Google Scholar]
- Brent RL, Beckman DA, Jensen M, Koszalka TR. Experimental yolk sac dysfunction as a model for studying nutritional disturbances in the embryo during early organogenesis. Teratology. 1990;41:405–413. doi: 10.1002/tera.1420410406. [DOI] [PubMed] [Google Scholar]
- Burton GJ, Hempstock J, Jauniaux E. Nutrition of the human fetus during the first trimester–a review. Placenta. 2001;22(Suppl A):S70–77. doi: 10.1053/plac.2001.0639. [DOI] [PubMed] [Google Scholar]
- Chen WS, Manova K, Weinstein DC, Duncan SA, Plump AS, Prezioso VR, Bachvarova RF, Darnell JE., Jr. Disruption of the HNF-4 gene, expressed in visceral endoderm, leads to cell death in embryonic ectoderm and impaired gastrulation of mouse embryos. Genes Dev. 1994;8:2466–2477. doi: 10.1101/gad.8.20.2466. [DOI] [PubMed] [Google Scholar]
- Copp AJ. Death before birth: clues from gene knockouts and mutations. Trends Genet. 1995;11:87–93. doi: 10.1016/S0168-9525(00)89008-3. [DOI] [PubMed] [Google Scholar]
- Copp AJ. Neurulation in the cranial region–normal and abnormal. J Anat. 2005;207:623–635. doi: 10.1111/j.1469-7580.2005.00476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copp AJ, Brook FA, Roberts HJ. A cell-type-specific abnormality of cell proliferation in mutant (curly tail) mouse embryos developing spinal neural tube defects. Development. 1988;104:285–295. doi: 10.1242/dev.104.2.285. [DOI] [PubMed] [Google Scholar]
- Courtemanche C, Elson-Schwab I, Mashiyama ST, et al. Folate deficiency inhibits the proliferation of primary human CD8+ T lymphocytes in vitro. J Immunol. 2004a;173:3186–3192. doi: 10.4049/jimmunol.173.5.3186. [DOI] [PubMed] [Google Scholar]
- Courtemanche C, Huang AC, Elson-Schwab I, et al. Folate deficiency and ionizing radiation cause DNA breaks in primary human lymphocytes: a comparison. FASEB J. 2004b;18:209–211. doi: 10.1096/fj.03-0382fje. [DOI] [PubMed] [Google Scholar]
- Cross JC, Werb Z, Fisher SJ. Implantation and the placenta: key pieces of the development puzzle. Science. 1994;266:1508–1518. doi: 10.1126/science.7985020. [DOI] [PubMed] [Google Scholar]
- D'Anci KE, Rosenberg IH. Folate and brain function in the elderly. Curr Opin Clin Nutr Metab Care. 2004;7:659–664. doi: 10.1097/00075197-200411000-00011. [DOI] [PubMed] [Google Scholar]
- Dong JM, Li F, Chiu JF. Induction of F9 cell differentiation by transient exposure to retinoic acid. Biochem Biophys Res Commun. 1990;170:147–152. doi: 10.1016/0006-291x(90)91252-n. [DOI] [PubMed] [Google Scholar]
- Doucette MM, Stevens VL. Folate receptor function is regulated in response to different cellular growth rates in cultured mammalian cells. J Nutr. 2001;131:2819–2825. doi: 10.1093/jn/131.11.2819. [DOI] [PubMed] [Google Scholar]
- Duncan SA, Manova K, Chen WS, Hoodless P, Weinstein DC, Bachvarova RF, Darnell JE., Jr. Expression of transcription factor HNF-4 in the extraembryonic endoderm, gut, and nephrogenic tissue of the developing mouse embryo: HNF-4 is a marker for primary endoderm in the implanting blastocyst. Proc Natl Acad Sci U S A. 1994;91:7598–7602. doi: 10.1073/pnas.91.16.7598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan SA, Nagy A, Chan W. Murine gastrulation requires HNF-4 regulated gene expression in the visceral endoderm: tetraploid rescue of Hnf-4(−/−) embryos. Development. 1997;124:279–287. doi: 10.1242/dev.124.2.279. [DOI] [PubMed] [Google Scholar]
- Eeckhoute J, Moerman E, Bouckenooghe T, et al. Hepatocyte nuclear factor 4 alpha isoforms originated from the P1 promoter are expressed in human pancreatic beta-cells and exhibit stronger transcriptional potentials than P2 promoter-driven isoforms. Endocrinology. 2003;144:1686–1694. doi: 10.1210/en.2002-0024. [DOI] [PubMed] [Google Scholar]
- Elwood PC. Molecular cloning and characterization of the human folate-binding protein cDNA from placenta and malignant tissue culture (KB) cells. J Biol Chem. 1989;264:14893–14901. [PubMed] [Google Scholar]
- Elwood PC, Nachmanoff K, Saikawa Y, et al. The divergent 5′ termini of the alpha human folate receptor (hFR) mRNAs originate from two tissue-specific promoters and alternative splicing: characterization of the alpha hFR gene structure. Biochemistry. 1997;36:1467–1478. doi: 10.1021/bi962070h. [DOI] [PubMed] [Google Scholar]
- Galmozzi E, Tomassetti A, Sforzini S, et al. Exon 3 of the alpha folate receptor gene contains a 5′ splice site which confers enhanced ovarian carcinoma specific expression. FEBS Lett. 2001;502:31–34. doi: 10.1016/s0014-5793(01)02659-x. [DOI] [PubMed] [Google Scholar]
- Gelineau-van Waes J, Finnell RH. Genetics of neural tube defects. Semin Pediatr Neurol. 2001;8:160–164. doi: 10.1053/spen.2001.26449. [DOI] [PubMed] [Google Scholar]
- Hogan BL, Costantini F, Lacy E. Manipulating the mouse embryo: a laboratory manual. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1996. [Google Scholar]
- Jiang G, Nepomuceno L, Hopkins K, Sladek FM. Exclusive homodimerization of the orphan receptor hepatocyte nuclear factor 4 defines a new subclass of nuclear receptors. Mol Cell Biol. 1995;15:5131–5143. doi: 10.1128/mcb.15.9.5131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappen C, Mello MA, Finnell RH, Salbaum JM. Folate modulates Hox gene-controlled skeletal phenotypes. Genesis. 2004;39:155–166. doi: 10.1002/gene.20036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley KM, Rowan BG, Ratnam M. Modulation of the folate receptor alpha gene by the estrogen receptor: mechanism and implications in tumor targeting. Cancer Res. 2003;63:2820–2828. [PubMed] [Google Scholar]
- Kim JM, Stewart R, Kim SW, et al. Changes in folate, vitamin B12 and homocysteine associated with incident dementia. J Neurol Neurosurg Psychiatry. 2008;79:864–868. doi: 10.1136/jnnp.2007.131482. [DOI] [PubMed] [Google Scholar]
- Lacey SW, Sanders JM, Rothberg KG, et al. Complementary DNA for the folate binding protein correctly predicts anchoring to the membrane by glycosyl-phosphatidylinositol. J Clin Invest. 1989;84:715–720. doi: 10.1172/JCI114220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladipo OA. Nutrition in pregnancy: mineral and vitamin supplements. Am J Clin Nutr. 2000;72(1 Suppl):280S–290S. doi: 10.1093/ajcn/72.1.280S. [DOI] [PubMed] [Google Scholar]
- Locksmith GJ, Duff P. Preventing neural tube defects: the importance of periconceptional folic acid supplements. Obstet Gynecol. 1998;91:1027–1034. doi: 10.1016/s0029-7844(98)00060-x. [DOI] [PubMed] [Google Scholar]
- Loots GG, Ovcharenko I. rVISTA 2.0: evolutionary analysis of transcription factor binding sites. Nucleic Acids Res. 2004;32(Web Server issue):W217–221. doi: 10.1093/nar/gkh383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loots GG, Ovcharenko I, Pachter L, et al. rVista for comparative sequence-based discovery of functional transcription factor binding sites. Genome Res. 2002;12:832–839. doi: 10.1101/gr.225502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukaski HC. Vitamin and mineral status: effects on physical performance. Nutrition. 2004;20:632–644. doi: 10.1016/j.nut.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Maddox DM, Manlapat A, Roon P, et al. Reduced-folate carrier (RFC) is expressed in placenta and yolk sac, as well as in cells of the developing forebrain, hindbrain, neural tube, craniofacial region, eye, limb buds and heart. BMC Dev Biol. 2003;3:6. doi: 10.1186/1471-213X-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milunsky A, Jick H, Jick SS, et al. Multivitamin/folic acid supplementation in early pregnancy reduces the prevalence of neural tube defects. JAMA. 1989;262:2847–2852. doi: 10.1001/jama.262.20.2847. [DOI] [PubMed] [Google Scholar]
- Molloy AM, Scott JM. Folates and prevention of disease. Public Health Nutr. 2001;4:601–609. doi: 10.1079/phn2001144. [DOI] [PubMed] [Google Scholar]
- Moscow JA, Gong M, He R, et al. Isolation of a gene encoding a human reduced folate carrier (RFC1) and analysis of its expression in transport-deficient, methotrexate-resistant human breast cancer cells. Cancer Res. 1995;55:3790–3794. [PubMed] [Google Scholar]
- Nilsson TK, Borjel AK. Novel insertion and deletion mutations in the 5′-UTR of the folate receptor-alpha gene: an additional contributor to hyperhomocysteinemia? Clin Biochem. 2004;37:224–229. doi: 10.1016/j.clinbiochem.2003.11.013. [DOI] [PubMed] [Google Scholar]
- Page ST, Owen WC, Price K, Elwood PC. Expression of the human placental folate receptor transcript is regulated in human tissues. Organization and full nucleotide sequence of the gene. J Mol Biol. 1993;229:1175–1183. doi: 10.1006/jmbi.1993.1116. [DOI] [PubMed] [Google Scholar]
- Piedrahita JA, Oetama B, Bennett GD, et al. Mice lacking the folic acid-binding protein Folbp1 are defective in early embryonic development. Nat Genet. 1999;23:228–232. doi: 10.1038/13861. [DOI] [PubMed] [Google Scholar]
- Prinz-Langenohl R, Fohr I, Pietrzik K. Beneficial role for folate in the prevention of colorectal and breast cancer. Eur J Nutr. 2001;40:98–105. doi: 10.1007/pl00007387. [DOI] [PubMed] [Google Scholar]
- Qiu A, Jansen M, Sakaris A, et al. Identification of an intestinal folate transporter and the molecular basis for hereditary folate malabsorption. Cell. 2006;127:917–928. doi: 10.1016/j.cell.2006.09.041. [DOI] [PubMed] [Google Scholar]
- Qiu A, Min SH, Jansen M, et al. Rodent intestinal folate transporters (SLC46A1): secondary structure, functional properties, and response to dietary folate restriction. Am J Physiol Cell Physiol. 2007;293:C1669–1678. doi: 10.1152/ajpcell.00202.2007. [DOI] [PubMed] [Google Scholar]
- Reddy JA, Haneline LS, Srour EF, et al. Expression and functional characterization of the beta-isoform of the folate receptor on CD34(+) cells. Blood. 1999;93:3940–3948. [PubMed] [Google Scholar]
- Reece EA, Eriksson UJ. The pathogenesis of diabetes-associated congenital malformations. Obstet Gynecol Clin North Am. 1996;23:29–45. doi: 10.1016/s0889-8545(05)70243-6. [DOI] [PubMed] [Google Scholar]
- Reece EA, Homko CJ, Wu YK, Wiznitzer A. Metabolic fuel mixtures and diabetic embryopathy. Clin Perinatol. 1993;20:517–532. [PubMed] [Google Scholar]
- Reece EA, Ji I, Wu YK, Zhao Z. Characterization of differential gene expression profiles in diabetic embryopathy using DNA microarray analysis. Am J Obstet Gynecol. 2006;195:1075–1080. doi: 10.1016/j.ajog.2006.05.054. [DOI] [PubMed] [Google Scholar]
- Ross JF, Chaudhuri PK, Ratnam M. Differential regulation of folate receptor isoforms in normal and malignant tissues in vivo and in established cell lines. Physiologic and clinical implications. Cancer. 1994;73:2432–2443. doi: 10.1002/1097-0142(19940501)73:9<2432::aid-cncr2820730929>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Ross JF, Wang H, Behm FG, et al. Folate receptor type beta is a neutrophilic lineage marker and is differentially expressed in myeloid leukemia. Cancer. 1999;85:348–357. doi: 10.1002/(sici)1097-0142(19990115)85:2<348::aid-cncr12>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Ryan BM, Weir DG. Relevance of folate metabolism in the pathogenesis of colorectal cancer. J Lab Clin Med. 2001;138:164–176. doi: 10.1067/mlc.2001.117161. [DOI] [PubMed] [Google Scholar]
- Sadasivan E, Cedeno MM, Rothenberg SP. Characterization of the gene encoding a folate-binding protein expressed in human placenta. Identification of promoter activity in a G-rich SP1 site linked with the tandemly repeated GGAAG motif for the ets encoded GA-binding protein. J Biol Chem. 1994;269:4725–4735. [PubMed] [Google Scholar]
- Said HM, Nguyen TT, Dyer DL, et al. Intestinal folate transport: identification of a cDNA involved in folate transport and the functional expression and distribution of its mRNA. Biochem Biophys Acta. 1996;1281:164–172. doi: 10.1016/0005-2736(96)00005-3. [DOI] [PubMed] [Google Scholar]
- Saikawa Y, Price K, Hance KW, et al. Structural and functional analysis of the human KB cell folate receptor gene P4 promoter: cooperation of three clustered Sp1-binding sites with initiator region for basal promoter activity. Biochemistry. 1995;34:9951–9961. doi: 10.1021/bi00031a018. [DOI] [PubMed] [Google Scholar]
- Saitsu H, Ishibashi M, Nakano H, Shiota K. Spatial and temporal expression of folate-binding protein 1 (Fbp1) is closely associated with anterior neural tube closure in mice. Dev Dyn. 2003;226:112–117. doi: 10.1002/dvdy.10203. [DOI] [PubMed] [Google Scholar]
- Salbaum JM. Punc, a novel mouse gene of the immunoglobulin superfamily, is expressed predominantly in the developing nervous system. Mech Dev. 1998;71:201–204. doi: 10.1016/s0925-4773(98)00005-7. [DOI] [PubMed] [Google Scholar]
- Shaw GM, Schaffer D, Velie EM, et al. Periconceptional vitamin use, dietary folate, and the occurrence of neural tube defects. Epidemiology. 1995;6:219–226. doi: 10.1097/00001648-199505000-00005. [DOI] [PubMed] [Google Scholar]
- Shen F, Ross JF, Wang X, Ratnam M. Identification of a novel folate receptor, a truncated receptor, and receptor type beta in hematopoietic cells: cDNA cloning, expression, immunoreactivity, and tissue specificity. Biochemistry. 1994;33:1209–1215. doi: 10.1021/bi00171a021. [DOI] [PubMed] [Google Scholar]
- Singh M. Role of micronutrients for physical growth and mental development. Indian J Pediatr. 2004;71:59–62. doi: 10.1007/BF02725658. [DOI] [PubMed] [Google Scholar]
- Smith SB, Kekuda R, Gu X, et al. Expression of folate receptor alpha in the mammalian retinol pigmented epithelium and retina. Invest Ophthalmol Vis Sci. 1999;40:840–848. [PubMed] [Google Scholar]
- Smithells RW, Ankers C, Carver ME, et al. Maternal nutrition in early pregnancy. Br J Nutr. 1977;38:497–506. doi: 10.1079/bjn19770115. [DOI] [PubMed] [Google Scholar]
- Smithells RW, Sheppard S, Schorah CJ. Vitamin deficiencies and neural tube defects. Arch Dis Child. 1976;51:944–950. doi: 10.1136/adc.51.12.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smithells RW, Sheppard S, Schorah CJ, et al. Apparent prevention of neural tube defects by periconceptional vitamin supplementation. Arch Dis Child. 1981;56:911–918. doi: 10.1136/adc.56.12.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegelstein O, Eudy JD, Finnell RH. Identification of two putative novel folate receptor genes in humans and mouse. Gene. 2000;258:117–125. doi: 10.1016/s0378-1119(00)00418-2. [DOI] [PubMed] [Google Scholar]
- Spiegelstein O, Mitchell LE, Merriweather MY, et al. Embryonic development of folate binding protein-1 (Folbp1) knockout mice: Effects of the chemical form, dose, and timing of maternal folate supplementation. Dev Dyn. 2004;231:221–231. doi: 10.1002/dvdy.20107. [DOI] [PubMed] [Google Scholar]
- Stoffel M, Duncan SA. The maturity-onset diabetes of the young (MODY1) transcription factor HNF4alpha regulates expression of genes required for glucose transport and metabolism. Proc Natl Acad Sci U S A. 1997;94:13209–13214. doi: 10.1073/pnas.94.24.13209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam PP, Behringer RR. Mouse gastrulation: the formation of a mammalian body plan. Mech Dev. 1997;68:3–25. doi: 10.1016/s0925-4773(97)00123-8. [DOI] [PubMed] [Google Scholar]
- Thomas P, Beddington R. Anterior primitive endoderm may be responsible for patterning the anterior neural plate in the mouse embryo. Curr Biol. 1996;6:1487–1496. doi: 10.1016/s0960-9822(96)00753-1. [DOI] [PubMed] [Google Scholar]
- Tomassetti A, Mangiarotti F, Mazzi M, et al. The variant hepatocyte nuclear factor 1 activates the P1 promoter of the human alpha-folate receptor gene in ovarian carcinoma. Cancer Res. 2003;63:696–704. [PubMed] [Google Scholar]
- Trippett TM, Bertino JR. Therapeutic strategies targeting proteins that regulate folate and reduced folate transport. J Chemother. 1999;11:3–10. doi: 10.1179/joc.1999.11.1.3. [DOI] [PubMed] [Google Scholar]
- van Straaten HW, Hekking JW, Consten C, Copp AJ. Intrinsic and extrinsic factors in the mechanism of neurulation: effect of curvature of the body axis on closure of the posterior neuropore. Development. 1993;117:1163–1172. doi: 10.1242/dev.117.3.1163. [DOI] [PubMed] [Google Scholar]
- Wald N, Sneddon J, Densem J, et al. Prevention of neural tube defects: results of the Medical Research Council Vitamin Study. MRC Vitamin Study Research Group. Lancet. 1991;338:131–137. [PubMed] [Google Scholar]
- Walker LS. Regulatory T cells: Folate receptor 4: a new handle on regulation and memory? Immunol Cell Biol. 2007;85:506–507. doi: 10.1038/sj.icb.7100115. [DOI] [PubMed] [Google Scholar]
- Wang X, Jansen G, Fan J, et al. Variant GPI structure in relation to membrane-associated functions of a murine folate receptor. Biochemistry. 1996;35:16305–16312. doi: 10.1021/bi961098q. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhao R, Russell RG, Goldman ID. Localization of the murine reduced folate carrier as assessed by immunohistochemical analysis. Biochim Biophys Acta. 2001;1513:49–54. doi: 10.1016/s0005-2736(01)00340-6. [DOI] [PubMed] [Google Scholar]
- Wong SC, Proefke SA, Bhushan A, Matherly LH. Isolation of human cDNAs that restore methotrexate sensitivity and reduced folate carrier activity in methotrexate transport-defective Chinese hamster ovary cells. J Biol Chem. 1995;270:17468–17475. doi: 10.1074/jbc.270.29.17468. [DOI] [PubMed] [Google Scholar]
- Wotton D, Lo RS, Lee S, Massague J. A Smad transcriptional core-pressor. Cell. 1999a;97:29–39. doi: 10.1016/s0092-8674(00)80712-6. [DOI] [PubMed] [Google Scholar]
- Wotton D, Lo RS, Swaby LA, Massague J. Multiple modes of repression by the Smad transcriptional corepressor TGIF. J Biol Chem. 1999b;274:37105–37110. doi: 10.1074/jbc.274.52.37105. [DOI] [PubMed] [Google Scholar]
- Wu M, Fan J, Gunning W, Ratnam M. Clustering of GPI-anchored folate receptor independent of both cross-linking and association with caveolin. J Membr Biol. 1997;159:137–147. doi: 10.1007/s002329900277. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T, Hirota K, Nagahama K, et al. Control of immune responses by antigen-specific regulatory T cells expressing the folate receptor. Immunity. 2007;27:145–159. doi: 10.1016/j.immuni.2007.04.017. [DOI] [PubMed] [Google Scholar]
- Yates JR, Ferguson-Smith MA, Shenkin A, et al. Is disordered folate metabolism the basis for the genetic predisposition to neural tube defects? Clin Genet. 1987;31(5):279–287. doi: 10.1111/j.1399-0004.1987.tb02809.x. [DOI] [PubMed] [Google Scholar]