Abstract
OBJECTIVE
An increased expression of RELM-β (resistin-like molecule-β), a gut-derived hormone, is observed in animal models of insulin resistance/obesity and intestinal inflammation. Intestinal sugar absorption is modulated by dietary environment and hormones/cytokines. The aim of this study was to investigate the effect of RELM-β on intestinal glucose absorption.
RESEARCH DESIGN AND METHODS
Oral glucose tolerance test was performed in mice and rats in the presence and the absence of RELM-β. The RELM-β action on glucose transport in rat jejunal sacs, everted rings, and mucosal strips was explored as well as downstream kinases modulating SGLT-1 and GLUT2 glucose transporters.
RESULTS
Oral glucose tolerance test carried out in rodents showed that oral administration of RELM-β increased glycemia. Studies in rat jejunal tissue indicated that mucosal RELM-β promoted absorption of glucose from the gut lumen. RELM-β had no effect on paracellular mannitol transport, suggesting a transporter-mediated transcellular mechanism. In studies with jejunal mucosa mounted in Ussing chamber, luminal RELM-β inhibited SGLT-1 activity in line with a diminished SGLT-1 abundance in brush border membranes (BBMs). Further, the potentiating effect of RELM-β on jejunal glucose uptake was associated with an increased abundance of GLUT2 at BBMs. The effects of RELM-β were associated with an increased amount of protein kinase C βII in BBMs and an increased phosphorylation of AMP-activated protein kinase (AMPK).
CONCLUSIONS
The regulation of SGLT-1 and GLUT2 by RELM-β expands the role of gut hormones in short-term AMPK/protein kinase C mediated control of energy balance.
The family of proteins called RELMs (resistin-like molecules) has been reported to be involved with insulin resistance, diabetes, and inflammatory processes. Resistin was initially identified as an adipokine that inhibits insulin action and adipocyte differentiation (1). RELM-β is a protein homologous to resistin that is localized mainly within the digestive tract (2,3). RELM-β is highly expressed in goblet cells of murine colon and is secreted in response to bacterial colonization. It plays an important role in host defense and innate immunity (4,5). We have shown that RELM-β may have a direct effect on intestinal goblet cell secretion (6) and others have shown that RELM-β can also act as a hormone. An acute perfusion of RELM-β in rat induced a hepatic insulin resistance (7).
Recently, a concomitant increase of serum concentration and intestinal expression of RELM-β has been reported in insulin-resistant models such as obese db/db mice and high-fat–fed mice (8). The intestinal expression of RELM-β in mice is controlled by fasting and by various nutritive elements such as glucose and saturated free fatty acids (9). Glucose reduces the enterocyte expression of RELM-β, while insulin and tumor necrosis factor-α can upregulate its expression (9). This suggests that intestinal RELM-β may not only be associated with inflammation but can also be a regulator of energy homeostasis.
Glucose, the main source of energy in humans, comes from the digestion of carbohydrates and is absorbed in the small intestine. Intestinal sugar absorption constantly adapts to the dietary environment (10). One risk factor for developing noninsulin-dependent diabetes (type 2 diabetes) is the excessive consumption of diets containing high levels of carbohydrates. An important defect in type 2 diabetes is the increased ability of intestine to absorb monosaccharides by intestinal sugar transporters (11). Intestinal glucose is actively transported by the Na+/glucose cotransporter-1 (SGLT-1) and passively by GLUT2 (10). Moreover, it is also becoming increasingly evident that the gut is not just site of nutrient absorption but is also an active endocrine organ (12,13). A paracrine regulation of hexose absorption by intestinal hormones such as glucose-dependent insulinotropic polypeptide and proglucagon-derived peptides GLP-1 and GLP-2 has been shown (10). Indeed, certain gastro-intestinal peptides secreted at the luminal side of intestine such as leptin, angiotensin II, and epidermal growth factor have a mucosal effect on hexose transport (14–17). Even though glucose is the main regulator of its own absorption, the modulatory effect of gut-derived molecules on intestinal sugar absorption plays a critical role in the adaptation to dietary environment. However, to our knowledge, nothing is known concerning the effect of RELM-β on intestinal absorption of glucose. A local action of RELM-β expressed in the jejunum is likely even though the highest expression of RELM-β is found in colonic goblet cells (2). There is also evidence that RELM-β expression can be upregulated in rat (18) and mice (19) goblet cells of proximal intestine. Considering that RELM-β expression is regulated by nutrients/insulin and inflammatory cytokines, it seems important to explore whether RELM-β can regulate glucose jejunal absorption.
In the present study, we show that exogenous RELM-β acted as a luminal effector in enhancing glycemia during oral glucose tolerance test (OGTT) carried out in mice and rats. The use of rat jejunal strips mounted in Ussing chamber indicated that RELM-β could directly inhibit SGLT-1 activity induced by glucose. The activation of AMP-activated protein kinase (AMPK) has been shown to downregulate SGLT-1 transport and upregulate glucose uptake by GLUT2 (20). The in vitro and in situ experiments performed with rat jejunal segments indicate that RELM-β increases transepithelial glucose transport by switching the active transport into passive entry. The mechanism involved activation of AMPK and protein kinase C (PKC) and an insertion of GLUT2 transporters in jejunal brush border membranes (BBMs). These data suggest that RELM-β, a gut-derived hormone, can directly modulate intestinal glucose transport.
RESEARCH DESIGN AND METHODS
Male Wistar rats weighing 220–240 g and male C57BL/6J mice weighing 20–25 g were from Centre Elevage Janvier, Le Genest-St. Isle, France. The animals had free access to tap water and standard food and were treated in accordance with European Community guidelines concerning care and use of laboratory animals.
Glucose tolerance test.
Gavages of conscious rats or mice with 1 g of glucose per kilogram of body weight were performed after a 16-h fast using a d-glucose solution without (control) or with RELM-β. The total bolus volume for mice and rats was 0.25 ml and 1 ml, respectively, and the amount of glucose in each bolus was adjusted for the animal weight. The recombinant murine RELM-β (18 kDa; PeproTech, Neuilly-sur-Seine, France) used was highly purified by high-performance liquid chromatography and was endotoxin-free. RELM-β, at a final concentration of 0.1 and 1 nmol/l, results in dose range of 0.01–0.1 μg/kg of body weight. RELM-β is a stable molecule as high amounts of the homodimer form have been detected in mice and human stools (21). Preliminary experiments showed that the peptide given by gavage was found in proximal small intestine after 5 min with a recovery of 70%. Before starting the OGTT, blood samples were taken from the tail and fasting blood glucose levels (milligram per deciliter) were determined (ACCU-CHEK; Roche Diagnostic, Meylan, France). The bleeds were further taken at 15, 30, 60, and 120 min after oral glucose administration. These experiments were performed at least with 6–10 individual animals.
Tissue preparation and short-circuit measurement.
Rats were fasted for 16 h and were killed by pentobarbital overdose. The proximal jejunum was dissected out and four adjacent samples were mounted in Ussing chambers as described previously (22). The tissues were bathed with 4 ml of Krebs-Ringer bicarbonate (KRB) solution (pH 7.4) with 10 mmol/l glucose at 37°C. In the solution bathing the mucosal side of the tissue, glucose was replaced by mannitol. Both solutions were gassed with 95% O2/5% CO2. Electrogenic ion transport was monitored continuously as short-circuit current (Isc) by using an automated voltage clamp apparatus (DVC 1000; WPI, Aston, England) linked through a MacLab 8 to a MacIntosh computer. KRB alone (vehicle) or containing RELM-β (0.001–1 nmol/l) was added in the mucosal bath 2 min before a glucose challenge. Carbachol (100 mmol/l) was added at the end of each experiment as a control. Further, similar tests were performed with RELM-β incubated overnight at 4°C with a rabbit polyclonal antibody raised against RELM-β. Results were expressed as the intensity of the Isc (μA/cm2) or as the percentage of the peak Isc obtained after glucose challenge (measured after 3 min) over basal Isc (measured just before the addition of glucose).
Transmural transport of hexoses.
The experiments were performed using jejunal sacs from adult Wistar rats. Animals were fasted for 16 h and were killed by pentobarbital overdose. The proximal jejunum was dissected out and rinsed in cold saline solution. Jejunal loops (4 cm long) were prepared and 0.5 ml of KRB solution without (control) or with 1 nmol/l RELM-β was inserted inside the jejunal lumen. The jejunal loops were incubated for 15 min in oxygenized KRB at 37°C and conditions were maintained during hexose transport assay. The corresponding jejunal loops were filled with 1 ml of KRB solution without (control) or with 1 nmol/l RELM-β and containing 0.02 μCi/ml of the isotopic tracer d-[1-14C] glucose (49.5 mCi/mmol) and glucose to obtain a final concentration of 10, 30, and 100 mmol/l. Similarly, we studied the paracellular transport with 30 mmol/l mannitol and the isotopic tracer d-[1-14C] mannitol (59 mCi/mmol) at 0.2 μCi/ml. All the jejunal loops were incubated in 10 ml of KRB solution during the indicated time. The radioactivity was measured in the collected samples of serosal KRB solution and used to calculate glucose or mannitol transport as picomoles per milligram of jejunal protein. Five independent experiments were performed and significance is expressed as *P < 0.05,**P < 0.01.
Western blot analysis.
Rats were anesthetized by pentobarbital and laparotomized for in situ experiments. Three jejunal segments (5 cm long) were tied and filled with 3 ml of KRB without (control) or with 1 nmol/l RELM-β. After 3 min of in situ incubation, 3 ml of 60 mmol/l glucose solution were injected in the lumen to obtain a final concentration of 30 mmol/l. After a further 3 min, these loops were removed and opened along the mesenteric border and the mucosa was scraped off on ice with a glass blade. Total cell protein extracts and BBMs were prepared from the scrapings as previously described (23), and enrichment was estimated by determination of alkaline phosphatase activity (20-fold increase of activity in BBMs). Solubilized proteins were resolved by electrophoresis on 10% SDS-PAGE gels and transferred onto nitrocellulose membranes for immunoblot analysis. The following rabbit antibodies were used at a 1:1,000 dilution: SGLT-1 (AB 1352; Chemicon International, Temecula, CA), GLUT2, PKC βII (sc-9117, sc-210; Santa Cruz Biotechnology, Tebu-Bio, France), phospho-AMPK-α (Thr172), AMPK-α (23A3), and phospho-PKC (pan (2531, 2603, 190D10; Cell Signaling Technology, Ozyme, France). The intensity of the specific immunoreactive bands was quantified using National Institutes of Health Image (Scion, Frederick, MD).
Glucose uptake experiments.
Uptake experiments were performed using rat intestinal everted rings as previously described (24). Groups of eight intestinal rings were incubated at 37°C for 15 min in oxygenized KRB buffer in the absence (control) and the presence of RELM-β (1 nmol/l) and cytochalasin B as indicated. Then the rings were incubated for 2 min in an uptake solution corresponding to a KRB buffer containing 30 mmol/l glucose and 0.1 μCi/ml of the isotopic tracer d-[1-14C] glucose. After adding the uptake solution, the rings were washed in ice-cold KRB solution, and radioactivity incorporated in the tissue was quantified by liquid scintillation. Data were not corrected for extracellular substrate because RELM-β was found not to affect paracellular diffusion. Total protein from homogenized tissue with a Dounce homogenizer was determined with bicinchoninic acid reagent from Pierce (Thermo Scientific, Brebières, France). Results are expressed as micromoles glucose per gram of tissue protein.
Chemicals.
Recombinant murine RELM-β was purchased from PeproTech (Neuilly-sur-Seine, France). Antibody raised against RELM-β was a gift from Dr. Blagoy Blagoev (University of Southern Denmark). d-[1-14C] Mannitol was from GE Healthcare Amersham Biosciences (les Ulis, France), d-[1-14C] glucose was from Perkin Elmer (Boston, MA), and compound C from Merck Sharp & Dohme-Chibret (Paris, France). All other chemical reagents were purchased from Sigma (St. Louis, MO).
Statistical analysis.
All results were expressed as means ± SE. One-way ANOVA with Turkey-Kramer multiple comparisons post hoc test was performed using GraphPad Prism version 4.0 for Windows (GraphPad Software, San Diego, CA). The level of significance was set at P < 0.05.
RESULTS
Effect of RELM-β on glucose tolerance tests.
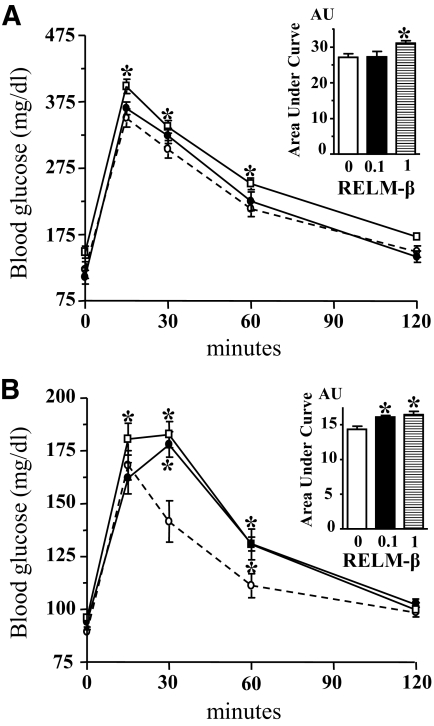
Oral administration of RELM-β increased glycemia during OGTT carried out in mice (Fig. 1A) and rats (Fig. 1B) as compared with control groups. The area under the curve was significantly (P < 0.05) increased in mice when RELM-β was used at 1 nmol/l and the two doses used (0.1 and 1 nmol/l) were effective in rats (Fig. 1A and B, insets). A similar 15% increase in blood glucose as compared with control was observed in both mice and rats. These data show that luminal administration of exogenous RELM-β is active in vivo as previously described (6).
FIG. 1.
OGTT in mice and rats. OGTT (1 g/kg) was performed in overnight-fasted mice (A) or rats (B) with a 15% d-glucose solution without (○, dotted lines) or with RELM-β (●, 0.1 nmol/l; □, 1 nmol/l). Glucose concentration was determined in blood samples from the tail and is expressed as milligram per deciliter over the time points (minutes). Each point is the means ± SE; n = 6–10. *P < 0.05 vs. control. Area under the curve (inset) is expressed in arbitrary units. In absence (0, □) and in presence of 0.1 nmol/l (■) and 1 nmol/l (▤) RELM-β.
Mucosal RELM-β inhibits Na+-dependent glucose transport.
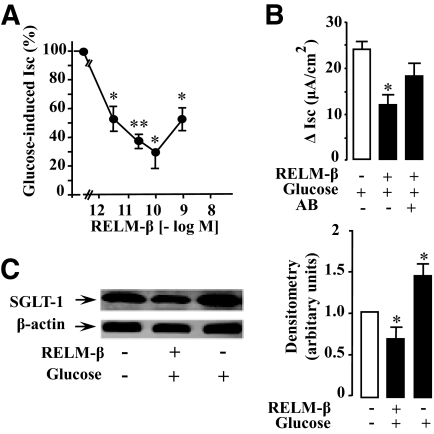
The route of glucose entry can involve active Na+-dependent GLUT (SGLT-1) or diffusive transporter GLUT2. We used the Ussing chamber to characterize the effect of mucosal RELM-β on rat intestinal active glucose transport (SGLT-1). Rat jejunal mucosa mounted in the chamber was allowed to reach a steady state (usually 40 min). Addition of 10 mmol/l glucose in the mucosal bath induced a rise in Isc (maximum after 3 min) representing an increase in SGLT-1 activity. Addition of RELM-β to the mucosal side 2 min before glucose challenge induced a marked and dose-dependent inhibition of glucose-induced Isc. As shown in Fig. 2A, addition of RELM-β to the mucosal side inhibited glucose transport by jejunal mucosa in a concentration-dependent manner. Maximal inhibition was achieved with 0.1 nmol/l RELM-β. The concentration that produced a half-maximal inhibition (IC50) of glucose transport was 3 pmol/l. Further, an overnight incubation of 0.1 nmol/l RELM-β with an antibody raised against RELM-β countered the inhibition of glucose-induced Isc by RELM-β as shown in Fig. 2B. Next, we investigated if the observed inhibition by RELM-β was associated with an altered abundance of SGLT-1 in jejunal BBMs. A typical immunoblot of SGLT-1 protein in BBMs after glucose challenge in the presence or the absence of RELM-β is shown in Fig. 2C. The mean densitometry of three separate blots shows that glucose increased the amount of SGLT-1 protein in BBMs by 1.5-fold as compared with control. This increase is reduced by half in the presence of RELM-β.
FIG. 2.
Effect of luminal RELM-β on glucose-induced Isc. Rat jejunal mucosa was mounted in Ussing chamber and the increase in Isc was studied at steady state. Electrogenic (Na+) transport was followed as an index of the active glucose transport by cotransporter SGLT-1. RELM-β was added in the mucosal bath 2 min before challenging the tissues with 10 mmol/l glucose. Values for Isc were standardized to control values and expressed as percentage of controls. A: Dose-dependent inhibition. B: The effect of 0.1 nmol/l RELM-β after an overnight incubation with an antibody against RELM-β. C: Representative SGLT-1 immunoblot of solubilized BBMs. BBMs were prepared from rat jejunum loops incubated in situ with and without luminal RELM-β during 6 min. Densitometric analysis of immunoreactive bands was performed using National Institutes of Health Image analysis program. The densitometry represents the amount of SGLT-1 relative to β-actin and is representative of at least three separate experiments. Each point of the Isc study represents the means ± SE of four to eight noncumulative values from five separate experiments. Significant differences from control, *P < 0.05, **P < 0.01.
RELM-β modulates jejunal glucose transport.
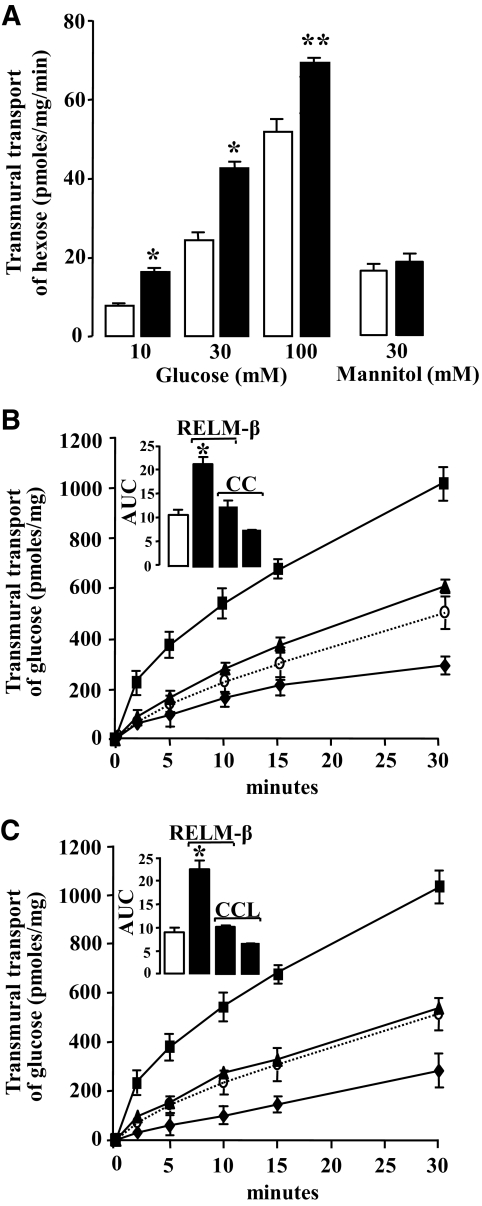
We isolated rat jejunum and performed in vitro studies of transmural glucose transport to directly evaluate the effect of RELM-β on intestinal transport. As shown in Fig. 3A, luminal glucose in jejunal loops significantly increased net mucosal to serosal glucose flux. A dose-dependent effect on glucose transport was observed with increasing glucose concentrations 10, 30, and 100 mmol/l. Further, RELM-β, at 1 nmol/l, significantly enhanced the jejunal transport of 10, 30, and 100 mmol/l glucose but not that of 30 mmol/l mannitol. Thus, increased jejunal glucose transport induced by RELM-β is unlikely to have been caused by changes in paracellular permeability. This is in line with histological studies of the jejunum tissues used in these experiments that did not show any visible mucosal deterioration (data not shown). Further, experiments were performed to identify if the effect of RELM-β implicated potential downstream kinases, PKC, and AMPK, which are known as key effectors of intestinal glucose transport (6,20,23,25). The AMPK inhibitor, compound C, and the PKC inhibitor, chelerythrine, inhibited the 30 mmol/l glucose-induced jejunal glucose transport as shown, respectively, in Fig. 3B and C. The insets represent the area under the curve of the 30-min glucose transport kinetics. RELM-β significantly increased glucose transport by approximately twofold and this effect was blunted by compound C and chelerythrine as shown, respectively, in Fig. 3B and C. These results indicate a likely involvement of PKC and AMPK in the luminal effect of RELM-β on glucose uptake.
FIG. 3.
Effect of luminal RELM-β on transmural transport of hexoses in rat jejunal loops. Transmural transport of glucose or mannitol was performed in jejunal sacs from adult Wistar rats. A: Intestinal sacs were incubated at 37°C during 15 min with 1 nmol/l RELM-β (■) or vehicle (□) in oxygenized KRB buffer with glucose at 10, 30, and 100 mmol/l and the isotopic tracer d-[1-14C] glucose. The radioactivity measured in the collected samples was used to calculate glucose transport as picomoles per milligram of jejunal protein per minute. Results from a similar experiment using mannitol (30 mmol/l). The kinetics of glucose (30 mmol/l) transmural transport is shown in the absence (○, dotted line) and in the presence (■) of 1 nmol/l RELM-β in B and C. B: The AMPK inhibitor (compound C or CC) was incubated without (♦) or with (▲) RELM-β. C: Similarly, the PKC inhibitor (chelerythrine chloride or CCL) is incubated without (♦) and with (▲) RELM-β. The insets show the corresponding area under the curve. The data are representative of the means ± SE of at least four individual experiments, *P < 0.05; **P < 0.01 vs. control.
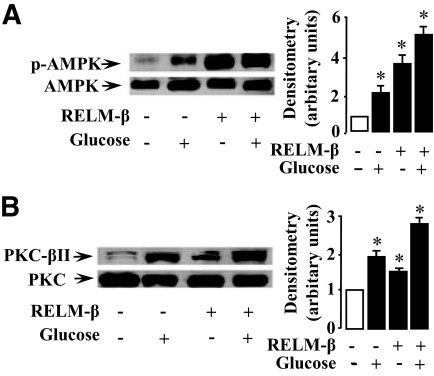
RELM-β stimulates phosphorylation of AMPK and translocation of PKC βII.
The above results prompted us to assess by in situ experiments the cellular effects of RELM-β on AMPK and PKC. Jejunal segments were injected with a KRB solution alone or containing 30 mmol/l glucose in the absence and the presence of 1 nmol/l RELM-β. The mucosal scrapings of jejunum were examined by Western blot analysis. The results indicated that glucose as well as RELM-β stimulated AMPK phosphorylation over control values as shown in Fig. 4A. The corresponding mean densitometric analysis indicates that RELM-β induced a threefold increase in AMPK phosphorylation. A further increase in the phosphorylation of AMPK occurred when glucose and RELM-β were added together. Similarly, RELM-β induced a threefold increase in PKC phosphorylation in line with our data (6) in the mouse colon (data not shown). This prompted us to study the translocation of cytosolic PKC βII of enterocytes to BBMs, a mechanism that is associated with intestinal glucose transport (20,23). We performed Western blot analysis to determine the expression of PKC βII in BBMs obtained from intestinal segments that were incubated with glucose (30 mmol/l) or RELM-β (1 nmol/l) as described above. As shown in Fig. 4B, RELM-β or glucose induced, respectively, 1.5- or 2-fold increase of PKC βII at the BBMs as compared with control values. A further increase (2.7-fold compared with the control) of PKC βII at the BBMs was observed when RELM-β and glucose were added together. These results suggest that RELM-β stimulates the phosphorylation of AMPK and PKC as well as an increased shift of PKC βII to the BBMs of rat jejunal tissue.
FIG. 4.
RELM-β induces phosphorylation of AMPK and translocation of PKC βII. Rats were anesthetized and their jejunal loops were used for in situ experiments. The loops were filled with KRB buffer with or without 1 nmol/l RELM-β. After 3 min, glucose (30 mmol/l) was added in this mucosal bath. After a further 3 min, loops were excised and kept on ice before scraping off the mucosa. Total protein extraction and BBMs preparation were performed immediately as described in research design and methods. Representative immunoblots for phospho-AMPK (A) in mucosal extracts and PKC βII (B) in BBMs are shown. The densitometry in A represents the amount of phosphorylated kinases relative to total AMPK. The amount of PKC βII in BBMs in B is relative to total PKC. The data are representative of three separate experiments. Significantly different from control, *P < 0.05.
RELM-β increases GLUT2 activity and its expression at BBMs.
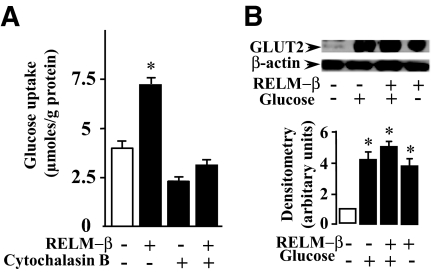
We explored whether RELM-β could enhance the glucose-induced GLUT2 translocation to the apical membrane of enterocytes. To this end, we measured glucose uptake in rat everted jejunal rings in the presence of RELM-β and without or with cytochalasin B, a competitive inhibitor of GLUT2 (26). As shown in Fig. 5A, cytochalasin B inhibited glucose uptake by 50% in agreement with the implication of GLUT2 in glucose uptake (27,28). RELM-β enhanced glucose uptake by twofold and this effect was also strongly inhibited by cytochalasin B. Further, we performed Western blot analysis to determine the expression of GLUT2 in BBMs obtained from intestinal segments that had been incubated with RELM-β under the same conditions as above. We observed that glucose or RELM-β alone induced, respectively, a four- or threefold increase in the amount of GLUT2 found in the brush-border fraction, respectively (Fig. 5B). When glucose and RELM-β were added together, a further increase in the amount of GLUT2 was observed (fivefold as compared with control). This suggests that RELM-β enhanced glucose uptake is a result of an increased insertion of GLUT2 into the BBMs.
FIG. 5.
Effect of RELM-β on glucose uptake and GLUT2 trafficking in BBMs. A: Rat jejunal everted rings were incubated in oxygenized KRB buffer without (control) or with 1 nmol/l RELM-β. The presence of cytochalasin B is indicated. The rings were incubated for 2 min in a KRB buffer containing 30 mmol/l glucose and 0.1 μCi/ml of the isotopic tracer d-[1-14C] glucose). The radioactivity incorporated in the tissue was determined by liquid scintillation. The amount of glucose incorporated is expressed as millimole per gram of tissue protein. B: Rat jejunal loops were treated 6 min with a mucosal bath containing 30 mmol/l glucose with or without 1 nmol/l RELM-β and BBMs were prepared as described in research design and methods. A representative Western blot analysis of the corresponding protein lysates with GLUT2 antibody is shown. Densitometric analysis of immunoblots indicates an increase of GLUT2 in the presence of glucose or RELM-β. The data are expressed relative to β-actin and is representative of at least three separate experiments. Significantly different from control, *P < 0.05.
DISCUSSION
In the present study, we show that RELM-β, a resistin-like molecule, can directly increase jejunal absorption of glucose in the rat. Several lines of evidence suggest that transepithelial transport of glucose in the small intestine can be mediated by an active absorption through Na+/glucose cotransporter (SGLT-1) as well as by a diffusive component GLUT2 at the apical membrane (27). We found that RELM-β inhibited the activity of SGLT-1, whereas enhancing the presence of GLUT2 at the BBM of enterocytes. Moreover, the increased jejunal glucose transport induced by RELM-β was inhibited by cytochalasin B in agreement with a functional role of GLUT2. The underlying molecular mechanism involves the activation of PKC βII and AMPK kinases as described for such reciprocal regulation of glucose transporters (20).
The acute treatment of rat jejunum with RELM-β had no significant effect on passive mannitol movement suggesting that RELM-β increases glucose transport by the use of transporters. We found that mucosal RELM-β inhibited the activity and the translocation of cytosolic SGLT-1 to cell membranes. This effect was blunted by an antibody raised against RELM-β. Other peptides such as leptin, angiotensin II, and CCK-8 have also been shown to inhibit SGLT-1 activity (14,16,28). We show that in contrast to CKK-8, which seems to have no effect on GLUT2 (28), RELM-β can induce GLUT2 translocation. Indeed, in response to RELM-β the amount of GLUT2 was increased in the BBMs. Similarly, a rapid insertion of GLUT2 to apical membrane in response to another gut-peptide, GLP-2, has been reported (29,30). Taken together, these findings suggest the involvement of GLUT2 in RELM-β stimulated glucose uptake.
The mechanisms responsible for RELM-β effect may involve the activation of PKC (6) and AMPK (25) that have been shown to regulate jejunal glucose transporters (20). This is sustained by the report that luminal epidermal growth factor increases jejunal glucose transport in rabbit through PKC activity (31). We found that chelerythrine, an inhibitor of PKC, blocked RELM-β stimulation of glucose uptake. The effect of RELM-β is accompanied by an increase in the amount of PKC βII at the BBMs. The activation of PKC has been shown to inhibit SGLT-1 mediated transport of hexoses (14,32). In oocytes expressing rat and rabbit SGLT-1, the activation of PKC decreases the maximum rate of transport for both isoforms. This change is accompanied by proportional change in the number of SGLT-1 molecules at the plasma membrane, indicating that PKC regulates endocytosis of the vesicles containing the transporter (33). Further, SGLT-1 contains a consensus site of PKC phosphorylation and thus PKC phosphorylation of the transporter could control its activity (34). These data are in line with our results showing that RELM-β inhibits SGLT-1 activity and its translocation to BBMs in rat small intestine.
The activation of AMPK has been shown to downregulate SGLT-1–dependent glucose transport but also to enhance GLUT2 translocation to the apical membrane of the jejunum (20). This effect of AMPK leads to an increased glucose uptake in jejunum (20) as well as in muscle (35). Interestingly, several hormones have been shown to regulate AMPK in a strictly tissue-specific manner (36). We demonstrate that the compound C blocked RELM-β stimulation of glucose transport in rat intestine suggesting the involvement of AMPK. Humans and rodents express two isoforms of the catalytic subunit (α1, α2) that form the heterotrimeric complex AMPK (α, β, and γ) known to serve as a regulator of energy balance (37). We showed that RELM-β could increase the phosphorylation of the conserved threonine residue (Thr-172) of α1 in the jejunal mucosa that is crucial for the AMPK activity. As evoked above, activated PKC decreases the number of SGLT-1 transporters but can also activate the translocation of GLUT2 to BBMs as described elsewhere (23). Thus, luminal RELM-β can directly enhance glucose transport by mustering GLUT2 at BBMs through PKC and AMPK activation.
This inverse regulation of SGLT-1 and GLUT2 by luminal RELM-β may be important when enterocytes require energy as shown in stress-induced pathology (38). The energy sensor molecule AMPK as well as PKC can increase the GLUT2 energy-independent pathway to override that of SGLT-1 that requires energy (20,32). In agreement with this concept, the SGLT-1-mediated absorption of nutrients such as galactose and glucose is decreased during either systemic (39) or intestinal inflammation (40). Further, to meet the increased metabolic demand of inflamed tissue, it has been shown that proinflammatory cytokines (interleukin [IL]-8, IL-6, and IL-1) can increase jejunal absorption of glucose without changes in BBM SGLT-1 content (41). An increased expression of RELM-β has been described in the intestine during jejunal inflammation (19,42,43). The expression of RELM-β is increased by several proinflammatory cytokines and by lipopolysaccharide (9,21) and they may act together to modulate intestinal glucose absorption. Thus, the enhanced glucose absorption by RELM-β in response to inflammatory stimulus may contribute to the associated energy demand.
Obese and insulin-resistant rodent models that are characterized by a low-grade inflammation are associated with an increased expression of the gut-derived RELM-β (8). Expression of RELM-β has been shown in rat (18) and mice (19) proximal intestine and the peptide may act locally in a paracrine manner or as a circulating hormone linking the gut to the liver. When given by gavage, it is possible that RELM-β could be partly absorbed by the small intestine to reach the blood as demonstrated for leptin (44). In favor of a hormonal effect, the infusion of intestinal RELM-β in mice has been shown to promote a marked increase in the rate of hepatic glucose production (7). This was associated with a rapidly induced hepatic but not peripheral insulin resistance. We observed that acute RELM-β administration in the intestinal lumen of rodents resulted in an increased glycemia in OGTT. This could result from the observed increased intestinal glucose absorption and an acute hepatic insulin resistance (7). This is in line with a local intestinal and distant action of this gut peptide. As to whether RELM-β may also counteract the described insulin inhibition of intestinal sugar absorption remains to be established (45). There may be different RELM-β thresholds in insulin target tissues as described for resistin (46). A better understanding of RELM-β action on different tissues could emerge if putative RELM-β receptors were identified. In this context, the effect of RELM-β may even involve rapid neuronal activation because RELM family members RELM-α (47) and RELM-β (4) can bind to neurons. Other than short-regulation, RELM-β can have a chronic effect as shown in diet-induced metabolic disorders (25). Transgenic mice over-expressing circulating RELM-β exhibited significant hyperglycemia, hyperlipedemia when fed on high-fat diet. In conclusion, our study shows that RELM-β increases intestinal glucose transport. Further studies may reveal if this process in conjunction with an adverse nutritional and inflammatory status, can participate in the onset of diabetes.
Acknowledgments
This work was supported by the Institut National de la Recherche Médicale (INSERM). R.B.K. received a fellowship from Nestlé, France.
No potential conflicts of interest relevant to this article were reported.
This study was presented in part at the 44th annual meeting of the European Association for the Study of Diabetes, Rome, Italy, 7–11 September 2008.
We thank C. Magnan, Jan Mester, and A. Bado for helpful discussions.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, Patel HR, Ahima RS, Lazar MA: The hormone resistin links obesity to diabetes. Nature 2001; 409: 307– 312 [DOI] [PubMed] [Google Scholar]
- 2.Steppan CM, Brown EJ, Wright CM, Bhat S, Banerjee RR, Dai CY, Enders GH, Silberg DG, Wen X, Wu GD, Lazar MA: A family of tissue-specific resistin-like molecules. Proc Natl Acad Sci U S A 2001; 98: 502– 506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gerstmayer B, Kusters D, Gebel S, Muller T, Van Miert E, Hofmann K, Bosio A: Identification of RELM-γ, a novel resistin-like molecule with a distinct expression pattern. Genomics 2003; 81: 588– 595 [DOI] [PubMed] [Google Scholar]
- 4.Artis D, Wang ML, Keilbaugh SA, He W, Brenes M, Swain GP, Knight PA, Donaldson DD, Lazar MA, Miller HR, Schad GA, Scott P, Wu GD: RELM-β/FIZZ2 is a goblet cell-specific immune-effector molecule in the gastrointestinal tract. Proc Natl Acad Sci U S A 2004; 101: 13596– 13600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nair MG, Guild KJ, Du Y, Zaph C, Yancopoulos GD, Valenzuela DM, Murphy A, Stevens S, Karow M, Artis D: Goblet cell-derived resistin-like molecule β augments CD4+ T cell production of IFN-γ and infection-induced intestinal inflammation. J Immunol 2008; 181: 4709– 15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krimi RB, Kotelevets L, Dubuquoy L, Plaisancie P, Walker F, Lehy T, Desreumaux P, Van Seuningen I, Chastre E, Forgue-Lafitte ME, Marie JC: Resistin-like molecule β regulates intestinal mucous secretion and curtails TNBS-induced colitis in mice. Inflamm Bowel Dis 2008; 14: 931– 941 [DOI] [PubMed] [Google Scholar]
- 7.Rajala MW, Obici S, Scherer PE, Rossetti L: Adipose-derived resistin and gut-derived resistin-like molecule-β selectively impair insulin action on glucose production. J Clin Invest 2003; 111: 225– 230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shojima N, Ogihara T, Inukai K, Fujishiro M, Sakoda H, Kushiyama A, Katagiri H, Anai M, Ono H, Fukushima Y, Horike N, Viana AY, Uchijima Y, Kurihara H, Asano T: Serum concentrations of resistin-like molecules β and γ are elevated in high-fat-fed and obese db/db mice, with increased production in the intestinal tract and bone marrow. Diabetologia 2005; 48: 984– 992 [DOI] [PubMed] [Google Scholar]
- 9.Fujio J, Kushiyama A, Sakoda H, Fujishiro M, Ogihara T, Fukushima Y, Anai M, Horike N, Kamata H, Uchijima Y, Kurihara H, Asano T: Regulation of gut-derived resistin-like molecule β expression by nutrients. Diabetes Res Clin Pract 2008; 79: 2– 10 [DOI] [PubMed] [Google Scholar]
- 10.Kellett GL, Brot-Laroche E, Mace OJ, Leturque A: Sugar asorption in the intestine: the role of GLUT2. Annu Rev Nutr 2008; 28: 35– 54 [DOI] [PubMed] [Google Scholar]
- 11.Dyer J, Wood IS, Palejwala A, Ellis A, Shirazi-Beechey SP: Expression of monosaccharide transporters in intestine of diabetic humans. Am J Physiol 2002; 282: G241– G248 [DOI] [PubMed] [Google Scholar]
- 12.Tschop M, Smiley DL, Heiman ML: Ghrelin induces adiposity in rodents. Nature 2000; 407: 908– 913 [DOI] [PubMed] [Google Scholar]
- 13.Chuadhri O, Small C, Bloom S: Gastrointestinal hormones regulating appetite. Philo Trans R Soc Lond B Biol Sci 2006; 361: 1187– 209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ducroc R, Guilmeau S, Akasbi K, Devaud H, Buyse M, Bado A: Luminal leptin induces rapid inhibition of active intestinal absorption of glucose mediated by sodium-glucose cotransporter 1. Diabetes 2005; 54: 348– 354 [DOI] [PubMed] [Google Scholar]
- 15.Inigo C, Patel N, Kellett GL, Barber A, Lostao MP: Luminal leptin inhibits intestinal sugar absorption in vivo. Acta Physiol (Oxf) 2007; 190: 303– 310 [DOI] [PubMed] [Google Scholar]
- 16.Wong TP, Debnam ES, Leung PS: Involvement of an enterocyte renin-angiotensin system in the local control of SGLT1-dependent glucose uptake across the rat small intestinal brush border membrane. J Physiol 2007; 584: 613– 623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hardin JA, Wong JK, Cheeseman CI, Gall DG: Effect of luminal epidermal growth factor on enterocyte glucose and proline transport. Am J Physiol 1996; 271: G509– G515 [DOI] [PubMed] [Google Scholar]
- 18.Yamauchi J, Kawai Y, Yamada M, Uchikawa R, Tegoshi T, Arizono N: Altered expression of goblet cell- and mucin glycosylation-related genes in the intestinal epithelium during infection with the nematode Nippostrongylus brasiliensis in rat. APMIS 2008; 114: 270– 278 [DOI] [PubMed] [Google Scholar]
- 19.Norkina O, Kaur S, Ziemer D, De Lisle RC: Inflammation of the cystic fibrosis mouse small intestine. Am J Physiol 2004; 286: G1032– 1041 [DOI] [PubMed] [Google Scholar]
- 20.Walker J, Jijon HB, Diaz H, Salehi P, Churchill T, Madsen KL: 5-Aminoimidazole-4-carboxamide riboside (AICAR) enhances GLUT2-dependent jejunal glucose transport: a possible role for AMPK. Biochem J 2005; 385: 485– 491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He W, Wang ML, Jiang HQ, Steppan CM, Shin ME, Thurnheer MC, Cebra JJ, Lazar MA, Wu GD: Bacterial colonization leads to the colonic secretion of RELM-β/FIZZ2, a novel goblet cell-specific protein. Gastroenterology 2003; 125: 1388– 1397 [DOI] [PubMed] [Google Scholar]
- 22.Ducroc R, Voisin T, El Firar A, Laburthe M: Orexins control intestinal glucose transport by distinct neuronal, endocrine, and direct epithelial pathways. Diabetes 2007; 56: 2494– 2500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Helliwell PA, Rumsby MG, Kellett GL: Intestinal sugar absorption is regulated by phosphorylation and turnover of protein kinase C βII mediated by phosphatidylinositol 3-kinase and mammalian target of rapamycin-dependent pathways. J Biol Chem 2003; 278: 28644– 28650 [DOI] [PubMed] [Google Scholar]
- 24.Lostao MP, Urdaneta E, Martinez-Anso E, Barber A, Martinez JA: Presence of leptin receptors in rat small intestine and leptin effect on sugar absorption. FEBS Lett 1998; 423: 302– 306 [DOI] [PubMed] [Google Scholar]
- 25.Kushiyama A, Shojima N, Ogihara T, Inukai K, Sakoda H, Fujishiro M, Fukushima Y, Anai M, Ono H, Horike N, Viana AY, Uchijima Y, Nishiyama K, Shimosawa T, Fujita T, Katagiri H, Oka Y, Kurihara H, Asano T: Resistin-like molecule β activates MAPKs, suppresses insulin signaling in hepatocytes, and induces diabetes, hyperlipidemia, and fatty liver in transgenic mice on a high fat diet. J Biol Chem 2005; 280: 42016– 42025 [DOI] [PubMed] [Google Scholar]
- 26.Kellett GL, Helliwell PA: The diffusive component of intestinal glucose absorption is mediated by the glucose-induced recruitment of GLUT2 to the brush-border membrane. Biochem J 2000; 350: 155– 162 [PMC free article] [PubMed] [Google Scholar]
- 27.Kellett GL, Brot-Laroche E: Apical GLUT2: a major pathway of intestinal sugar absorption. Diabetes 2005; 54: 3056– 3062 [DOI] [PubMed] [Google Scholar]
- 28.Hirsh AJ, Cheeseman CI: Cholecystokinin decreases intestinal hexose absorption by a parallel reduction in SGLT1 abundance in the brush-border membrane. J Biol Chem 1998; 273: 14545– 14549 [DOI] [PubMed] [Google Scholar]
- 29.Affleck JA, Helliwell PA, Kellett GL: Immunocytochemical detection of GLUT2 at the rat intestinal brush-border membrane. J Histochem Cytochem 2003; 51: 1567– 1574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Au A, Gupta A, Schembri P, Cheeseman CI: Rapid insertion of GLUT2 into the rat jejunal brush-border membrane promoted by glucagon-like peptide 2. Biochem J 2002; 367: 247– 254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Millar GA, Hardin JA, Johnson LR, Gall DG: The role of PI 3-kinase in EGF-stimulated jejunal glucose transport. Can J Physiol Pharmacol 2002; 80: 77– 84 [DOI] [PubMed] [Google Scholar]
- 32.Barrenetxe J, Sainz N, Barber A, Lostao MP: Involvement of PKC and PKA in the inhibitory effect of leptin on intestinal galactose absorption. Biochem Biophys Res Commun 2004; 317: 717– 721 [DOI] [PubMed] [Google Scholar]
- 33.Wright EM, Hirsch JR, Loo DD, Zampighi GA: Regulation of Na+/glucose cotransporters. J Exp Biol 1997; 200: 287– 293 [DOI] [PubMed] [Google Scholar]
- 34.Kennelly PJ, Krebs EG: Consensus sequences as substrate specificity determinants for protein kinases and protein phosphatases. J Biol Chem 1991; 266: 15555– 15558 [PubMed] [Google Scholar]
- 35.Lemieux K, Konrad D, Klip A, Marette A: The AMP-activated protein kinase activator AICAR does not induce GLUT4 translocation to transverse tubules but stimulates glucose uptake and p38 mitogen-activated protein kinases α and β in skeletal muscle. Faseb J 2003; 17: 1658– 1665 [DOI] [PubMed] [Google Scholar]
- 36.van Thuijl H, Kola B, Korbonits M: Appetite and metabolic effects of ghrelin and cannabinoids: involvement of AMP-activated protein kinase. Vitam Horm 2008; 77: 121– 148 [DOI] [PubMed] [Google Scholar]
- 37.Hardie DG: AMPK: a key regulator of energy balance in the single cell and the whole organism. Int J Obes (Lond) 2008; 32: S7– S12 [DOI] [PubMed] [Google Scholar]
- 38.Boudry G, Cheeseman CI, Perdue MH: Physiological stress impairs Na+-dependent glucose absorption and increases GLUT2 expression in the rat jejunal brush-border membrane. Am J Physiol 2007; 292: R862– R867 [DOI] [PubMed] [Google Scholar]
- 39.Amador P, García-Herrera J, Marca MC, de la Osada J, Acín S, Navarro MA, Salvador MT, Lostao MP, Rodríguez-Yoldi MJ: Intestinal d-galactose transport in an endotoxemia model in the rabbit. J Membr Biol 2007; 215: 125– 133 [DOI] [PubMed] [Google Scholar]
- 40.Sundaram U, Coon S, Wisel S, West AB: Corticosteroids reverse the inhibition of Na-glucose cotransport in the chronically inflamed rabbit ileum. Am J Physiol 1999; 276: G211– G218 [DOI] [PubMed] [Google Scholar]
- 41.Hardin J, Kroeker K, Chung B, Gall DG: Effect of proinflammatory interleukins on jejunal nutrient transport. Gut 2000; 47: 184– 191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McVay LD, Keilbaugh SA, Wong TM, Kierstein S, Shin ME, Lehrke M, Lefterova MI, Shifflett DE, Barnes SL, Cominelli F, Cohn SM, Hecht G, Lazar MA, Haczku A, Wu GD: Absence of bacterially induced RELM-β reduces injury in the dextran sodium sulfate model of colitis. J Clin Invest 2006; 116: 2914– 2923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Knight PA, Pemberton AD, Robertson KA, Roy DJ, Wright SH, Miller HR: Expression profiling reveals novel innate and inflammatory responses in the jejunal epithelial compartment during infection with Trichinella spiralis. Infect Immun 72: 6076– 6086, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cammisotto PG, Gingras D, Bendayan M: Transcytosis of gastric leptin through the rat duodenal mucosa. Am J Physiol 2007; 293: G773– G779, 2007 [DOI] [PubMed] [Google Scholar]
- 45.Tobin V, Le Gall M, Fioramonti X, Stolarczyk E, Blazquez AG, Klein C, Prigent M, Serradas P, Cuif MH, Magnan C, Leturque A, Brot-Laroche E: Insulin internalizes GLUT2 in the enterocytes of healthy but not insulin-resistant mice. Diabetes 2008; 57: 555– 562 [DOI] [PubMed] [Google Scholar]
- 46.Qi Y, Nie Z, Lee YS, Singhal NS, Scherer PE, Lazar MA, Ahima RS: Loss of resistin improves glucose homeostasis in leptin deficiency. Diabetes 2006; 55: 3083– 3090 [DOI] [PubMed] [Google Scholar]
- 47.Holcomb IN, Kabakoff RC, Chan B, Baker TW, Gurney A, Henzel W, Nelson C, Lowman HB, Wright BD, Skelton NJ, Frantz GD, Tumas DB, Peale FV, Jr, Shelton DL, Hébert CC: FIZZ1, a novel cysteine-rich secreted protein associated with pulmonary inflammation, defines a new gene family. EMBO J 2000; 19: 4046– 4055 [DOI] [PMC free article] [PubMed] [Google Scholar]