Abstract
OBJECTIVE
We investigated the effects of 18 confirmed type 2 diabetes risk single nucleotide polymorphisms (SNPs) on insulin sensitivity, insulin secretion, and conversion of proinsulin to insulin.
RESEARCH DESIGN AND METHODS
A total of 5,327 nondiabetic men (age 58 ± 7 years, BMI 27.0 ± 3.8 kg/m2) from a large population-based cohort were included. Oral glucose tolerance tests and genotyping of SNPs in or near PPARG, KCNJ11, TCF7L2, SLC30A8, HHEX, LOC387761, CDKN2B, IGF2BP2, CDKAL1, HNF1B, WFS1, JAZF1, CDC123, TSPAN8, THADA, ADAMTS9, NOTCH2, KCNQ1, and MTNR1B were performed. HNF1B rs757210 was excluded because of failure to achieve Hardy-Weinberg equilibrium.
RESULTS
Six SNPs (TCF7L2, SLC30A8, HHEX, CDKN2B, CDKAL1, and MTNR1B) were significantly (P < 6.9 × 10−4) and two SNPs (KCNJ11 and IGF2BP2) were nominally (P < 0.05) associated with early-phase insulin release (InsAUC0–30/GluAUC0–30), adjusted for age, BMI, and insulin sensitivity (Matsuda ISI). Combined effects of these eight SNPs reached −32% reduction in InsAUC0–30/GluAUC0–30 in carriers of ≥11 vs. ≤3 weighted risk alleles. Four SNPs (SLC30A8, HHEX, CDKAL1, and TCF7L2) were significantly or nominally associated with indexes of proinsulin conversion. Three SNPs (KCNJ11, HHEX, and TSPAN8) were nominally associated with Matsuda ISI (adjusted for age and BMI). The effect of HHEX on Matsuda ISI became significant after additional adjustment for InsAUC0–30/GluAUC0–30. Nine SNPs did not show any associations with examined traits.
CONCLUSIONS
Eight type 2 diabetes–related loci were significantly or nominally associated with impaired early-phase insulin release. Effects of SLC30A8, HHEX, CDKAL1, and TCF7L2 on insulin release could be partially explained by impaired proinsulin conversion. HHEX might influence both insulin release and insulin sensitivity.
Impaired insulin secretion and insulin resistance, two main pathophysiological mechanisms leading to type 2 diabetes, have a significant genetic component (1). Recent studies have confirmed 20 genetic loci reproducibly associated with type 2 diabetes (2–13). Three were previously known (PPARG, KCNJ11, and TCF7L2), whereas 17 loci were recently discovered either by genome-wide association studies (SLC30A8, HHEX-IDE, LOC387761, CDKN2A/2B, IGF2BP2, CDKAL1, FTO, JAZF1, CDC123/CAMK1D, TSPAN8/LGR5, THADA, ADAMTS9, NOTCH2, KCNQ1, and MTNR1B), or candidate gene approach (WFS1 and HNF1B). The mechanisms by which these genes contribute to the development of type 2 diabetes are not fully understood.
PPARG is the only gene from the 20 confirmed loci previously associated with insulin sensitivity (14,15). Association with impaired β-cell function has been reported for 14 loci (KCNJ11, SLC30A8, HHEX-IDE, CDKN2A/2B, IGF2BP2, CDKAL1, TCF7L2, WFS1, HNF1B, JAZF1, CDC123/CAMK1D, TSPAN8/LGR5, KCNQ1, and MTNR1B) (6,12,13,16–38). Although associations of variants in HHEX (16–22), CDKAL1 (6,21–26), TCF7L2 (22,27–30), and MTNR1B (13,31,32) with impaired insulin secretion seem to be consistent across different studies, information concerning other genes is limited (12,18–25,27,33–38). The mechanisms by which variants in these genes affect insulin secretion are unknown. However, a few recent studies suggested that variants in TCF7L2 (22,39–42), SLC30A8 (22), CDKAL1 (22), and MTNR1B (31) might influence insulin secretion by affecting the conversion of proinsulin to insulin. Variants of FTO have been shown to confer risk for type 2 diabetes through their association with obesity (7,16) and therefore were not included in this study.
Large population-based studies can help to elucidate the underlying mechanisms by which single nucleotide polymorphisms (SNPs) of different risk genes predispose to type 2 diabetes. Therefore, we investigated confirmed type 2 diabetes–related loci for their associations with insulin sensitivity, insulin secretion, and conversion of proinsulin to insulin in a population-based sample of 5,327 nondiabetic Finnish men.
RESEARCH DESIGN AND METHODS
A total of 5,327 nondiabetic men from the ongoing population-based cross-sectional METSIM (Metabolic Syndrome in Men) study (10,26,43) were included in the study (age 58 ± 7 years, BMI 27.0 ± 3.8 kg/m2). Of these, 3,594 (68%) subjects had normal glucose tolerance, 884 (17%) had isolated impaired fasting glucose, 503 (9%) had isolated impaired glucose tolerance, and 346 (6%) had both impaired fasting glucose and impaired glucose tolerance. Subjects with type 2 diabetes (n = 898) were excluded from the analyses. Subjects aged from 45 to 70 years were randomly selected from the population register of Kuopio, Eastern Finland (population of 95,000) for the METSIM study. Every participant had a 1-day outpatient visit to the Clinical Research Unit at the University of Kuopio. Blood samples were drawn after 12 h of fasting followed by an oral glucose tolerance test (OGTT). The study was approved by the Ethics Committee of the University of Kuopio and Kuopio University Hospital and carried out in accordance with the Helsinki Declaration.
Clinical measurements.
Height and weight were measured to the nearest 0.5 cm and 0.1 kg, respectively. BMI was calculated as weight (killograms) divided by height (meters) squared.
OGTT.
A 2-h OGTT (75 g of glucose) was performed, with samples for plasma glucose, insulin, and proinsulin drawn at 0, 30, and 120 min. Glucose tolerance was evaluated according to the World Health Organization criteria (44).
Laboratory measurements.
Plasma glucose was measured by enzymatic hexokinase photometric assay (Konelab Systems Reagents; Thermo Fischer Scientific, Vantaa, Finland), insulin by immunoassay (ADVIA Centaur Insulin IRI, No. 02230141; Siemens Medical Solutions Diagnostics, Tarrytown, NY), and proinsulin by immunoassay (Human Proinsulin Ria kit; Linco Research, St. Charles, MO). Proinsulin data were available for 2,697 subjects.
Genotyping.
Genotyping of 19 SNPs was performed with the TaqMan Allelic Discrimination Assay (Applied Biosystems) (PPARG rs1801282, KCNJ11 rs5219, TCF7L2 rs7903146, SLC30A8 rs13266634, HHEX rs1111875, LOC387761 rs7480010, CDKN2B rs10811661, IGF2BP2 rs4402960, CDKAL1 rs7754840, HNF1B rs757210, WFS1 rs10010131, JAZF1 rs864745, CDC123 rs12779790, TSPAN8 rs7961581, THADA rs7578597, ADAMTS9 rs4607103, NOTCH2 rs10923931, KCNQ1 rs2283228), and Sequenom iPlex gold SBE (Sequenom) (MTNR1B rs10830963). TaqMan genotyping call rate was 100% and error rate 0% among 4.5% of DNA samples genotyped in duplicate. Sequenom iPlex call rate for MTNR1B rs10830963 was 96.8% and error rate 0% among 4.2% of DNA samples genotyped in duplicate. All SNPs were consistent with Hardy-Weinberg equilibrium (P > 0.05) except for HNF1B rs757210 (P < 0.0001). This SNP was omitted from all statistical analyses.
Calculations.
The trapezoidal method was used to calculate glucose, insulin, and proinsulin area under the curve (AUC) during OGTT. Early-phase insulin release (InsAUC0–30/GluAUC0–30) was calculated as the total insulin area under the curve divided by the total glucose area under the curve during the first 30 min of an OGTT. Matsuda index of insulin sensitivity (Matsuda ISI) was calculated as reported previously (45). In our previous validation study, InsAUC0–30/GluAUC0–30 had the highest correlation (r = 0.666) with the first-phase insulin secretion in an intravenous glucose tolerance test among 11 different indexes tested, and Matsuda ISI had the highest correlation with lean body mass adjusted M value from the euglycemic-hyperinsulinemic clamp (r = 0.776) among six different indexes tested (46). Four indexes of proinsulin conversion were calculated: proinsulin/insulin ratio in the fasting state (Proins0/Ins0), an index of proinsulin conversion to insulin during the first 30 min (ProinsAUC0–30/InsAUC0–30), 30–120 min (ProinsAUC30–120/InsAUC30–120), and 0–120 min (ProinsAUC0–120/InsAUC0–120) of an OGTT. All indexes of proinsulin conversion were multiplied by 100. All calculations were based on glucose, insulin, and proinsulin concentrations at 0, 30, and 120 min of an OGTT. Disposition index was calculated as InsAUC0–30/GluAUC0–30 × Matsuda ISI. To estimate a combined impact of multiple type 2 diabetes risk alleles (denoted as the risk allele throughout the text) on InsAUC0–30/GluAUC0–30 we calculated a genetic risk score as a sum of weighted risk alleles (47) at SNPs significantly or nominally associated with InsAUC0–30/GluAUC0–30 in initial analyses. For each subject, the number of risk alleles (0,1,2) per SNP was weighted for their effect sizes (shown in Table 1; average effect size per allele among eight SNPs was 1.58, which was considered as one weighted risk allele), and the sum of weighted alleles for each subject was rounded to closest integer. Subjects with ≤3 and ≥11 weighted risk alleles were pooled to obtain larger numbers.
TABLE 1.
Associations of 18 SNPs with early-phase insulin release (InsAUC0–30/GluAUC0–30), proinsulin conversion (ProinsAUC0–30/InsAUC0–30), insulin sensitivity (Matsuda ISI), and disposition index (disposition index = InsAUC0–30/GluAUC0–30 × Matsuda ISI) in nondiabetic subjects
| Gene SNP | Alleles MAF (%) | InsAUC0–30 / GluAUC0–30 |
ProinsAUC0–30 / InsAUC0–30 |
Matsuda ISI |
Disposition index |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Effect size B (SE) | P values | *P values | Effect size B (SE) | P values | *P values | Effect size B (SE) | P values | †P values | Effect size B (SE) | P values | †P values | ||
| PPARG rs1801282 | C/G 15.5 | 0.63 (0.57) | 0.316 | 0.664 | 0.14 (0.45) | 0.991 | 0.560 | −0.11 (0.11) | 0.364 | 0.054 | −0.30 (1.99) | 0.958 | 0.810 |
| KCNJ11 rs5219 | G/A 47.7 | −1.14 (0.41) | 3.8E-04 | 0.025 | 0.49 (0.32) | 0.115 | 0.531 | 0.25 (0.08) | 0.005 | 0.008 | −1.32 (1.40) | 0.362 | 0.231 |
| TCF7L2 rs7903146 | C/T 17.7 | −1.78 (0.53) | 3.9E-05 | 9.8E-07 | 0.75 (0.42) | 0.002 | 6.0E-04 | 0.12 (0.11) | 0.228 | 0.920 | −6.51 (1.87) | 8.3E-05 | 3.4E-06 |
| SLC30A8 rs13266634 | C/T 39.1 | −0.83 (0.41) | 0.013 | 3.2E-04 | 0.73 (0.33) | 1.9E-05 | 1.2E-05 | −0.00 (0.08) | 0.871 | 0.679 | −4.19 (1.46) | 0.001 | 4.2E-04 |
| HHEX rs1111875 | C/T 46.9 | −2.73 (0.40) | 3.2E-12 | 1.4E-14 | 0.80 (0.32) | 9.7E-06 | 6.5E-06 | 0.17 (0.08) | 0.010 | 0.017 | −8.89 (1.42) | 2.5E-09 | 1.2E-10 |
| LOC387761 rs7480010 | A/G 17.5 | −0.51 (0.54) | 0.540 | 0.290 | −0.33 (0.44) | 0.829 | 0.194 | 0.17 (0.11) | 0.087 | 0.345 | 3.57 (1.91) | 0.094 | 0.189 |
| CDKN2B rs10811661 | A/G 14.5 | −1.15 (0.58) | 0.021 | 1.7E-04 | 0.31 (0.47) | 0.285 | 0.211 | −0.03 (0.12) | 0.847 | 0.413 | −6.30 (1.99) | 4.3E-04 | 0.001 |
| IGF2BP2 rs4402960 | C/A 32.1 | −1.34 (0.43) | 0.004 | 0.004 | 0.14 (0.34) | 0.263 | 0.368 | 0.08 (0.09) | 0.182 | 0.440 | −4.22 (1.53) | 0.038 | 0.014 |
| CDKAL1 rs7754840 | G/C 37.0 | −1.68 (0.42) | 3.4E-05 | 2.2E-06 | 0.32 (0.34) | 3.1E-04 | 0.001 | 0.12 (0.08) | 0.181 | 0.176 | −5.25 (1.48) | 1.6E-04 | 6.4E-05 |
| WFS1 rs10010131 | G/A 45.0 | −0.56 (0.41) | 0.048 | 0.397 | 0.01 (0.33) | 0.402 | 0.081 | 0.14 (0.08) | 0.055 | 0.100 | 0.23 (1.44) | 0.986 | 0.808 |
| JAZF1 rs864745 | A/G 48.5 | 0.15 (0.41) | 0.551 | 0.554 | −0.25 (0.32) | 0.792 | 0.968 | −0.10 (0.08) | 0.198 | 0.067 | −1.31 (1.43) | 0.301 | 0.241 |
| CDC123 rs12779790 | A/G 21.5 | −0.82 (0.49) | 0.059 | 0.062 | −0.07 (0.39) | 0.486 | 0.598 | 0.07 (0.10) | 0.369 | 0.433 | −2.36 (1.73) | 0.196 | 0.043 |
| TSPAN8 rs7961581 | A/G 19.4 | 0.23 (0.51) | 0.525 | 0.891 | −0.29 (0.41) | 0.120 | 0.310 | −0.15 (0.10) | 0.343 | 0.008 | −0.75 (1.80) | 0.635 | 0.308 |
| THADA rs7578597 | A/G 5.0 | −2.09 (0.93) | 0.263 | 0.232 | −1.24 (0.73) | 0.425 | 0.267 | 0.04 (0.18) | 0.659 | 0.373 | −3.51 (3.27) | 0.355 | 0.410 |
| ADAMTS9 rs4607103 | G/A 26.1 | −0.66 (0.47) | 0.335 | 0.221 | −0.04 (0.37) | 0.087 | 0.069 | −0.04 (0.09) | 0.809 | 0.587 | −2.13 (1.65) | 0.308 | 0.332 |
| NOTCH2 rs10923931 | C/A 13.8 | −0.56 (0.59) | 0.228 | 0.668 | −0.95 (0.47) | 0.360 | 0.080 | 0.16 (0.12) | 0.054 | 0.060 | 1.21 (2.09) | 0.244 | 0.300 |
| KCNQ1 rs2283228 | A/C 6.2 | −1.03 (0.84) | 0.161 | 0.093 | 0.31 (0.66) | 0.176 | 0.353 | 0.10 (0.17) | 0.701 | 0.284 | −3.29 (2.96) | 0.162 | 0.221 |
| MTNR1B rs10830963 | C/G 36.0 | −2.02 (0.42) | 1.4E-07 | 1.0E-13 | −0.21 (0.33) | 0.301 | 0.189 | 0.03 (0.08) | 0.577 | 0.436 | −9.65 (1.47) | 6.7E-11 | 3.8E-13 |
Effect size shown is B-coefficient (SE) per copy of the type 2 diabetes risk allele, and was calculated using untransformed variables adjusted for age by linear regression. P values were calculated using log-transformed variables (because of their skewed distribution) by linear regression. P values are adjusted for age;
P values are adjusted for age, BMI, and Matsuda ISI;
†P values are adjusted for age and BMI. In the entire cohort, means ± SE of examined parameters and the number of subjects with available data were as follows: InsAUC0–30/GluAUC0–30 30.4 ± 0.29 pmol/mmol (n = 5,298), ProinsAUC0–30/InsAUC0–30 12.5 ± 0.23 (n = 2,697), Matsuda ISI 7.03 ± 0.06 (mg/dl, mU/l; n = 5,295), and disposition index 163.7 ± 1.02 (n = 5,295). P values significant after correction for multiple testing (P < 6.9 × 10−4) are in bold. Risk alleles are underlined. Results for the additive model are presented.
Statistical analysis.
Effect sizes [B (SE)] per copy of the risk allele were estimated by linear regression adjusted for age, using untransformed dependent variables, as previously described (13). P values were calculated using logarithmically transformed variables (all except for age) because of their skewed distribution and were adjusted for age in the primary analyses. In the secondary analyses, additional adjustment was performed as follows: effects of SNPs on InsAUC0–30/GluAUC0–30 and ProinsAUC0–30/InsAUC0–30 were adjusted for age, BMI, and Matsuda ISI (to examine effects independent of obesity and insulin sensitivity), and effects of SNPs on Matsuda ISI and disposition index were adjusted for age and BMI. Effect of genetic risk score on InsAUC0–30/GluAUC0–30 was analyzed by linear regression adjusted for age, BMI, and Matsuda ISI because of significant association of genetic risk score with these covariates. Hardy-Weinberg equilibrium was tested by χ2 test. Statistical analyses were conducted with the SPSS 14 programs (SPSS, Chicago, IL). P < 0.05 was considered nominally significant, and P < 6.9 × 10−4 calculated using Bonferroni correction for multiple comparisons was considered statistically significant, given 72 independent tests for 18 SNPs and four outcomes measured (obesity [BMI], insulin release [InsAUC0–30/GluAUC0–30], insulin sensitivity [Matsuda ISI], and proinsulin conversion [ProinsAUC0–30/InsAUC0–30]). Power of the current sample was estimated using the Bioconductor's GeneticsDesign package version 1.1 (http://www.bioconductor.org/packages/2.3/bioc/html/GeneticsDesign.html). We had power ≥80% to detect changes from 5 to 8% per copy of the risk allele for InsAUC0–30/GluAUC0–30, Matsuda ISI, and disposition index for SNPs with minor allele frequency >30%, and power ≥80% to detect a change of ∼15% in ProinsAUC0–30/InsAUC0–30 for SNPs with minor allele frequency larger than 30%.
RESULTS
Primary analyses.
Primary analyses were carried out under the additive model adjusted for age.
Obesity.
None of the 18 SNPs was significantly associated with BMI, although for 4 SNPs (TCF7L2 rs7903146, CDC123 rs12779790, TSPAN8 rs7961581, and MTNR1B rs10830963) the association was nominally significant (P = 0.018, 0.006, 0.031, and 0.035). The effect sizes were <−1% per type 2 diabetes risk allele. To examine obesity-independent effects of all SNPs, we additionally adjusted their effects for BMI.
Insulin sensitivity.
None of the 18 SNPs had significant effect on Matsuda ISI in a primary analysis. Two SNPs, HHEX rs1111875 and KCNJ11 rs5219, were nominally associated with Matsuda ISI, with effect sizes ranging from +2 to +4% per risk allele (P = 0.010 and 0.005) (Table 1). Adjustment for BMI did not have a major impact on these associations but revealed another nominal association between TSPAN8 rs7961581 and Matsuda ISI (P = 0.008, effect size −2% per risk allele). However, both KCNJ11 rs5219 and HHEX rs1111875 were also associated with InsAUC0–30/GluAUC0–30. Adjustment for InsAUC0–30/GluAUC0–30 abolished the effect of KCNJ11 rs5219 (P = 0.906) but strengthened the effect of HHEX rs1111875 on Matsuda ISI (P = 3.6 × 10−5).
Insulin release.
Altogether, eight SNPs (in or near KCNJ11, TCF7L2, SLC30A8, HHEX, CDKN2B, IGF2BP2, CDKAL1, and MTNR1B) were nominally or significantly associated with InsAUC0–30/GluAUC0–30. The largest effects on InsAUC0–30/GluAUC0–30 (from −6 to −9% per risk allele) were observed for TCF7L2 rs7903146, HHEX rs1111875, CDKAL1 rs7754840, and MTNR1B rs10830963 and were statistically significant in both primary analyses and analyses adjusted for age, BMI, and Matsuda ISI (Table 1). Effect sizes of the SNPs in or near KCNJ11, SLC30A8, CDKN2B, and IGF2BP2 were <−5% per risk allele. Adjustment of effects of these SNPs for BMI and Matsuda ISI in addition to age attenuated the initially significant effect of KCNJ11 rs5219 (P = 0.024), strengthened the associations of SLC30A8 rs13266634 and CDKN2B rs10811661 to significant level (P = 3.2 × 10−4 and 1.7 × 10−4), and did not change nominal association of IGF2BP2 rs4402960 with InsAUC0–30/GluAUC0–30 (P = 0.004) (Table 1).
Proinsulin conversion.
Four SNPs (in or near HHEX, SLC30A8, TCF7L2, and CDKAL1) were associated with ProinsAUC0–30/InsAUC0–30, with effect sizes ranging from +3 to +6% per risk allele (Tables 1 and 2). For HHEX rs1111875 and SLC30A8 rs13266634 the effects were significant regardless of adjustments used (adjusted for age: P = 9.7 × 10−6 and 1.9 × 10−5; adjusted for age, BMI, and Matsuda ISI: P = 6.5 × 10−6 and 1.2 × 10−5). In contrast, adjustment for BMI and Matsuda ISI attenuated the significant effect of CDKAL1 rs7754840 to nominal level (P = 0.002), and strengthened nominal effect of TCF7L2 rs7903146 to significant level (P = 6.0 × 10−4). Similar results, although slightly attenuated, were obtained when alternative indexes of proinsulin conversion based on proinsulin and insulin AUCs during 0–120 min or 30–120 min of an OGTT were used (ProinsAUC0–120/InsAUC0–120 and ProinsAUC30–120/InsAUC30–120, Table 2). SLC30A8 rs13266634 and TCF7L2 rs7903146 were also nominally associated with fasting proinsulin/insulin ratio (Proins0/Ins0, Table 2). Overall, these results were consistent with associations of TCF7L2, SLC30A8, HHEX, and CDKAL1 with insulin release, because the risk alleles associated with lower insulin release were associated with higher proinsulin/insulin ratio.
TABLE 2.
Associations of four SNPs with proinsulin/insulin ratio at fasting state (Proins0/Ins0), during 0–30 min (ProinsAUC0–30/InsAUC0–30), 30–120 min (ProinsAUC30–120/InsAUC30–120), and 0–120 min (ProinsAUC0–120/InsAUC0–120) of an OGTT in nondiabetic subjects
| Gene SNP | Alleles MAF (%) | Proins0/Ins0 |
ProinsAUC0–30/InsAUC0–30 |
ProinsAUC30–120/InsAUC30–120 |
ProinsAUC0–120/InsAUC0–120 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Effect size B (SE) | P values | *P values | Effect size B (SE) | P values | *P values | Effect size B (SE) | P values | *P values | Effect size B (SE) | P values | *P values | ||
| TCF7L2 rs7903146 | C/T 17.7 | 1.20 (1.22) | 0.042 | 0.021 | 0.75 (0.42) | 0.002 | 6.0E-04 | 0.55 (0.44) | 0.005 | 1.1E-03 | 0.57 (0.43) | 0.004 | 0.001 |
| SLC30A8 rs13266634 | C/T 39.1 | 1.59 (0.96) | 0.006 | 0.003 | 0.73 (0.33) | 1.9E-05 | 1.2E-05 | 0.64 (0.35) | 1.1E-04 | 4.2E-05 | 0.64 (0.34) | 8.2E-05 | 2.8E-05 |
| HHEX rs1111875 | C/T 46.9 | 0.74 (0.94) | 0.365 | 0.622 | 0.80 (0.32) | 9.7E-06 | 6.5E-06 | 0.69 (0.34) | 0.002 | 0.002 | 0.71 (0.33) | 0.001 | 6.6E-04 |
| CDKAL1 rs7754840 | G/C 37.0 | −0.39 (0.98) | 0.313 | 0.775 | 0.32 (0.34) | 3.1E-04 | 0.001 | 0.36 (0.35) | 0.003 | 0.009 | 0.35 (0.35) | 0.002 | 0.005 |
Effect size shown is B-coefficient (SE) per copy of the type 2 diabetes risk allele and was calculated using untransformed variables adjusted for age by linear regression. P values were calculated using log-transformed variables (because of their skewed distribution) by linear regression. P values are adjusted for age;
*P values are adjusted for age, BMI, and Matsuda ISI. In the entire cohort, means ± SE of examined parameters and the number of subjects with available data were as follows: Proins0/Ins0 36.3 ± 0.67 (n = 2,712), ProinsAUC0–30/InsAUC0–30 12.5 ± 0.23 (n = 2,697), ProinsAUC30–120/InsAUC30–120 14.1 ± 0.24 (n = 2,693), ProinsAUC0–120/InsAUC0–120 13.8 ± 0.24 (n = 2,692). P values significant after correction for multiple testing (P < 6.9 × 10−4) are in bold. Risk alleles are underlined. Results for the additive model are presented.
Disposition index.
Most of the insulin release–related SNPs (in or near TCF7L2, SLC30A8, HHEX, CDKN2B, IGF2BP2, CDKAL1, and MTNR1B) were also significantly or nominally associated with disposition index (Table 1). The largest effects ranging from −3 to −6% per risk allele were observed for MTNR1B rs10830963 (P = 6.7 × 10−11), HHEX rs1111875 (P = 2.5 × 10−9), TCF7L2 rs7903146 (P = 8.3 × 10−5), CDKN2B rs10811661 (P = 4.3 × 10−4), and CDKAL1 rs7754840 (P = 1.6 × 10−4). Adjustment for BMI did not attenuate these associations, except for that of CDKN2B (P = 0.001).
Given the number of tests (18 tests for each variable), we would expect 0.9 P values <0.05 per variable at random. The number of associations with P < 0.05 was larger than expected (nine for InsAUC0–30/GluAUC0–30, four for ProinsAUC0–30/InsAUC0–30, two for Matsuda ISI, and seven for disposition index in primary analyses), suggesting that the associations we found were not likely to occur by chance. However, it should be mentioned that despite the large sample size we did not have sufficient power (>80%) to detect small effects (<6% per risk allele) on different traits examined for 9 of 18 SNPs investigated.
We repeated all analyses in the subgroup of subjects with normal glucose tolerance (n = 3,594) (supplemental Table 1, available in an online appendix at http://diabetes.diabetesjournals.org/cgi/content/full/db09-0117/DC1). The effect sizes were mostly similar, although associations were generally slightly weaker because of a smaller sample size. In contrast, in analyses including both nondiabetic subjects and 442 subjects with newly diagnosed type 2 diabetes the associations described above were somewhat more statistically significant with similar effect sizes and revealed nominal associations of CDC123 rs12779790 and ADAMTS9 rs4607103 with disposition index (P = 0.001 and 0.043, adjusted for age and BMI, effect sizes ∼−2% per risk allele, supplemental Table 2).
Combined effect of risk alleles on insulin release.
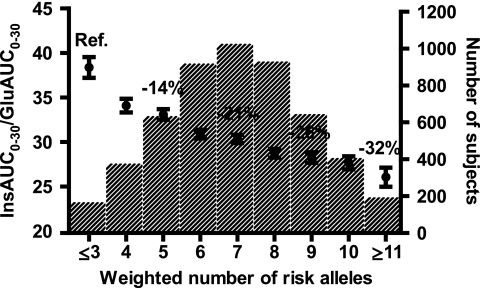
We combined the risk alleles at eight SNPs significantly or nominally associated with InsAUC0–30/GluAUC0–30 (KCNJ11, TCF7L2, SLC30A8, HHEX, CDKN2B, IGF2BP2, CDKAL1, and MTNR1B) to evaluate their combined effects on insulin release. InsAUC0–30/GluAUC0–30 gradually decreased with an increasing number of risk alleles (relative effect size −4% per allele, P = 9.3 × 10−44 adjusted for age, BMI, and Matsuda ISI). Subjects with ≥11 weighted risk alleles (n = 190) had decreased InsAUC0–30/GluAUC0–30 by −32% compared with subjects with ≤3 weighted risk alleles (n = 163) (Fig. 1). We also performed similar analysis using nonweighted risk alleles. The difference in InsAUC0–30/GluAUC0–30 between subjects with ≤3 and ≥11 risk alleles was −37% (relative effect size −4% per risk allele, P = 3.8 × 10−28).
FIG. 1.
Early-phase insulin release (InsAUC0–30/GluAUC0–30) according to the number of risk alleles in eight insulin secretion–related SNPs (KCNJ11 rs5219, TCF7L2 rs7903146, SLC30A8 rs13266634, HHEX rs1111875, CDKN2B rs10811661, IGF2BP2 rs4402960, CDKAL1 rs7754840, and MTNR1B rs10830963). For each subject, the number of type 2 diabetes risk alleles (0, 1, 2) per SNP was weighted for their effect sizes (shown in Table 1; average effect size per risk allele among eight SNPs was 1.58, which was considered as one weighted risk allele). Effect of the number of the risk alleles on InsAUC0–30/GluAUC0–30 was significant (P = 9.3 × 10−44, adjusted for age, BMI, and Matsuda ISI). Data are shown as means ± SE (adjusted for age, BMI, and Matsuda ISI). Bars show numbers of subjects in each category.
DISCUSSION
In this large population-based study, we investigated the effects of confirmed type 2 diabetes risk variants on insulin secretion, insulin sensitivity, and proinsulin processing. We showed in 5,327 nondiabetic Finnish men that 8 of 18 type 2 diabetes–related variants were significantly (TCF7L2, SLC30A8, HHEX, CDKN2B, CDKAL1, and MTNR1B) or nominally (KCNJ11 and IGF2BP2) associated with early-phase insulin release (InsAUC0–30/GluAUC0–30) after adjustment for age, BMI, and Matsuda ISI. InsAUC0–30/GluAUC0–30 decreased gradually with increasing number of type 2 diabetes risk alleles in these SNPs and was −32% less in subjects with ≥11 than with ≤3 risk alleles. Furthermore, four variants (TCF7L2, SLC30A8, HHEX, and CDKAL1) were also associated with proinsulin conversion (ProinsAUC0–30/InsAUC0–30). SNPs in or near KCNJ11, HHEX, and TSPAN8 were nominally associated with Matsuda ISI (adjusted for age and BMI).
Insulin secretion has an important genetic component, as suggested by twin studies reporting heritability estimates >50% (1), and a majority of diabetes susceptibility genes have been shown to associate with parameters of insulin secretion (48). Our finding of eight SNPs associated with insulin secretion, alone or in combination, provides additional evidence on the importance of the genes regulating insulin secretion as risk genes for type 2 diabetes. An observation similar to our results was reported in a study by Pascoe et al. (27), where carriers of nine or more risk alleles in seven genes exhibited reduced insulin secretion (assessed by the insulinogenic index) by −21.8% and reduced glucose sensitivity of β-cells by −26.6% compared with carriers of four or less risk alleles. In our study, the largest effects on InsAUC0–30/GluAUC0–30 were observed for HHEX, MTNR1B, TCF7L2, and CDKAL1 (effect sizes ranging from −6 to −9% per risk allele). This finding is in agreement with previous studies, which have also quite consistently reported associations of these genes with impaired insulin secretion (6,13,17,30–32). Effects of SNPs in KCNJ11, SLC30A8, IGF2BP2, and CDKN2B on insulin secretion were <5% in our study. Previous studies examining these SNPs for an association with insulin secretion have been inconclusive (6,18–19,22–25,35), most probably because of insufficient power to detect modest effects of these SNPs. A few studies have reported associations of variants of WFS1 (36), TSPAN8 (33), JAZF1 (33), CDC123 (33), LOC387761 (24), and KCNQ1 (12) with insulin secretion, but our study failed to confirm such an association.
The mechanisms by which the insulin secretion–related genes influence insulin release have remained largely unknown. One of the plausible mechanisms proposed by previous studies is impaired conversion of proinsulin to insulin. In our study, four SNPs were significantly (SLC30A8 rs13266634, HHEX rs1111875, and TCF7L2 rs7903146) or nominally (CDKAL1 rs7754840) associated with the proinsulin/insulin ratio during the first 30 min of an OGTT (adjusted for age, BMI, and Matsuda ISI). Variants in SLC30A8 and TCF7L2 were also nominally associated with fasting proinsulin/insulin ratio. Association of TCF7L2 rs7903146 with proinsulin levels (40,41) or proinsulin/insulin ratio (39,42) has been previously reported. Although the mechanisms behind this association are not clear, impaired glucagon-like peptide 1 signaling seems to be involved (49). In a recent study (22), the association of SLC30A8 rs13266634, CDKAL1 rs7754840, and TCF7L2 rs7903146 with the proinsulin/insulin AUC ratio during OGTT was also shown. Our finding that HHEX variant is associated with impaired proinsulin conversion has not previously been reported. Our results suggest that SNPs in or near TCF7L2, CDKAL1, SLC30A8, and HHEX may affect insulin secretion, at least partially, through impaired proinsulin conversion. Although we had proinsulin data from almost 2,700 subjects, the power of our study was limited to detect effect sizes <15% in the ProinsAUC0–30/InsAUC0–30 ratio. Therefore, even larger studies are needed to identify SNPs significantly associated with defects in proinsulin conversion.
PPARG has been the only clear insulin sensitivity–related gene among 20 diabetes susceptibility loci confirmed by genome-wide association studies. We observed only a small effect (−2% per risk allele) of PPARG rs1801282 (Pro12Ala) on Matsuda ISI, which was close to be nominally significant (P = 0.054, adjusted for age and BMI). Similar small effects (∼2% per risk allele) on Matsuda ISI were observed for variants in or near KCNJ11, HHEX, and TSPAN8 in our study, but none of them reached significant level after adjustment for age and BMI. In a recent study by Staiger et al. (34), a trend for association of TSPAN8 rs7961581 with Matsuda ISI and homeostasis model assessment of insulin resistance indexes of insulin sensitivity or resistance has also been reported. However, association of HHEX rs1111875 became significant after additional adjustment for InsAUC0–30/GluAUC0–30 in our study, and the risk allele was associated with higher Matsuda ISI. Although HHEX is primarily a candidate gene for impaired insulin secretion, it remains to be elucidated whether it also affects tissue-specific insulin sensitivity independently of changes in insulin secretion.
HHEX rs1111875 was associated with all traits examined in our study, and particularly its effects on InsAUC0–30/GluAUC0–30 and ProinsAUC0–30/InsAUC0–30 ratios were the most significant among all examined SNPs. Although the association of the HHEX locus with insulin secretion is well established (16–22), its association with insulin sensitivity and proinsulin conversion has not been previously reported. Further studies are needed to elucidate the molecular mechanisms of SNPs of the HHEX gene (or other genes near rs1111875) in regulating glucose homeostasis.
Our study has limitations. Only Finnish men were included in our study, and therefore we cannot be sure whether our results are applicable to women and to different ethnic or racial groups. However, no evidence exists that the sex could modify the effects of diabetes susceptibility genes on glucose metabolism. We used surrogate markers of insulin secretion and insulin sensitivity derived from an OGTT, because the application of more accurate methods (intravenous glucose tolerance test, euglycemic clamp) is not feasible in a study having thousands of participants. Finally, despite the large sample size we did not have sufficient power (>80%) to detect small effects (<6% per allele) of examined SNPs on Matsuda ISI and InsAUC0–30/GluAUC0–30, which may explain negative findings for 9 of 18 SNPs in PPARG, LOC387761, WFS1, JAZF1, CDC123, THADA, ADAMTS9, NOTCH2, and KCNQ1.
In summary, we showed in a large cohort of nondiabetic Finnish men that 8 of 18 type 2 diabetes–related loci were significantly (TCF7L2, SLC30A8, HHEX, CDKN2B, CDKAL1, and MTNR1B) or nominally (KCNJ11 and IGF2BP2) associated with impaired early-phase insulin release, which decreased by −32% in carriers of ≥11 vs. ≤3 weighted type 2 diabetes risk alleles at these loci. Effects of TCF7L2, SLC30A8, HHEX, and CDKAL1 on insulin secretion could be explained, at least in part, by impaired conversion of proinsulin to insulin. HHEX might influence both insulin release and insulin sensitivity.
Supplementary Material
Acknowledgments
This study was supported by a grant from the Academy of Finland (contract no. 124243), The Finnish Heart Foundation, The Finnish Diabetes Foundation, TEKES (contract no. 1510/31/06), Commission of the European Community (LSHM-CT-2004-512013 EUGENE2, and HEALTH-F2-2007[-201681) (to M.L.), National Institutes of Health Grant DK-62370 (to M.B.), and The National Human Genome Research Institute Intramural project no. 1 Z01 HG000024 (to F.S.C.).
No potential conflicts of interest relevant to this article were reported.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Schousboe K, Visscher PM, Henriksen JE, Hopper JL, Sørensen TI, Kyvik KO: Twin study of genetic and environmental influences on glucose tolerance and indices of insulin sensitivity and secretion. Diabetologia 2003; 46: 1276– 1283 [DOI] [PubMed] [Google Scholar]
- 2.Sladek R, Rocheleau G, Rung J, Dina C, Shen L, Serre D, Boutin P, Vincent D, Belisle A, Hadjadj S, Balkau B, Heude B, Charpentier G, Hudson TJ, Montpetit A, Pshezhetsky AV, Prentki M, Posner BI, Balding DJ, Meyre D, Polychronakos C, Froguel P: A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature 2007; 445: 881– 885 [DOI] [PubMed] [Google Scholar]
- 3.Diabetes Genetics Initiative of Broad Institute of Harvard and MIT, Lund University, and Novartis Institutes of BioMedical Research Saxena R, Voight BF, Lyssenko V, Burtt NP, de Bakker PI, Chen H, Roix JJ, Kathiresan S, Hirschhorn JN, Daly MJ, Hughes TE, Groop L, Altshuler D, Almgren P, Florez JC, Meyer J, Ardlie K, Bengtsson Boström K, Isomaa B, Lettre G, Lindblad U, Lyon HN, Melander O, Newton-Cheh C, Nilsson P, Orho-Melander M, Råstam L, Speliotes EK, Taskinen MR, Tuomi T, Guiducci C, Berglund A, Carlson J, Gianniny L, Hackett R, Hall L, Holmkvist J, Laurila E, Sjögren M, Sterner M, Surti A, Svensson M, Svensson M, Tewhey R, Blumenstiel B, Parkin M, Defelice M, Barry R, Brodeur W, Camarata J, Chia N, Fava M, Gibbons J, Handsaker B, Healy C, Nguyen K, Gates C, Sougnez C, Gage D, Nizzari M, Gabriel SB, Chirn GW, Ma Q, Parikh H, Richardson D, Ricke D, Purcell S: Genome-wide association analysis identifies loci for type 2 diabetes and triglyceride levels. Science 2007; 316: 1331– 1336 [DOI] [PubMed] [Google Scholar]
- 4.Zeggini E, Weedon MN, Lindgren CM, Frayling TM, Elliott KS, Lango H, Timpson NJ, Perry JR, Rayner NW, Freathy RM, Barrett JC, Shields B, Morris AP, Ellard S, Groves CJ, Harries LW, Marchini JL, Owen KR, Knight B, Cardon LR, Walker M, Hitman GA, Morris AD, Doney AS, the Wellcome Trust Case Control Consortium (WTCCC) McCarthy MI, Hattersley AT: Replication of genome-wide association signals in UK samples reveals risk loci for type 2 diabetes. Science 2007; 316: 1336– 1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scott LJ, Mohlke KL, Bonnycastle LL, Willer CJ, Li Y, Duren WL, Erdos MR, Stringham HM, Chines PS, Jackson AU, Prokunina-Olsson L, Ding CJ, Swift AJ, Narisu N, Hu T, Pruim R, Xiao R, Li XY, Conneely KN, Riebow NL, Sprau AG, Tong M, White PP, Hetrick KN, Barnhart MW, Bark CW, Goldstein JL, Watkins L, Xiang F, Saramies J, Buchanan TA, Watanabe RM, Valle TT, Kinnunen L, Abecasis GR, Pugh EW, Doheny KF, Bergman RN, Tuomilehto J, Collins FS, Boehnke M: A genome-wide association study of type 2 diabetes in Finns detects multiple susceptibility variants. Science 2007; 316: 1341– 1345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steinthorsdottir V, Thorleifsson G, Reynisdottir I, Benediktsson R, Jonsdottir T, Walters GB, Styrkarsdottir U, Gretarsdottir S, Emilsson V, Ghosh S, Baker A, Snorradottir S, Bjarnason H, Ng MC, Hansen T, Bagger Y, Wilensky RL, Reilly MP, Adeyemo A, Chen Y, Zhou J, Gudnason V, Chen G, Huang H, Lashley K, Doumatey A, So WY, Ma RC, Andersen G, Borch-Johnsen K, Jorgensen T, van Vliet-Ostaptchouk JV, Hofker MH, Wijmenga C, Christiansen C, Rader DJ, Rotimi C, Gurney M, Chan JC, Pedersen O, Sigurdsson G, Gulcher JR, Thorsteinsdottir U, Kong A, Stefansson K: A variant in CDKAL1 influences insulin response and risk of type 2 diabetes. Nat Genet 2007; 39: 770– 775 [DOI] [PubMed] [Google Scholar]
- 7.Frayling TM, Timpson NJ, Weedon MN, Zeggini E, Freathy RM, Lindgren CM, Perry JR, Elliott KS, Lango H, Rayner NW, Shields B, Harries LW, Barrett JC, Ellard S, Groves CJ, Knight B, Patch AM, Ness AR, Ebrahim S, Lawlor DA, Ring SM, Ben-Shlomo Y, Jarvelin MR, Sovio U, Bennett AJ, Melzer D, Ferrucci L, Loos RJ, Barroso I, Wareham NJ, Karpe F, Owen KR, Cardon LR, Walker M, Hitman GA, Palmer CN, Doney AS, Morris AD, Smith GD, Hattersley AT, McCarthy MI: A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science 2007; 316: 889– 894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gudmundsson J, Sulem P, Steinthorsdottir V, Bergthorsson JT, Thorleifsson G, Manolescu A, Rafnar T, Gudbjartsson D, Agnarsson BA, Baker A, Sigurdsson A, Benediktsdottir KR, Jakobsdottir M, Blondal T, Stacey SN, Helgason A, Gunnarsdottir S, Olafsdottir A, Kristinsson KT, Birgisdottir B, Ghosh S, Thorlacius S, Magnusdottir D, Stefansdottir G, Kristjansson K, Bagger Y, Wilensky RL, Reilly MP, Morris AD, Kimber CH, Adeyemo A, Chen Y, Zhou J, So WY, Tong PC, Ng MC, Hansen T, Andersen G, Borch-Johnsen K, Jorgensen T, Tres A, Fuertes F, Ruiz-Echarri M, Asin L, Saez B, van Boven E, Klaver S, Swinkels DW, Aben KK, Graif T, Cashy J, Suarez BK, van Vierssen Trip O, Frigge ML, Ober C, Hofker MH, Wijmenga C, Christiansen C, Rader DJ, Palmer CN, Rotimi C, Chan JC, Pedersen O, Sigurdsson G, Benediktsson R, Jonsson E, Einarsson GV, Mayordomo JI, Catalona WJ, Kiemeney LA, Barkardottir RB, Gulcher JR, Thorsteinsdottir U, Kong A, Stefansson K: Two variants on chromosome 17 confer prostate cancer risk, and the one in TCF2 protects against type 2 diabetes. Nat Genet 2007; 39: 977– 983 [DOI] [PubMed] [Google Scholar]
- 9.Sandhu MS, Weedon MN, Fawcett KA, Wasson J, Debenham SL, Daly A, Lango H, Frayling TM, Neumann RJ, Sherva R, Blech I, Pharoah PD, Palmer CN, Kimber C, Tavendale R, Morris AD, McCarthy MI, Walker M, Hitman G, Glaser B, Permutt MA, Hattersley AT, Wareham NJ, Barroso I: Common variants in WFS1 confer risk of type 2 diabetes. Nat Genet 2007; 39: 951– 953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zeggini E, Scott LJ, Saxena R, Voight BF, Marchini JL, Hu T, de Bakker PI, Abecasis GR, Almgren P, Andersen G, Ardlie K, Boström KB, Bergman RN, Bonnycastle LL, Borch-Johnsen K, Burtt NP, Chen H, Chines PS, Daly MJ, Deodhar P, Ding CJ, Doney AS, Duren WL, Elliott KS, Erdos MR, Frayling TM, Freathy RM, Gianniny L, Grallert H, Grarup N, Groves CJ, Guiducci C, Hansen T, Herder C, Hitman GA, Hughes TE, Isomaa B, Jackson AU, Jørgensen T, Kong A, Kubalanza K, Kuruvilla FG, Kuusisto J, Langenberg C, Lango H, Lauritzen T, Li Y, Lindgren CM, Lyssenko V, Marvelle AF, Meisinger C, Midthjell K, Mohlke KL, Morken MA, Morris AD, Narisu N, Nilsson P, Owen KR, Palmer CN, Payne F, Perry JR, Pettersen E, Platou C, Prokopenko I, Qi L, Qin L, Rayner NW, Rees M, Roix JJ, Sandbaek A, Shields B, Sjögren M, Steinthorsdottir V, Stringham HM, Swift AJ, Thorleifsson G, Thorsteinsdottir U, Timpson NJ, Tuomi T, Tuomilehto J, Walker M, Watanabe RM, Weedon MN, Willer CJ, Wellcome Trust Case Control Consortium. Illig T, Hveem K, Hu FB, Laakso M, Stefansson K, Pedersen O, Wareham NJ, Barroso I, Hattersley AT, Collins FS, Groop L, McCarthy MI, Boehnke M, Altshuler D: Meta-analysis of genome-wide association data and large-scale replication identifies additional susceptibility loci for type 2 diabetes. Nat Genet 2008; 40: 638– 645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Unoki H, Takahashi A, Kawaguchi T, Hara K, Horikoshi M, Andersen G, Ng DP, Holmkvist J, Borch-Johnsen K, Jørgensen T, Sandbæk A, Lauritzen T, Hansen T, Nurbaya S, Tsunoda T, Kubo M, Babazono T, Hirose H, Hayashi M, Iwamoto Y, Kashiwagi A, Kaku K, Kawamori R, Tai ES, Pedersen O, Kamatani N, Kadowaki T, Kikkawa R, Nakamura Y, Maeda S: SNPs in KCNQ1 are associated with susceptibility to type 2 diabetes in East Asian and European populations. Nat Genet 2008; 40: 1098– 1102 [DOI] [PubMed] [Google Scholar]
- 12.Yasuda K, Miyake K, Horikawa Y, Hara K, Osawa H, Furuta H, Hirota Y, Mori H, Jonsson A, Sato Y, Yamagata K, Hinokio Y, Wang HY, Tanahashi T, Nakamura N, Oka Y, Iwasaki N, Iwamoto Y, Yamada Y, Seino Y, Maegawa H, Kashiwagi A, Takeda J, Maeda E, Shin HD, Cho YM, Park KS, Lee HK, Ng MC, Ma RC, So WY, Chan JC, Lyssenko V, Tuomi T, Nilsson P, Groop L, Kamatani N, Sekine A, Nakamura Y, Yamamoto K, Yoshida T, Tokunaga K, Itakura M, Makino H, Nanjo K, Kadowaki T, Kasuga M: Variants in KCNQ1 are associated with susceptibility to type 2 diabetes mellitus. Nat Genet 2008; 40: 1092– 1097 [DOI] [PubMed] [Google Scholar]
- 13.Prokopenko I, Langenberg C, Florez JC, Saxena R, Soranzo N, Thorleifsson G, Loos RJ, Manning AK, Jackson AU, Aulchenko Y, Potter SC, Erdos MR, Sanna S, Hottenga JJ, Wheeler E, Kaakinen M, Lyssenko V, Chen WM, Ahmadi K, Beckmann JS, Bergman RN, Bochud M, Bonnycastle LL, Buchanan TA, Cao A, Cervino A, Coin L, Collins FS, Crisponi L, de Geus EJ, Dehghan A, Deloukas P, Doney AS, Elliott P, Freimer N, Gateva V, Herder C, Hofman A, Hughes TE, Hunt S, Illig T, Inouye M, Isomaa B, Johnson T, Kong A, Krestyaninova M, Kuusisto J, Laakso M, Lim N, Lindblad U, Lindgren CM, McCann OT, Mohlke KL, Morris AD, Naitza S, Orrù M, Palmer CN, Pouta A, Randall J, Rathmann W, Saramies J, Scheet P, Scott LJ, Scuteri A, Sharp S, Sijbrands E, Smit JH, Song K, Steinthorsdottir V, Stringham HM, Tuomi T, Tuomilehto J, Uitterlinden AG, Voight BF, Waterworth D, Wichmann HE, Willemsen G, Witteman JC, Yuan X, Zhao JH, Zeggini E, Schlessinger D, Sandhu M, Boomsma DI, Uda M, Spector TD, Penninx BW, Altshuler D, Vollenweider P, Jarvelin MR, Lakatta E, Waeber G, Fox CS, Peltonen L, Groop LC, Mooser V, Cupples LA, Thorsteinsdottir U, Boehnke M, Barroso I, Van Duijn C, Dupuis J, Watanabe RM, Stefansson K, McCarthy MI, Wareham NJ, Meigs JB, Abecasis GR: Variants in MTNR1B influence fasting glucose levels. Nat Genet 2009; 41: 77– 81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ek J, Andersen G, Urhammer SA, Hansen L, Carstensen B, Borch-Johnsen K, Drivsholm T, Berglund L, Hansen T, Lithell H, Pedersen O: Studies of the Pro12Ala polymorphism of the peroxisome proliferator-activated receptor-γ2 (PPAR-γ2) gene in relation to insulin sensitivity among glucose tolerant Caucasians. Diabetologia 2001; 44: 1170– 1176 [DOI] [PubMed] [Google Scholar]
- 15.Fritsche A, Madaus A, Tschritter O, Ozeker M, Wulle EL, Machicao F, Häring H, Stumvoll M: Polymorphism of pro12Ala in peroxisome proliferator activated receptor-γ2 (PPAR-γ2): β-cell function and insulin sensitivity. Dtsch Med Wochenschr 2001; 126: 580– 584 [DOI] [PubMed] [Google Scholar]
- 16.Freathy RM, Timpson NJ, Lawlor DA, Pouta A, Ben-Shlomo Y, Ruokonen A, Ebrahim S, Shields B, Zeggini E, Weedon MN, Lindgren CM, Lango H, Melzer D, Ferrucci L, Paolisso G, Neville MJ, Karpe F, Palmer CN, Morris AD, Elliott P, Jarvelin MR, Smith GD, McCarthy MI, Hattersley AT, Frayling TM: Common variation in the FTO gene alters diabetes-related metabolic traits to the extent expected given its effect on BMI. Diabetes 2008; 57: 1419– 1426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Staiger H, Stančáková A, Zilinskaite J, Vänttinen M, Hansen T, Marini MA, Hammarstedt A, Jansson PA, Sesti G, Smith U, Pedersen O, Laakso M, Stefan N, Fritsche A, Häring HU: A candidate type 2 diabetes polymorphism near the HHEX locus affects acute glucose-stimulated insulin release in European populations: results from the EUGENE2 study. Diabetes 2008; 57: 514– 517 [DOI] [PubMed] [Google Scholar]
- 18.Staiger H, Machicao F, Stefan N, Tschritter O, Thamer C, Kantartzis K, Schäfer SA, Kirchhoff K, Fritsche A, Häring HU: Polymorphisms within novel risk loci for type 2 diabetes determine β-cell function. PLoS ONE 2007; 2: e832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moore AF, Jablonski KA, McAteer JB, Saxena R, Pollin TI, Franks PW, Hanson RL, Shuldiner AR, Knowler WC, Altshuler D, Florez JC: Diabetes Prevention Program Research Group: extension of type 2 diabetes genome-wide association scan results in the Diabetes Prevention Program. Diabetes 2008; 57: 2503– 2510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grarup N, Rose CS, Andersson EA, Andersen G, Nielsen AL, Albrechtsen A, Clausen JO, Rasmussen SS, Jørgensen T, Sandbaek A, Lauritzen T, Schmitz O, Hansen T, Pedersen O: Studies of association of variants near the HHEX, CDKN2A/B, and IGF2BP2 genes with type 2 diabetes and impaired insulin release in 10,705 Danish subjects: validation and extension of genome-wide association studies. Diabetes 2007; 56: 3105– 3111 [DOI] [PubMed] [Google Scholar]
- 21.Pascoe L, Tura A, Patel SK, Ibrahim IM, Ferrannini E, Zeggini E, Weedon MN, Mari A, Hattersley AT, McCarthy MI, Frayling TM, Walker M: the RISC Consortium, the UK Type 2 Diabetes Genetics Consortium. Common variants of the novel type 2 diabetes genes CDKAL1 and HHEX/IDE are associated with decreased pancreatic β-cell function. Diabetes 2007; 56: 3101– 3104 [DOI] [PubMed] [Google Scholar]
- 22.Kirchhoff K, Machicao F, Haupt A, Schäfer SA, Tschritter O, Staiger H, Stefan N, Häring HU, Fritsche A: Polymorphisms in the TCF7L2, CDKAL1 and SLC30A8 genes are associated with impaired proinsulin conversion. Diabetologia 2008; 51: 597– 601 [DOI] [PubMed] [Google Scholar]
- 23.Rong R, Hanson RL, Ortiz D, Wiedrich C, Kobes S, Knowler WC, Bogardus C, Baier LJ: Association analysis of variation in or near FTO, CDKAL1, SLC30A8, HHEX, EXT2, IGF2BP2, LOC387761, and CDKN2B with type 2 diabetes and related quantitative traits in Pima Indians. Diabetes 2008; 58: 478– 488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palmer ND, Goodarzi MO, Langefeld CD, Ziegler J, Norris JM, Haffner SM, Bryer-Ash M, Bergman RN, Wagenknecht LE, Taylor KD, Rotter JI, Bowden DW: Quantitative trait analysis of type 2 diabetes susceptibility loci identified from whole genome association studies in the Insulin Resistance Atherosclerosis Family Study. Diabetes 2008; 57: 1093– 1100 [DOI] [PubMed] [Google Scholar]
- 25.Groenewoud MJ, Dekker JM, Fritsche A, Reiling E, Nijpels G, Heine RJ, Maassen JA, Machicao F, Schäfer SA, Häring HU, 't Hart LM, van Haeften TW: Variants of CDKAL1 and IGF2BP2 affect first-phase insulin secretion during hyperglycaemic clamps. Diabetologia 2008; 51: 1659– 1663 [DOI] [PubMed] [Google Scholar]
- 26.Stančáková A, Pihlajamäki J, Kuusisto J, Stefan N, Fritsche A, Häring H, Andreozzi F, Succurro E, Sesti G, Boesgaard TW, Hansen T, Pedersen O, Jansson PA, Hammarstedt A, Smith U, Laakso M: the EUGENE2 Consortium. Single-nucleotide polymorphism rs7754840 of CDKAL1 is associated with impaired insulin secretion in nondiabetic offspring of type 2 diabetic subjects and in a large sample of men with normal glucose tolerance. J Clin Endocrinol Metab 2008; 93: 1924– 1930 [DOI] [PubMed] [Google Scholar]
- 27.Pascoe L, Frayling TM, Weedon MN, Mari A, Tura A, Ferrannini E, Walker M: the RISC Consortium. β-Cell glucose sensitivity is decreased by 39% in non-diabetic individuals carrying multiple diabetes-risk alleles compared with those with no risk alleles. Diabetologia 2008; 51: 1989– 1992 [DOI] [PubMed] [Google Scholar]
- 28.Palmer ND, Lehtinen AB, Langefeld CD, Campbell JK, Haffner SM, Norris JM, Bergman RN, Goodarzi MO, Rotter JI, Bowden DW: Association of TCF7L2 gene polymorphisms with reduced acute insulin response in Hispanic Americans. J Clin Endocrinol Metab 2008; 93: 304– 309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Munoz J, Lok KH, Gower BA, Fernandez JR, Hunter GR, Lara-Castro C, De Luca M, Garvey WT: Polymorphism in the transcription factor 7-like 2 (TCF7L2) gene is associated with reduced insulin secretion in nondiabetic women. Diabetes 2006; 55: 3630– 3634 [DOI] [PubMed] [Google Scholar]
- 30.Saxena R, Gianniny L, Burtt NP, Lyssenko V, Giuducci C, Sjögren M, Florez JC, Almgren P, Isomaa B, Orho-Melander M, Lindblad U, Daly MJ, Tuomi T, Hirschhorn JN, Ardlie KG, Groop LC, Altshuler D: Common single nucleotide polymorphisms in TCF7L2 are reproducibly associated with type 2 diabetes and reduce the insulin response to glucose in nondiabetic individuals. Diabetes 2006; 55: 2890– 2895 [DOI] [PubMed] [Google Scholar]
- 31.Lyssenko V, Nagorny CL, Erdos MR, Wierup N, Jonsson A, Spégel P, Bugliani M, Saxena R, Fex M, Pulizzi N, Isomaa B, Tuomi T, Nilsson P, Kuusisto J, Tuomilehto J, Boehnke M, Altshuler D, Sundler F, Eriksson JG, Jackson AU, Laakso M, Marchetti P, Watanabe RM, Mulder H, Groop L: Common variant in MTNR1B associated with increased risk of type 2 diabetes and impaired early insulin secretion. Nat Genet 2009; 41: 82– 88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Staiger H, Machicao F, Schäfer SA, Kirchhoff K, Kantartzis K, Guthoff M, Silbernagel G, Stefan N, Häring HU, Fritsche A: Polymorphisms within the novel type 2 diabetes risk locus MTNR1B determine β-cell function. PLoS ONE 2008; 3: e3962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grarup N, Andersen G, Krarup NT, Albrechtsen A, Schmitz O, Jørgensen T, Borch-Johnsen K, Hansen T, Pedersen O: Association testing of novel type 2 diabetes risk alleles in the JAZF1, CDC123/CAMK1D, TSPAN8, THADA, ADAMTS9, and NOTCH2 loci with insulin release, insulin sensitivity, and obesity in a population-based sample of 4,516 glucose-tolerant middle-aged Danes. Diabetes 2008; 57: 2534– 2540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Staiger H, Machicao F, Kantartzis K, Schäfer SA, Kirchhoff K, Guthoff M, Silbernagel G, Stefan N, Fritsche A, Häring HU: Novel meta-analysis-derived type 2 diabetes risk loci do not determine prediabetic phenotypes. PLoS ONE 2008; 3: e3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nielsen EM, Hansen L, Carstensen B, Echwald SM, Drivsholm T, Glümer C, Thorsteinsson B, Borch-Johnsen K, Hansen T, Pedersen O: The E23K variant of Kir6.2 associates with impaired post-OGTT serum insulin response and increased risk of type 2 diabetes. Diabetes 2003; 52: 573– 577 [DOI] [PubMed] [Google Scholar]
- 36.Sparsø T, Andersen G, Albrechtsen A, Jørgensen T, Borch-Johnsen K, Sandbaek A, Lauritzen T, Wasson J, Permutt MA, Glaser B, Madsbad S, Pedersen O, Hansen T: Impact of polymorphisms in WFS1 on prediabetic phenotypes in a population-based sample of middle-aged people with normal and abnormal glucose regulation. Diabetologia 2008; 51: 1646– 1652 [DOI] [PubMed] [Google Scholar]
- 37.Florez JC, Jablonski KA, McAteer J, Sandhu MS, Wareham NJ, Barroso I, Franks PW, Altshuler D, Knowler WC: Diabetes Prevention Program Research Group: testing of diabetes-associated WFS1 polymorphisms in the Diabetes Prevention Program. Diabetologia 2008; 51: 451– 457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wegner L, Hussain MS, Pilgaard K, Hansen T, Pedersen O, Vaag A, Poulsen P: Impact of TCF7L2 rs7903146 on insulin secretion and action in young and elderly Danish twins. J Clin Endocrinol Metab 2008; 93: 4013– 4019 [DOI] [PubMed] [Google Scholar]
- 39.González-Sánchez JL, Martínez-Larrad MT, Zabena C, Pérez-Barba M, Serrano-Ríos M: Association of variants of the TCF7L2 gene with increases in the risk of type 2 diabetes and the proinsulin: insulin ratio in the Spanish population. Diabetologia 2008; 51: 1993– 1997 [DOI] [PubMed] [Google Scholar]
- 40.Dahlgren A, Zethelius B, Jensevik K, Syvänen AC, Berne C: the ULSAM Cohort. Variants of the TCF7L2 gene are associated with beta cell dysfunction and confer an increased risk of type 2 diabetes mellitus in the ULSAM cohort of Swedish elderly men. Diabetologia 2007; 50: 1852– 1857 [DOI] [PubMed] [Google Scholar]
- 41.Loos RJ, Franks PW, Francis RW, Barroso I, Gribble FM, Savage DB, Ong KK, O'Rahilly S, Wareham NJ: TCF7L2 polymorphisms modulate proinsulin levels and β-cell function in a British Europid population. Diabetes 2007; 56: 1943– 1947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stolerman ES, Manning AK, McAteer JB, Fox CS, Dupuis J, Meigs JB, Florez JC: TCF7L2 variants are associated with increased proinsulin/insulin ratios but not obesity traits in the Framingham Heart Study. Diabetologia 2009; 52: 614– 620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang J, Kuusisto J, Vänttinen M, Kuulasmaa T, Lindström J, Tuomilehto J, Uusitupa M, Laakso M: Variants of the transcription 7-like 2 (TCF7L2) gene predict conversion to type 2 diabetes in the Finnish Diabetes Prevention Study and are associated with impaired glucose regulation and impaired glucose tolerance. Diabetologia 2007; 50: 1192– 1200 [DOI] [PubMed] [Google Scholar]
- 44.World Health Organization. Definition, Diagnosis and Classification of Diabetes Mellitus and its Complications. Part 1: Diagnosis and Classification of Diabetes Mellitus Geneva, Department of Noncommunicable Disease Surveillance, 1999 [Google Scholar]
- 45.Matsuda M, DeFronzo RA: Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 1999; 22: 1462– 1470 [DOI] [PubMed] [Google Scholar]
- 46.Stančáková A, Javorský M, Kuulasmaa T, Haffner SM, Kuusisto J, Laakso M: Changes in insulin sensitivity and insulin release in relation to glycemia and glucose tolerance in 6,414 Finnish men. Diabetes 2009; 58: 1212– 1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Willer CJ, Speliotes EK, Loos RJ, Li S, Lindgren CM, Heid IM, Berndt SI, Elliott AL, Jackson AU, Lamina C, Lettre G, Lim N, Lyon HN, McCarroll SA, Papadakis K, Qi L, Randall JC, Roccasecca RM, Sanna S, Scheet P, Weedon MN, Wheeler E, Zhao JH, Jacobs LC, Prokopenko I, Soranzo N, Tanaka T, Timpson NJ, Almgren P, Bennett A, Bergman RN, Bingham SA, Bonnycastle LL, Brown M, Burtt NP, Chines P, Coin L, Collins FS, Connell JM, Cooper C, Smith GD, Dennison EM, Deodhar P, Elliott P, Erdos MR, Estrada K, Evans DM, Gianniny L, Gieger C, Gillson CJ, Guiducci C, Hackett R, Hadley D, Hall AS, Havulinna AS, Hebebrand J, Hofman A, Isomaa B, Jacobs KB, Johnson T, Jousilahti P, Jovanovic Z, Khaw KT, Kraft P, Kuokkanen M, Kuusisto J, Laitinen J, Lakatta EG, Luan J, Luben RN, Mangino M, McArdle WL, Meitinger T, Mulas A, Munroe PB, Narisu N, Ness AR, Northstone K, O'Rahilly S, Purmann C, Rees MG, Ridderstråle M, Ring SM, Rivadeneira F, Ruokonen A, Sandhu MS, Saramies J, Scott LJ, Scuteri A, Silander K, Sims MA, Song K, Stephens J, Stevens S, Stringham HM, Tung YC, Valle TT, Van Duijn CM, Vimaleswaran KS, Vollenweider P, Waeber G, Wallace C, Watanabe RM, Waterworth DM, Watkins N, Wellcome Trust Case Control Consortium. Witteman JC, Zeggini E, Zhai G, Zillikens MC, Altshuler D, Caulfield MJ, Chanock SJ, Farooqi IS, Ferrucci L, Guralnik JM, Hattersley AT, Hu FB, Jarvelin MR, Laakso M, Mooser V, Ong KK, Ouwehand WH, Salomaa V, Samani NJ, Spector TD, Tuomi T, Tuomilehto J, Uda M, Uitterlinden AG, Wareham NJ, Deloukas P, Frayling TM, Groop LC, Hayes RB, Hunter DJ, Mohlke KL, Peltonen L, Schlessinger D, Strachan DP, Wichmann HE, McCarthy MI, Boehnke M, Barroso I, Abecasis GR, Hirschhorn JN: Genetic Investigation of ANthropometric Traits Consortium: six new loci associated with body mass index highlight a neuronal influence on body weight regulation. Nat Genet 2009; 41: 25– 34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Perry JR, Frayling TM: New gene variants alter type 2 diabetes risk predominantly through reduced β-cell function. Curr Opin Clin Nutr Metab Care 2008; 11: 371– 377 [DOI] [PubMed] [Google Scholar]
- 49.Schäfer SA, Tschritter O, Machicao F, Thamer C, Stefan N, Gallwitz B, Holst JJ, Dekker JM, T'hart LM, Nijpels G, van Haeften TW, Häring HU, Fritsche A: Impaired glucagon-like peptide-1-induced insulin secretion in carriers of transcription factor 7-like 2 (TCF7L2) gene polymorphisms. Diabetologia 2007; 50: 2443– 2450 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.