Iatrogenic hypoglycemia is the limiting factor in the glycemic management of diabetes (1,2). It causes recurrent morbidity in most people with type 1 diabetes as well as many with advanced type 2 diabetes and is sometimes fatal. It precludes maintenance of euglycemia over a lifetime of diabetes and, therefore, full realization of the benefits of glycemic control. It compromises physiological and behavioral defenses against subsequent falling plasma glucose concentrations and thus causes a vicious cycle of recurrent hypoglycemia. Insight into the pathophysiology of glucose counterregulation will continue to lead to understanding of the frequency and impact of, risk factors for, and prevention of iatrogenic hypoglycemia (1,2).
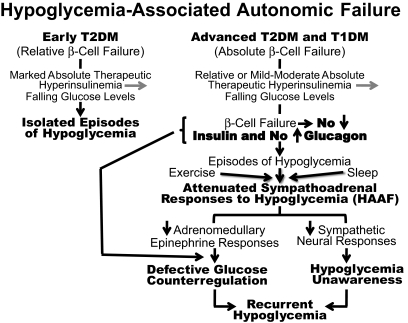
Marked absolute therapeutic hyperinsulinemia can cause isolated episodes of hypoglycemia in people with diabetes. However, episodes of hypoglycemia are more typically the result of the interplay of relative or mild-moderate absolute therapeutic hyperinsulinemia and compromised physiological and behavioral defenses against falling plasma glucose concentrations (1,2) (Fig. 1). The compromised physiological defenses include loss of decrements in β-cell insulin, loss of increments in α-cell glucagon, and, given the latter, attenuation of increments in adrenomedullary epinephrine. The compromised behavioral defense is the failure of carbohydrate ingestion resulting from loss of symptoms, which is largely the result of an attenuated sympathetic neural response. The concept of hypoglycemia-associated autonomic failure (HAAF) in diabetes posited, initially, that recent antecedent hypoglycemia causes both defective glucose counterregulation (by attenuating the epinephrine response in the setting of absent insulin and glucagon responses) and hypoglycemia unawareness (by attenuating largely the sympathetic neural and resulting symptomatic responses) and thus also causes a vicious cycle of recurrent hypoglycemia (1,2). The original concept of hypoglycemia-related HAAF was subsequently expanded to include exercise-related and sleep-related HAAF (1,2).
FIG. 1.
Schematic representation of the components of hypoglycemia-associated autonomic failure in diabetes (1,2). TD1M, type 1 diabetes; T2DM, type 2 diabetes.
The mechanism(s) of the key feature of these three types of HAAF in diabetes—an attenuated sympathoadrenal response to falling plasma glucose concentrations—is not known. That of hypoglycemia-related HAAF has been hypothetically attributed to 1) increased cortisol secretion during recent antecedent hypoglycemia, 2) increased blood-to-brain glucose transport resulting from recent antecedent hypoglycemia, or 3) effects of recent antecedent hypoglycemia on hypothalamic, or perhaps a cerebral network, functions (1,2).
With respect to the former mechanism, Davis et al. (3) reported that cortisol elevations, produced by cortisol infusions in a dose of 2.0 μg · kg−1 · min−1 over 2 h two times reduced the plasma epinephrine, norepinephrine, muscle sympathetic nerve activity, and pancreatic polypeptide responses to hypoglycemia the following day in nondiabetic humans. Therefore, they suggested that it is the increase in circulating cortisol, acting on the brain, during recent antecedent hypoglycemia that reduces the sympathoadrenal (adrenal medullae and sympathetic neural) as well as the parasympathetic neural responses to subsequent hypoglycemia. That notion was seemingly supported by the finding that pharmacological cortisol hypersecretion, produced by α1–24ACTH infusions, reduced the sympathoadrenal (plasma epinephrine, norepinephrine, and symptomatic) and parasympathetic (plasma pancreatic polypeptide) responses to hypoglycemia the following day (4). However, the interpretation of the original data (3) was undercut by the findings 1) that cortisol elevations comparable with those that occur during hypoglycemia did not reduce the sympathoadrenal and symptomatic responses to subsequent hypoglycemia (5,6) and 2) that blockade of cortisol secretion (with metyrapone) did not prevent the effect of recent antecedent hypoglycemia to reduce the plasma epinephrine responses to hypoglycemia the following day in humans (6).
Obviously, the mechanisms of hypoglycemia-related, exercise-related, and sleep-related HAAF need not be the same. For example, recent antecedent hypoglycemia and prior exercise involve previous cortisol elevations but sleep-related HAAF does not (1,2).
The risk of hypoglycemia during or shortly after exercise, particularly in people with insulin-treated diabetes, is generally recognized (1,2). The risk of hypoglycemia occurring several hours after exercise, and thus often during the night (1,2,7,8), is less widely appreciated. In one series of patients with type 1 diabetes, one-quarter suffered nocturnal hypoglycemia in the absence of afternoon exercise and one-half suffered nocturnal hypoglycemia in the presence of afternoon exercise (8). That follows directly from the physiology of glucose counterregulation in nondiabetic individuals (9) and its pathophysiology during exercise in absolutely endogenous insulin-deficient diabetes: type 1 or advanced type 2 diabetes (10,11). In the setting of absent insulin and glucagon responses to falling glucose levels, attenuated sympathoadrenal responses are reduced further after exercise (10). Such patients have exercise-related HAAF.
In this issue of Diabetes, Bao et al. (12) report that cortisol elevations comparable with those that occur during hypoglycemia produced by cortisol infusions in a dose of 1.0 μg · kg−1 · min−1 over 2 h two times, reduce the plasma epinephrine, norepinephrine, and pancreatic polypeptide responses to exercise the following day in patients with type 1 diabetes. These data suggest that prior stress-related cortisol elevations, such as those during hypoglycemia, might be one factor contributing to the pathogenesis of exercise-related HAAF. However, an effect of prior cortisol elevations to reduce symptoms, an important feature of HAAF, was not reported. In addition, an effect of inhibition of prior cortisol synthesis to improve defenses against subsequent exercise-related HAAF was not provided.
Clearly, iatrogenic hypoglycemia is a problem for people with diabetes that has not been solved. Pending the prevention and cure of diabetes and the development of methods that provide plasma glucose–regulated insulin replacement or secretion, innovative research in humans as well as in experimental animals is needed if we are to eliminate hypoglycemia from the lives of people affected by diabetes.
Acknowledgments
The author's original research cited was supported, in part, by National Institutes of Health Grants R37 DK27085, MO1 RR00036 (now UL1 RR24992), P60 DK20579, and T32 DK07120 and by a fellowship award from the American Diabetes Association.
The author has served as a consultant to MannKind Corporation, Marcadia Biotech, and Merck and Co. in the past year. He receives no research support from, holds no stock in, and serves as a spokesperson for no pharmaceutical or device firm. No other potential conflicts of interest relevant to this article were reported.
This manuscript was prepared by Janet Dedeke.
Footnotes
See accompanying original article, p. 2100.
REFERENCES
- 1.Cryer PE: Hypoglycemia in Diabetes American Diabetes Association, Alexandria, VA, 2009 [Google Scholar]
- 2.Cryer PE: The barrier of hypoglycemia in diabetes. Diabetes 2008; 57: 3169– 3176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davis SN, Shavers C, Costa F, Mosqueda-Garcia R: Role of cortisol in the pathogenesis of deficient counterregulation after antecedent hypoglycemia in normal humans. J Clin Invest 1996; 98: 680– 691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McGregor VP, Banarer S, Cryer PE: Elevated endogenous cortisol reduces autonomic neuroendocrine and symptom responses to subsequent hypoglycemia. Am J Physiol Endocrinol Metab 2002; 282: E770– E777 [DOI] [PubMed] [Google Scholar]
- 5.Raju B, McGregor VP, Cryer PE: Cortisol elevations comparable to those that occur during hypoglycemia do not cause hypoglycemia-associated autonomic failure. Diabetes 2003; 52: 2083– 2089 [DOI] [PubMed] [Google Scholar]
- 6.Goldberg PA, Weiss R, McCrimmon RJ, Hintz EV, Dziura JD, Sherwin RS: Antecedent hypercortisolemia is not primarily responsible for generating hypoglycemia-associated autonomic failure. Diabetes 2006; 55: 1121– 1126 [DOI] [PubMed] [Google Scholar]
- 7.MacDonald MJ: Postexercise late-onset hypoglycemia in insulin-dependent diabetic patients. Diabetes Care 1987; 10: 584– 588 [DOI] [PubMed] [Google Scholar]
- 8.Tsalikian E, Mauras N, Beck RW, Tamborlane WV, Janz KF, Chase HP, Wysocki T, Weinzimer SA, Buckingham BA, Kollman C, Xing D, Ruedy KJ: the Diabetes Research In Children Network (DirecNet) Study Group. Impact of exercise on overnight glycemic control in children with type 1 diabetes mellitus. J Pediatr 2005; 147: 528– 534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galassetti P, Mann S, Tate D, Neill RA, Costa F, Wasserman DH, Davis SN: Effects of antecedent prolonged exercise on subsequent counterregulatory responses to hypoglycemia. Am J Physiol Endocrinol Metab 2001; 280: E908– E917 [DOI] [PubMed] [Google Scholar]
- 10.Sandoval DA, Guy DLA, Richardson MA, Ertl AC, Davis SN: Effects of low and moderate antecedent exercise on counterregulatory responses to subsequent hypoglycemia in type 1 diabetes. Diabetes 2004; 53: 1798– 1806 [DOI] [PubMed] [Google Scholar]
- 11.Ertl AC, Davis SN: Evidence for a vicious cycle of exercise and hypoglycemia in type 1 diabetes mellitus. Diabete Metab Res Rev 2004; 20: 124– 130 [DOI] [PubMed] [Google Scholar]
- 12.Bao S, Briscoe VJ, Tate DB, Davis SN: Effects of differing antecedent increases of plasma cortisol on counterregulatory responses during subsequent exercise in type 1 diabetes. Diabetes 2009; 58: 2100– 2108 [DOI] [PMC free article] [PubMed] [Google Scholar]