Antigen-based therapies (ABTs) seek to prevent or inhibit autoimmune diseases by inducing regulatory T-cell responses (active tolerance) or anergizing/deleting pathogenic T-cells (passive tolerance). The theoretical appeal of this therapeutic approach is that it may promote tolerance with little debilitation of the immune system. The clinical application of ABTs for autoimmune disease is still in its infancy. Although initial attempts to apply this therapeutic strategy in multiple sclerosis, rheumatoid arthritis, and type 1 diabetes met with failure, recent results from clinical studies hold promise that this approach may be able to delay the onset of type 1 diabetes as well as preserve β-cell function in latent autoimmune diabetes in adults (LADA) patients and in children newly diagnosed with type 1 diabetes. If verified, it would be an important translation of NOD mouse findings to clinical applications. NOD mouse studies, however, indicate that the immunological impact of ABTs is much more dynamic and complex than previously appreciated. The differences in type 1 diabetes pathogenesis between rodents and humans, as well as other immunotherapeutic approaches, have been extensively reviewed elsewhere (e.g., [1–3]). Here, we will focus on ABTs and discuss what has been learned about their immunological impact, the theoretical factors affecting their efficacy and safety, as well as potential markers of their therapeutic efficacy.
Inflammatory T-cell responses spread among β-cell antigens during the development of murine and human type 1 diabetes.
Type 1 diabetes is mediated by autoreactive T-cells recognizing β-cell autoantigens (β-CAAs). In NOD mice, autoreactive CD4+ T-cells are generally Th1 type (interferonγ secreting), and autoimmunity progressively spreads intra- and intermolecularly among β-CAAs such as insulin, GAD, heat shock protein (HSP), and IGRP during the disease process (4,5). Similarly, spontaneous CD8+ T-cell responses develop to β-CAAs such as insulin, GAD, IGRP, and others (6). Paralleling observations in NOD mice, autoreactive Th1-biased CD4+ and CD8+ T-cells arise in human type 1 diabetic patients to many of the same β-CAAs (6–9). This spreading of inflammatory T-cell responses creates a proinflammatory cascade, driving disease progression (10,11).
ABT-induced active tolerance.
Autoantigen administration in modes that induce regulatory T-cell responses such as Th2 (secreting interleukin [IL]-4 and IL-5), Th3 (secreting TGFβ), Tr1 (secreting IL-10), and CD4+CD25+Foxp3+ Treg (secreting IL-10 and TGFβ) can inhibit proinflammatory Th1 and Th17 responses and prevent disease in animal models of type 1 diabetes, multiple sclerosis, rheumatoid arthritis, and uveitis. This induction of active tolerance does not require information regarding the initiating target antigen or the specificity of effector T-cells. When therapy-induced regulatory T-cells encounter the antigen at sites of inflammation, they release anti-inflammatory cytokines, which locally suppress effector T-cells regardless of their specificity in a process termed bystander suppression (12). Even though inflammatory autoreactive T-cell responses persist, ABT-induced regulatory responses can establish a tissue-specific long-term functional tolerance without debilitating immune competence.
ABTs not only induce regulatory responses to the administered autoantigen, they also promote the spreading of regulatory responses to other target tissue autoantigens (13). For example, NOD mice treated with a single β-CAA in incomplete Freund's adjuvant (IFA, a Th2-promoting adjuvant) developed Th2 responses to the injected autoantigen as well as all the other tested β-CAAs (13,14). This spreading of regulatory responses is limited to β-CAA target determinants and does not occur in wild-type mice (13,15). ABT-induced Tr1 and Treg responses have also been observed to spread in the experimental autoimmune encephalomyelitis (EAE) model and after organ transplantation (16,17). Thus, like inflammatory responses, regulatory T-cell responses can spread, presumably through their secretion of cytokines and their effects on antigen-presenting cells (APCs) that promote the activation/expansion of similar T-cell responses (13,15,18–20).
This ABT-induced spreading of regulatory responses can be very broad and rapid. For example, when neonatal NOD mice were immunized with a single β-CAA in IFA, splenic Th2-type cell autoimmunity arose precociously to every tested β-CAA by the time the mice were 4 weeks old, which is much sooner than when autoreactivity to these β-CAAs becomes detectable in unmanipulated NOD mice (21). Evidently, prior to the onset of insulitis NOD mice have a broad array of β-CAA–reactive T-cells that can quickly activate and/or expand when sufficient costimulatory signals are provided. Because Th2 responses spread to every tested β-CAA target determinant, the number of regulatory T-cell responses resulting from spreading may far outnumber those primed to the administered β-CAA itself.
Besides increasing bystander suppression, the spreading of regulatory T-cell responses can exhaust naïve autoantigen-specific T-cell pools that could be recruited into the pathogenic response (22). Moreover, their anti-inflammatory cytokines can educate and modulate APCs into tolerogenic APCs, which can further promote regulatory T-cell responses (18–20). These coinciding effects may underlie the protective effects of ABT.
Factors affecting the efficacy of ABTs in NOD mice
Size matters.
A small antigen may contain only one determinant that is presented only by a particular major histocompatability complex, whereas a large protein contains many determinants presented by each major histocompatability complex allele product. Accordingly, the size of the autoantigen used in ABTs affects the magnitude of induced T-cell responses. Indeed, GAD but not insulin B-chain or HSPp277 vaccination prolonged syngenic islet graft survival in diabetic NOD mice (23), and treatment with a combination of GAD peptides prevented disease more effectively in NOD mice than the individual peptides (14).
Antigen expression pattern.
Each self-protein has a unique expression pattern leading to differences in central and peripheral tolerance induction and the number and avidity of antigen-specific (cognate) T-cells remaining in the repertoire (10). In humans, the expression level of proinsulin in the thymus is regulated by allelic variations that are linked with type 1 diabetes susceptibility (24). In NOD mice, proinsulin 2, but not proinsulin 1, is expressed in the thymus, and knocking out proinsulin 2 expression accelerates their disease process (25). Whether the GAD65 gene is transcribed in the NOD mouse thymus is controversial, and there is no detectable GAD enzymatic activity in their thymus (D.L.K., unpublished data). GADs are fairly rare proteins in neurons, and although GAD65 is expressed at very low levels in mouse β-cells (26), it is sufficient to allow a GAD65-specific CD8+ T-cell clone to rapidly destroy β-cells in NOD/scid mice (27). The low expression of GAD in tissues may underlie the large high-avidity repertoire of naïve GAD65-reactive T-cells in preautoimmune NOD mice, which is indicative of little negative selection (11). The size and avidity of naïve antigen-specific T-cell pools determines in large part the ability of that antigen to prime regulatory responses (28).
Stage of the disease process.
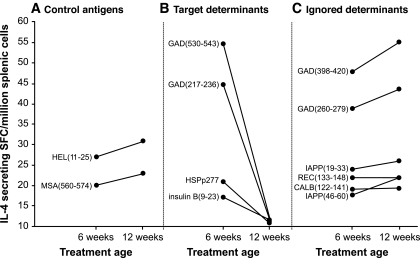
Although ABTs can be highly effective when administered early in the NOD mouse disease process, they become less effective as the autoimmune process progresses. The ability of foreign antigens and non–β-cell self-antigens to induce immune responses in NOD mice is unaffected by their disease process (Fig. 1A) (22,28). In contrast, there is a dramatic decline in the ability of whole β-CAAs and peptides containing β-CAA target determinants to prime Th2 immunity and Th2 spreading with disease progression (Fig. 1B) (22,28). This attenuation in the ability of β-CAAs to induce regulatory responses is likely to reflect the progressive recruitment of naïve β-CAA–reactive T-cells into the pathogenic response, which reduces the availability of naïve cognate T-cells that can be primed by ABT.
FIG. 1.
The ability of target determinants but not ignored determinants to induce Th2 responses attenuates with disease progression in NOD mice. NOD mice were immunized either at 6 or 12 weeks of age with a peptide containing a control immunogenic foreign (hen egg lysozyme [HEL]) or a self-determinant (mouse serum albumin [MSA]) (A), a major target determinant of a β-CAA (B), or an absolute cryptic determinant from within GAD or from within other β-cell antigens that are completely ignored by the autoimmune response (islet amyloid polypeptide [IAPP], reduced expression in cancer [REC], or calbindin [CALB]) (C) in IFA. Subsequently, the frequency of IL-4–secreting splenic T-cells responding to the injected antigen was determined by enzyme-linked immunosorbent spot. Data shown is the mean number of IL-4–secreting spot-forming colonies (SFC). Details about the ignored determinants are provided in (28).
After NOD mice develop mild hyperglycemia, ABTs have little ability to slow disease progression. NOD mice often progress from mild to severe hyperglycemia within 1–2 weeks, but it takes about 10–14 days for ABTs to induce maximal immune responses to the administered β-CAA and longer for regulatory T-cell responses to spread to other β-CAAs. By the time ABT-induced regulatory responses peak, insufficient β-cell mass remains. In contrast, anti-CD3 and other systemic immunosuppressants have an immediate effect and can reverse hyperglycemia in newly diabetic NOD mice (29). Even with anti-CD3 treatment, however, NOD mice must be treated before their blood glucose exceeds 400 mg/dl to be effective (30). This suggests that sufficient residual β-cell mass is required for the treatment to be beneficial, a notion supported by results from clinical trials with anti-CD3 and GAD vaccination (31,32).
Although ABTs do not efficiently reverse hyperglycemia in NOD mice, ABTs can induce potent regulatory responses after type 1 diabetes onset, evidenced by the ability of ABTs to prolong the survival of syngenic islet grafts in NOD mice (23,33). In these studies, diabetic NOD mice were given ABT, maintained on insulin, and implanted several weeks later with syngenic islets, thereby allowing sufficient time for the induction of regulatory responses. Vaccination with GAD, but not insulin B-chain or HSPp277, prolonged islet graft survival (23). This may reflect GAD's larger size and numerous immunogenic determinants as well as greater immunogenicity in NOD mice with advanced disease (22). Additionally, the size of the ABT-induced CD4+CD25+ Treg response was an important factor (33). These findings suggest that late in the disease process, regulatory responses may be best elicited with target tissue antigens that have large pools of uncommitted cognate T-cells available for priming.
Targeted versus ignored β-cell antigens.
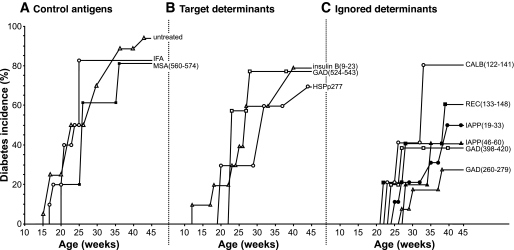
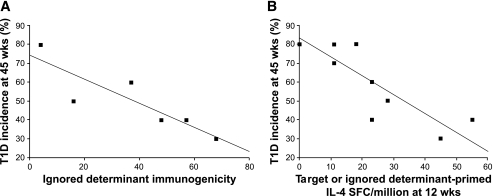
ABTs have focused on administering the autoantigens themselves. However, because the ability of β-CAAs to prime immune responses attenuates with disease progression, β-cell antigens that are completely uninvolved in the autoimmune process can, depending on their immunogenicity, prevent disease more effectively in NOD mice with an advanced disease process (Figs. 1–3; [28]). Such ABT-induced neoautoimmunity, however, may have unforeseen dangers. Likewise, the immunogenicity of synthetic peptides containing β-CAA target determinants attenuates, but the immunogenicity of determinants within the same β-CAA that are ignored by the autoimmune process (i.e., absolute cryptic determinants; Table 1; [34,35]) is unaffected by disease progression (Fig. 1C; [28]). Because absolute cryptic determinants are poorly presented from whole antigen and have had little impact on T-cell selection, there are large pools of cognate T-cells available for priming late in the disease process by treatment with a synthetic peptide containing the determinant (which bypasses whole antigen processing) (10,34,35). Vaccination of 12-week-old NOD mice with absolute cryptic determinants primed stronger regulatory T-cell responses and prevented type 1 diabetes more effectively than vaccination with the major target determinants of β-CAAs (Figs. 1 and 2). The immunogenicity of targeted and ignored determinants as well as the magnitude of induced Th2 responses were positively correlated with their ability to prevent type 1 diabetes in NOD mice (Fig. 3; [28]). These observations suggest that fragmenting a large autoantigen (e.g., by endopeptidases or by using a set of synthetic peptides) may expose many cryptic determinants and prime more regulatory responses than whole autoantigen. Inducing regulatory responses to absolute cryptic β-CAA determinants may be safe because 1) regulatory autoreactivity spreads only to β-CAA target determinants, like what occurs after traditional ABTs (15,28), and 2) it is less likely to boost pathogenic T-cell responses because cognate T-cells are not already activated in vivo. In the cancer vaccine field, immunization with cryptic determinants can elicit larger and higher-avidity T-cell responses than tumor antigen–dominant determinants in mice, and clinical trials using cryptic tumor antigen determinants are in progress (35,36).
FIG. 2.
Type 1 diabetes incidence in NOD mice immunized with a control (A), target (B), or ignored (C) antigen. Mice were immunized at 12 weeks of age with the indicated antigen. *P < 0.05 and **P < 0.01 relative to the unmanipulated NOD mouse group. (Details are provided in ref. 28). CALB, calbindin; IAPP, islet amyloid polypeptide; MSA, mouse serum albumin; REC, reduced expression in cancer.
FIG. 3.
Correlation between a β-CAA's ability to prime responses in NOD mice and its therapeutic efficacy. A: Correlation between a β-CAA ignored determinant's immunogenicity (as determined in ref. 28) and its therapeutic efficacy (type 1 diabetes incidence at 45 weeks in Fig. 2) (R = 0.87; P = 0.02). B: Correlation between the ability of a target or an ignored determinant to prime IL-4–secreting Th2 responses at 12 weeks of age and its therapeutic efficacy (R = 0.86; P = 0.002). (Details are provided in ref. 28). SFC, spot-forming colonies; T1D, type 1 diabetes.
TABLE 1.
Glossary
| Active tolerance | Active tolerance is based on the induction of immune responses that can suppress pathogenic responses, achieving a functional tolerance. |
| Passive tolerance | Passive tolerance is based on deleting/anergizing autoantigen-specific T-cells. |
| Determinant | A short continuous amino acid stretch within a peptide that binds to MHC class I or II, which is recognized by T-cell receptors. In contrast, an epitope comprises continuous or noncontinuous parts of a macromolecule and is recognized by an antibody. |
| Dominant determinant | Dominant determinants are efficiently processed from whole antigens and presented by MHC. |
| Cryptic determinant | These are immunogenic determinants that are poorly processed and presented from whole antigen but can elicit immune responses when delivered in synthetic peptides (which bypasses whole-antigen processing). Cryptic determinants often become targets of autoimmune responses because their presentation becomes sufficient to activate cognate T-cells in the context of inflammation (ref. 34). Other cryptic determinants are immunogenic but never become involved in the autoimmune response and are termed absolute cryptic determinants (ref. 35). Because these determinants have had little impact on T-cell selection, there are large pools of cognate T-cells available for priming by ABT (ref. 28). |
| LADA | LADA describes adults with a slowly progressive form of type 1 diabetes. The diagnosis of LADA is based on 1) adult onset of diabetes, 2) circulating islet autoantibodies, and 3) insulin independence at diagnosis. About 10% of adults with non–insulin-requiring diabetes have LADA. |
Prior immunological history affects subsequent immune responses.
In mice, preexisting Th1 responses can modify subsequent immune responses to another antigen administered in a Th2-promoting adjuvant (20). This modulation may be due to cytokines produced by the first wave of effector and memory cells or by education of APCs (19,20). Children vaccinated with Bacillus Calmette-Guerin have reduced incidence of atopic disease, indicating that an individual's prior immune responses influence subsequent responses (37). Interestingly, GAD/alum vaccination (20 μg) did not boost GAD autoantibodies in LADA patients who have a slowly progressing form of type 1 diabetes (Table 1), but greatly increased GAD autoantibodies in newly diabetic children, suggesting that the aggressiveness of an autoimmune response influences immune responses to ABT (see below). If preexisting autoimmune responses modulate ABT-induced responses, it may be beneficial to reduce pathogenic responses prior to ABT, to coadminister factors (e.g., cytokines) that will help enforce the induction of regulatory responses, and to optimize ABT dose and frequency for different stages of the autoimmune process.
ABT dose and route.
The subcutaneous administration of soluble self-antigen is generally only weakly immunogenic, but repeated treatments can prime regulatory responses (38,39). Mucosal delivery favors the generation of regulatory responses (12,40). Inhalation of antigen induces regulatory T-cell responses more effectively than delivering the antigen orally. The most efficient way to induce robust responses to self-antigens is to deliver them in adjuvants. Immunization with adjuvant provides a depot of antigen that can promote tolerogenic T-cells and APCs long-term in vivo. However, a more robust immune response has the inherent danger of inducing more undesired responses.
DNA vectors that express a β-CAA are stable and can codeliver other genes encoding immune-modulating proteins. Since CpG motifs in DNA act as proinflammatory adjuvants by binding to a Toll-like receptor, codelivery of immunoregulatory genes may be necessary to drive functionally dominant regulatory responses (e.g., [33]).
The effect of ABT dose and frequency in humans with ongoing autoimmunity is only beginning to be studied and remains a key issue for developing ABTs. We should presume that ABTs will evoke a mixture of immune responses including inducing regulatory T-cells, boosting pathogenic responses, and causing T-cell exhaustion, anergy, and deletion. The extent of each of these responses may depend on the dose, route, and frequency of treatment. Such mixed responses to ABT may explain why different dosages of oral insulin gave variable results in a small clinical trial with new-onset type 1 diabetic patients (41), why the beneficial effect of GAD/alum vaccination in LADA patients was confined to a particular dose and did not display dose dependency (42), and why GAD/alum-treated new-onset type 1 diabetic patients displayed a very diverse array of cytokine responses to GAD ([32], see below).
Passive tolerance.
ABTs can induce passive tolerance by anergizing/deleting autoreactive T-cells. Passive tolerance can also be created in transgenic animals by suppressing autoantigen expression or by eliminating target determinants from an autoantigen. Passive tolerance to insulin (43,44) and GAD (4,45) in NOD mice prevents the development of insulitis and type 1 diabetes. These observations may define primary β-cell autoantigen(s) or point to autoreactivities that are necessary drivers of the autoimmune response without which other nascent autoreactivities succumb to activation-induced cell death or regulation. Transgenic NOD mice that are largely, but incompletely, passively tolerized to GAD (46) initially have a broad reduction in T-cell responses to other β-CAAs, indicating that early T-cell autoreactivities require mutual support for expansion (J.T., D.L.K., unpublished data). As the mice grow older, however, they develop supernormal T-cell responses to other β-CAAs, such that disease incidence is unabated. Thus, deleting/anergizing many GAD-reactive T-cells allowed other β-CAA–reactive T-cells to eventually expand to a greater extent, perhaps by reducing competition for APCs or homeostatic proliferation in the target tissue. These findings underscore the dynamic nature of autoimmune responses after altering the T-cell repertoire to a single self-antigen and the potential for unexpected consequences.
It is unknown whether an initiating antigen exits in human type 1 diabetes. Central and peripheral tolerance induction mechanisms should only leave T-cells that interact with β-CAAs at subactivation thresholds. Theoretically, when a perturbation in antigen presentation arises from islet remodeling, metabolic demands, or local inflammation, T-cells (reactive to multiple β-CAAs) that were previously interacting with cognate β-CAAs at just below activation thresholds should receive sufficient stimulation to activate and expand, causing multiple initial beaks in self-tolerance. In this case, enforcing passive tolerance to a single β-CAA in preautoimmune humans may have little benefit, unless there are key driver T-cell autoreactivities.
The administration of self-antigens intravenously in the absence of sufficient costimulatory signals is a classic modality to induce passive tolerance. However, continued antigen treatments may be necessary to enforce passive tolerance because new naive T-cells will emerge from the thymus. Moreover, the induction of passive tolerance to single autoantigen after the onset of autoimmunity is unlikely to be effective because T-cell reactivities to other β-CAAs can drive disease progression. Accordingly, after the onset of autoimmunity, the induction of long-lived regulatory responses is, theoretically, a better therapeutic strategy.
Prevention trials of antigen-based therapies for those at risk for type 1 diabetes.
The Diabetes Prevention Trial (DPT)-1 tested whether daily subcutaneous insulin injections and annual intravenous insulin infusions could prevent disease in individuals at high risk for developing type 1 diabetes. This treatment was geared to induce β-cell rest, but immunological mechanisms might also be evoked because insulin treatment can boost insulin autoantibodies and induce Th2 responses (39). The DPT-1 found no effect on type 1 diabetes incidence (47). Because the treatment did not alter the subject's insulin autoantibody (IAA) levels, its immunological impact has been questioned. Another arm of the DPT-1 found that oral insulin treatment had no overall effect (48) but did delay type 1 diabetes onset in a subpopulation with high IAA (49). Understanding the beneficial effect of treatment in this subpopulation may help in designing more efficacious ABTs. Reasons for the overall lack of efficacy could include suboptimal dose, degradation of the immunogen, weak immunogenicity via this route, and simultaneous induction of regulatory and pathogenic responses.
The Type 1 Diabetes Prediction and Prevention Study concluded that daily intranasal insulin treatment could not prevent or delay diabetes, even when treatment began soon after autoantibodies arose (50). There were no changes in β-cell–reactive antibodies associated with treatment. In contrast, the Intranasal Insulin Trial showed that intranasal insulin administration to individuals at high risk for developing type 1 diabetes increased antibodies and decreased T-cell responses to insulin, and a subsequent clinical trial is in progress (40). Together, these initial prevention studies have been reassuring in terms of safety, but have raised questions about immunological impact, optimal mode of antigen delivery, and antigen dose.
Intervention trials of ABTs in recent-onset type 1 diabetic patients.
Disease prevention is preferable to intervention after disease onset, but requires screening individuals for type 1 diabetes risk markers and runs the risk of exacerbating the disease process. Intervention trials are more affordable because potential subjects are readily identified and efficacy can be evaluated within a shorter time frame. Preserving residual β-cell function after type 1 diabetes onset may prevent or delay long-term complications.
Insulin-based intervention trials.
There have been four clinical trials of oral insulin and one trial of an altered insulin B-chain peptide (NBI-6024, subcutaneous) in newly diabetic individuals (41,51–53). Most have observed no protective effect on residual β-cell function and no study-related change in autoantibodies to β-CAAs (51–53). One study observed a slower decline in plasma C-peptide levels in a subpopulation of patients (41). However, two studies found tendencies for basal C-peptide to decline faster in an oral insulin–treated subgroup (41,52), raising concern that treatment could in some cases accelerate the disease process. In other studies, intradermal treatment with a low dose (30 μg), but not a high dose (300 μg), of a soluble DR4-restricted proinsulin peptide induced transient IL-10–secreting T-cell responses in patients with long-standing type 1 diabetes (54).
Intervention trials using HSPp277.
There have been several small clinical trials of vaccination with an altered HSPp277 peptide in an adjuvant (Diapep), which induces IL-10– (predominantly), IL-4–, and IL-13 antigen–specific responses (rev. in [55]). Most of these studies have reported a beneficial effect. Individuals with higher IL-10 before treatment responded better to treatment. Paralleling this finding, another study found that new-onset type 1 diabetic patients with more IL-10 production are more likely to experience a honeymoon phase (56). These observations suggest that the individual's predispositions to develop regulatory responses and/or preexisting immune responses may influence ABT-induced responses.
GAD/alum vaccine in LADA and new-onset type 1 diabetes.
Clinical trials of GAD/alum vaccination were first conducted in individuals with LADA, a slowly progressing form of type 1 diabetes (Table 1) (42). Subjects received placebo or GAD/alum (4, 20, 100, or 500 μg) subcutaneously, twice. Only the 500-μg dose boosted GAD autoantibody levels. After 6 months, the CD4+CD25+/CD4+CD25− cell ratio, as well as serum C-peptide levels, increased from baseline only in the 20-μg–dose group. Evidently, ABT can have a beneficial immunomodulatory effect without changing humoral responses to the administered antigen, at least in LADA patients. A 5-year follow-up reported no significant study-related adverse effects and that C-peptide levels were significantly higher only in the 20-μg–dose group (57). If confirmed, this treatment may provide a safe long-term therapy to preserve β-cell function in LADA patients.
The mechanisms underlying the apparent effectiveness of the 20-μg dose, but not other dosages, are not yet evident. Multiple low doses of antigen often induce tolerance by priming regulatory responses. High antigen/adjuvant dosages often induce tolerance by exhausting antigen-specific T-cells but can promote humoral responses because some antigen escapes from the adjuvant and can directly activate memory B-cells, which may explain why the high GAD/alum dose was ineffective but boosted GAD autoantibodies. Alternatively, each GAD/alum dose may have evoked a different mixture of immune responses (i.e., regulatory responses, boosting of pathogenic responses, exhaustion, anergy, and deletion). The 20-μg dose may have induced the most favorable ratio of beneficial/pathogenic responses to promote functional tolerance. The notion that treatment induced a mixture of immune responses is supported by observations that compared with PBMCs from placebo-treated controls, PBMCs from GAD/alum-treated type 1 diabetic subjects secreted higher levels of a diverse array of cytokines in response to GAD (see below).
The beneficial effect of the 20-μg GAD/alum dose was further supported by a subsequent larger clinical trial with newly diabetic children (32). This study found that GAD/alum (20 μg) vaccination preserved β-cell function in patients treated within 6 months of type 1 diabetes onset but not in those treated >6 months after type 1 diabetes onset. The effectiveness of treatment in more recently diagnosed patients is likely to reflect greater remaining β-cell mass. This parallels findings with anti-CD3 treatment in which the treatment was most effective in those with the highest residual β-cell function at the time of treatment (31). The treatment induced higher levels of IL-5, IL-10, and IL-13 GAD-specific responses accompanied by higher frequency of Foxp3+ and TGFβ-secreting T-cells, even after 15 months (32). Interestingly, treatment also promoted higher levels of some proinflammatory cytokine responses to GAD. It is possible that these proinflammatory factors were counteracted by the enhanced regulatory responses or that these factors participated in regulatory circuits. In contrast to the LADA study, GAD/alum treatment greatly boosted GAD autoantibodies in new-onset type 1 diabetic patients, indicating a difference in immune responses to GAD/alum vaccination depending on whether the individual had a slow or more aggressive disease process. Further analysis revealed that the best responders to GAD/alum were individuals with higher initial levels of GAD autoantibody (58), an observation reminiscent of oral insulin's beneficial effects in individuals with high IAA at entry into the DPT-1 (49).
In this study, the major factor affecting a subject's responsiveness to therapy was time to treatment, suggesting that residual β-cell mass at the time of treatment is a major factor influencing GAD/alum effectiveness. In this regard, it is notable that LADA patients, who are likely to have more β-cell mass than new-onset type 1 diabetic patients (i.e., they are not yet insulin dependent), actually increased their C-peptide secretion over baseline 1 year after 20-μg GAD/alum treatment (42). Importantly, although insulin secretion gradually decreased in GAD/alum-treated new-onset type 1 diabetic patients, the rate of decline was similar to that reported after anti-CD3 treatment (31,32,59). However, GAD/alum therapy did not have significant adverse side effects. This, together with the relative stability of GAD/alum preparations, may make this treatment well suited for use in developing countries.
The safety of ABTs.
There is abundant evidence from animal models that ABTs can have potentially deleterious effects. For example, when neonatal NOD mice are vaccinated with any β-CAA in IFA, they develop precocious Th2 (predominantly) and some Th1 responses to the administered β-CAA by 4 weeks of age (21). Thus, despite treating NOD mice well before the onset of insulitis and using a Th2-promoting adjuvant, ABT promoted some Th1 responses to the injected β-CAA. Apparently, partially or fully activated Th1 cells reactive to many different β-CAAs are already present in NOD mice well before the development of insulitis, which can rapidly expand to detectable levels in the context of increased autoantigen stimulation (21). Additionally, oral insulin exacerbated disease in BB rats, insulin B(9–23)/IFA boosted cognate Th1 responses in older NOD mice, mucosal autoantigen delivery can prime pathogenic CD8+ T-cell responses (40), repeated administration of β-CAA peptides can induce anaphylaxis, Th2 cells can induce disease in immunodeficient mice, and induced autoimmunity to HSPs can lead to destruction of retinal cells in rats. Finally, GAD autoimmunity is often found in individuals with a rare neurological disorder called stiff-person's syndrome and sometimes in other rare neurodegenerative diseases, raising concerns that ABTs that boost GAD autoimmunity could have deleterious neurological consequences.
In type 1 diabetes clinical trials thus far, there is some indication that oral insulin can accelerate the reduction in C-peptide levels in some new-onset type 1 diabetic patient subgroups (41,52) and that depending on the dose, oral insulin may be beneficial or deleterious (41). Although the lack of ABT beneficial effects in most type 1 diabetes clinical trials has been ascribed to an insufficient immunological impact, it could also reflect ABTs inducing both beneficial and pathogenic responses that counteracted one another. Due to the potential to accelerate β-cell damage, ABT trials should first be tested for their ability to preserve residual β-cell mass in new-onset type 1 diabetic patients and assessed for their long-term safety before extending the treatment to the pre-diabetic population.
Markers of therapeutic efficacy.
Studies of ABTs in NOD mice, as well as studies of cancer vaccines in humans, have found that the magnitude of the antigen-induced immune response and the extent of determinant spreading of T-cell responses to other target tissue antigens are associated with better clinical outcomes (28,60). Currently, there are no surrogate immunological markers of ABT efficacy in humans. These are urgently needed to quickly assess candidate treatments rather than relying on the long-term clinical outcome. Although induction antigen–specific IgG1 is associated with the induction of regulatory Th2 responses in mice (e.g., [23]), such autoantibody isotype associations are not clear in humans. Moreover, humoral responses may not be associated with therapeutic efficacy, as observed in GAD/alum-treated LADA patients (42).
Determining the magnitude and functional phenotype of T-cell responses in humans currently requires multiple labor-intensive assays. Autoantigen-specific T-cells are present in blood at very low frequency, such that the development of assays that can reproducibly measure T-cell responses to multiple β-CAAs simultaneously at the single T-cell level will be valuable. These assays, together with the measurement of different subclasses of β-CAA–specific antibodies, may allow assessments of the efficacy of ABTs in clinical trials. In addition, imaging technologies such as magnetic resonance imaging and positron emission tomography have growing potential to noninvasively image islet inflammation and β-cell mass in humans, but are costly.
Future prospects.
ABTs have the advantage of being less immune invasive than immunosuppressive therapies and are relatively easy and inexpensive to manufacture, administer, and store. ABT clinical trials are largely focused on intervention after type 1 diabetes onset as a test platform. However, no ABT or systemic immune–modulating clinical intervention trial has achieved long-term euglycemia. Accordingly, there is great interest in combination therapies that combine an immune modulator (antigen specific or systemic immunosuppressant) with a factor that promotes β-cell regeneration. However, these treatments may need repeating, and permanent remission may be hard to safely establish in the context of established autoimmunity and greatly diminished β-cell mass.
Long-term goals should be fixed on preventing type 1 diabetes for multiple reasons: 1) there are excellent genetic and autoantibody markers of susceptibility, and type 1 diabetes has a long prodomal phase; 2) less invasive treatments may be efficacious at earlier stages of the disease process; 3) prevention costs may be less than those of insulin therapy and treating long-term complications. An ABT that bolsters regulatory responses to β-CAAs long-term in at-risk individuals appears to be the most promising and safe monotherapy. As preventive therapies become available, it will be important to have inexpensive high-throughput screening methods to identify those at risk for type 1 diabetes. Development of a blood spot collection card that can be mailed for autoantibody detection may enable cost-effective surveillance and disease prevention.
Areas of research that might yield insights for optimizing ABTs are presented in Table 2. The success of some ABTs in phase II clinical trials engenders cautious optimism that new treatments will enable the prevention of type 1 diabetes and help preserve β-cell function after type 1 diabetes onset.
TABLE 2.
Outstanding issues for ABTs for type 1 diabetes
| How does antigen dose, frequency, and route affect induction of regulatory T-cells and how can these parameters be optimized to avoid boosting preexisting pathogenic responses or de novo priming of pathogenic responses? |
| Will coadministration of other immune modulators with ABT help induce regulatory responses and minimize boosting of pathogenic responses? |
| What are reliable immunological surrogate markers of ABT therapeutic efficacy and safety? |
| Why did individuals with high IAA and GAD autoantibodies respond better to oral insulin treatment and GAD/alum vaccination, respectively (refs. 49,58)? |
| How can beneficial ABT-induced immune responses be maintained? |
| What are the noninvasive imaging markers of ABT therapeutic efficacy? |
Acknowledgments
D.L.K. is on the scientific advisory board of Diamyd Medical and is a coinventor of GAD-related patents. No other potential conflicts of interest relevant to this article were reported.
We regret that reference limitations preclude our citing many reports that provided important contributions to the field. We thank Angelica Olcott and other members of the Kaufman lab for their contributions as well as Michael Clare-Salzler for his comments on the manuscript.
REFERENCES
- 1.Roep BO, Atkinson M, von Herrath M: Satisfaction (not) guaranteed: re-evaluating the use of animal models of type 1 diabetes. Nat Rev Immunol 2004; 4: 989– 997 [DOI] [PubMed] [Google Scholar]
- 2.Akirav E, Kushner JA, Herold KC: β-Cell mass and type 1 diabetes: going, going, gone? Diabetes 2008; 57: 2883– 2888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Staeva-Vieira T, Peakman M, von Herrath M: Translational mini-review series on type 1 diabetes: immune-based therapeutic approaches for type 1 diabetes. Clin Exp Immunol 2007; 148: 17– 31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaufman DL, Clare-Salzler M, Tian J, Forsthuber T, Ting GS, Robinson P, Atkinson MA, Sercarz EE, Tobin AJ, Lehmann PV: Spontaneous loss of T-cell tolerance to glutamic acid decarboxylase in murine insulin-dependent diabetes. Nature 1993; 366: 69– 72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tisch R, Yang XD, Singer SM, Liblau RS, Fugger L, McDevitt HO: Immune response to glutamic acid decarboxylase correlates with insulitis in non-obese diabetic mice. Nature 1993; 366: 72– 75 [DOI] [PubMed] [Google Scholar]
- 6.Di Lorenzo TP, Peakman M, Roep BO: Translational mini-review series on type 1 diabetes: systematic analysis of T-cell epitopes in autoimmune diabetes. Clin Exp Immunol 2007; 148: 1– 16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arif S, Tree TI, Astill TP, Tremble JM, Bishop AJ, Dayan CM, Roep BO, Peakman M: Autoreactive T-cell responses show proinflammatory polarization in diabetes but a regulatory phenotype in health. J Clin Invest 2004; 113: 451– 463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ott PA, Herzog BA, Quast S, Hofstetter HH, Boehm BO, Tary-Lehmann M, Durinovic-Bello I, Berner BR, Lehmann PV: Islet-cell antigen-reactive T-cells show different expansion rates and Th1/Th2 differentiation in type 1 diabetic patients and healthy controls. Clin Immunol 2005; 115: 102– 114 [DOI] [PubMed] [Google Scholar]
- 9.Ott PA, Dittrich MT, Herzog BA, Guerkov R, Gottlieb PA, Putnam AL, Durinovic-Bello I, Boehm BO, Tary-Lehmann M, Lehmann PV: T-cells recognize multiple GAD65 and proinsulin epitopes in human type 1 diabetes, suggesting determinant spreading. J Clin Immunol 2004; 24: 327– 339 [DOI] [PubMed] [Google Scholar]
- 10.Lehmann PV, Targoni OS, Forsthuber TG: Shifting T-cell activation thresholds in autoimmunity and determinant spreading. Immunol Rev 1998; 164: 53– 61 [DOI] [PubMed] [Google Scholar]
- 11.Tian J, Gregori S, Adorini L, Kaufman DL: The frequency of high avidity T-cells determines the hierarchy of determinant spreading. J Immunol 2001; 166: 7144– 7150 [DOI] [PubMed] [Google Scholar]
- 12.Weiner HL: Oral tolerance: immune mechanisms and treatment of autoimmune diseases. Immunol Today 1997; 18: 335– 343 [DOI] [PubMed] [Google Scholar]
- 13.Tian J, Lehmann PV, Kaufman DL: Determinant spreading of T helper 2 (TH2) responses to pancreatic islet autoantigens. J Exp Med 1997; 186: 2039– 2044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tisch R, Wang B, Serreze DV: Induction of glutamic acid decarboxylase 65-specific Th2 cells and suppression of autoimmune diabetes at late stages of disease is epitope dependent. J Immunol 1999; 163: 1178– 1187 [PubMed] [Google Scholar]
- 15.Tian J, Olcott AP, Hanssen LR, Zekzer D, Middleton B, Kaufman DL: Infectious Th1 and Th2 immunity in diabetes prone mice. Immunol Rev 1998; 164: 119– 127 [DOI] [PubMed] [Google Scholar]
- 16.Yin L, Yu M, Edling AE, Kawczak JA, Mathisen PM, Nanavati T, Johnson JM, Tuohy VK: Pre-emptive targeting of the epitope spreading cascade with genetically modified regulatory T-cells during autoimmune demyelinating disease. J Immunol 2001; 167: 6105– 6112 [DOI] [PubMed] [Google Scholar]
- 17.Waldmann H, Adams E, Fairchild P, Cobbold S: Infectious tolerance and the long-term acceptance of transplanted tissue. Immunol Rev 2006; 212: 301– 313 [DOI] [PubMed] [Google Scholar]
- 18.Tang Q, Adams JY, Tooley AJ, Bi M, Fife BT, Serra P, Santamaria P, Locksley RM, Krummel MF, Bluestone JA: Visualizing regulatory T-cell control of autoimmune responses in nonobese diabetic mice. Nat Immunol 2006; 7: 83– 92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alpan O, Bachelder E, Isil E, Arnheiter H, Matzinger P: ‘Educated’ dendritic cells act as messengers from memory to naive T helper cells. Nat Immunol 2004; 5: 615– 622 [DOI] [PubMed] [Google Scholar]
- 20.Tian J, Lu Y, Hanssen L, Dang H, Kaufman DL: Memory and effector T-cells modulate subsequently primed immune responses to unrelated antigens. Cell Immunol 2003; 224: 74– 85 [DOI] [PubMed] [Google Scholar]
- 21.Tian J, Olcott AP, Kaufman DL: Antigen-based immunotherapy drives the precocious development of autoimmunity. J Immunol 2002; 169: 6564– 6569 [DOI] [PubMed] [Google Scholar]
- 22.Tian J, Kaufman DL: Attenuation of inducible Th2 immunity with autoimmune disease progression. J Immunol 1998; 161: 5399– 5403 [PubMed] [Google Scholar]
- 23.Tian J, Clare-Salzler M, Herschenfeld A, Middleton B, Newman D, Mueller R, Arita S, Evans C, Atkinson MA, Mullen Y, Sarvetnick N, Tobin AJ, Lehmann PV, Kaufman DL: Modulating autoimmune responses to GAD inhibits disease progression and prolongs islet graft survival in diabetes-prone mice. Nat Med 1996; 2: 1348– 1353 [DOI] [PubMed] [Google Scholar]
- 24.Pugliese A, Zeller M, Fernandez A, Jr, Zalcberg LJ, Bartlett RJ, Ricordi C, Pietropaolo M, Eisenbarth GS, Bennett ST, Patel DD: The insulin gene is transcribed in the human thymus and transcription levels correlated with allelic variation at the INS VNTR-IDDM2 susceptibility locus for type 1 diabetes. Nat Genet 1997; 15: 293– 297 [DOI] [PubMed] [Google Scholar]
- 25.Thebault-Baumont K, Dubois-Laforgue D, Krief P, Briand JP, Halbout P, Vallon-Geoffroy K, Morin J, Laloux V, Lehuen A, Carel JC, Jami J, Muller S, Boitard C: Acceleration of type 1 diabetes mellitus in proinsulin 2-deficient NOD mice. J Clin Invest 2003; 111: 851– 857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim J, Richter W, Aanstoot HJ, Shi Y, Fu Q, Rajotte R, Warnock G, Baekkeskov S: Differential expression of GAD65 and GAD67 in human, rat, and mouse pancreatic islets. Diabetes 1993; 42: 1799– 1808 [DOI] [PubMed] [Google Scholar]
- 27.Severe S, Gauvrit A, Vu AT, Bach JM: CD8+ T lymphocytes specific for glutamic acid decarboxylase 90–98 epitope mediate diabetes in NOD SCID mouse. Mol Immunol 2007; 44: 2950– 2960 [DOI] [PubMed] [Google Scholar]
- 28.Olcott AP, Tian J, Walker V, Dang H, Middleton B, Adorini L, Washburn L, Kaufman DL: Antigen-based therapies using ignored determinants of beta cell antigens can more effectively inhibit late-stage autoimmune disease in diabetes-prone mice. J Immunol 2005; 175: 1991– 1999 [DOI] [PubMed] [Google Scholar]
- 29.Chatenoud L, Thervet E, Primo J, Bach JF: Anti-CD3 antibody induces long-term remission of overt autoimmunity in nonobese diabetic mice. Proc Natl Acad Sci U S A 1994; 91: 123– 127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.von Herrath M: Can we learn from viruses how to prevent type 1 diabetes?: the role of viral infections in the pathogenesis of type 1 diabetes and the development of novel combination therapies. Diabetes 2009; 58: 2– 11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Keymeulen B, Vandemeulebroucke E, Ziegler AG, Mathieu C, Kaufman L, Hale G, Gorus F, Goldman M, Walter M, Candon S, Schandene L, Crenier L, De Block C, Seigneurin JM, De Pauw P, Pierard D, Weets I, Rebello P, Bird P, Berrie E, Frewin M, Waldmann H, Bach JF, Pipeleers D, Chatenoud L: Insulin needs after CD3-antibody therapy in new-onset type 1 diabetes. N Engl J Med 2005; 352: 2598– 2608 [DOI] [PubMed] [Google Scholar]
- 32.Ludvigsson J, Faresjo M, Hjorth M, Axelsson S, Cheramy M, Pihl M, Vaarala O, Forsander G, Ivarsson S, Johansson C, Lindh A, Nilsson NO, Aman J, Ortqvist E, Zerhouni P, Casas R: GAD treatment and insulin secretion in recent-onset type 1 diabetes. N Engl J Med 2008; 359: 1909– 1920 [DOI] [PubMed] [Google Scholar]
- 33.Pop SM, Wong CP, He Q, Wang Y, Wallet MA, Goudy KS, Tisch R: The type and frequency of immunoregulatory CD4+ T-cells govern the efficacy of antigen-specific immunotherapy in nonobese diabetic mice. Diabetes 2007; 56: 1395– 1402 [DOI] [PubMed] [Google Scholar]
- 34.Sercarz EE, Lehmann PV, Ametani A, Benichou G, Miller A, Moudgil K: Dominance and crypticity of T-cell antigenic determinants. Annu Rev Immunol 1993; 11: 729– 766 [DOI] [PubMed] [Google Scholar]
- 35.Moudgil KD, Sercarz EE: The self-directed T-cell repertoire: its creation and activation. Rev Immunogenet 2000; 2: 26– 37 [PubMed] [Google Scholar]
- 36.Bolonaki I, Kotsakis A, Papadimitraki E, Aggouraki D, Konsolakis G, Vagia A, Christophylakis C, Nikoloudi I, Magganas E, Galanis A, Cordopatis P, Kosmatopoulos K, Georgoulias V, Mavroudis D: Vaccination of patients with advanced non-small-cell lung cancer with an optimized cryptic human telomerase reverse transcriptase peptide. J Clin Oncol 2007; 25: 2727– 2734 [DOI] [PubMed] [Google Scholar]
- 37.Shirakawa T, Enomoto T, Shimazu S, Hopkin JM: The inverse association between tuberculin responses and atopic disorder. Science 1997; 275: 77– 79 [DOI] [PubMed] [Google Scholar]
- 38.Guéry JC, Galbiati F, Smiroldo S, Adorini L: Selective development of T helper (Th)2 cells induced by continuous administration of low dose soluble proteins to normal and beta(2)-microglobulin-deficient BALB/c mice. J Exp Med 1996; 183: 485– 497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tian J, Kaufman DL: Insulin selectively primes Th2 responses and induces regulatory tolerance to insulin in pre-diebetic mice. Diabetologia 1998; 41: 237– 240 [DOI] [PubMed] [Google Scholar]
- 40.Harrison LC: Vaccination against self to prevent autoimmune disease: the type 1 diabetes model. Immunol Cell Biol 2008; 86: 139– 145 [DOI] [PubMed] [Google Scholar]
- 41.Ergun-Longmire B, Marker J, Zeidler A, Rapaport R, Raskin P, Bode B, Schatz D, Vargas A, Rogers D, Schwartz S, Malone J, Krischer J, Maclaren NK: Oral insulin therapy to prevent progression of immune-mediated (type 1) diabetes. Ann N Y Acad Sci 2004; 1029: 260– 277 [DOI] [PubMed] [Google Scholar]
- 42.Agardh CD, Cilio CM, Lethagen A, Lynch K, Leslie RD, Palmer M, Harris RA, Robertson JA, Lernmark A: Clinical evidence for the safety of GAD65 immunomodulation in adult-onset autoimmune diabetes. J Diabetes Complications 2005; 19: 238– 246 [DOI] [PubMed] [Google Scholar]
- 43.French MB, Allison J, Cram DS, Thomas HE, Dempsey-Collier M, Silva A, Georgiou HM, Kay TW, Harrison LC, Lew AM: Transgenic expression of mouse proinsulin II prevents diabetes in nonobese diabetic mice. Diabetes 1997; 46: 34– 39 [DOI] [PubMed] [Google Scholar]
- 44.Nakayama M, Abiru N, Moriyama H, Babaya N, Liu E, Miao D, Yu L, Wegmann DR, Hutton JC, Elliott JF, Eisenbarth GS: Prime role for an insulin epitope in the development of type 1 diabetes in NOD mice. Nature 2005; 435: 220– 223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yoon JW, Yoon CS, Lim HW, Huang QQ, Kang Y, Pyun KH, Hirasawa K, Sherwin RS, Jun HS: Control of autoimmune diabetes in NOD mice by GAD expression or suppression in beta cells. Science 1999; 284: 1183– 1187 [DOI] [PubMed] [Google Scholar]
- 46.Jaeckel E, Klein L, Martin-Orozco N, von Boehmer H: Normal incidence of diabetes in NOD mice tolerant to glutamic acid decarboxylase. J Exp Med 2003; 197: 1635– 1644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Effects of insulin in relatives of patients with type 1 diabetes mellitus. N Engl J Med 2002; 346: 1685– 1691 [DOI] [PubMed] [Google Scholar]
- 48.Barker JM, McFann KK, Orban T: Effect of oral insulin on insulin autoantibody levels in the Diabetes Prevention Trial Type 1 oral insulin study. Diabetologia 2007; 50: 1603– 1606 [DOI] [PubMed] [Google Scholar]
- 49.Skyler JS, Krischer JP, Wolfsdorf J, Cowie C, Palmer JP, Greenbaum C, Cuthbertson D, Rafkin-Mervis LE, Chase HP, Leschek E: Effects of oral insulin in relatives of patients with type 1 diabetes: the Diabetes Prevention Trial–Type 1. Diabetes Care 2005; 28: 1068– 1076 [DOI] [PubMed] [Google Scholar]
- 50.Nanto-Salonen K, Kupila A, Simell S, Siljander H, Salonsaari T, Hekkala A, Korhonen S, Erkkola R, Sipila JI, Haavisto L, Siltala M, Tuominen J, Hakalax J, Hyoty H, Ilonen J, Veijola R, Simell T, Knip M, Simell O: Nasal insulin to prevent type 1 diabetes in children with HLA genotypes and autoantibodies conferring increased risk of disease: a double-blind, randomised controlled trial. Lancet 2008; 372: 1746– 1755 [DOI] [PubMed] [Google Scholar]
- 51.Chaillous L, Lefevre H, Thivolet C, Boitard C, Lahlou N, Atlan-Gepner C, Bouhanick B, Mogenet A, Nicolino M, Carel JC, Lecomte P, Marechaud R, Bougneres P, Charbonnel B, Sai P: Oral insulin administration and residual beta-cell function in recent-onset type 1 diabetes: a multicentre randomised controlled trial. Diabete Insuline Orale group. Lancet 2000; 356: 545– 549 [DOI] [PubMed] [Google Scholar]
- 52.Pozzilli P, Pitocco D, Visalli N, Cavallo MG, Buzzetti R, Crino A, Spera S, Suraci C, Multari G, Cervoni M, Manca Bitti ML, Matteoli MC, Marietti G, Ferrazzoli F, Cassone Faldetta MR, Giordano C, Sbriglia M, Sarugeri E, Ghirlanda G: No effect of oral insulin on residual beta-cell function in recent-onset type I diabetes (the IMDIAB VII). IMDIAB Group. Diabetologia 2000; 43: 1000– 1004 [DOI] [PubMed] [Google Scholar]
- 53.Monetini L, Cavallo MG, Sarugeri E, Sentinelli F, Stefanini L, Bosi E, Thorpe R, Pozzilli P: Cytokine profile and insulin antibody IgG subclasses in patients with recent onset type 1 diabetes treated with oral insulin. Diabetologia 2004; 47: 1795– 1802 [DOI] [PubMed] [Google Scholar]
- 54.Thrower SL, James L, Hall W, Green KM, Arif S, Allen JS, Van-Krinks C, Lozanoska-Ochser B, Marquesini L, Brown S, Wong FS, Dayan CM, Peakman M: Proinsulin peptide immunotherapy in type 1 diabetes: report of a first-in-man Phase I safety study. Clin Exp Immunol, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huurman VA, van der Meide PE, Duinkerken G, Willemen S, Cohen IR, Elias D, Roep BO: Immunological efficacy of heat shock protein 60 peptide DiaPep277 therapy in clinical type I diabetes. Clin Exp Immunol 2008; 152: 488– 497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sanda S, Roep BO, von Herrath M: Islet antigen specific IL-10+ immune responses but not CD4+CD25+FoxP3+ cells at diagnosis predict glycemic control in type 1 diabetes. Clin Immunol 2008; 127: 138– 143 [DOI] [PubMed] [Google Scholar]
- 57.Agardh CD, Lynch K, Palmer M, Link K, Lernmark A: GAD65 vaccination: 5 years of follow-up in a randomised dose-escalating study in adult-onset autoimmune diabetes. Diabetologia 2009; 52: 1363– 1368 [DOI] [PubMed] [Google Scholar]
- 58.Cheramy M, Ludvigsson J, Casas R: GAD65 treatment induces high GADA levels in type 1 diabetic children-a relation to C-peptide response, but not adverse effects. Diabetologia 2008; 51: S231 [Google Scholar]
- 59.Herold KC, Gitelman SE, Masharani U, Hagopian W, Bisikirska B, Donaldson D, Rother K, Diamond B, Harlan DM, Bluestone JA: A single course of anti-CD3 monoclonal antibody hOKT3γ1(Ala-Ala) results in improvement in C-peptide responses and clinical parameters for at least 2 years after onset of type 1 diabetes. Diabetes 2005; 54: 1763– 1769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ribas A, Timmerman JM, Butterfield LH, Economou JS: Determinant spreading and tumor responses after peptide-based cancer immunotherapy. Trends Immunol 2003; 24: 58– 61 [DOI] [PubMed] [Google Scholar]