Abstract
OBJECTIVE
The aim of this analysis was to assess the prospective association of serum testosterone and dehydroepiandrosterone sulfate (DHEAS) levels with incident metabolic syndrome (MetS) in men.
RESEARCH DESIGN AND METHODS
Data were obtained from the Study of Health in Pomerania (SHIP), a population-based prospective cohort of adults aged 20–79 years. Analyses were conducted in 1,004 men without baseline MetS defined by National Cholesterol Education Program Adult Treatment Panel III guidelines. Testosterone and DHEAS were categorized by age-specific quartiles and Poisson regression models with relative risks (RRs) and 95% CIs were estimated.
RESULTS
After a median follow-up time of 5.0 years, 480 men (47.8%) developed MetS. Testosterone levels decreased with increasing number of MetS components. Testosterone in the lowest quartile predicted MetS (RR 1.38 [95% CI 1.13–1.69]), particularly among men aged 20–39 years (2.06 [1.29–3.29]), even after adjustment for age, smoking, alcohol consumption, physical activity, waist circumference, self-related health, and time of blood sampling. DHEAS levels were not related to incident MetS (0.99 [0.83–1.19]).
CONCLUSIONS
Low testosterone but not DHEAS predicts development of MetS in a population-based cohort of 1,004 men aged 20–79 years. Especially in young men aged 20–39 years, results suggest low testosterone as a strong predictor for incident MetS. Assessment of testosterone in young and middle-age men may allow early interventions in the general population.
In men, a decline in serum total testosterone and adrenal androgens like dehydroepiandrosterone sulfate (DHEAS) with increasing age is well documented (1) and has been linked to a variety of physiological changes including abdominal obesity, insulin resistance, diabetes, and cardiovascular disease (CVD) (1,2). The metabolic syndrome (MetS), a concept of clustered metabolic disorders, was established to identify subjects with increased risk of developing CVD end points. Previous findings suggest that low serum testosterone might be directly associated with MetS, in both cross-sectional (2,3) and longitudinal studies (4) and consistent across race and ethnic groups (5). These studies, however, are somewhat limited by cross-sectional design (2,3,5), definition of MetS without adherence to Adult Treatment Panel III (ATP III) guidelines (6), or study population's age structure (>40 years of age) (2–4). Low DHEAS levels are associated with impaired glucose tolerance and insulin resistance (7). The aim of the present analysis was to investigate the prospective association of testosterone and DHEAS levels with incident MetS in a large population-based sample of 1,004 men aged 20–79 years.
RESEARCH DESIGN AND METHODS
Data from the Study of Health in Pomerania (SHIP) were used (8,9). The target population was adult German residents of West Pomerania in northeastern Germany. From 2,117 male baseline participants (response 69%), 1,589 were repeatedly examined (response 83.6%). Men not followed-up (n = 528) or with baseline MetS (n = 450) were excluded. Another 77 men who used opiates (anatomic-therapeutical-chemical [ATC] code N02AA, A07DA02, R05DA, and R05FA0; n = 11), glucocorticoids (ATC code R03BA, n = 31; H02AB, n = 28), sexual hormones (ATC code G03; n = 2), testosterone 5α reductase inhibitors (ATC code G04CB; n = 4), or sexual hormone antagonists (ATC code L02B; n = 1) were also excluded. Among the remaining 1,062 men, 1 man with testosterone >55.5 nmol/l (>1,599.5 ng/dl), 9 men with testosterone <0.69 nmol/l (<19.9 ng/dl), and a further 48 men with missing data on testosterone, DHEAS, or confounders were excluded. Thus, the final study population comprised 1,004 men.
Sociodemographic and behavioral characteristics were assessed by computer-assisted personal interviews. Mean daily alcohol consumption was calculated using beverage-specific pure ethanol volume proportions (10). Riskful alcohol consumption was classified as >30 g alcohol/day. Smoking habits were assessed by dividing men into categories of current, former, and never smokers. Men who participated in physical training during summer or winter for at least 1 h/week were classified as being physically active. Self-related health was assessed by the single-item question: “Over the last 12 months, would you say your health has been very good, good, fair, poor, or very poor?” The definition of diabetes was based on self-reported physicians diagnosis or self-reported use of antidiabetic medication (ATC code A10). Waist circumference was measured to the nearest 0.1 cm using an inelastic tape midway between the lower rib margin and the iliac crest in the horizontal plane with the subject standing comfortably with weight distributed evenly on both feet. Height was measured to the nearest 1 cm using a digital ultrasound instrument, and weight was measured to the nearest 0.1 kg in light clothing and without shoes using standard digital scales (Soehnle-Waagen, Nassau, Germany). BMI was calculated as weight in kilograms divided by the square of height in meters. After a 5-min rest period, systolic and diastolic blood pressure was measured three times in the right arm of seated subjects using a digital blood pressure monitor (HEM-705CP; Omron, Tokyo, Japan) with each reading being followed by a further rest period of 3 min. The last two readings were averaged to give the mean diastolic and systolic blood pressure. Hypertension was defined as elevated mean systolic (≥130 mmHg) and diastolic (≥85 mmHg) blood pressure or use of antihypertensive medication (ATC code C02). MetS was defined by any three or more of the five components proposed by ATP III (6) and recently updated with minor modifications by the American Heart Association (AHA) and the National Heart, Lung, and Blood Institute (NHLBI) (11) and were modified for the use of nonfasting blood samples (12): 1) abdominal obesity, waist circumference ≥94 cm in men; 2) elevated triglycerides, ≥2.0 mmol/l or lipid medication (ATC code C10ab); 3) low HDL cholesterol, <1.03 mmol/l in men; 4) high blood pressure, ≥130/85 mmHg or antihypertensive medication (ATC code C02); 5) high blood glucose, ≥8.0 mmol/or diabetes medication (ATC code A10).
Nonfasting blood samples were drawn from the cubital vein in the supine position. The samples were taken between 7:00 a.m. and 04:00 p.m., and serum aliquots were prepared for immediate analysis and for storage at −80°C for further analysis. Testosterone and DHEAS levels were measured from frozen serum aliquots using competitive chemiluminescent enzyme immunoassays on an Immulite 2500 analyzer (Siemens Healthcare Medical Diagnostics, Bad Nauheim, Germany) (13). Baseline total and HDL cholesterol were measured photometrically (Hitachi 704; Roche, Mannheim, Germany). Follow-up HDL cholesterol was quantified by lipid electrophoresis (HELENA SAS-3 system; Helena 7 BioSciences Europe, Tyne & Wear, U.K.). To ensure comparability, we applied a previously published conversion factor (14) with virtually no differences in the estimates (data not shown). Dyslipidemia was defined by ratio of total to HDL cholesterol ≥5.0 mmol/l (15). Triglycerides and glucose were determined enzymatically using reagents from Roche Diagnostics (Hitachi 717; Roche Diagnostics, Mannheim, Germany).
Statistical Analysis.
Descriptive statistics, proportions for categorical variables, and means ± SD or medians (interquartile range) for continuous variables were used to describe the study population. Univariate statistics were performed with χ2 testing for categorical variables and Wilcoxon test for continuous variables. Hormone levels were assessed by age-specific (decades) quartiles. Effects were estimated using generalized linear models with Poisson distribution, log link function, and robust error variances presented in relative risks (RRs) and 95% CIs. Adjustments included age, smoking habits, alcohol consumption, physical activity, waist circumference, self-related health, and blood sampling time. The P value for trend was calculated to test for linearity of RRs. Receiver operating characteristic analysis was evaluated if the measurement of testosterone added significantly to the prediction of MetS by standard risk factors. All P values were two tailed, and P < 0.05 was considered statistically significant. All analyses were performed with Stata 9.0 (Stata, College Station, TX).
RESULTS
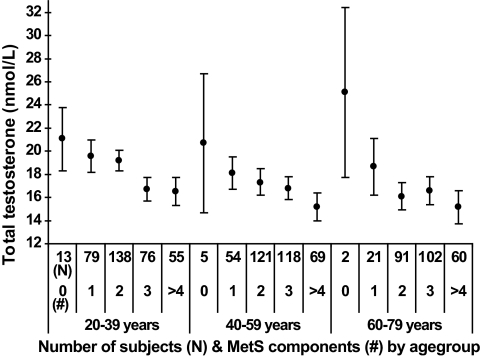
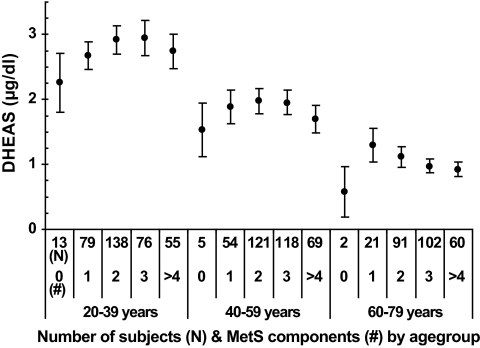
After a median follow-up time of 5.0 years (range 4.4–8.3), 480 men (47.8%) developed MetS. The analytical sample appeared to be healthier than the full sample, whereas men with incident MetS were significantly older, exposed to lower testosterone and DHEAS levels, scored worse for self-related health, were physically less active showed higher body fat accumulation, and exposed significant differences in all MetS components than men without incident MetS (Table 1). We detected an overall trend of increasing risk of incident MetS with decreasing levels of both testosterone and DHEAS in unadjusted analyses (Table 2). The risk of incident MetS was highest for men with baseline testosterone and DHEAS levels in the lowest quartile (unadjusted RR 1.52 [95% CI 1.25–1.85]) and 1.38 [1.16–1.65], respectively) than the highest quartile. P for trend statistics revealed that RRs were also linearly elevated (P for trend < 0.001). Stratifying analyses by 20-year age-groups revealed that in particular, young (20–39 years) (2.50 [1.58–3.96]) and middle-aged (40–59 years) (1.41 [1.06–1.89]) men were exposed to elevated risk of incident MetS from low baseline testosterone. Further adjustment for age, smoking, alcohol consumption, physical activity, waist circumference, self-related health, and time of blood sampling confirmed the prospective association between low testosterone and incident MetS, whereas the association between low DHEAS and MetS was no longer consistent (Table 2). Adjusted RRs for lowest compared with highest quartile of testosterone by 20-year age-groups was 2.06 (95% CI 1.29–3.29) for men aged 20–39 years, 1.34 (1.00–1.81) for men aged 40–59 years, and 1.00 (0.75–1.37) for men aged 60–79 years. In receiver operating characteristic analysis, the inclusion of continuous testosterone in the multivariable basic model improved prediction of incident MetS significantly (P < 0.001). Furthermore, a distinct trend of decreasing testosterone with increasing number of incident MetS components was observed in all three age-groups (Fig. 1). With regard to DHEAS, Fig. 2 displayed an inverted “U” shape over increasing number of MetS components.
TABLE 1.
Baseline characteristics of men by full and analytical sample
| Full sample | Analytical sample | Without MetS at follow-up | Incident MetS at follow-up | |
|---|---|---|---|---|
| n | 2,117 | 1,004 | 524 | 480 |
| Age (years) | 51.3 ± 16.6 | 48.7 ± 15.9* | 45.6 ± 15.7 | 51.9 ± 15.5* |
| Total testosterone (nmol/l) | 15.9 (12.7–20.1) | 16.6 (13.4–20.6)* | 17.7 (14.4–21.5) | 15.5 (12.6–19.1)* |
| DHEAS (μg/dl) | 1.63 (0.96–2.56) | 1.74 (1.09–2.69)* | 1.98 (1.25–2.83) | 1.58 (0.94–2.52)* |
| Daily alcohol consumption (g/day) | 11.9 (1.5–28.2) | 13.6 (2.5–28.9) | 16.5 (2.7–30.3) | 10.0 (0.0–27.2)* |
| Riskful alcohol consumption | 23.0 | 23.9 | 25.2 | 22.5 |
| Self-related health | ||||
| Very good | 2.1 | 2.3* | 2.5 | 2.1* |
| Good | 15.4 | 19.1* | 21.4 | 16.7* |
| Fair | 64.0 | 64.6* | 66.4 | 62.7* |
| Poor/very poor | 18.4 | 13.9* | 9.7 | 18.5* |
| Smoking | ||||
| never smoker | 21.0 | 24.2* | 26.5 | 21.7 |
| ex-smoker | 45.3 | 42.2* | 39.3 | 45.4 |
| current smoker | 33.7 | 33.6* | 34.2 | 32.9 |
| Physical activity | 41.0 | 46.7* | 50.4 | 42.7* |
| BMI (kg/m2) | 27.6 ± 4.0 | 26.4 ± 3.3* | 25.5 ± 3.2 | 27.4 ± 3.0* |
| Waist circumference (cm) | 102.0 (97.6–107.2) | 100.3 (96.4–104.3)* | 98.8 (94.8–103.3) | 101.9 (98.3–105.7)* |
| Systolic blood pressure (mmHg) | 143.6 ± 19.5 | 139.2 ± 17.9* | 134.6 ± 16.3 | 141.2 ± 17.8* |
| Diastolic blood pressure (mmHg) | 86.3 ± 11.3 | 85.0 ± 10.9* | 83.0 ± 10.8 | 86.8 ± 10.4* |
| Hypertension | 62.4 | 59.8* | 56.1 | 63.8 |
| Glucose (mmol/l) | 5.9 ± 1.9 | 5.4 ± 1.3* | 5.2 ± 0.63 | 5.6 ± 1.7* |
| Triglycerides (mmol/l) | 2.13 ± 1.61 | 1.77 ± 1.42* | 1.41 ± 0.83 | 2.12 ± 1.80 |
| HDL cholesterol (mmol/l) | 1.30 ± 0.37 | 1.38 ± 0.34* | 1.49 ± 0.35 | 1.28 ± 0.28 |
| Diabetes | 8.8 | 7.2* | 6.7 | 7.7 |
| Dyslipidemia | 62.0 | 56.5* | 33.4 | 81.7 |
Data are percentages, means ± SD, or medians (interquartile range). To convert the values of serum testosterone to ng/dl multiply by 28.82. To convert the values of serum DHEAS to μmol/l multiply by 0.027.
*P < 0.05 using χ2 test (nominal data) and Wilcoxon test (continuous data) for bivariate comparisons between analytical sample and full sample as well as between subjects with and without metabolic syndrome at follow-up, respectively.
TABLE 2.
Poisson regression models for the association of serum total testosterone and DHEAS levels at baseline with incident MetS at follow-up
| Unadjusted RR (95% CI) |
Adjusted RR (95% CI)* |
|||||||
|---|---|---|---|---|---|---|---|---|
| All men | 20–39 years of age | 40–59 years of age | 60–79 years of age | All men | 20–39 years of age | 40–59 years of age | 60–79 years of age | |
| Quartiles of total total testosterone | n = 1,004 | 361 | 367 | 276 | 1,004 | 361 | 367 | 276 |
| <25th | 1.52 (1.25–1.85)§ | 2.50 (1.58–3.96)§ | 1.41 (1.06–1.89)† | 1.15 (0.86–1.54) | 1.38 (1.13–1.69)‡ | 2.06 (1.29–3.29)‡ | 1.34 (1.00–1.81)† | 1.00 (0.75–1.37) |
| 25–50th | 1.41 (1.15–1.72)‡ | 2.20 (1.38–3.53)‡ | 1.30 (0.96–1.75) | 1.13 (0.84–1.52) | 1.43 (1.18–1.75)§ | 2.05 (1.27–3.31)‡ | 1.31 (0.96–1.78) | 1.00 (0.74–1.35) |
| 50–75th | 1.14 (0.92–1.42) | 1.38 (0.82–2.34) | 1.04 (0.75–1.45) | 1.15 (0.86–1.54) | 1.10 (0.89–1.36) | 1.27 (0.77–2.11) | 1.03 (0.74–1.43) | 1.10 (0.83–1.48) |
| >75th | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) |
| P for trend | <0.001 | <0.001 | 0.020 | 0.397 | <0.001 | <0.001 | 0.041 | 0.912 |
| Quartiles of DHEAS | ||||||||
| <25th | 1.38 (1.16–1.65)§ | 0.89 (0.61–1.30) | 1.06 (0.79–1.40) | 1.20 (0.82–1.47) | 0.99 (0.83–1.19) | 0.82 (0.55–1.23) | 1.03 (0.75–1.40) | 1.13 (0.84–1.52) |
| 25–50th | 1.23 (1.02–1.47)† | 0.74 (0.49–1.11) | 1.09 (0.82–1.44) | 1.18 (0.88–1.59) | 1.01 (0.84–1.21) | 0.70 (0.47–1.04) | 1.09 (0.82–1.46) | 1.17 (0.87–1.57) |
| 50–75th | 0.98 (0.80–1.19) | 1.00 (0.70–1.43) | 0.98 (0.72–1.32) | 1.06 (0.77–1.46) | 1.02 (0.85–1.22) | 0.98 (0.70–1.38) | 0.96 (0.71–1.30) | 1.09 (0.80–1.50) |
| >75th | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) |
| P for trend | <0.001 | 0.588 | 0.470 | 0.018 | 0.812 | 0.317 | 0.536 | 0.053 |
*Adjusted for age, smoking, alcohol consumption, physical activity, waist circumference, self-related health, and blood sampling time.
†P < 0.05;
‡P < 0.01;
§P < 0.00.
FIG. 1.
Means with 95% CI in the analytical sample (n = 1,004) for total testosterone levels according to zero, one, two, three, four, or more components of MetS at baseline by 20-year age-groups. To convert the values of serum testosterone to ng/dl multiply by 28.82.
FIG. 2.
Means with 95% CI in the analytical sample (n = 1,004) for DHEAS levels according to zero, one, two, three, four, or more components of MetS at baseline by 20-year age-groups. To convert the values of serum DHEAS to μmol/l multiply by 0.027.
DISCUSSION
Our results from a population-based sample of 1,004 men aged 20–79 years revealed an inverse association between baseline testosterone and the development of MetS independent of important confounding factors. Especially in young men aged 20–39 years, low testosterone was a strong predictor for incident MetS. Baseline DHEAS did not show an independent prospective association with incident MetS. The association of low testosterone not only with components of MetS but also with MetS itself was previously reported from different cross-sectional studies in varying populations (2,3,5) as well as from longitudinal studies (4). Our finding of an independent prospective association of testosterone with MetS in young men has not been reported previously. Most remarkably, the association appeared to be stronger in young and middle-age men than in the elderly.
Although the temporal sequence of low testosterone preceding the development of MetS suggests a causal relationship between the two phenomena, we cannot prove whether low testosterone contributes to or is a very early consequence of mechanisms finally leading to MetS. There is evidence for both views because, on the one hand, weight loss in obese men and in men with MetS increased free and total testosterone as well as sex hormone–binding globulin levels (16) and, on the other hand, interventional studies with testosterone in men with low serum levels decreased fat mass, total cholesterol, and LDL cholesterol (17,18). Thus, the possibility exists that there is a vicious cycle between low testosterone and metabolic alterations leading to MetS and consequently contributing to more severe complications such as type 2 diabetes and CVD. Given reports of greatly increased MetS prevalence in hypogonadism from Klinefelter's syndrome with little effect of testosterone treatment on body composition (19) and higher cardiovascular risk among men undergoing long-term androgen-deprivation therapy (20), we recommend interventional studies of exogenous testosterone supplementation in men with MetS to further delineate the causal relationship of testosterone and MetS.
The present findings suggest that DHEAS is not associated with MetS. Although previous observational studies were able to detect an association of DHEAS and ischemic heart disease (21), the clinical significance of DHEAS in CVD remains uncertain (22). So, the possibilities to explain why in our study testosterone is a predictor of MetS whereas DHEAS is not are broad, ranging from differences in study population's age structure, time of blood sampling, or laboratory methodology. However, data from prospective randomized trials are needed to illuminate the basic physiological role of DHEAS in CVD and to clarify whether DHEAS supplementation has any cardiovascular benefit.
Limitations arise from the lack of measured free testosterone, sex hormone–binding globulin, or albumin for calculation of bioavailable testosterone as well as from single measurement of testosterone. Due to logistical impossibilities, we used nonfasting blood samples for the definition of MetS. However, because differences between fasting and nonfasting participants are reported to be negligible (23), fasting status is unlikely to cause associations. In summary, our results suggest that testosterone may serve as an early indicator for future metabolic risk. Therefore, further studies should examine whether testosterone supplementation protects against the development of MetS, especially in younger and middle-aged men.
Acknowledgments
SHIP is part of the Community Medicine Net (http://www.medizin.uni-greifswald.de/cm) of the University of Greifswald, which is funded by grants from the German Federal Ministry of Education and Research (BMBF, Grant 01ZZ0403); the Ministry for Education, Research, and Cultural Affairs; and the Ministry for Social Affairs of the Federal State of Mecklenburg–West Pomerania. The analyses were further supported by the Competence Network Diabetes of Germany Federal Ministry of Education and Research.
The authors disclose that the testosterone and DHEAS reagents used were sponsored by Siemens Healthcare Diagnostics, Eschborn, formerly DPC Biermann, Bad Nauheim, Germany. Novo Nordisk provided partial grant support for the determination of plasma samples and data analysis. No other potential conflicts of interest relevant to this article were reported.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Kaufman JM, Vermeulen A: The decline of androgen levels in elderly men and its clinical and therapeutic implications. Endocr Rev 2005; 26: 833– 876 [DOI] [PubMed] [Google Scholar]
- 2.Laaksonen DE, Niskanen L, Punnonen K, Nyyssonen K, Tuomainen TP, Salonen R, Rauramaa R, Salonen JT: Sex hormones, inflammation and the metabolic syndrome: a population-based study. Eur J Endocrinol 2003; 149: 601– 608 [DOI] [PubMed] [Google Scholar]
- 3.Muller M, Grobbee DE, den Tonkelaar I, Lamberts SW, van der Schouw YT: Endogenous sex hormones and metabolic syndrome in aging men. J Clin Endocrinol Metab 2005; 90: 2618– 2623 [DOI] [PubMed] [Google Scholar]
- 4.Laaksonen DE, Niskanen L, Punnonen K, Nyyssonen K, Tuomainen TP, Valkonen VP, Salonen R, Salonen JT: Testosterone and sex hormone–binding globulin predict the metabolic syndrome and diabetes in middle-aged men. Diabetes Care 2004; 27: 1036– 1041 [DOI] [PubMed] [Google Scholar]
- 5.Kupelian V, Hayes FJ, Link CL, Rosen R, McKinlay JB: Inverse association of testosterone and the metabolic syndrome in men is consistent across race and ethnic groups. J Clin Endocrinol Metab 2008; 93: 3403– 3410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Third report of the National Cholesterol Education Program (NCEP) Expert Panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation 2002; 106: 3143– 421 [PubMed] [Google Scholar]
- 7.Haffner SM, Valdez RA, Mykkanen L, Stern MP, Katz MS: Decreased testosterone and dehydroepiandrosterone sulfate concentrations are associated with increased insulin and glucose concentrations in nondiabetic men. Metabolism 1994; 43: 599– 603 [DOI] [PubMed] [Google Scholar]
- 8.Haring R, Alte D, Volzke H, Sauer S, Wallaschofski H, John U, Schmidt CO: Extended recruitment efforts minimize attrition but not necessarily bias. J Clin Epidemiol 2009; 62: 252– 260 [DOI] [PubMed] [Google Scholar]
- 9.John U, Greiner B, Hensel E, Ludemann J, Piek M, Sauer S, Adam C, Born G, Alte D, Greiser E, Haertel U, Hense HW, Haerting J, Willich S, Kessler C: Study of Health In Pomerania (SHIP): a health examination survey in an east German region: objectives and design. Soz Praventivmed 2001; 46: 186– 194 [DOI] [PubMed] [Google Scholar]
- 10.Alte D, Luedemann J, Rose HJ, John U: Laboratory markers carbohydrate-deficient transferrin, gamma-glutamyltransferase, and mean corpuscular volume are not useful as screening tools for high-risk drinking in the general population: results from the Study of Health in Pomerania (SHIP). Alcohol Clin Exp Res 2004; 28: 931– 940 [DOI] [PubMed] [Google Scholar]
- 11.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, Jr, Spertus JA, Costa F: Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Circulation 2005; 112: 2735– 2752 [DOI] [PubMed] [Google Scholar]
- 12.Lidfeldt J, Nyberg P, Nerbrand C, Samsioe G, Schersten B, Agardh CD: Socio-demographic and psychosocial factors are associated with features of the metabolic syndrome: the Women's Health in the Lund Area (WHILA) study. Diabetes Obes Metab 2003; 5: 106– 112 [DOI] [PubMed] [Google Scholar]
- 13.Friedrich N, Volzke H, Rosskopf D, Steveling A, Krebs A, Nauck M, Wallaschofski H: Reference ranges for serum dehydroepiandrosterone sulfate and testosterone in adult men. J Androl 2008; 29: 610– 617 [DOI] [PubMed] [Google Scholar]
- 14.Nauck M, Winkler K, Marz W, Wieland H: Quantitative determination of high-, low-, and very-low-density lipoproteins and lipoprotein(a) by agarose gel electrophoresis and enzymatic cholesterol staining. Clin Chem 1995; 41: 1761– 1767 [PubMed] [Google Scholar]
- 15.Koenig W, Meisinger C: Uric acid, type 2 diabetes, and cardiovascular diseases: fueling the common soil hypothesis? Clin Chem 2008; 54: 231– 233 [DOI] [PubMed] [Google Scholar]
- 16.Niskanen L, Laaksonen DE, Punnonen K, Mustajoki P, Kaukua J, Rissanen A: Changes in sex hormone-binding globulin and testosterone during weight loss and weight maintenance in abdominally obese men with the metabolic syndrome. Diabetes Obes Metab 2004; 6: 208– 215 [DOI] [PubMed] [Google Scholar]
- 17.Page ST, Amory JK, Bowman FD, Anawalt BD, Matsumoto AM, Bremner WJ, Tenover JL: Exogenous testosterone (T) alone or with finasteride increases physical performance, grip strength, and lean body mass in older men with low serum T. J Clin Endocrinol Metab 2005; 90: 1502– 1510 [DOI] [PubMed] [Google Scholar]
- 18.Saad F, Gooren L, Haider A, Yassin A: An exploratory study of the effects of 12 month administration of the novel long-acting testosterone undecanoate on measures of sexual function and the metabolic syndrome. Arch Androl 2007; 53: 353– 357 [DOI] [PubMed] [Google Scholar]
- 19.Bojesen A, Kristensen K, Birkebaek NH, Fedder J, Mosekilde L, Bennett P, Laurberg P, Frystyk J, Flyvbjerg A, Christiansen JS, Gravholt CH: The metabolic syndrome is frequent in Klinefelter's syndrome and is associated with abdominal obesity and hypogonadism. Diabetes Care 2006; 29: 1591– 1598 [DOI] [PubMed] [Google Scholar]
- 20.Braga-Basaria M, Dobs AS, Muller DC, Carducci MA, John M, Egan J, Basaria S: Metabolic syndrome in men with prostate cancer undergoing long-term androgen-deprivation therapy. J Clin Oncol 2006; 24: 3979– 3983 [DOI] [PubMed] [Google Scholar]
- 21.Feldman HA, Johannes CB, Araujo AB, Mohr BA, Longcope C, McKinlay JB: Low dehydroepiandrosterone and ischemic heart disease in middle-aged men: prospective results from the Massachusetts Male Aging Study. Am J Epidemiol 2001; 153: 79– 89 [DOI] [PubMed] [Google Scholar]
- 22.Thijs L, Fagard R, Forette F, Nawrot T, Staessen JA: Are low dehydroepiandrosterone sulphate levels predictive for cardiovascular diseases?: a review of prospective and retrospective studies. Acta Cardiol 2003; 58: 403– 410 [DOI] [PubMed] [Google Scholar]
- 23.Hildrum B, Mykletun A, Hole T, Midthjell K, Dahl AA: Age-specific prevalence of the metabolic syndrome defined by the International Diabetes Federation and the National Cholesterol Education Program: the Norwegian HUNT 2 study. BMC Public Health 2007; 7: 220. [DOI] [PMC free article] [PubMed] [Google Scholar]