Abstract
The Vma21p protein in yeast is an essential assembly chaperone for the vacuolar ATPase, the major proton pump of cellular membranes. In this issue, Ramachandran et al. (2009) report that mutations in the gene encoding the human homolog VMA21 cause the disease X-linked myopathy with excessive autophagy through an unexpected mechanism.
Tight regulation of pH in intracellular compartments is critical for multiple cellular processes, including membrane trafficking, coupled transport of molecules, and protein degradation (Forgac, 2007). Transport of hydrogen ions across cellular membranes is predominantly conducted by a proton pump, the vacuolar ATPase (V-ATPase). This rotary nanomachine comprises 13 subunits that form an ATP-hydrolytic domain (V1) and a proton-translocator domain (V0). V-ATPases are also expressed in the plasma membranes of specialized cells and contribute to multiple functions, such as renal acidification, bone resorption, sperm maturation, and control of cytoplasmic pH. Mutations in cell-specific isoforms of V-ATPase subunits cause tissue-specific diseases. For example, mutations of the a2 isoform (in the Golgi and early endosomes of endothelial cells) cause developmental delays and wrinkled skin (Kornak et al., 2008); mutations in a3 (in the osteoclast plasma membrane) cause osteopetrosis (Frattini et al., 2000); mutations in a4 (in the plasma membrane of distal nephron and inner ear cells) cause renal tubular acidosis and deafness (Karet et al., 1999). In this issue, Ramachandran et al. (2009) now characterize a general defect in V-ATPase function caused by mutations in an essential chaperone, VMA21, that result in muscle-specific disease in humans. The authors show that VMA21 deficiency decreases V-ATPase activity, resulting in an increase in intralysosomal pH. This increase in pH impairs lysosomal action and blocks the autophagic degradation of proteins leading to accumulation of autophagosomes and autophagic vacuoles.
Through linkage studies and candidate gene sequencing, Ramachandran et al. (2009) identified six single-nucleotide substitutions in the LOC203547 gene in male patients from 14 families with a rare disease called X-linked myopathy with excessive autophagy (XMEA). This childhood-onset disorder is characterized by the progressive atrophy and vacuolation of skeletal muscle (Kalimo et al., 1988). Because all of the mutations resulted in down-regulation of the LOC203547 transcript and its protein product in the muscle cells of XMEA patients, the defective genes were considered to be hypomorphic alleles. To confirm that the mutations were pathogenic, the authors documented that the abnormalities in autophagy they observed in fibroblasts from XMEA patients could be corrected by stable transfection with a retrovirus expressing the normal gene. Although the LOC203547 gene product has only ~20% identity with yeast Vma21p, the authors posit that the proteins are orthologs and accordingly designate the human version VMA21. In support of this notion, the human gene rescued the growth defects of yeast vma21 deletion mutants cultured under both normal and stress conditions.
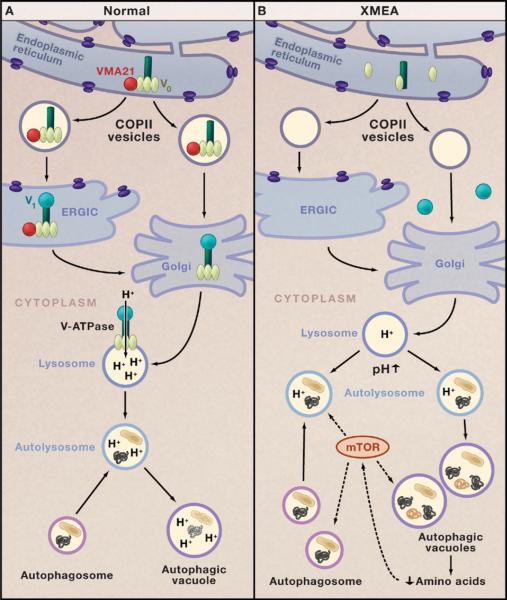
Ramachandran and colleagues demonstrate that the functions of human VMA21 are similar, but not identical, to those of yeast Vma21p. Both proteins are required to initiate assembly of the proton-translocator domain V0 of the V-ATPase in the endoplasmic reticulum (ER), but Vma21p interacts with subunit c′ of V0, whereas VMA21 interacts with subunit c″ (Figure 1A). Both proteins chaperone the V0 domain in COPII vesicles during transport from the ER to the ER-Golgi intermediate compartment (ERGIC) and to the Golgi apparatus, where V-ATPase assembly is completed by addition of the V1 ATP-hydrolytic domain (Figure 1). However, in contrast to Vma21p, which is recycled back to the ER by COPI vesicles via a carboxy-terminal dilysine signal, VMA21 lacks this signal and does not return to the ER.
Figure 1. V-ATPase and Autophagy in Health and Disease.
(A) The chaperone VMA21 (red circle) binds to the c′ subunit of the vacuolar-ATPase (V-ATPase; dark green) in the endoplasmic reticulum and initiates assembly of the proton-translocator domain (V0; pale green) (Ramachandran et al., 2009). VMA21 chaperones V0 in COPII vesicles to the endoplasmic reticulum Golgi intermediate complex (ERGIC). VMA21 is not present in the Golgi apparatus, but it is here that V-ATPase assembly is completed by addition of the ATP-hydrolytic domain (V1; turquoise). Proton pumping by the V-ATPase is critical for regulating the pH of lysosomes. Fusion of lysosomes with autophagosomes generates autolysosomes that degrade target proteins and organelles.
(B) In the disease X-linked myopathy with excessive autophagy (XMEA), deficiency of VMA21 leads to failure of V0 assembly, reduced levels of V-ATPase, and a decrease in lysosomal pH that impairs lysosomal and autophagic digestion of cellular proteins and organelles. Decreased protein degradation in XMEA may lower intracellular levels of amino acids that modulate the mTOR signaling pathway, which, in turn, upregulates macroautophagy. This leads to excessive accumulation of autophagosomes, autolysosomes, and autophagic vacuoles and a muscle-specific disease phenotype.
Lysosomes are critical for the degradation of excess or damaged proteins and organelles by autophagy. There are three types of autophagy: chaperone-mediated autophagy, microautophagy, and macroautophagy (Mizushima et al., 2008). Chaperone-mediated autophagy results in degradation of soluble proteins, whereas micro- and macroautophagy result in engulfment of large structures via selective and nonselective mechanisms to produce autophagosomes. In chaperone-mediated autophagy, chaperone-bound proteins are targeted to lysosomes, to which they gain access through a membrane receptor called LAMP2A (lysosome-associated membrane protein 2A). In microautophagy, cytosolic components are pocketed by lysosomes through invaginations of the organellar membrane. In macroautophagy, double-membrane structures called phagophores surround the targets for degradation to form double-membrane autophagosomes. Autophagosomes then fuse with lysosomes to generate autolysosomes, which, in turn, coalesce to form autophagic vacuoles (Figure 1A).
Given the functions of VMA21, it is not surprising that deficiency of this protein would cause marked loss of V-ATPase complexes in the organelles of muscle and of cultured fibroblasts and lympho-blasts from XMEA patients (Figure 1B). As a consequence of V-ATPase deficiency, the lysosomal pH is 0.5 units higher in fibroblasts from XMEA patients than in those from healthy individuals. The lysosomal pH is altered enough to decrease the autophagic degradation of long-lived proteins, but not the maturation of three lysosomal enzymes. This led Ramachandran et al. to hypothesize that the higher lysosomal pH reduces enzymatic activities in the autophagic pathway selectively, thus resulting in the accumulation of autophagic vacuoles in XMEA. This concept is supported by reports that pharmacological inhibition of V-ATPase in cells impairs autophagy and increases the numbers of autolysosomes and autophagic vacuoles (Shacka et al., 2006). Nevertheless, direct evidence of altered autolysosomal pH and of its effects on macroautophagic protein degradation in XMEA is still wanting. Although not noted by the authors, lack of lysosomal upregulation in response to the overabundance of autophagosomes may also contribute to the delayed degradation of the contents of autophagic vacuoles.
Ramachandran et al. further postulate that the block in autophagy would result in upregulation of macroautophagy. In agreement with this notion, they did observe an increase in early components of the macroautophagic activation pathway including beclin-1, beclin-1-hVps34 complexes, and LC3. They further propose that decreased recycling of excess and degraded proteins and the reduced liberation of amino acids enhances autophagy because a decrease in amino acids in the cytoplasm modulates the mTOR signaling pathway, which then upregulates macroautophagy (Figure 1B). In support of this hypothesis, the authors noted prominent dephosphorylation of p70S6 kinase, a key component of the mechanism by which mTOR upregulates macroautophagy. They also observed that partial inhibition of lysosomal hydrolases by leupeptin in control fibroblasts and lymphoblasts resulted in mTOR-mediated upregulation of microautophagy, reflected by proliferation of autolysosomes and autophagic vacuoles. If the authors' notion that macroautophagy in XMEA is enhanced by activation of mTOR is correct, then blocking this pathway to reduce autophagy may prove beneficial to patients. This potentially useful therapeutic strategy can be assessed in cell culture models using pharmacological blockade or genetic interventions such as RNA interference.
An issue that could not be addressed experimentally in the Ramachandran et al. study is why a general defect in V-ATPase, a housekeeping protein complex, should cause a skeletal muscle-specific phenotype. The authors speculate that the preferential involvement of muscle may be explained by the greater macroautophagic response of this tissue to decreased amino acids. As the authors note in their paper, other disorders of lysosomal function produce autophagic vacuolar myopathies in humans that closely resemble XMEA. Prolonged use of chloroquine, a drug for treating malaria and rheumatoid arthritis, and Danon disease, an X-linked dominant myopathy due to LAMP2 deficiency, cause skeletal myopathies that closely resemble XMEA (although brain and heart are also clinically affected in Danon disease) (Kalimo et al., 1988; Nishino et al., 2000). Chloroquine accumulates in lysosomes, where, just like V-ATPase deficiency, it elevates lysosomal pH and blocks autophagy. By contrast, LAMP2 deficiency affects chaperone-mediated autophagy, lysosomal biogenesis, lysosome-autophagosome fusion, and lysosomal movement along microtubules. Defects in multiple lysosomal functions could easily account for the more widespread organ involvement seen in Danon disease. Development of an animal model of VMA21 deficiency may reveal the reasons for the muscle-specific phenotype of XMEA.
Overall, the work of Ramachandran et al. is praiseworthy, not only for identifying primary VMA21 deficiency as the cause of XMEA, but also for the elegant characterization of the mechanism resulting in pathogenic autophagic vacuoles. The findings confirm the importance of V-ATPase in the regulation of lysosomal pH, which appears to impact autophagy directly by impairing lysosomal degradation and indirectly through modulation of the mTOR pathway in skeletal muscle. As the authors suggest, elucidation of V-ATPase functions and the consequences of dysfunction may be useful in the design of V-ATPase modulators to treat not only XMEA but also malaria, HIV, osteoporosis, and cancer metastasis.
Acknowledgments
We thank I. Nishino, S. Noguchi, and E. Area Gomez for helpful comments. The authors are supported by the National Institutes of Health (R01 HD057543; P01 HD32062), the Muscular Dystrophy Association, and the Marriot Mitochondrial Disorder Clinical Research Fund.
References
- Forgac M. Nat. Rev. Mol. Cell Biol. 2007;8:917–929. doi: 10.1038/nrm2272. [DOI] [PubMed] [Google Scholar]
- Frattini A, Orchard PJ, Sobacchi C, Giliani S, Abinun M, Mattsson JP, Keeling DJ, Anders-son AK, Wallbrandt P, Zecca L, et al. Nat. Genet. 2000;25:343–346. doi: 10.1038/77131. [DOI] [PubMed] [Google Scholar]
- Kalimo H, Savontaus ML, Lang H, Paljarvi L, Sonninen V, Dean PB, Katevuo K, Salminen A. Ann. Neurol. 1988;23:258–265. doi: 10.1002/ana.410230308. [DOI] [PubMed] [Google Scholar]
- Karet FE, Finberg KE, Nelson RD, Nayir A, Mocan H, Sanjad SA, Rodriguez-Soriano J, Santos F, Cremers CW, Di Pietro A, et al. Nat. Genet. 1999;21:84–90. doi: 10.1038/5022. [DOI] [PubMed] [Google Scholar]
- Kornak U, Reynders E, Dimopoulou A, van Reeuwijk J, Fischer B, Rajab A, Budde B, Nurnberg P, Foulquier F, Lefeber D, et al. Nat. Genet. 2008;40:32–34. doi: 10.1038/ng.2007.45. [DOI] [PubMed] [Google Scholar]
- Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Nature. 2008;451:1069–1075. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishino I, Fu J, Tanji K, Yamada T, Shimojo S, Koori T, Mora M, Riggs JE, Oh SJ, Koga Y, et al. Nature. 2000;406:906–910. doi: 10.1038/35022604. [DOI] [PubMed] [Google Scholar]
- Ramachandran N, Munteanu I, Wang P, Aubourg P, Rilstone J, Israelian N, Naranian T, Paroutis P, Guo R, Ren Z-P, et al. Cell. 2009 doi: 10.1016/j.cell.2009.01.054. this issue. [DOI] [PubMed] [Google Scholar]
- Shacka JJ, Klocke BJ, Shibata M, Uchiyama Y, Datta G, Schmidt RE, Roth KA. Mol. Pharmacol. 2006;69:1125–1136. doi: 10.1124/mol.105.018408. [DOI] [PubMed] [Google Scholar]