Abstract
OBJECTIVE
Apolipoprotein CIII (apoCIII) is an independent risk factor for cardiovascular disease, but the molecular mechanisms involved are poorly understood. We investigated potential proatherogenic properties of apoCIII-containing LDL from hypertriglyceridemic patients with type 2 diabetes.
RESEARCH DESIGN AND METHODS
LDL was isolated from control subjects, subjects with type 2 diabetes, and apoB transgenic mice. LDL-biglycan binding was analyzed with a solid-phase assay using immunoplates coated with biglycan. Lipid composition was analyzed with mass spectrometry. Hydrolysis of LDL by sphingomyelinase was analyzed after labeling plasma LDL with [3H]sphingomyelin. ApoCIII isoforms were quantified after isoelectric focusing. Human aortic endothelial cells were incubated with desialylated apoCIII or with LDL enriched with specific apoCIII isoforms.
RESULTS
We showed that enriching LDL with apoCIII only induced a small increase in LDL-proteoglycan binding, and this effect was dependent on a functional site A in apoB100. Our findings indicated that intrinsic characteristics of the diabetic LDL other than apoCIII are responsible for further increased proteoglycan binding of diabetic LDL with high-endogenous apoCIII, and we showed alterations in the lipid composition of diabetic LDL with high apoCIII. We also demonstrated that high apoCIII increased susceptibility of LDL to hydrolysis and aggregation by sphingomyelinases. In addition, we demonstrated that sialylation of apoCIII increased with increasing apoCIII content and that sialylation of apoCIII was essential for its proinflammatory properties.
CONCLUSIONS
We have demonstrated a number of features of apoCIII-containing LDL from hypertriglyceridemic patients with type 2 diabetes that could explain the proatherogenic role of apoCIII.
Apolipoprotein CIII (apoCIII) is a protein secreted mostly by the liver and, to a lesser extent, by the intestine (1). In the circulation, it is associated mainly with triglyceride-rich lipoproteins (TRLs), HDLs, and, to a lower degree, LDLs (1–3). Total plasma apoCIII levels have been identified as a major determinant of serum triglycerides, and epidemiological studies have demonstrated that apoCIII and apoB lipoproteins that have apoCIII as a component independently predict coronary heart disease (4,5). It has been shown that lifelong deficiency of apoCIII has a cardioprotective effect (6) and that the content of apoCIII in LDL is an independent risk factor for coronary events in diabetic patients: those with the quartile of LDL with the highest apoCIII have a more than sixfold higher relative risk of new coronary events than the quartile with the lowest apoCIII content (5).
The molecular mechanisms that explain why apoCIII is a strong risk factor for cardiovascular disease are incompletely understood. Experiments in vitro show that apoCIII induces hypertriglyceridemia by inhibition of lipoprotein lipase (LPL) (7) and hepatic lipase (8) activity, disruption of the interaction of TRLs with heparan sulfate proteoglycans, and reduction of the clearance of apoB-containing lipoproteins (9). Kinetic studies in humans also indicate that apoCIII affects TRL metabolism (10,11). In addition, apoCIII alone, or as a component of TRL and LDL, induces activation of adhesion molecules and proinflammatory nuclear factor-κB in monocytes and endothelial cells (12,13), showing that it is directly involved in atherogenesis.
ApoCIII has also been shown to increase the binding of LDL to artery wall proteoglycans and increase accumulation of lipoproteins in the vessel wall (3,14). The mechanisms involved are unclear, as apoCIII does not bind directly to proteoglycans (14). Binding between LDLs and proteoglycans involves an ionic interaction between clusters of basic amino acids in apoB100 (site A at residues 3148–3158 and site B at residues 3359–3369) and negatively charged sulfate groups on the glycosaminoglycan chains of proteoglycans (15). Following subendothelial retention, LDL is exposed to several enzymes, including sphingomyelinases (SMase), which promote aggregation and fusion of retained lipoproteins (16). ApoCIII increases SMase-catalyzed hydrolysis by three- to fourfold in vitro (17).
ApoCIII is present in three isoforms, termed apoCIII0, apoCIII1, and apoCIII2 according to the number of sialic acid molecules bound to the protein (18). Each isoform has been shown to contribute, respectively, to ∼10, 55, and 35% of the total apoCIII levels in circulation (19). The degree of sialylation of apoCIII seems to influence its function as apoCIII2 inhibits LPL-mediated hydrolysis of TRLs less efficiently than apoCIII1 (20), despite having an apparent twofold greater affinity for TRL than the other two apoCIII isoforms (21). Furthermore, the kinetics of the sialylated isoforms shows the strongest associations with the expression of many features of the metabolic syndrome, including hypertriglyceridemia (22).
In this study, we investigated potential proatherogenic properties of apoCIII-containing LDL from patients with type 2 diabetes. In particular, we elucidated how apoCIII induces increased binding of LDL to artery wall proteoglycans. We also identified unique characteristics of LDL with the highest apoCIII content: for example, the lipoproteins associated with the highest relative risk of new coronary events in patients with type 2 diabetes (5).
RESEARCH DESIGN AND METHODS
Study population.
In the Helsinki Centre, 270 subjects with type 2 diabetes aged 50–75 years were recruited to the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study (23). Of these subjects, 101 volunteered for this substudy. A group of 93 healthy subjects aged 50–75 years (44 women) was recruited as control subjects. See online supplement for details available at http://diabetes.diabetesjournals.org/cgi/content/full/db09-0206/DC1.
Isolation of human LDL.
Blood samples were obtained after an overnight fast. Lipoproteins were isolated from fresh serum by sequential ultracentrifugation (24), and LDL subclasses were separated (25). See online supplement for details.
Isolation of recombinant LDL.
Recombinant LDL (d = 1.02–1.05 g/ml) were isolated from human apoB100 transgenic mice expressing RK3148–3158SQ LDL (site A mutant), RK3359–3369SA LDL (site B mutant), or recombinant control LDL by sequential ultracentrifugation and dialyzed against 150 mmol/l NaCl and 0.01% EDTA, pH 7.4 (26).
ApoB and apoCIII measurements.
ApoB was determined by an immunoprecipitation method (Thermo, Vantaa, Finland) and apoCIII by an immunoturbidimetric method (Kamiya, Seattle, WA). All analyses were performed on a Konelab 20 autoanalyzer (Thermo).
Immunoaffinity isolation of apoCIII-containing LDL.
LDL was isolated from 0.5 ml of serum from eight subjects with type 2 diabetes with buffers of physiological ionic strength and pH prepared with deuterium oxide (D2O) and sucrose (27). The LDL was subjected to immunoaffinity isolation using Dynabeads Protein A (Invitrogen, Carlsbad, CA) coated with Rabbit Anti-Human ApoCIII (Academy-Medical, Houston, TX).
Enrichment of LDL with apoCIII.
Enrichment of human or recombinant LDL with human purified apoCIII (Chemicon, Temecula, CA) was performed as described previously for apoE (28). ApoCIII-free LDL used for enrichment studies was isolated after immunoaffinity isolation.
Proteoglycan- and receptor-binding assays.
The binding of LDL to biglycan was assessed with a solid-phase assay using Maxisorp immunoplates (NUNC) coated with biglycan (28).
Lipid extraction and lipid class separation.
Lipid extraction and lipid class separation were performed as described previously (29,30). Lipid classes were detected and quantified using a PL-ELS 1000 detector (Polymer Laboratories, Amherst, MA). See online supplement for details.
Mass spectrometry.
The lipids were determined from a total lipid extract using a QSTAR XL QqTOF mass spectrometer (MDS Sciex, Concord, Canada) and normalized against the apoB protein value. See online supplement for details.
Isolation and quantification of GM1.
The GM1 ganglioside was extracted as previously described (31) and measured using a microtiter well-binding assay (32). See online supplement for details.
[3H]sphingomyelin labeling of LDL and SMase treatment of [N-palmitoyl-9,10-3H]sphingomyelin-LDL.
Plasma LDL was labeled with [N-palmitoyl-9,10-3H]sphingomyelin as described by Schissel et al. (16,33). SMase treatment of LDL was performed as described by Shissel et al. (16) with minor modifications according to Oorni et al. (34). See online supplement for details.
Quantitation of apoCIII isoforms.
ApoCIII isoforms were quantified in LDL after electric focusing and Western blot analysis as described by Wopereis et al. (19). Samples with LDL-apoCIII/apoB molar ratios >0.20 were analyzed. Five samples with higher LDL-apoCIII/apoB molar ratios were excluded from the analysis because of insufficient sample size.
Desialylation of apoCIII using neuroaminidase.
ApoCIII (Chemicon, Temecula, CA) was desialylated as described (21). See online supplement for details.
Incubation of human aortic endothelial cells with apoCIII.
Human aortic endothelial cells (HAECs) were seeded onto 6-well tissue culture plates between passages five and six. HAECs were incubated for 24 h without apoCIII (control subjects) or with 20 μg/ml neuraminidase-treated or untreated apoCIII. See online supplement for details.
Statistical analysis.
The statistical analysis was performed using ANOVA with all pair-wise multiple comparison procedures (Tukey's test). Binding parameters and their standard errors were determined using the nonlinear regression (curve-fitting) function of GraphPad Prism (GraphPad Software, San Diego, CA).
RESULTS
Increased binding of diabetic LDL to biglycan is only partially mediated by apoCIII.
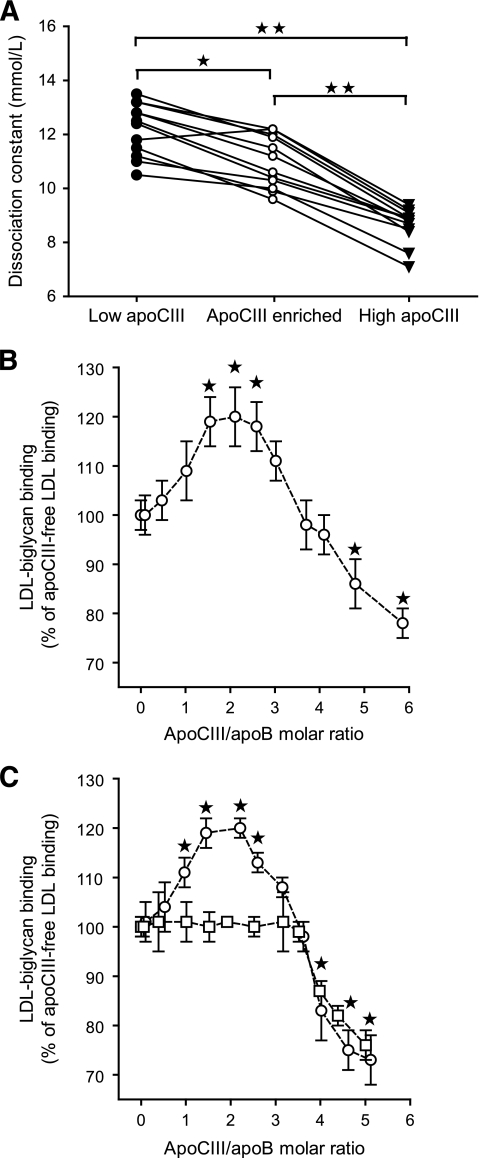
To investigate the mechanism for the increased proteoglycan binding of apoCIII-containing LDLs isolated from subjects with type 2 diabetes, we divided the patients into two groups according to median value of LDL-apoCIII. We paired each patient with high LDL-apoCIII with a patient with low LDL-apoCIII according to age, A1C, LDL size, and LDL cholesterol levels and identified 12 matched pairs. The matched pairs had comparable particle composition in all three LDL subclasses (supplementary Table 1). The LDL-apoCIII/apoB molar ratios in the low– and high–LDL-apoCIII groups were 0.56 ± 0.23 and 2.04 ± 0.72 (means ± SD), respectively (P < 0.001). LDL with high apoCIII displayed higher binding to biglycan than LDL with low apoCIII in every matched pair (Fig. 1A). The dissociation constants (Kd) of LDL in the low- and high-apoCIII groups were 12.2 ± 1.0 nmol/l and 8.6 ± 0.7 nmol/l (means ± SD), respectively (P < 0.001).
FIG. 1.
Effect of apoCIII content on binding of LDL to biglycan in vitro. A: The binding of LDL isolated from patients with type 2 diabetes was analyzed using solid-phase assays for biglycan in vitro. Twelve pairs of LDL with low (●) or high (▾) LDL-apoCIII were analyzed. The low– LDL-apoCIII samples were also enriched in vitro with human apoCIII (○) so they contained approximately as much apoCIII as the high LDL-apoCIII samples, and their binding was analyzed. The dissociation constants (Kd) of the corresponding samples in each matched pair are shown. *P = 0.005, **P < 0.001. B: LDL with no apoCIII was isolated from subjects with type 2 diabetes and enriched in vitro with increasing amounts of human purified apoCIII, and LDL binding was analyzed by solid-phase assays for biglycan. C: Recombinant wild-type LDL (○) and site A mutant LDL (□) were isolated from human apoB100 transgenic mice and enriched with increasing amounts of human purified apoCIII. The LDL binding was analyzed by solid-phase assays for biglycan and is expressed as a percentage of apoCIII-free LDL binding to biglycan (means ± SD, n = 4–5). *P < 0.05 versus apoCIII-free LDL.
To elucidate if increased binding of high LDL-apoCIII to proteoglycans is mediated by apoCIII or by other intrinsic properties of the LDL, we then enriched the low–LDL-apoCIII samples with apoCIII in vitro so that they on average contained as much apoCIII as the high–LDL-apoCIII samples (the maximal difference in each pair was 16%). Binding studies to biglycan showed that apoCIII-enriched LDL displayed higher binding to biglycan than LDL with low apoCIII but significantly lower binding than LDL with endogenously high apoCIII (Kd = 11.0 ± 1.0 nmol/l) (Fig. 1A). We also performed the identical binding study with nondiabetic control LDL. The results showed that increasing the apoCIII content of control LDL did not significantly increase its binding to biglycan (supplementary Fig. 1).
Taken together, these data indicate that apoCIII induces a small increase in binding of diabetic LDL to artery proteoglycans. However, other intrinsic characteristics of diabetic LDL are essential for further increased proteoglycan binding of apoCIII-containing LDL. We tested this hypothesis by analyzing the binding to biglycan of four of the 12 pairs of diabetic LDL with high or low apoCIII/apoB molar ratio after immunoaffinity removal of the apoCIII-containing LDL. The results showed that apoCIII-free LDL isolated from LDL with high apoCIII/apoB molar ratio bound significantly better than apoCIII-free LDL isolated from LDL with low apoCIII/apoB molar ratio (Kd = 9.8 ± 0.29 vs. 12.8 ± 0.57 nmol/l, respectively).
ApoCIII-induced increase in LDL-proteoglycan binding is mediated by site A in apoB.
To further elucidate the mechanism for how apoCIII induces increased proteoglycan binding, apoCIII-free LDL isolated from subjects with type 2 diabetes was enriched in vitro with increasing amounts of human apoCIII. Analysis showed that 41 ± 8% of the apoCIII-free LDL became enriched with apoCIII. Solid-phase proteoglycan binding studies showed that there was not a simple linear relationship between apoCIII content and binding of human LDL to biglycan (Fig. 1B). Initially, apoCIII enrichment increased the binding of LDL to biglycan, and maximal binding was achieved with an apoCIII/apoB molar ratio of approximately two: for example, the molar ratio seen in diabetic LDL with high apoCIII. Additional enrichment of LDL with apoCIII negatively influenced the binding to biglycan, and an apoCIII/apoB molar ratio higher than four inhibited binding of LDL to biglycan compared with apoCIII-free LDL.
We next performed an identical binding study with recombinant control LDL or recombinant LDL with apoB mutated at proteoglycan binding site A or site B. The binding of recombinant control LDL was almost identical to binding of human LDL (Fig. 1C). In contrast, site A mutant LDL did not display increased binding to biglycan after enrichment with apoCIII but displayed the same diminished binding to biglycan when the apoCIII/apoB molar ratio was higher than four (Fig. 1C). Recombinant site B mutant LDL failed to interact with biglycan both in the absence and presence of apoCIII (data not shown). Thus, the increased LDL-proteoglycan binding induced by apoCIII is dependent on a functional site A in apoB100, and enrichment with apoCIII does not substitute for the lack of a functional site B.
Altered lipid composition in diabetic LDL with high apoCIII/apoB molar ratio.
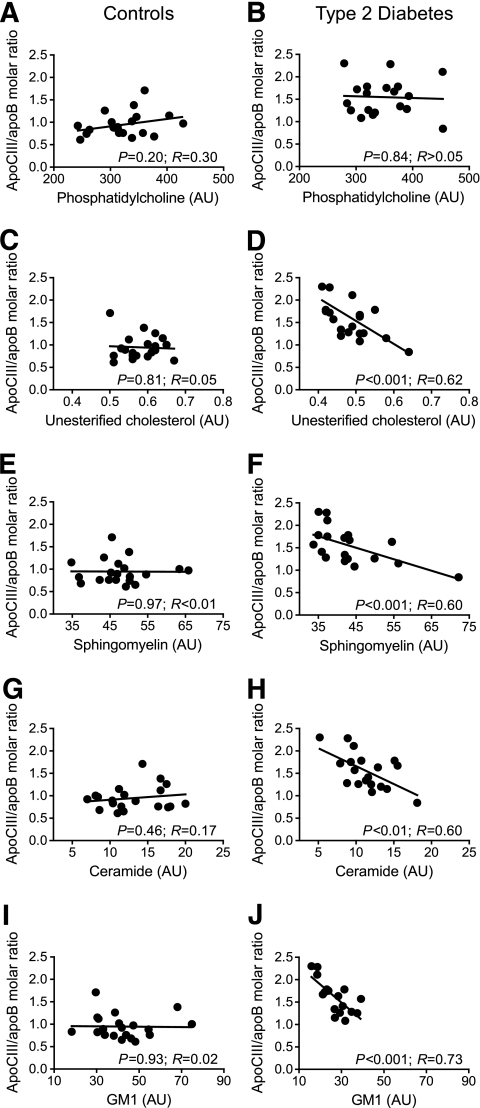
As our results showed that the increased binding of apoCIII-containing diabetic LDL to biglycan was only partially explained by apoCIII, other intrinsic characteristics of diabetic LDL with endogenously high apoCIII/apoB molar ratio must explain its increased proteoglycan binding compared with LDL enriched with apoCIII in vitro. We tested the hypothesis that the apoCIII content of diabetic LDL was associated with alterations in the lipid composition of the LDL particles by analyzing LDL isolated from control subjects (n = 20) or patients with type 2 diabetes (n = 20) (see supplementary Tables 2 and 3, available in an online appendix, for characteristics of these subjects).
The results showed that the content of phosphatidylcholine, the major membrane lipid on LDL, did not correlate with the apoCIII/apoB molar ratio in either LDL isolated from control subjects or that from subjects with type 2 diabetes (Fig. 2A and B). In contrast, we showed a highly significant negative correlation between the apoCIII/apoB molar ratio and the content of unesterified cholesterol, sphingomyelin, ceramide, and the ceramide-containing ganglioside GM1 in LDL isolated from subjects with type 2 diabetes but not in LDL isolated from control subjects (Fig. 2C–J). Furthermore, LDL isolated from subjects with type 2 diabetes contained significantly less GM1 than LDL isolated from control subjects (26.5 vs. 42.3 μmol/mg apoB, respectively, P < 0.001).
FIG. 2.
Correlation between the LDL-apoCIII/apoB molar ratio and lipid content of apoCIII-containing LDL isolated from control subjects (n = 20) or subjects with type 2 diabetes (n = 20). The levels of phosphatidylcholine (A and B), unesterified cholesterol (C and D), sphingomyelin (E and F), ceramide (G and H), and GM1 (I and J) were analyzed as described in research design and methods and normalized against the apoB level.
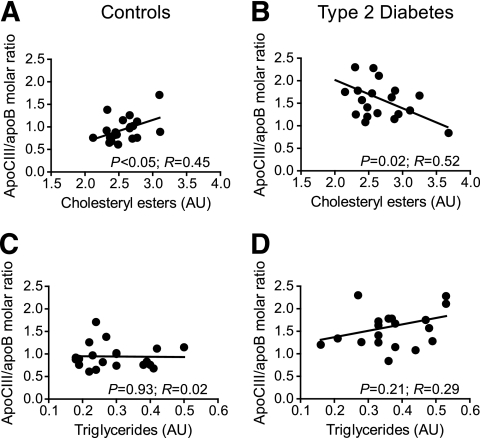
Analysis of core lipids showed a significant negative correlation between cholesteryl esters and the apoCIII/apoB molar ratio in LDL isolated from subjects with type 2 diabetes and a significant positive correlation in LDL isolated from control subjects (Fig. 3A and B). In contrast, triglycerides did not correlate with the apoCIII/apoB molar ratio in either LDL isolated from control subjects or that from patients with type 2 diabetes (Fig. 3C and D). However, there was a nonsignificant positive trend in LDL isolated from subjects with type 2 diabetes. We analyzed the association between LDL-apoCIII and LDL-triglycerides (TG) in all subjects. The results showed no significant correlation in the control group (n = 93) but a positive correlation in subjects with type 2 diabetes (r = 0.451, P = 0.001, n = 101). The correlations for the three LDL subclasses were LDL1-TG (r = 0.208, P = 0.047); LDL2-TG (r = 0.304, P = 0.003); and LDL3-TG (r = 0.200, P = 0.056).
FIG. 3.
Correlation between the LDL-apoCIII/apoB molar ratio and lipid content of apoCIII-containing LDL isolated from control subjects (n = 20) or subjects with type 2 diabetes (n = 20). The levels of cholesteryl esters (A and B) and triacylglycerol (C and D) were analyzed as described in research design and methods and normalized against the apoB level.
Not all LDL in the circulation contains apoCIII. We complemented the studies where we analyzed the total LDL fraction by comparing the lipid composition in apoCIII-containing and apoCIII-free LDL isolated from subjects with type 2 diabetes. The total LDL was isolated and subjected to anti-apoCIII immunoaffinity isolation. The results showed that 29 ± 5% of the LDL particles contained apoCIII and that the apoCIII-containing LDL contained significantly less unesterified and esterified cholesterol and sphingomyelin and ceramide as well as significantly more triglycerides than apoCIII-free LDL (Table 1).
TABLE 1.
Lipid composition of apoCIII-containing LDL and apoCIII-free LDL
| ApoCIII-containing LDL | ApoCIII-free LDL | P | |
|---|---|---|---|
| CE/phosphatidylcholine (ratio weight) | 2.2 ± 0.3 | 3.2 ± 1.0 | 0.03 |
| Triglycerides/phosphatidylcholine (ratio weight) | 0.21 ± 0.04 | 0.15 ± 0.03 | 0.008 |
| FC/phosphatidylcholine (ratio weight) | 0.44 ± 0.05 | 0.55 ± 0.09 | 0.007 |
| Sphingomyelin/phosphatidylcholine (ratio weight) | 0.30 ± 0.05 | 0.36 ± 0.06 | 0.007 |
| Ceramide (pmol/μg phosphatidylcholine) | 3.5 ± 1.0 | 4.7 ± 1.1 | 0.037 |
Data are means ± SD. ApoCIII-containing and apoCIII-free LDL was isolated from subjects with type 2 diabetes. The lipid composition of the isolated LDL was analyzed using mass spectrometry-based lipid analysis. The amounts of triglycerides, unesterified cholesterol, sphingomyelin, and ceramide were correlated to the amount of phosphatidylcholine (n = 8). CE, esterified cholesterol; FC, unesterified cholesterol.
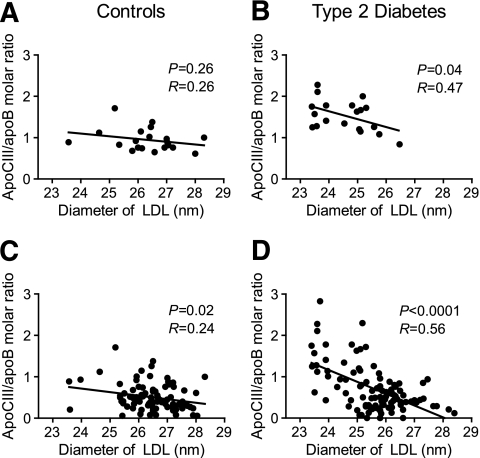
Analysis of the LDL-apoCIII/apoB molar ratio versus the LDL diameter of the analyzed samples shown in Fig. 2A–J showed a significant negative correlation in subjects with type 2 diabetes (P < 0.05) but not in control subjects (Fig. 4A and B). However, analysis of the LDL-apoCIII/apoB molar ratio versus the LDL diameter in all available samples showed a significant negative correlation in both control subjects (n = 93; P < 0.05) and subjects with type 2 diabetes (n = 101; P < 0.0001) (Fig. 4C and D).
FIG. 4.
Analysis of the LDL-apoCIII/apoB molar ratio versus the LDL diameter of the samples analyzed in Fig. 2 showed a significant negative correlation in subjects with type 2 diabetes (P < 0.05) but not in control subjects (Fig. 4A and B). Analysis of the LDL-apoCIII/apoB molar ratio versus the LDL diameter in all available samples showed a significant negative correlation in both control subjects (n = 93; P < 0.05) and subjects with type 2 diabetes (n = 101; P < 0.0001) (Fig. 4C and D).
High apoCIII increases susceptibility of LDL to hydrolysis and aggregation by sphingomyelinase.
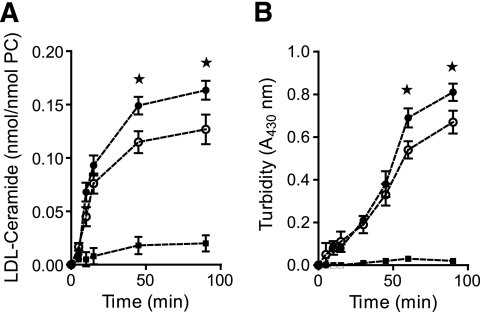
ApoCIII interacts with sphingomyelin and has been proposed to activate SMase (16). We analyzed the kinetics of SMase-induced hydrolysis of LDL and the kinetics of lipoprotein aggregation. LDL was isolated from subjects with type 2 diabetes and two groups were analyzed: apoCIII-free LDL and LDL enriched with apoCIII in vitro with an average of 4.3 apoCIII molecules per LDL particle. The kinetics showed that LDL enriched with apoCIII was significantly more hydrolyzed than apoCIII-free LDL (Fig. 5A). Furthermore, apoCIII-containing LDL aggregated more efficiently than apoCIII-free LDL (Fig. 5B). The results also showed that SMase-induced hydrolysis of LDL preceded the LDL aggregation.
FIG. 5.
Kinetics of SMase hydrolysis and LDL aggregation induced by SMase and the effects of enzymatic inhibition. A: LDL (0.5 mg/ml) was incubated with 50 ng/ml Bacillus cereus SMase/ml at 37°C. At the indicated time points, aliquots of the apoCIII-free LDL (○) or apoCIII-enriched LDL (●) were harvested then assayed for extent of SM hydrolysis (A) and for aggregation (B). As a negative control, LDL was incubated in the absence of SMase (■).
Increased apoCIII2 in subjects with type 2 diabetes.
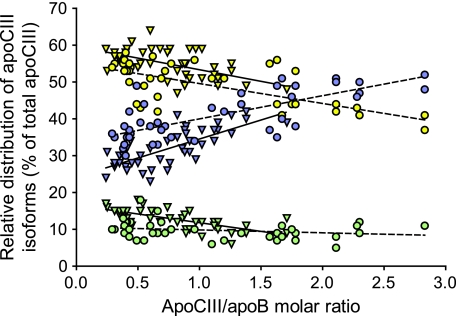
We analyzed the relative distribution of apoCIII isoforms in LDL isolated from control subjects (n = 44) and subjects with type 2 diabetes (n = 42). At comparable apoCIII/apoB molar ratios, LDL from patients with type 2 diabetes had more apoCIII2 and less apoCIII1 than that from control subjects (Fig. 6). Furthermore, the proportion of apoCIII0 was almost constant at 10%, whereas the apoCIII1 decreased and the apoCIII2 increased with increasing apoCIII/apoB molar ratio. Thus, apoCIII2 was the dominating form of apoCIII on LDL with more than two molecules apoCIII per LDL particle.
FIG. 6.
The relative distribution of apoCIII isoforms in LDL versus the LDL-apoCIII/apoB molar ratio. ApoCIII isoforms were quantified in LDL isolated from control subjects (n = 44; ▾) and subjects with type 2 diabetes (n = 42; ●), using isoelectric focusing and Western blot analysis. Disialylated apoCIII are shown in blue, monosialylated apoCIII are yellow, and nonsialylated apoCIII0 are green. The linear regression is shown separately for control subjects (filled line) and subjects with type 2 diabetes (dotted line). ApoCIII0 control subjects (r = −0.67, P < 0.0001); apoCIII0 DM2 (r = 0.24, NS); apoCIII1 control subjects (r = −0.59, P < 0.0001); apoCIII1 DM2 (r = −0.69, P < 0.0001); apoCIII2 control subjects (r = 0.76, P < 0.0001); and apoCIII2 DM2 (r = 0.73, P < 0.0001).
Sialylation of apoCIII is necessary for causing a cellular response in HAECs.
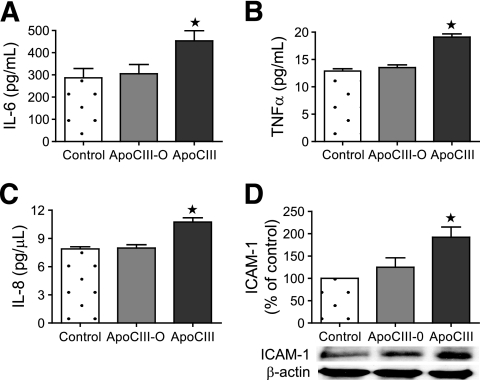
To test if the sialylation of apoCIII influences secretion of proinflammatory mediators and expression of adhesion molecules in HAECs, apoCIII was treated with neuroaminidase to remove the sialic acid residues. HAECs were incubated for 24 h without apoCIII (control subjects) with 20 μg/ml untreated apoCIII or with 20 μg/ml apoCIII-treated neuraminidase (desialylated). The untreated apoCIII contained ∼12% apoCIII0, 54% apoCIII1, and 34% apoCIII2. Immunoanalysis of the medium from cells incubated with untreated apoCIII showed significantly higher concentrations of IL-6, tumor necrosis factor (TNF)-α, and interleukin (IL)-8 than that in the medium from control cells (Fig. 6). In contrast, incubation of the cells with desialylated apoCIII did not influence the concentrations of IL-6, TNFα, or IL-8 in the medium (Fig. 7). Immunoblot analysis showed that untreated but not desialylated apoCIII induced significantly increased expression of the cellular protein intracellular adhesion molecule (ICAM)-1 compared with control (Fig. 7D).
FIG. 7.
Effect of desialylation of apoCIII on cellular response in HAECs. HAECs were incubated for 24 h without apoCIII (control subjects), with 20 μg/ml apoCIII desialylated apoCIII (ApoCIII-0), or with 20 μg/ml untreated apoCIII (ApoCIII). Levels of IL-6, TNF-α, and IL-8 in the cell culture medium were analyzed by multiplex analysis (A–C). Levels of ICAM-1 in total cell lysates were measured by immunoblot and corrected against β-actin level (means ± SD, n = 12). *P < 0.001.
We then isolated apoCIII0, apoCIII1, and apoCIII2; enriched apoCIII-free LDL particles with these apoCIII-isoforms at an apoCIII/apoB molar ratio of approximately four; and incubated HAECs for 36 h with 80 μg/ml LDL. The result showed that the medium from cells incubated with LDL containing apoCIII2 showed significantly higher concentrations of IL-6 (294 ± 34 vs. 221 ± 29 pg/ml), TNF-α (9.2 ± 2.1 vs. 6.8 ± 1.3 pg/ml), and IL-8 (6.4 ± 0.4 vs. 5.2 ± 0.3 pg/μl) than medium from cells incubated with apoCIII-free LDL (means ± SD, n = 5, P < 0.05). In contrast, incubation of cells with LDL enriched with apoCIII0 or apoCIII1 did not significantly increase the concentration of Il-6, TNFα, or IL-8 in the medium compared with media from cells incubated with apoCIII-free LDL (see supplementary Table 4, available in an online appendix). Immunoblot analysis showed that LDL enriched with apoCIII1 and apoCIII2, but not apoCIII0, induced significantly increased expression of ICAM-1 compared with apoCIII-free LDL (187 ± 18%; 224 ± 21 and 142 ± 28%, means ± SD, n = 5, P < 0.05).
DISCUSSION
In this study, we demonstrated that apoCIII induced a small increase only in proteoglycan binding for LDL from patients with type 2 diabetes. Our findings indicated that intrinsic characteristics of diabetic LDL other than apoCIII are essential for further increased proteoglycan binding of apoCIII-containing LDL, and we showed that the amount of LDL-apoCIII in diabetic LDL was associated with a greatly altered lipid composition of the LDL. We also showed that high apoCIII increased susceptibility of LDL to hydrolysis and aggregation by SMase. In addition, we demonstrated that sialylation of apoCIII increased with increasing LDL-apoCIII content and that the sialylation of apoCIII is essential for inducing a cellular response in HAECs.
To further elucidate the mechanism for how apoCIII induces increased proteoglycan binding we analyzed the impact of increasing LDL-apoCIII on binding to biglycan and observed that there was not a simple linear relationship: an increase in LDL-apoCIII at moderate levels increased the binding, whereas supraphysiological levels of LDL-apoCIII inhibited the binding. Interestingly, very high levels of LDL-apoCIII (apoCIII/apoB ration of 4.6) negatively affected the binding of LDL to the LDL receptor (data not shown), indicating that supraphysiological levels of LDL-apoCIII disturb the conformation of site B, the combined principal proteoglycan binding site (15), and the LDL receptor binding site of apoB (26), resulting in a diminished LDL receptor and proteoglycan binding.
We used recombinant LDL with apoB mutated at site A to show that the increased proteoglycan binding was dependent on a functional site A, and we propose that moderate levels of LDL-apoCIII induce a conformational change in apoB that activates site A. This proteoglycan binding site has been shown to become active in modified LDL and in small dense LDL (35).
We tested the hypothesis that an altered lipid composition in diabetic LDL with high endogenous apoCIII content could account for its increased proteoglycan binding compared with LDL enriched with apoCIII in vitro. The finding that apoCIII-containing LDL contained less unesterified cholesterol and more triglycerides than apoCIII-free LDL is in agreement with earlier studies (2,36). The triglyceride content of LDL is reciprocally related to the number of exposed free lysine amino groups of apoB100 (37), and we have earlier shown that the triglyceride content of LDL decreases the affinity for proteoglycans (35). Therefore, it is unlikely that the increased proteoglycan binding of apoCIII-containing LDL is mediated by the increased LDL triglycerides.
We analyzed the lipid monolayer in human LDL. This comprises mainly unesterified cholesterol, phosphatidylcholine, and sphingomyelin at ∼55, 30, and 15 mol%, respectively (38). We showed that a high apoCIII content in diabetic LDL was associated with a reduction in unesterified cholesterol, sphingomyelin, ceramide, and the ceramide-containing ganglioside GM1 but not in phosphatidylcholine. Cholesterol has been shown to positively affect a closer lateral packing in LDL (39), and ceramide induces a less fluid monolayer membrane (40). Therefore, the change in lipid composition observed in diabetic LDL with high apoCIII/apoB molar ratio could be associated with higher membrane fluidity and higher freedom in lateral moving, thus allowing apoB to acquire a conformation that is more favorable for proteoglycan binding. Furthermore, a monolayer rich of phosphatidylcholine and poor of unesterified cholesterol, in contrast to a monolayer rich in sphingomyelin and unesterified cholesterol, is likely to favor penetration of core lipids toward the water environment, thus making it possible for the water-soluble enzymes to reach the hydrophobic core lipids in the LDL particles (41).
Previous studies have reported that lipoproteins extracted from human atherosclerotic lesions are enriched in ceramide (33). Our results show that apoCIII-containing LDL contains less ceramide. A possible explanation for this discrepancy might be that ceramide could leave the LDL particles in the plasma but that ceramide generated locally by hydrolysis of SMase in atherosclerotic lesions remain bound to the LDL particle.
It is not known if the lipid composition of diabetic LDL is altered by increased apoCIII or if the high membrane fluidity of diabetic LDL simply allows more apoCIII molecules to bind to LDL. However, it has been reported that secretory SMase activity is elevated in the serum of patients with type 2 diabetes (42). In addition, depletion of cell surface sphingomyelin with SMase has been shown to result in increased efflux of unesterified cholesterol (43), which could explain the parallel decrease of sphingomyelin and unesterified cholesterol in diabetic LDL with high apoCIII. We showed that enrichment with apoCIII in vitro increased the susceptibility of LDL to hydrolysis and aggregation by SMase, indicating that diabetic LDL with high apoCIII content is particularly susceptible to proatherosclerotic modifications by SMase. Thus, it is possible that the lipid alterations we demonstrated in diabetic LDL with high apoCIII could be induced by both increased SMase activity and increased susceptibility of LDL to SMase. However, we cannot exclude the possibility that other mechanisms could induce the altered lipid composition of diabetic LDL with high apoCIII.
The results showed that the apoCIII content of control LDL did not significantly increase its binding to biglycan. However, apoCIII enrichment of recombinant LDL isolated from human apoB transgenic mice increased binding to biglycan. A possible explanation for this is that the recombinant LDL was isolated from mice fed a high-fat diet, and high-fat feeding is known to induce hyperlipidemia and impaired glucose tolerance in mice (44).
The increased susceptibility of apoCIII-enriched LDL to hydrolysis and aggregation by SMase could also contribute to the increased inflammatory response observed in atherosclerosis. For example, modification of LDL by SMase in the artery wall promotes the release of ceramide and arachidonic acid (45). It is not known how apoCIII stimulates the hydrolysis of sphingomyelin. However, results by Schissel et al. (16) indicate that apoCIII acts as a bridge between SMase and membrane sphingomyelin, thereby stimulating sphingomyelin hydrolysis. In addition, our lipid composition studies showed that LDL isolated from subjects with type 2 diabetes contained significantly less GM1 than LDL isolated from control subjects and that high apoCIII was associated with lower GM1 content. Interestingly, GM1 is an inhibitor of SMase (46). Thus, we showed that apoCIII-containing LDL both had enhanced susceptibility for SMase and contained a reduced amount of a SMase inhibitor. This combination appears to increase proatherogenic features of LDL. Furthermore, sphingomyelin appears to inhibit hydrolysis of LDL phospholipids by group IIa and group V sPLA2 (47), two enzymes known to promote atherosclerosis (48,49). Thus, the apoCIII-containing diabetic LDL with reduced sphingomyelin content likely displays increased susceptibility for hydrolysis by group IIa and group V sPLA2.
We also showed that LDL isolated from subjects with type 2 diabetes had more disialylated apoCIII and less monosialylated apoCIII compared with control subjects. Furthermore, the relative contribution of apoCIII2 increased with increasing apoCIII content. The results also showed an inverse correlation in diabetics between apoCIII/apoB molar ratio in LDL and LDL diameter. ApoCIII-containing LDL has been shown to be larger in size and richer in triglyceride than LDL without apoCIII (2,36). Thus, the results might indicate that diabetics with high apoCIII in LDL also have apoCIII-free small dense LDL. Indeed, recent results show that the kinetics of apoCIII1 and apoCIII2 are the strongest correlate of the expression of the small, dense LDL phenotype (22). We also showed that the sialylation of apoCIII was important for its proinflammatory properties. The sialylation of proteins, and especially of apolipoproteins, is poorly understood, and the molecular mechanism(s) that explains how increased sialylation influences the cellular response remains to be elucidated.
Lee et al. (50) showed that apoCIII was associated with plasma triglyceride concentration but that VLDL, intermediate-density lipoprotein, and LDL with apoCIII were similar in diabetic and nondiabetic groups. Because we did not include a comparable hypertriglyceridemic control group in this study, we cannot discriminate if the alterations in LDL are because of either the diabetes or the hypertriglyceridemia.
In conclusion, we have demonstrated features of diabetic LDL with high apoCIII that could explain the proatherogenic role of apoCIII. We showed that increases in apoCIII content only play a minor role in the increased proteoglycan binding of diabetic LDL with high apoCIII and propose that alterations in lipid composition in LDL with a high endogenous apoCIII/apoB molar ratio allow apoB to acquire a conformation that is more favorable for proteoglycan binding. We also propose that our observed alterations in lipid composition could be caused by an increased susceptibility of diabetic LDL with high apoCIII to SMase. Finally, our results suggest that increased levels of apoCIII sialylation on LDL could also increase the proatherogenic impact of diabetic LDL with high apoCIII content.
Supplementary Material
Acknowledgments
This work was supported by The Swedish Research Council, The Swedish Heart-Lung Foundation, the Swedish Foundation for Strategic Research, The Sigrid Juselius Foundation, the Helsinki University Central Hospital Research Foundation, and the EU-funded project ETHERPATHS (Contract FP7-KBBE-222639).
No potential conflicts of interest relevant to this article were reported.
We thank German Camejo and Kevin J. Williams for stimulating discussions and constructive criticism; Elin Björk, Hannele Hilden, Helinä Perttunen-Nio, Maria Heyden, and Kristina Skålén for expert technical assistance; and Rosie Perkins for editing the manuscript.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Jong MC, Hofker MH, Havekes LM: Role of ApoCs in lipoprotein metabolism: functional differences between ApoC1, ApoC2, and ApoC3. Arterioscler Thromb Vasc Biol 1999; 19: 472– 484 [DOI] [PubMed] [Google Scholar]
- 2.Campos H, Perlov D, Khoo C, Sacks FM: Distinct patterns of lipoproteins with apoB defined by presence of apoE or apoC-III in hypercholesterolemia and hypertriglyceridemia. J Lipid Res 2001; 42: 1239– 1249 [PubMed] [Google Scholar]
- 3.Davidsson P, Hulthe J, Fagerberg B, Olsson BM, Hallberg C, Dahllof B, Camejo G: A proteomic study of the apolipoproteins in LDL subclasses in patients with the metabolic syndrome and type 2 diabetes. J Lipid Res 2005; 46: 1999– 2006 [DOI] [PubMed] [Google Scholar]
- 4.Sacks FM, Alaupovic P, Moye LA, Cole TG, Sussex B, Stampfer MJ, Pfeffer MA, Braunwald E: VLDL, apolipoproteins B, CIII, and E, and risk of recurrent coronary events in the Cholesterol and Recurrent Events (CARE) trial. Circulation 2000; 102: 1886– 1892 [DOI] [PubMed] [Google Scholar]
- 5.Lee SJ, Campos H, Moye LA, Sacks FM: LDL containing apolipoprotein CIII is an independent risk factor for coronary events in diabetic patients. Arterioscler Thromb Vasc Biol 2003; 23: 853– 858 [DOI] [PubMed] [Google Scholar]
- 6.Pollin TI, Damcott CM, Shen H, Ott SH, Shelton J, Horenstein RB, Post W, McLenithan JC, Bielak LF, Peyser PA, Mitchell BD, Miller M, O'Connell JR, Shuldiner AR: A null mutation in human APOC3 confers a favorable plasma lipid profile and apparent cardioprotection. Science 2008; 322: 1702– 1705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang C-S, McConathy WJ, Kloer HU, Alaupovic P: Modulation of lipoprotein lipase activity by apolipoproteins. Effect of apolipoprotein C-III. J Clin Invest 1985; 75: 384– 390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kinnunen PKJ, Ehnholm C: Effect of serum and C-apoproteins from very low density lipoproteins on human postheparin plasma hepatic lipase. FEBS Lett 1976; 65: 354– 357 [DOI] [PubMed] [Google Scholar]
- 9.Ebara T, Ramakrishnan R, Steiner G, Shachter NS: Chylomicronemia due to apolipoprotein CIII overexpression in apolipoprotein E-null mice. Apolipoprotein CIII-induced hypertriglyceridemia is not mediated by effects on apolipoprotein E. J Clin Invest 1997; 99: 2672– 2681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zheng C, Khoo C, Ikewaki K, Sacks FM: Rapid turnover of apolipoprotein C-III-containing triglyceride-rich lipoproteins contributing to the formation of LDL subfractions J Lipid Res 2007; 48: 1190– 1203 [DOI] [PubMed] [Google Scholar]
- 11.Cohn JS, Patterson BW, Uffelman KD, Davignon J, Steiner G: Rate of production of plasma and very-low-density lipoprotein (VLDL) apolipoprotein C-III is strongly related to the concentration and level of production of VLDL triglyceride in male subjects with different body weights and levels of insulin sensitivity. J Clin Endocrinol Metab 2004; 89: 3949– 3955 [DOI] [PubMed] [Google Scholar]
- 12.Kawakami A, Aikawa M, Alcaide P, Luscinskas FW, Libby P, Sacks FM: Apolipoprotein CIII induces expression of vascular cell adhesion molecule-1 in vascular endothelial cells and increases adhesion of monocytic cells. Circulation 2006; 114: 681– 687 [DOI] [PubMed] [Google Scholar]
- 13.Kawakami A, Aikawa M, Nitta N, Yoshida M, Libby P, Sacks FM: Apolipoprotein CIII-induced THP-1 cell adhesion to endothelial cells involves pertussis toxin-sensitive G protein- and protein kinase C α-mediated nuclear factor-κB activation. Arterioscler Thromb Vasc Biol 2007; 27: 219– 225 [DOI] [PubMed] [Google Scholar]
- 14.Olin-Lewis K, Krauss RM, La Belle M, Blanche PJ, Barrett PH, Wight TN, Chait A: ApoC-III content of apoB-containing lipoproteins is associated with binding to the vascular proteoglycan biglycan. J Lipid Res 2002; 43: 1969– 1977 [DOI] [PubMed] [Google Scholar]
- 15.Boren J, Olin K, Lee I, Chait A, Wight TN, Innerarity TL: Identification of the principal proteoglycan-binding site in LDL. A single-point mutation in apo-B100 severely affects proteoglycan interaction without affecting LDL receptor binding. J Clin Invest 1998; 101: 2658– 2664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schissel SL, Jiang X, Tweedie-Hardman J, Jeong T, Camejo EH, Najib J, Rapp JH, Williams KJ, Tabas I: Secretory sphingomyelinase, a product of the acid sphingomyelinase gene, can hydrolyze atherogenic lipoproteins at neutral pH. Implications for atherosclerotic lesion development. J Biol Chem 1998; 273: 2738– 2746 [DOI] [PubMed] [Google Scholar]
- 17.Ahmad TY, Beaudet AL, Sparrow JT, Morrisett JD: Human lysosomal sphingomyelinase: substrate efficacy of apolipoprotein/sphingomyelin complexes. Biochemistry 1986; 25: 4415– 4420 [DOI] [PubMed] [Google Scholar]
- 18.Ito Y, Breslow JL, Chait BT: Apolipoprotein C-III0 lacks carbohydrate residues: use of mass spectrometry to study apolipoprotein structure. J Lipid Res 1989; 30: 1781– 1787 [PubMed] [Google Scholar]
- 19.Wopereis S, Grunewald S, Morava E, Penzien JM, Briones P, Garcia-Silva MT, Demacker PN, Huijben KM, Wevers RA: Apolipoprotein C-III isofocusing in the diagnosis of genetic defects in O-glycan biosynthesis. Clin Chem 2003; 49: 1839– 1845 [DOI] [PubMed] [Google Scholar]
- 20.Holdsworth G, Stocks J, Dodson P, Galton DJ: An abnormal triglyceride-rich lipoprotein containing excess sialylated apolipoprotein C-III. J Clin Invest 1982; 69: 932– 939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mann CJ, Troussard AA, Yen FT, Hannouche N, Najib J, Fruchart JC, Lotteau V, Andre P, Bihain BE: Inhibitory effects of specific apolipoprotein C-III isoforms on the binding of triglyceride-rich lipoproteins to the lipolysis-stimulated receptor. J Biol Chem 1997; 272: 31348– 31354 [DOI] [PubMed] [Google Scholar]
- 22.Mauger JF, Couture P, Bergeron N, Lamarche B: Apolipoprotein C-III isoforms: kinetics and relative implication in lipid metabolism. J Lipid Res 2006; 47: 1212– 1218 [DOI] [PubMed] [Google Scholar]
- 23.Hiukka A, Leinonen E, Jauhiainen M, Sundvall J, Ehnholm C, Keech AC, Taskinen MR: Long-term effects of fenofibrate on VLDL and HDL subspecies in participants with type 2 diabetes mellitus. Diabetologia 2007; 50: 2067– 2075 [DOI] [PubMed] [Google Scholar]
- 24.Taskinen MR, Kuusi T, Helve E, Nikkila EA, Yki-Jarvinen H: Insulin therapy induces antiatherogenic changes of serum lipoproteins in noninsulin-dependent diabetes. Arteriosclerosis 1988; 8: 168– 177 [DOI] [PubMed] [Google Scholar]
- 25.Griffin BA, Caslake MJ, Yip B, Tait GW, Packard CJ, Shepherd J: Rapid isolation of low density lipoprotein (LDL) subfractions from plasma by density gradient ultracentrifugation. Atherosclerosis 1990; 83: 59– 67 [DOI] [PubMed] [Google Scholar]
- 26.Boren J, Lee I, Zhu W, Arnold K, Taylor S, Innerarity TL: Identification of the low density lipoprotein receptor-binding site in apolipoprotein B100 and the modulation of its binding activity by the carboxyl terminus in familial defective apo-B100. J Clin Invest 1998; 101: 1084– 1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stahlman M, Davidsson P, Kanmert I, Rosengren B, Boren J, Fagerberg B, Camejo G: Proteomics and lipids of lipoproteins isolated at low salt concentrations in D2O/sucrose or in KBr. J Lipid Res 2008; 49: 481– 490 [DOI] [PubMed] [Google Scholar]
- 28.Skalen K, Gustafsson M, Rydberg EK, Hulten LM, Wiklund O, Innerarity TL, Boren J: Subendothelial retention of atherogenic lipoproteins in early atherosclerosis. Nature 2002; 417: 750– 754 [DOI] [PubMed] [Google Scholar]
- 29.Ekroos K, Chernushevich IV, Simons K, Shevchenko A: Quantitative profiling of phospholipids by multiple precursor ion scanning on a hybrid quadrupole time-of-flight mass spectrometer. Anal Chem 2002; 74: 941– 949 [DOI] [PubMed] [Google Scholar]
- 30.Homan R, Anderson MK: Rapid separation and quantitation of combined neutral and polar lipid classes by high-performance liquid chromatography and evaporative light-scattering mass detection. J Chromatogr B Biomed Sci Appl 1998; 708: 21– 26 [DOI] [PubMed] [Google Scholar]
- 31.Wang WQ, Gustafson A: Ganglioside extraction from erythrocytes: a comparison study. Acta Chem Scand 1995; 49: 929– 936 [DOI] [PubMed] [Google Scholar]
- 32.Teneberg S, Hirst TR, Angstrom J, Karlsson KA: Comparison of the glycolipid-binding specificities of cholera toxin and porcine Escherichia coli heat-labile enterotoxin: identification of a receptor-active non-ganglioside glycolipid for the heat-labile toxin in infant rabbit small intestine. Glycoconj J 1994; 11: 533– 540 [DOI] [PubMed] [Google Scholar]
- 33.Schissel SL, Tweedie-Hardman J, Rapp JH, Graham G, Williams KJ, Tabas I: Rabbit aorta and human atherosclerotic lesions hydrolyze the sphingomyelin of retained low-density lipoprotein. Proposed role for arterial-wall sphingomyelinase in subendothelial retention and aggregation of atherogenic lipoproteins. J Clin Invest 1996; 98: 1455– 1464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oorni K, Posio P, Ala-Korpela M, Jauhiainen M, Kovanen PT: Sphingomyelinase induces aggregation and fusion of small very low-density lipoprotein and intermediate-density lipoprotein particles and increases their retention to human arterial proteoglycans. Arterioscler Thromb Vasc Biol 2005; 25: 1678– 1683 [DOI] [PubMed] [Google Scholar]
- 35.Flood C, Gustafsson M, Pitas RE, Arnaboldi L, Walzem RL, Boren J: Molecular mechanism for changes in proteoglycan binding on compositional changes of the core and the surface of low-density lipoprotein-containing human apolipoprotein B100. Arterioscler Thromb Vasc Biol 2004; 24: 564– 570 [DOI] [PubMed] [Google Scholar]
- 36.Khoo C, Campos H, Judge H, Sacks FM: Effects of estrogenic oral contraceptives on the lipoprotein B particle system defined by apolipoproteins E and C-III content. J Lipid Res 1999; 40: 202– 212 [PubMed] [Google Scholar]
- 37.Aviram M, Lund-Katz S, Phillips MC, Chait A: The influence of the triglyceride content of low density lipoprotein on the interaction of apolipoprotein B-100 with cells. J Biol Chem 1988; 263: 16842– 16848 [PubMed] [Google Scholar]
- 38.Gupta AK, Rudney H: Sphingomyelinase treatment of low density lipoprotein and cultured cells results in enhanced processing of LDL which can be modulated by sphingomyelin. J Lipid Res 1992; 33: 1741– 1752 [PubMed] [Google Scholar]
- 39.Ibdah JA, Lund-Katz S, Phillips MC: Molecular packing of high-density and low-density lipoprotein surface lipids and apolipoprotein A-I binding. Biochemistry 1989; 28: 1126– 1133 [DOI] [PubMed] [Google Scholar]
- 40.Sola R, Baudet MF, Motta C, Maille M, Boisnier C, Jacotot B: Effects of dietary fats on the fluidity of human high-density lipoprotein: influence of the overall composition and phospholipid fatty acids. Biochim Biophys Acta 1990; 1043: 43– 51 [DOI] [PubMed] [Google Scholar]
- 41.Hevonoja T, Pentikinen MO, Hyvönen MT, Kovanen PT, Ala-Korpela M: Structure of low density lipoprotein (LDL) particles: basis for understanding molecular changes in modified LDL. Biochimica Biophysica Acta 2000; 1488: 189– 210 [DOI] [PubMed] [Google Scholar]
- 42.Gorska M, Baranczuk E, Dobrzyn A: Secretory Zn2+-dependent sphingomyelinase activity in the serum of patients with type 2 diabetes is elevated. Horm Metab Res 2003; 35: 506– 507 [DOI] [PubMed] [Google Scholar]
- 43.Ohvo H, Olsio C, Slotte JP: Effects of sphingomyelin and phosphatidylcholine degradation on cyclodextrin-mediated cholesterol efflux in cultured fibroblasts. Biochim Biophys Acta 1997; 1349: 131– 141 [DOI] [PubMed] [Google Scholar]
- 44.Van Heek M, Compton DS, France CF, Tedesco RP, Fawzi AB, Graziano MP, Sybertz EJ, Strader CD, Davis HR, Jr: Diet-induced obese mice develop peripheral, but not central, resistance to leptin. J Clin Invest 199799: 385– 390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oestvang J, Bonnefont-Rousselot D, Ninio E, Hakala JK, Johansen B, Anthonsen MW: Modification of LDL with human secretory phospholipase A(2) or sphingomyelinase promotes its arachidonic acid-releasing propensity. J Lipid Res 2004; 45: 831– 838 [DOI] [PubMed] [Google Scholar]
- 46.Fanani ML, Maggio B: Mutual modulation of sphingomyelinase and phospholipase A2 activities against mixed lipid monolayers by their lipid intermediates and glycosphingolipids. Mol Membr Biol 1997; 14: 25– 29 [DOI] [PubMed] [Google Scholar]
- 47.Gesquiere L, Cho W, Subbaiah PV: Role of group IIa and group V secretory phospholipases A(2) in the metabolism of lipoproteins. Substrate specificities of the enzymes and the regulation of their activities by sphingomyelin. Biochemistry 2002; 41: 4911– 4920 [DOI] [PubMed] [Google Scholar]
- 48.Bostrom MA, Boyanovsky BB, Jordan CT, Wadsworth MP, Taatjes DJ, de Beer FC, Webb NR: Group v secretory phospholipase A2 promotes atherosclerosis: evidence from genetically altered mice. Arterioscler Thromb Vasc Biol 2007; 27: 600– 606 [DOI] [PubMed] [Google Scholar]
- 49.Sartipy P, Camejo G, Svensson L, Hurt-Camejo E: Phospholipase A(2) modification of low density lipoproteins forms small high density particles with increased affinity for proteoglycans and glycosaminoglycans. J Biol Chem 1999; 274: 25913– 25920 [DOI] [PubMed] [Google Scholar]
- 50.Lee SJ, Moye LA, Campos H, Williams GH, Sacks FM: Hypertriglyceridemia but not diabetes status is associated with VLDL containing apolipoprotein CIII in patients with coronary heart disease. Atherosclerosis 2003; 167: 293– 302 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.