Abstract
OBJECTIVE
The hypothalamic neuropeptide orexin influences (feeding) behavior as well as energy metabolism. Administration of exogenous orexin-A into the brain has been shown to increase both food intake and blood glucose levels. In the present study, we investigated the role of endogenous hypothalamic orexin release in glucose homeostasis in rats.
RESEARCH DESIGN AND METHODS
We investigated the effects of the hypothalamic orexin system on basal endogenous glucose production (EGP) as well as on hepatic and peripheral insulin sensitivity by changing orexinergic activity in the hypothalamus combined with hepatic sympathetic or parasympathetic denervation, two-step hyperinsulinemic-euglycemic clamps, immunohistochemistry, and RT-PCR studies.
RESULTS
Hypothalamic disinhibition of neuronal activity by the γ-aminobutyric acid receptor antagonist bicuculline (BIC) increased basal EGP, especially when BIC was administered in the perifornical area where orexin-containing neurons but not melanocortin-concentrating hormone–containing neurons were activated. The increased BIC-induced EGP was largely prevented by intracerebroventricular pretreatment with the orexin-1 receptor antagonist. Intracerebroventricular administration of orexin-A itself caused an increase in plasma glucose and prevented the daytime decrease of EGP. The stimulatory effect of intracerebroventricular orexin-A on EGP was prevented by hepatic sympathetic denervation. Plasma insulin clamped at two or six times the basal levels did not counteract the stimulatory effect of perifornical BIC on EGP, indicating hepatic insulin resistance. RT-PCR showed that stimulation of orexin neurons increased the expression of hepatic glucoregulatory enzymes.
CONCLUSIONS
Hypothalamic orexin plays an important role in EGP, most likely by changing the hypothalamic output to the autonomic nervous system. Disturbance of this pathway may result in unbalanced glucose homeostasis.
The hypothalamic neuropeptide orexin is involved in arousal and energy homeostasis (1). Lack of orexin results in narcolepsy and hypophagia. Despite the reduced food intake, both narcoleptic patients with orexin deficiency and the animal model with genetic ablation of orexin neurons tend to be obese (2,3). These findings indicate that the link between orexin and energy homeostasis is not only via appetite stimulation (4,5) but also involves additional mechanisms in the control of energy metabolism. In keeping with this notion, orexin-ataxin-3 transgenic mice show reduced metabolic rate, independent of other behavioral changes induced by orexin, such as arousal, locomotion, and food intake (6).
Orexin-A can regulate plasma glucose concentrations via both central and peripheral mechanisms (7,8), but the neurotransmitter(s) responsible for controlling the endogenous activity of the orexin neurons is (are) not evident. Besides the glutamatergic input that is derived from a local neuronal network (9), orexin neurons also receive γ-aminobutyric acid (GABA)ergic inputs from a variety of brain areas such as arcuate nucleus (ARC) neuropeptide Y neurons (9), basal forebrain (10), and preoptic area (11). During the light period (i.e., the sleeping/fasting period of rats), orexin neurons in the perifornical area are inhibited by GABA inputs originating from the biological clock neurons located in the suprachiasmatic nucleus (SCN) (12). Interestingly, hypothalamic application of the GABAα receptor antagonist bicuculline (BIC) not only activates orexin neurons (13) but also increases plasma glucose concentrations (14–16).
To test the hypothesis that GABA acts as an inhibitory neurotransmitter in upstream brain areas to control the glucoregulatory function of orexin, we activated the orexin neurons in the perifornical orexin area (PF-Oa) of rats by retrodialysis of GABAα receptor antagonist and investigated its effect on endogenous glucose production (EGP), using the stable isotope technique. The effect of BIC on hepatic and peripheral insulin sensitivity was investigated by performing euglycemic clamps at two different levels of hyperinsulinemia. The specific involvement of orexin neurons in the response of EGP to BIC was confirmed in several ways: 1) by comparing the EGP response from BIC administration in the PF-Oa with that in two nearby brain areas that do not contain orexin neurons (i.e., the dorsal part of the dorsomedial hypothalamus [dDMH] and the hypothalamic paraventricular nucleus [PVN]), 2) by comparing EGP responses with and without intracerebroventricular coinfusion of orexin-1 receptor (OX-1R) antagonist SB-408124 during BIC administration in the PF-Oa, and 3) by investigating the effects of intracerebroventricular administration of orexin-A and melanin-concentrating hormone (MCH) on EGP. To investigate the possible involvement of the autonomic nervous system in the glucoregulatory actions of orexin-A, we combined the intracerebroventricular administration of orexin-A with specific hepatic sympathetic or parasympathetic denervations. Furthermore, to elucidate the metabolic signaling pathway utilized by the orexin system to control hepatic insulin sensitivity, we also examined several hepatic glucoregulatory factors by quantitative real-time PCR.
RESEARCH DESIGN AND METHODS
All experiments were conducted under the approval of the animal care committee of the Royal Netherlands Academy of Arts and Sciences. Male Wistar rats weighing 300–350 g (Harlan Nederland, Horst, the Netherlands) were housed in individual cages (25 × 25 × 35 cm), with a 12/12-h light-dark schedule (lights on at 0700 h). Food and water were available ad libitum, unless stated otherwise.
Surgery preparation.
Animals underwent surgeries according to the different experimental designs under anesthesia, with 0.8 ml/kg i.m. Hypnorm (Janssen, High Wycombe, Buckinghamshire, U.K.) and 0.4 ml/kg s.c. Dormicum (Roche, Almere, the Netherlands).
Silicon catheters were inserted into the right jugular vein and left carotid artery for intravenous infusions and blood sampling, respectively. With a standard Kopf stereotaxic apparatus, bilateral microdialysis probes (14) were placed into the PF-Oa, dDMH, and the PVN. Intracerebroventricular guiding probes were placed into the lateral cerebral ventricle. All coordinates were adapted from the atlas of Paxinos and Watson (17) (see the online appendix data 1 and Table 1 [available at http://diabetes.diabetesjournals.org/cgi/content/full/db09-0385/DC1]). All catheters and probes were fixed on top of the head and secured with dental cement.
Hepatic sympathetic or parasympathetic branches were denervated according to our previously published methods (14). The effectiveness of the hepatic sympathetic denervation was checked by measurement of norepinephrine content in the liver as described before (18). After recovery, animals were connected to a multichannel fluid-infusion swivel (Instech Laboratories, Plymouth Meeting, PA) 1 day before the experiment for adaption. Food was removed at the beginning of the light period to exclude intestinal glucose absorption. The first experimental blood samples were collected 5 h later.
Tracer dilution study.
To study glucose kinetics, [6,6-2H2]glucose (as a primed [8.0 μmol in 5 min]-continuous [16.6 μmol/h] infusion) was used as tracer (>99% enriched; Cambridge Isotopes, Andover, MA). In experiments 1–4, blood samples were taken at t = −5 min for measuring background enrichment of [6,6-2H2]glucose, at t = 90, 95, and 100 min for determining enrichment during the equilibration state, and at t = 210, 220, 230, 240, and 250 min for determining enrichment during the retrodialysis and/or intracerebroventricular infusion of different drugs according to the different experimental designs.
In experiment 1, Ringer's dialysis (vehicle, 3 μl/min) was started together with [6,6-2H2]glucose infusion in both BIC and vehicle groups. After the t = 100 min blood sample, the Ringer's solution was changed to a BIC solution (100 μmol/l, 3 μl/min; Sigma) or Ringer's (vehicle control, 3 μl/min) again. After t = 250 min blood sampling, to validate the placement of the microdialysis probes, animals were perfused by 4% paraformaldehyde and went through Nissl and immunohistochemical staining.
In experiment 2, the primed-continuous [6,6-2H2]glucose infusion and Ringer's dialysis (vehicle) were started (after primary background blood sampling) together with OX-1R antagonist 1-(6,8-difluoro-2-methyl-quinolin-4-yl)-3-(4-dimethylamino-phenyl)-urea (SB-408124) (50 mmol/l, 5 μl/h; Sigma-Aldrich, St. Louis, MO) or 20% DMSO (vehicle, 5 μl/h); the dose of SB-408124 was adapted from SB-334867 in previously reported data (19,20). At the end of the equilibration state, BIC retrodialysis was applied in both groups. After the t = 250 min sampling, animals were perfused and intracerebroventricular and microdialysis probe placements were validated as described above.
In experiment 3, with liver nerve–intact rats, and in experiment 4, with liver sympathetic–, parasympathetic–, or sham-denervated rats, after the t = 100 min blood sample, a continuous intracerebroventricular infusion of orexin-A (1 mmol/l, 5 μl/h; Bachem, Weil am Rhein, Germany), MCH (1 mmol/l, 5 μl/h; Bachem) (only in experiment 3), or purified water (Milli-Q water, vehicle, 5 μl/h) (only in experiment 3) (21) was started. After t = 250 min blood sampling, animals were then deeply anesthetized and 2 μl colored dye was injected via the intracerebroventricular guiding probe to validate the probe placement.
In experiment 5, both hyperinsulinemic clamp 1 and clamp 2 consisted of a basal Ringer's (vehicle) equilibration period (t = 0–100 min), a primary Ringer's (hyperinsulinemic-euglycemic clamp) period (t = 110–140 min), and a secondary BIC (hyperinsulinemic-euglycemic clamp) period (t = 140–250 min). At t = 100 min, insulin was administered in a primed (7.2 mU · kg−1 · min−1 in 4 min for clamp 1 and 21.6 mU · kg−1 · min−1 in 4 min for clamp 2)-continuous (3 mU · kg−1 · min−1 for clamp 1 and 9 mU · kg−1 · min−1 for clamp 2) intravenous infusion. A variable infusion of a 25% glucose solution (containing 1% [6,6-2H2]glucose) was used to maintain euglycemia (5.5 ± 0.2 mmol/l), as determined by a 10-min carotid catheter blood sampling. Thirty minutes after the start of the primary insulin infusion, Ringer's perfusion of the microdialysis probes was replaced by the BIC solution in BIC group. At the end of the clamp, five blood samples were taken with a 10-min interval from t = 210–250 min. Liver tissue was then collected under deep anesthesia for real-time PCR study, and animals were perfused for Nissl and immunohistochemical staining.
Immunohistochemistry.
For immunohistochemical staining, animals were killed by an intra-atrial perfusion with saline, followed by a solution of 4% paraformaldehyde in 0.1 mol/l phosphate buffer (pH 7.4) at 4°C. After postfixation and equilibration for 48 h with 30% sucrose in 0.1 mol/l Tris-buffered saline, the brain tissue was cut into 30-μm sections and divided into three equal groups for Fos and orexin or MCH immunostaining (online appendix data 2).
Quantitative real-time PCR.
Total RNA from liver tissue was isolated with Trizol (Invitrogen), and single-stranded complementary DNA was synthesized. One microliter of each cDNA was incubated in a final volume of 20 μl real-time PCR containing 1 × SYBR Green master mix (Applied Biosystems) and 3 pmol reverse and 3 pmol forward primers (online appendix data 3 and Table 2). Efficiency of primer was determined on the basis of a cDNA dilution series. Quantitative real-time PCR was performed in an Applied Biosystems Prism Sequence Detection System (model ABI7300). Standard PCR conditions were used. The data were acquired and processed automatically by Sequence Detection Software 33 (Applied Biosystems).
Analytical methods.
Blood samples were immediately chilled on ice in tubes containing a 5 μl heparin solution and centrifuged at 4°C. Plasma was then stored at −20°C for analysis. Plasma glucose concentrations were determined using a glucose/glucose oxidase-perid method (Boehringer Mannheim, Mannheim, Germany). Plasma insulin, glucagon, and corticosterone concentrations were measured using radioimmunoassay kits (Linco Research, St. Charles, MO, and ICN Biomedicals, Costa Mesa, CA, respectively). Plasma [6,6-2H2]glucose enrichment was measured by gas chromatography–mass spectrometry.
Statistics.
Results are expressed by averaging values from three plasma samples at the end of the equilibration state and five plasma samples at the end of the experimental period. Student's t test was used to test orexin-A or BIC effects on EGP, comparing the mean of the equilibration state with the mean of the experimental period. ANOVA and post hoc Student's t tests were also performed to evaluate the BIC effects of different hypothalamic nuclei and to compare the different states of (hyper)insulinemia.
RESULTS
Removal of the endogenous GABA inhibition of perifornical orexin neurons stimulates EGP (experiment 1).
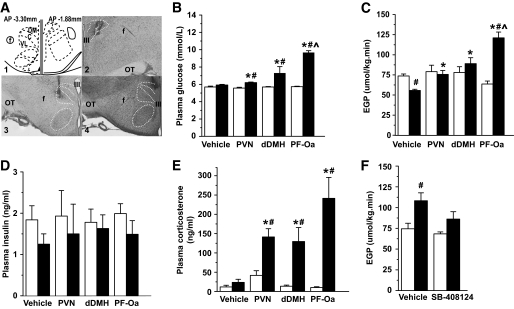
Experiment 1 was performed to investigate whether activation of orexin neurons during the light period would affect glucose metabolism. Histological analysis of the probe placements showed that the tip of the microdialysis probes was located either in the lateral part of the PVN (Fig. 1A2), at the upper borders of the PF-Oa (Fig. 1A3), or in the dDMH (Fig. 1A4).
FIG. 1.
GABAa receptor antagonist BIC administration in the PVN, dDMH, or PF-Oa causes different EGP responses that are independent of changes in plasma insulin and corticosterone. A: Representative microdialysis probe placements in PVN, PF-Oa, and dDMH. The left and right panels of graph A1 are located at AP coordinates −3.30 and −1.88 mm, respectively. Graphs A2, A3, and A4 show a Nissl-stained example of each placement. PVN, DMH, and VMH are outlined by a white dotted line. B–E: Display the plasma glucose concentration, EGP, plasma insulin, and corticosterone concentration before (equilibration state) and after BIC (or vehicle) in different hypothalamic nuclei. □, vehicle retrodialysis equilibration state; ■, BIC (or vehicle) retrodialysis state. F: Average EGP before (□) and after a 2-h infusion of BIC in the PF-Oa (■) with intracerebroventricular vehicle or orexin-1 receptor antagonist SB-408124 (pre)treatment. Intracerebroventricular (pre)treatment with the SB-408124 blocks the BIC-induced EGP increase. C: Compact zone of DMH. DM, dorsomedial DMH; f, fornix; OT, optic tract; VL, ventrolateral DMH; III, third ventricle; IV, fourth ventricle. Scale bar: 400 μm. Data are presented as means ± SE. #P < 0.05 vs. equilibration state; *P < 0.05 vs. vehicle control; ∧P < 0.05 vs. dDMH and PVN.
Rats with Ringer's retrodialysis (n = 6, vehicle) showed no changes in plasma glucose levels as compared with their own equilibration state (Fig. 1B); however, it showed a clear decline in EGP (Fig. 1C). This steady decline most likely is due to absence of food in the cages and not to the different brain infusions, as it was not observed in animals that remained in ad libitum conditions (online appendix data 4, online appendix Fig. 1). Retrodialysis of BIC in the PF-Oa (n = 5) caused a significant hyperglycemic response and a pronounced increase in EGP as compared with its own equilibration state as well as to that of the Ringer's infusion. Retrodialysis of BIC into the dDMH (n = 6) and PVN (n = 4) also caused a significant, albeit smaller, hyperglycemic response. Different from the PF-Oa group, both the dDMH and PVN group did not show a significant increase in EGP as compared with their own equilibration, but both groups differed significantly from the Ringer's group at the end of their BIC administration period. Plasma insulin levels were not affected by the BIC treatment (Fig. 1D). In addition BIC, but not Ringer's, significantly increased plasma corticosterone levels, without significant differences among the three groups (Fig. 1E). Moreover, no correlation was found between the area under the curve of the corticosterone response and the EGP increase in BIC-treated rats (r = 0.55, P = 0.10).
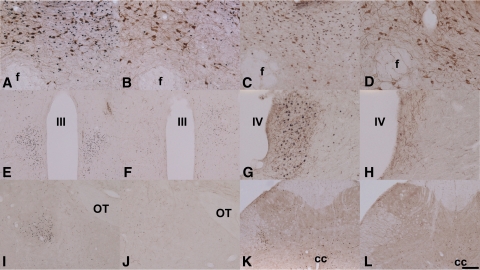
Immunohistochemical staining with the Fos antibody showed that in BIC-treated brains (n = 4–6), the major part of the activated neurons (as indicated by the expression of Fos in their nucleus) was limited to a restricted area around the dialysis probes (±500 μm spread from the edge of the dialysis probe). Double immunohistochemical stainings with Fos and orexin (n = 4–6) showed that when the Ringer's dialysis probes ended in the upper part of the PF-Oa, very few Fos positive nuclei (one to five per section) were observed and no colocalization of orexin and Fos was found. On the other hand, in animals with BIC dialysis probes ending in the upper part of the PF-Oa, double immunohistochemical staining showed that most of the orexin neurons (81 ± 14 single-labeled neurons per section) were activated (58 ± 9 Fos/orexin double-labeled per section [i.e., ∼71%]) (Fig. 2A). With the same BIC stimulation, most of the MCH neurons that are interspersed among the orexin neurons were not activated (Fig. 2C). However, when dialysis probes were placed in the dDMH, only a few orexin neurons (104 ± 6 single-labeled neurons per section) were activated (21 ± 2 Fos/orexin double-labeled neurons per section [i.e., ∼20%]) (Fig. 2B) (P < 0.001 vs. PF-Oa group). Fos expression after BIC administration into the PVN did not engage the orexin neurons. This specific activation of orexin neurons by BIC is consistent with a previous study that targeted BIC to the perifornical area (13). In addition, when most of the orexin neurons were Fos positive, Fos immunoreactivity was also found in orexin-targeting areas, such as PVN (Fig. 2E), locus coeruleus (Fig. 2G), central amygdaloid nucleus (Fig. 2I), and even as far as the intermediolateral cell column of the sacral spinal cord (Fig. 2K) (22), confirming the intensive activation of orexin neurons. Although the ARC has been suggested to be involved in the appetite-stimulating actions of orexin (23,24), BIC administration into the PF-Oa did not result in different numbers of Fos immunoreactive neurons in this nucleus as compared with the Ringer's control group (6.6 ± 1.5 vs. 3.3 ± 0.5 per section, P = 0.14).
FIG. 2.
A–D: Fos immunoreactivity around the microdialysis probes in the PF-Oa and dDMH area. Fos and orexin double stainings are shown for BIC administration in the PF-Oa (A) and dDMH (B). C: Fos and MCH double staining after BIC administration in the PF-Oa. D: Illustrates the Fos and orexin double staining in a Ringer's control. After BIC administration in the PF-Oa, Fos immunoreactivity is also present in orexin target areas such as PVN (E), locus coeruleus (G), central amygdaloid nucleus (I), and intermediolateral cell column of the sacral spinal cord (K). F, H, J, and L: The panels on their respective right side show the absence of Fos-ir in these areas with Ringer's dialysis in PF-Oa area. E–L: Double stained for Fos and orexin. The photos shown are representative for all the other animals in the same group, which are four to six rats depending on individual groups. Cc, central canal; f, fornix; III, third ventricle; IV, fourth ventricle; OT, optic tract. Scale bar: A–D, G, and H: 100 μm. E, F, I–L: 200 μm. (A high-quality digital representation of this figure is available in the online issue.)
Antagonizing the central orexin-1 receptor prevents the increase in EGP induced by BIC administration in the PF-Oa (experiment 2).
Retrodialysis of BIC in the PF-Oa, combined with intracerebroventricular vehicle treatment, significantly increased EGP (Fig. 1F) as well as plasma glucose concentration (6.1 ± 0.1 vs. 7.8 ± 0.3 mmol/l, P < 0.001) (i.e., similar to the results of experiment 1). However, intracerebroventricular (pre)treatment with SB-408124 prevented the BIC-induced increase in EGP. The BIC-induced increase in plasma glucose concentration was also attenuated, but it was still significantly higher than its own equilibration state (5.9 ± 0.1 vs. 6.9 ± 0.2 mmol/l, P = 0.002) and showed a trend to be significantly different from the BIC-induced increase in plasma glucose in the vehicle group (P = 0.055).
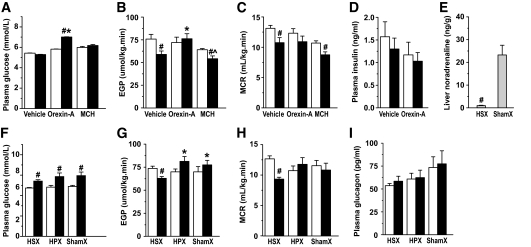
Central administration of orexin-A stimulates EGP (experiment 3).
To confirm that orexin neurons are indeed involved in the BIC-induced increase in EGP, in experiment 3 we performed an intracerebroventricular infusion of either orexin-A or vehicle. Intracerebroventricular administration of vehicle had no effect on plasma glucose levels (Fig. 3A). However, a clear decline in EGP (Fig. 3B) and metabolic clearance rate (MCR) (EGP/plasma glucose concentration) (Fig. 3C) was apparent (i.e., similar to what was found in the Ringer's animals of experiment 1). Intracerebroventricular infusion of orexin-A increased the plasma glucose level. In contrast to the vehicle group, no decrease in EGP and MCR was observed. EGP at the end of the orexin-A infusion was also significantly higher than that in the vehicle group at the end of the infusion state; thus, intracerebroventricular orexin-A prevented the endogenous decline of EGP. Plasma insulin concentrations did not change during intracerebroventricular infusion of either vehicle or orexin-A (Fig. 3D). In line with the absence of Fos immunoreactivity in MCH neurons after BIC stimulation, no significant effects of intracerebroventricular-administered MCH (n = 5) on plasma glucose levels, EGP, or MCR compared with the vehicle control animals were found (Fig. 3A–C).
FIG. 3.
A–C: Involvement of the autonomic nervous system in the EGP increase induced by BIC administration in the PF-Oa. Average plasma glucose concentration, EGP, and MCR before and after a 2-h infusion of vehicle, orexin-A, or MCH into the lateral cerebral ventricle. A–D: □, equilibrium state; ■, intracerebroventricular infusion state. D: Average plasma insulin concentration before (equilibration state) and after intracerebroventricular vehicle or orexin-A infusion. E: Hepatic sympathetic denervation abolishes 96% of the norepinephrine content in the liver. F–I: Plasma glucose concentration, EGP, MCR, and plasma glucagon concentration with intracerebroventricular orexin-A infusion after HSX, HPX, and shamX. F–I: □, equilibrium state; ■, orexin-A intracerebroventricular infusion state. Data are presented as means ± SE. #P < 0.05 infusion state vs. equilibration state (or shamX versus HSX in E); *P < 0.05 vs. vehicle control in A and B and vs. HSX in G; ∧P < 0.05 MCH vs. orexin-A group.
Hepatic sympathetic, but not parasympathetic, denervation blocks the effect of intracerebroventricular orexin-A infusion on EGP (experiment 4).
Successful hepatic sympathetic denervation (HSX) was validated by a significant decrease of liver norepinephrine concentrations compared with sham denervation (shamX) (Fig. 3E). After HSX, intracerebroventricular infusion of orexin-A no longer could prevent the endogenous decline of EGP and MCR as shown in experiment 3 (compare Fig. 3G and H with Fig. 3B and C). However, after hepatic parasympathetic denervation (HPX) or shamX the intracerebroventricular infusion of orexin-A was as effective as in liver-intact animals to prevent the endogenous drop in EGP and MCR (Fig. 3G and H). Plasma glucose changes, on the other hand, were not affected by any of the denervation procedures (i.e., plasma glucose levels increased after intracerebroventricular orexin-A infusion in all three groups [Fig. 3F]). No differences were found in plasma glucagon concentrations as a result of the intracerebroventricular orexin-A infusion or the denervation procedure (Fig. 3I).
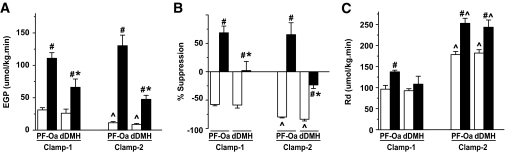
Removal of the endogenous GABA inhibition of perifornical orexin neurons reduces hepatic insulin sensitivity (experiment 5).
During clamp 1 (3 mU · kg−1 · min−1), retrodialysis of BIC in the PF-Oa (n = 6) and dDMH (n = 4) after the equilibration state still induced a significant increase in EGP in both groups, despite the presence of peripheral hyperinsulinemia (Fig. 4A). Furthermore, EGP in the dDMH group was significantly lower than that in the PF-Oa group. Thus, the insulin-mediated suppression of EGP (i.e., hepatic insulin sensitivity) was significantly attenuated in both BIC groups compared with their vehicle control groups (Fig. 4B), but the effect was significantly more pronounced in the PF-Oa group than in the dDMH group. The rate of glucose disappearance (Rd) was significantly increased by BIC in the PF-Oa group but not in the dDMH group (Fig. 4C) compared with their respective control groups.
FIG. 4.
Average EGP (A), insulin suppression of EGP (B), and Rd (C) under hyperinsulinemic-euglycemic clamp 1 (3 mU · kg−1 · min−1) and clamp 2 (9 mU · kg−1 · min−1) conditions. BIC administration in the PF-Oa induced the strongest increase in EGP as well as in Rd. Data are presented as means ± SE. #P < 0.05 vs. Ringer's control; *P < 0.05 vs. PF-Oa; ∧P < 0.05 vs. clamp 1. A: □, vehicle retrodialysis group; ■, BIC retrodialysis group.
To explore the effect of BIC on peripheral insulin sensitivity, clamp 2 with a higher plasma insulin level was performed. Despite the higher insulin levels, which resulted in a significant further decrease in EGP in the two Ringer's control groups, BIC still caused a significant increase in EGP in both the PF-Oa (n = 7) and the dDMH (n = 4) group compared with their respective control groups (Fig. 4A). Again, the effect was more pronounced in the PF-Oa group than in the dDMH group. With regard to Rd, the two Ringer's groups showed a significantly higher Rd, as compared with clamp 1, and both BIC groups showed a significantly higher Rd than the control groups (Fig. 4C).
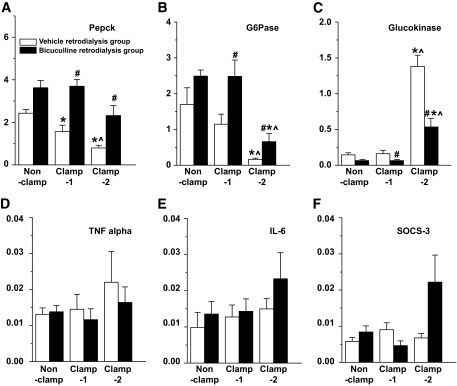
The real-time PCR study on the liver tissue revealed that BIC treatment significantly diminished the inhibitory effect of hyperinsulinemia on the expression of phosphoenolpyruvate cerboxykinase (PEPCK) and glucose-6-phosphatase (G6Pase), as well as its stimulatory effect on the expression of glucokinase (Fig. 5A–C). In addition to these glucoregulatory genes, we also checked three genes that have previously been associated with the development of hepatic insulin resistance (25). However, the BIC-induced activation of orexin neurons had no significant effects on the expression level of either tumor necrosis factor-α (TNFα), interleukin-6 (IL-6), or suppressor of cytokine signaling (SOCS)-3 (Fig. 5D–F).
FIG. 5.
Effects of BIC and hyperinsulinemia on the hepatic expression level of PEPCK, G6Pase, glucokinase, TNF-α, IL-6, and SOCS-3 mRNA. BIC administration in the perifornical orexin area counteracts the effects of hyperinsulinemia on the hepatic expression level of the PEPCK, G6Pase, and glucokinase genes and has no effects on that of the TNFα, IL-6, and SOCS-3 genes. □, Ringer's control; ■, BIC groups. Data are presented as means ± SE. #P < 0.05 vs. Ringer's; *P < 005 vs. nonclamp; ∧P < 0.05 vs. clamp 1.
DISCUSSION
Plasma glucose concentrations are kept within a narrow physiological range by dynamically balancing glucose production and utilization to avoid hypoglycemia and hyperglycemia and to guarantee substrate availability for energy production. Disturbances in the regulation of glucose metabolism can result in insulin resistance and type 2 diabetes. Accumulated animal data of recent years indicate that derangements of hypothalamic neural networks may act as a critical factor responsible for an unbalanced glucose metabolism (26). The present study identifies orexin as one of the critical players in such a glucoregulatory hypothalamic network. Activation of orexin neurons in the hypothalamic perifornical area increases blood glucose concentrations via a stimulation of EGP. For the stimulatory effect of orexin on glucose production to become apparent, an intact autonomic outflow from brain to liver via the hepatic sympathetic innervation is essential.
DMH and PVN have both been defined as being part of the endocrine and autonomic hypothalamic output network, with the general concept that the PVN functions as the hypothalamic integrating center for autonomic and endocrine information and serves as the final neuroendocrine and autonomic output nucleus from the hypothalamus (27), whereas the DMH has a more specific role in controlling the stress response and circadian rhythms of sleep (28). In comparison with the PVN, the separation between DMH and PF-Oa is less obvious. The rat DMH is a large complex nucleus, with its dorsomedial and ventrolateral parts divided by a compact central zone. Unlike the PVN, the different divisions of the DMH do not show a clear neurochemical separation. For instance, a large part of the PF orexin neurons extends into the ventrolateral DMH. In fact, in some (lesion) studies the DMH was considered as one area, often including part of the PF-Oa (29). In the present study, the glucoregulatory effects of BIC markedly differed between PF-Oa, dDMH, and PVN with respect to its effects on EGP and peripheral glucose uptake. Removal of the GABAergic inhibition clearly separated the PF-Oa, dDMH, and PVN.
Within the PF-Oa area, the distribution of orexin and MCH neurons strongly overlaps, without any colocalization. MCH and orexin both have been characterized as orexigenic peptides (30), and both orexin and MCH neurons receive GABAergic inputs (31), as well as being sensitive to other metabolic inputs such as leptin (32), NPY/agouti-related peptide, and proopiomelanocortin (33,34). Nevertheless, our and other studies (13) show that during the sleep period only orexin, but not MCH, neurons can be activated by removal of the GABAergic inhibition, indicating that the stimulatory effect of BIC on EGP probably involves activation of orexin, but not MCH, neurons. Indeed, intracerebroventricular orexin, but not intracerebroventricular MCH, was able to affect EGP, and intracerebroventricular administration of the orexin antagonist blocked a major part of the EGP stimulatory effect of BIC. Together, these data indicate that in the PF-Oa area, orexin neurons play a prominent role in the control of EGP.
Both neuronal and hormonal factors have been considered to relay the orexin signal to the periphery. Central administration of orexin can activate the hypothalamo-pituitary-adrenal axis and consequently increase plasma corticosterone levels (35). Corticosterone is able to stimulate hepatic glucose production, but in the present study EGP responses to BIC were site specific, whereas the corticosterone responses were not, suggesting that corticosterone is not the major factor responsible for the increase in EGP. In addition, also an altered pancreatic release of glucagon does not seem to be the determining factor. An alternative pathway for relaying orexin signaling to the liver is via the autonomic nervous system. Indeed, orexin can influence liver function via a multilevel sympathetic output pathway (36). Previous studies (14,21) have already shown that denervation of the sympathetic input to the liver abolishes a hypothalamic induced increase in EGP. Also in line with other studies (37,38), a purely hormonal effect of orexin on EGP is therefore not very likely. In the present study, we showed that central administration of orexin-A can only affect EGP when an intact sympathetic innervation of the liver is present, indicating a stimulatory effect of central orexin on sympathetic neuronal outflow. Remarkably, the sympathetic denervation of the liver did not prevent the stimulatory effect of intracerebroventricular orexin-A on plasma glucose concentrations. The separation of the plasma glucose and EGP responses indicates that increased central orexin signaling might affect plasma glucose concentrations not only through changes in the hepatic glucose production but also by affecting peripheral glucose uptake. A central control of peripheral glucose uptake, mediated by the autonomic nervous system, is also supported by previous studies showing that the increased glucose uptake in skeletal muscle and brown adipose tissue after the central administration of N-methyl-D-aspartate and leptin is abolished by sympathetic denervation (39,40). Since plasma insulin is not affected by orexin-A administration and hepatic sympathetic denervation (14), and our experiments were performed under fasting condition, the effect on glucose in experiment 4 seems to be mediated by a change in non–insulin-dependent glucose uptake.
The GABAergic input to the orexin neurons is timed by the central biological clock located in the SCN, with the most prominent inhibition occurring during the sleep period (13,41). This timed GABA input probably is responsible for switching the orexin activity on and off in its target areas, thereby controlling the sleep/wake cycle (42,43). Since the SCN not only times glucose metabolism (44) but also controls sympathetic preautonomic neurons (45), very possibly the SCN utilizes GABA as a timing signal to control orexin activity and consequently influence sympathetic outflow and regulate glucose metabolism. On the other hand, it is well accepted that insulin signaling in the arcuate AGRP/NPY neurons accounts for ∼40% of the insulin-mediated suppression of EGP via the autonomic output to liver (21,46,47). Therefore, it is possible that removal of GABA inhibition at the level of the PF-Oa could also block central insulin signaling by antagonizing the arcuate NPY/GABA projection to the orexin neurons or by activating the orexinergic projection to the arcuate NPY neurons (counteracting the suppressive effect of insulin on NPY neurons) (21,23,48). However, most of our experiments were performed under basal insulin conditions, making the contribution of such a pathway less prominent. Nevertheless, the present data support the conclusion from other studies using the orexin knockout mice model by showing that orexin is indeed an essential factor for maintaining hypothalamic insulin sensitivity for glucose metabolism (49). However, at which hypothalamic area and under which situation this interaction takes place needs further investigation.
In conclusion, we identified orexin as an important hypothalamic regulator of glucose homeostasis. The hypothalamic orexin system is possibly involved in incorporating timing information from the master brain clock into the control mechanism of EGP and also provides a possible molecular explanation for the previously observed correlation between short sleep duration and an increased risk for insulin resistance (50). The present results show that the inappropriate activation of orexin neurons during sleep deprivation will induce an increase in basal EGP and a reduction in hepatic insulin sensitivity.
Supplementary Material
Acknowledgments
This work was supported by a grant from the Dutch Diabetes Fonds (2004.00.027).
No potential conflicts of interest relevant to this article were reported.
We thank Wilma Verweij for her help with correcting the English, An Ruiter for her technical assistance, and Jilles Timmer for the husbandry of the experimental animals.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Yamanaka A, Beuckmann CT, Willie JT, Hara J, Tsujino N, Mieda M, Tominaga M, Yagami K, Sugiyama F, Goto K, Yanagisawa M, Sakurai T: Hypothalamic orexin neurons regulate arousal according to energy balance in mice. Neuron 2003; 38: 701– 713 [DOI] [PubMed] [Google Scholar]
- 2.Hara J, Beuckmann CT, Nambu T, Willie JT, Chemelli RM, Sinton CM, Sugiyama F, Yagami K, Goto K, Yanagisawa M, Sakurai T: Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron 2001; 30: 345– 354 [DOI] [PubMed] [Google Scholar]
- 3.Kok SW, Overeem S, Visscher TL, Lammers GJ, Seidell JC, Pijl H, Meinders AE: Hypocretin deficiency in narcoleptic humans is associated with abdominal obesity. Obes Res 2003; 11: 1147– 1154 [DOI] [PubMed] [Google Scholar]
- 4.Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M: Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 1998; 92: 573– 585 [DOI] [PubMed] [Google Scholar]
- 5.Cai XJ, Evans ML, Lister CA, Leslie RA, Arch JR, Wilson S, Williams G: Hypoglycemia activates orexin neurons and selectively increases hypothalamic orexin-B levels: responses inhibited by feeding and possibly mediated by the nucleus of the solitary tract. Diabetes 2001; 50: 105– 112 [DOI] [PubMed] [Google Scholar]
- 6.Zhang S, Zeitzer JM, Sakurai T, Nishino S, Mignot E: Sleep/wake fragmentation disrupts metabolism in a mouse model of narcolepsy. J Physiol 2007; 581: 649– 663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nowak KW, Mackowiak P, Switonska MM, Fabis M, Malendowicz LK: Acute orexin effects on insulin secretion in the rat: in vivo and in vitro studies. Life Sci 2000; 66: 449– 454 [DOI] [PubMed] [Google Scholar]
- 8.Matsumura K, Tsuchihashi T, Abe I: Central orexin-A augments sympathoadrenal outflow in conscious rabbits. Hypertension 2001; 37: 1382– 1387 [DOI] [PubMed] [Google Scholar]
- 9.Horvath TL, Gao XB: Input organization and plasticity of hypocretin neurons: possible clues to obesity's association with insomnia. Cell Metab 2005; 1: 279– 286 [DOI] [PubMed] [Google Scholar]
- 10.Henny P, Jones BE: Innervation of orexin/hypocretin neurons by GABAergic, glutamatergic or cholinergic basal forebrain terminals evidenced by immunostaining for presynaptic vesicular transporter and postsynaptic scaffolding proteins. J Comp Neurol 2006; 499: 645– 661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sakurai T, Nagata R, Yamanaka A, Kawamura H, Tsujino N, Muraki Y, Kageyama H, Kunita S, Takahashi S, Goto K, Koyama Y, Shioda S, Yanagisawa M: Input of orexin/hypocretin neurons revealed by a genetically encoded tracer in mice. Neuron 2005; 46: 297– 308 [DOI] [PubMed] [Google Scholar]
- 12.Kalsbeek A, Palm IF, La Fleur SE, Scheer FA, Perreau-Lenz S, Ruiter M, Kreier F, Cailotto C, Buijs RM: SCN outputs and the hypothalamic balance of life. J Biol Rhythms 2006; 21: 458– 469 [DOI] [PubMed] [Google Scholar]
- 13.Alam MN, Kumar S, Bashir T, Suntsova N, Methippara MM, Szymusiak R, McGinty D: GABA-mediated control of hypocretin- but not melanin-concentrating hormone-immunoreactive neurones during sleep in rats. J Physiol 2005; 563: 569– 582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalsbeek A, La Fleur S, Van Heijningen C, Buijs RM: Suprachiasmatic GABAergic inputs to the paraventricular nucleus control plasma glucose concentrations in the rat via sympathetic innervation of the liver. J Neurosci 2004; 24: 7604– 7613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lang CH: Inhibition of central GABAA receptors enhances hepatic glucose production and peripheral glucose uptake. Brain Res Bull 1995; 37: 611– 616 [DOI] [PubMed] [Google Scholar]
- 16.Nonogaki K, Iguchi A, Sakamoto N: Bicuculline methiodide influences the central nervous system to produce hyperglycemia in rats. J Neuroendocrinol 1994; 6: 443– 446 [DOI] [PubMed] [Google Scholar]
- 17.Paxinos G, Watson C: The Rat Brain in Stereotaxic Coordinates San Diego, CA, Academic Press, 1998 [Google Scholar]
- 18.Klieverik LP, Sauerwein HP, Ackermans MT, Boelen A, Kalsbeek A, Fliers E: Effects of thyrotoxicosis and selective hepatic autonomic denervation on hepatic glucose metabolism in rats. Am J Physiol Endocrinol Metab 2008; 294: E513– E520 [DOI] [PubMed] [Google Scholar]
- 19.Porter RA, Chan WN, Coulton S, Johns A, Hadley MS, Widdowson K, Jerman JC, Brough SJ, Coldwell M, Smart D, Jewitt F, Jeffrey P, Austin N: 1,3-Biarylureas as selective non-peptide antagonists of the orexin-1 receptor. Bioorg Med Chem Lett 2001; 11: 1907– 1910 [DOI] [PubMed] [Google Scholar]
- 20.Kushikata T, Hirota K, Yoshida H, Kudo M, Lambert DG, Smart D, Jerman JC, Matsuki A: Orexinergic neurons and barbiturate anesthesia. Neuroscience 2003; 121: 855– 863 [DOI] [PubMed] [Google Scholar]
- 21.van den Hoek AM, Van Heijningen C, Schro-van der Elst JP, Ouwens DM, Havekes LM, Romijn JA, Kalsbeek A, Pijl H: Intracerebroventricular administration of neuropeptide Y induces hepatic insulin resistance via sympathetic innervation. Diabetes 2008; 57: 2304– 2310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van den Pol AN: Hypothalamic hypocretin (orexin): robust innervation of the spinal cord. J Neurosci 1999; 19: 3171– 3182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horvath TL, Diano S, van den Pol AN: Synaptic interaction between hypocretin (orexin) and neuropeptide Y cells in the rodent and primate hypothalamus: a novel circuit implicated in metabolic and endocrine regulations. J Neurosci 1999; 19: 1072– 1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muroya S, Funahashi H, Yamanaka A, Kohno D, Uramura K, Nambu T, Shibahara M, Kuramochi M, Takigawa M, Yanagisawa M, Sakurai T, Shioda S, Yada T: Orexins (hypocretins) directly interact with neuropeptide Y, POMC and glucose-responsive neurons to regulate Ca 2+ signaling in a reciprocal manner to leptin: orexigenic neuronal pathways in the mediobasal hypothalamus. Eur J Neurosci 2004; 19: 1524– 1534 [DOI] [PubMed] [Google Scholar]
- 25.Muse ED, Lam TK, Scherer PE, Rossetti L: Hypothalamic resistin induces hepatic insulin resistance. J Clin Invest 2007; 117: 1670– 1678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horvath TL: The hardship of obesity: a soft-wired hypothalamus. Nat Neurosci 2005; 8: 561– 565 [DOI] [PubMed] [Google Scholar]
- 27.Swanson LW, Sawchenko PE: Paraventricular nucleus: a site for the integration of neuroendocrine and autonomic mechanisms. Neuroendocrinology 1980; 31: 410– 417 [DOI] [PubMed] [Google Scholar]
- 28.Saper CB: Staying awake for dinner: hypothalamic integration of sleep, feeding, and circadian rhythms. Prog Brain Res 2006; 153: 243– 252 [DOI] [PubMed] [Google Scholar]
- 29.Bellinger LL, Bernardis LL: The dorsomedial hypothalamic nucleus and its role in ingestive behavior and body weight regulation: lessons learned from lesioning studies. Physiol Behav 2002; 76: 431– 442 [DOI] [PubMed] [Google Scholar]
- 30.Shimada M, Tritos NA, Lowell BB, Flier JS, Maratos-Flier E: Mice lacking melanin-concentrating hormone are hypophagic and lean. Nature 1998; 396: 670– 674 [DOI] [PubMed] [Google Scholar]
- 31.Backberg M, Ultenius C, Fritschy JM, Meister B: Cellular localization of GABA receptor alpha subunit immunoreactivity in the rat hypothalamus: relationship with neurones containing orexigenic or anorexigenic peptides. J Neuroendocrinol 2004; 16: 589– 604 [DOI] [PubMed] [Google Scholar]
- 32.Hakansson M, De Lecea L, Sutcliffe JG, Yanagisawa M, Meister B: Leptin receptor- and STAT3-immunoreactivities in hypocretin/orexin neurones of the lateral hypothalamus. J Neuroendocrinol 1999; 11: 653– 663 [DOI] [PubMed] [Google Scholar]
- 33.Broberger C, De Lecea L, Sutcliffe JG, Hokfelt T: Hypocretin/orexin- and melanin-concentrating hormone-expressing cells form distinct populations in the rodent lateral hypothalamus: relationship to the neuropeptide Y and agouti gene-related protein systems. J Comp Neurol 1998; 402: 460– 474 [PubMed] [Google Scholar]
- 34.Elias CF, Saper CB, Maratos-Flier E, Tritos NA, Lee C, Kelly J, Tatro JB, Hoffman GE, Ollmann MM, Barsh GS, Sakurai T, Yanagisawa M, Elmquist JK: Chemically defined projections linking the mediobasal hypothalamus and the lateral hypothalamic area. J Comp Neurol 1998; 402: 442– 459 [PubMed] [Google Scholar]
- 35.Kuru M, Ueta Y, Serino R, Nakazato M, Yamamoto Y, Shibuya I, Yamashita H: Centrally administered orexin/hypocretin activates HPA axis in rats. Neuroreport 2000; 11: 1977– 1980 [DOI] [PubMed] [Google Scholar]
- 36.van den Top M, Nolan MF, Lee K, Richardson PJ, Buijs RM, Davies CH, Spanswick D: Orexins induce increased excitability and synchronisation of rat sympathetic preganglionic neurones. J Physiol 2003; 549: 809– 821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chiasson JL, Shikama H, Chu DT, Exton JH: Inhibitory effect of epinephrine on insulin-stimulated glucose uptake by rat skeletal muscle. J Clin Invest 1981; 68: 706– 713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rizza R, Haymond M, Cryer P, Gerich J: Differential effects of epinephrine on glucose production and disposal in man. Am J Physiol 1979; 237: E356– E362 [DOI] [PubMed] [Google Scholar]
- 39.Lang CH, Ajmal M, Baillie AG: Neural control of glucose uptake by skeletal muscle after central administration of NMDA. Am J Physiol 1995; 268: R492– R497 [DOI] [PubMed] [Google Scholar]
- 40.Sudo M, Minokoshi Y, Shimazu T: Ventromedial hypothalamic stimulation enhances peripheral glucose uptake in anesthetized rats. Am J Physiol 1991; 261: E298– E303 [DOI] [PubMed] [Google Scholar]
- 41.Estabrooke IV, McCarthy MT, Ko E, Chou TC, Chemelli RM, Yanagisawa M, Saper CB, Scammell TE: Fos expression in orexin neurons varies with behavioral state. J Neurosci 2001; 21: 1656– 1662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Deboer T, Overeem S, Visser NA, Duindam H, Frolich M, Lammers GJ, Meijer JH: Convergence of circadian and sleep regulatory mechanisms on hypocretin-1. Neuroscience 2004; 129: 727– 732 [DOI] [PubMed] [Google Scholar]
- 43.Taheri S, Sunter D, Dakin C, Moyes S, Seal L, Gardiner J, Rossi M, Ghatei M, Bloom S: Diurnal variation in orexin A immunoreactivity and prepro-orexin mRNA in the rat central nervous system. Neurosci Lett 2000; 279: 109– 112 [DOI] [PubMed] [Google Scholar]
- 44.La Fleur SE, Kalsbeek A, Wortel J, Buijs RM: A suprachiasmatic nucleus generated rhythm in basal glucose concentrations. J Neuroendocrinol 1999; 11: 643– 652 [DOI] [PubMed] [Google Scholar]
- 45.Kalsbeek A, Foppen E, Schalij I, Van HC, van d, V Fliers E, Buijs RM: Circadian control of the daily plasma glucose rhythm: an interplay of GABA and glutamate. PLoS ONE 2008; 3: e3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Konner AC, Janoschek R, Plum L, Jordan SD, Rother E, Ma X, Xu C, Enriori P, Hampel B, Barsh GS, Kahn CR, Cowley MA, Ashcroft FM, Bruning JC: Insulin Action in AgRP-Expressing Neurons Is Required for Suppression of Hepatic Glucose Production. Cell Metab 2007; 5: 438– 449 [DOI] [PubMed] [Google Scholar]
- 47.Pocai A, Lam TK, Gutierrez-Juarez R, Obici S, Schwartz GJ, Bryan J, Aguilar-Bryan L, Rossetti L: Hypothalamic K(ATP) channels control hepatic glucose production. Nature 2005; 434: 1026– 1031 [DOI] [PubMed] [Google Scholar]
- 48.Sakurai T: Orexins and orexin receptors: implication in feeding behavior. Regul Pept 1999; 85: 25– 30 [DOI] [PubMed] [Google Scholar]
- 49.Tsuneki H, Murata S, Anzawa Y, Soeda Y, Tokai E, Wada T, Kimura I, Yanagisawa M, Sakurai T, Sasaoka T: Age-related insulin resistance in hypothalamus and peripheral tissues of orexin knockout mice. Diabetologia 2008; 51: 657– 667 [DOI] [PubMed] [Google Scholar]
- 50.Van Cauter E, Holmback U, Knutson K, Leproult R, Miller A, Nedeltcheva A, Pannain S, Penev P, Tasali E, Spiegel K: Impact of sleep and sleep loss on neuroendocrine and metabolic function. Horm Res 2007; 67( Suppl. 1): 2– 9 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.