Abstract
Background and Purpose
Currently, the only FDA-approved therapy for acute ischemic stroke is the administration of recombinant tissue plasminogen activator (tPA). Echogenic liposomes (ELIP), phospholipid vesicles filled with gas and fluid, can be manufactured to incorporate tPA. Also, transcranial ultrasound-enhanced thrombolysis can increase the recanalization rate in stroke patients. However, there is little data on lytic efficacy of combining ultrasound, echogenic liposomes, and tPA treatment. In this study, we measure the effects of pulsed 120-kHz ultrasound on the lytic efficacy of tPA and tPA-incorporating ELIP (t-ELIP) in an in-vitro human clot model. It is hypothesized that t-ELIP exhibits similar lytic efficacy to that of rt-PA.
Methods:
Blood was drawn from 22 subjects after IRB approval. Clots were made in 20-μL pipettes, and placed in a water tank for microscopic visualization during ultrasound and drug treatment. Clots were exposed to combinations of [tPA] = 3.15 μg/ml, [t-ELIP] = 3.15 μg/ml, and 120-kHz ultrasound for 30 minutes at 37 °C in human plasma. At least 12 clots were used for each treatment. Clot lysis over time was imaged and clot diameter was measured over time, using previously developed imaging analysis algorithms. The fractional clot loss (FCL), which is the decrease in mean clot width at the end of lytic treatment, was used as a measure of lytic efficacy for the various treatment regimens.
Results:
The fractional clot loss FCL was 31% (95% CI: 26-37%) and 71% (56-86%) for clots exposed to tPA alone or tPA with 120 kHz ultrasound. Similarly, FCL was 48% (31-64%) and 89% (76-100%) for clots exposed to tELIP without or with ultrasound.
Conclusions:
The lytic efficacy of tPA containing echogenic liposomes is comparable to that of tPA alone. The addition of 120 kHz ultrasound significantly enhanced lytic treatment efficacy for both tPA and t-ELIP. Liposomes loaded with tPA may be a useful adjunct in lytic treatment with tPA.
Keywords: Acute ischemic stroke, Tissue plasminogen activator, Ultrasound, Echogenic liposomes
Introduction
Recombinant tissue plasminogen activator (tPA) is the only currently FDA approved lytic for treatment of acute ischemic stroke. However, there are contraindications to the use of this medication, it must be administered within 3 hours of symptom onset, and there can be substantial side effects such as intracerebral hemorrhage (ICH) [1-3]. As a result, there is much interest in potential adjunctive therapies, such as therapeutic hypothermia[4], ultrasound enhanced thrombolysis (UET) [5], and targeted drug delivery agents to minimize the total systemic lytic dose [6-8].
In-vitro and in-vivo studies have demonstrated the enhanced lytic efficacy of tPA with ultrasound [9-13] and in-vivo settings. These findings have led to clinical trials of UET in acute ischemic stroke patients [5,14,15]. For example, Alexandrov found that combined 2 MHz transcranial Doppler ultrasound and tPA improved the recanalization rate over that of tPA treatment alone in acute ischemic stroke patients [5]. However, a trial comparing 300 kHz UET with tPA treatment alone showed no improvement in recanalization rate and an increase in ICH in the UET treated patients [15]. Clearly, the “ideal” ultrasound and t-PA parameters for UET treatment of stroke are unknown.
Several mechanisms have been proposed to explain the phenomenon of ultrasound enhanced thrombolysis including thermal effects [16], microstreaming [17] and cavitation [18,19]. In recent studies by S. Datta et al, UET was found to be correlated with the presence of stable cavitation in in-vitro porcine [19] and human clots [20], as measured using an acoustic technique. In stable cavitation, small microbubbles are produced and oscillate in size in response to the ultrasound acoustic field, and persist in the fluid for long periods of time. This can lead to substantial shear forces in the plasma surrounding these microbubbles and may increase the permeation of tPA into the sample clot. Others have observed similar results [21].
Ultrasound contrast agents, which are micron-sized gas bubbles typically enclosed by either a protein or lipid shell are widely used in clinical practice to improve the signal-to-noise ratio in ultrasound image acquisition. Recent studies have shown that these agents also improve the lytic efficacy for UET [20,22,23]. These results suggest that increasing the likelihood of cavitation using ultrasound contrast agents could increase the efficacy of UET.
Echogenic liposomes (ELIP), an ultrasound contrast agent, are sub-micron sized phospholipid-bilayer vesicles which contain both gas and fluid [6,7,24,25]. ELIP can encapsulate drugs, such as tPA, thereby allowing localized drug delivery while minimizing systemic exposure [26,27]. This t-ELIP may be of great use as a lytic therapy for several reasons. First, by chemically targeting t-ELIP for thrombus, it may be possible to increase the local concentration of tPA around the thrombus while reducing the systemic tPA dose. This could possibly reduce the incidence of the hemorrhagic complications of tPA [28]. Second, the presence of a gas bubble may increase the lytic efficacy due to cavitation-related mechanisms, as discussed above. Finally, the gas bubble allows controlled rupture of the t-ELIP [25,29] and release of the tPA by an external ultrasound pulse. In-vitro studies have demonstrated that t-ELIP exhibits substantial lytic efficacy [7,30] and has a high affinity for fibrinogen [7], a major structural component of human clot. However, there is little data on the lytic efficacy of combined t-ELIP and ultrasound in the in-vitro or in-vivo setting.
The objective of this study was to determine the thrombolytic efficacy of combined tPA-incorporating echogenic liposomes (t-ELIP) and 120 kHz ultrasound treatment in a well-defined in-vitro human clot model. This model uses a previously developed microscopic imaging technique [31-33] to measure clot diameter as a function of time, while undergoing exposure to lytic treatment. This technique directly measures clot size; reducing clot size is the primary concern for any clinician treating a thrombotic disease with lytic therapy. The primary hypothesis is that the lytic efficacy of t-ELIP is comparable to that of rt-PA.
Materials and Methods
Preparation of free tPA and human plasma
Non-incorporated or “free” tPA was obtained from the manufacturer (tPA, Activase®, Genentech, San Francisco, CA) as a lyophilized powder. Each vial was mixed with sterile water to a concentration of 1 mg/ml as per manufacturer's instructions, aliquoted into 1.0 ml centrifuge tubes (Fisher Scientific), and stored at −80 °C. The enzymatic activity of tPA is stable for at least 1 year when stored in this fashion [34]. Human fresh-frozen plasma (hFFP) was procured from a blood bank in 250-300 ml units. Each unit was briefly thawed, aliquoted into 50 ml centrifuge tubes (Fisher Scientific), and stored at −80 °C. Aliquots of tPA and plasma were allowed to thaw for experiments, and the remaining amounts discarded following completion of each experiment.
Preparation of tPA-incorporating echogenic liposomes
The sonication-lyophilization-rehydration method by which ELIP were prepared has been described previously [35]. Composition for this formulation was DPPC:DOPC:DPPG:Chol in a 46:24:24:6 molar ratio (DPPC=dipalmitoylphosphatidylcholine; DOPC=dioleoylphosphatidylcholine; DPPG=dipalmitoylphosphatidylglycerol; Chol=Cholesterol). The component lipids were dissolved in chloroform and the solvent was allowed to evaporate completely under argon in a 50 °C water bath with constant rotation until a thin film of lipids was formed on the vial wall. The resulting lipid film was placed under vacuum for full removal of the solvent and then hydrated with distilled, deionized water. Incorporation of tPA into the echogenic liposomes was accomplished by adding 200 μl of 1 mg/ml tPA solution and 300 μl of 0.32 M mannitol for the rehydration of the lipid film [7]. This dispersion was sonicated for 5 min to achieve a mean size of 500 nm. The liposome suspension and the sample was then frozen at −70 °C. Frozen samples were lyophilized for 48 h and resuspended with deionized water. The final concentration used for dilution was 10 mg lipid/ml [7,30].
Entrapped tPA was separated from the free tPA by centrifugation prior to lyophilization at 16,000 rpm for 20 min at 37 °C [36]. Using this method, an entrapment efficiency of 50% could be achieved [30]. Entrapped tPA includes both the tPA associated with the lipid bilayer, as well as full encapsulation of the drug within the liposomal aqueous phase; this method results in 70% of the incorporated tPA being associated with the lipid bilayer, whereas 30% appear to be truly encapsulated within the liposomal core. For each experiment, a 0.6 mg sample of t-ELIP was reconstituted using air-saturated deionized water [25]. t-ELIP has been demonstrated to maintain high echogeni-city at frequencies of 3.5, 4.5, and 20 MHz [29,30].
Production of blood clots
Human whole blood was drawn from twenty-two volunteers by sterile venipuncture, following local Institutional Review Board approval and written informed consent. Samples of 1-2 ml were placed in sterile glass tubes and allowed to form clots in and around a small diameter (∼600 μm) micropipette (Becton, Dickinson and Company, Franklin Lakes, NJ; 20λ) through which a segment of 7-0 silk suture (Ethicon Industries, Cornelia, GA) had been threaded. The clots were incubated for three hours at 37 °C, and refrigerated at 5 °C for 3 days ensuring maximal clot retraction, lytic resistance and stability.
This technique yields clots on the order of 200-300 μm in diameter; comparable to the diameter of the intracerebral segments of the middle cerebral arteries (80 to 840 μm) in humans [37]. In addition, the initial lytic rate for clots exposed to human fresh-frozen plasma alone was found to be on the order of 1% clot width per minute or about 2 to 3 μm/minute [31]. Utilizing clots of 200-300 μm diameter allows resolution of these small values of lytic rate. Additional details and justification of the clot sample preparation are discussed elsewhere [32,33,38]. In the work presented here, the initial clot diameter for all clots measured was 236±35 μm (mean and standard deviation, N = 215 clots).
Clot Visualization
For each experiment, the clot attached to the suture was placed in a clean micropipette (Drummond Scientific Company, Broomall, PA), and inserted into a U-shaped sample holder composed of hollow luer lock connectors and silicone tubing (Cole Parmer, Vernon Hills, IL; outer diameter 0.125”). The sample holder was placed in an acrylic water tank with a microscope slide at the bottom allowing visualization of the clot diameter using an inverting microscope (see [32], Fig. 1). Images were recorded at a rate of 6 frames per minute, using a microscope CCD camera, and stored on a desktop computer for later analysis. The average clot diameter was determined for each image, and measured as a function of time as before [32,38]. A full description of the experimental setup and image processing techniques is provided in previous work [32,33,38].
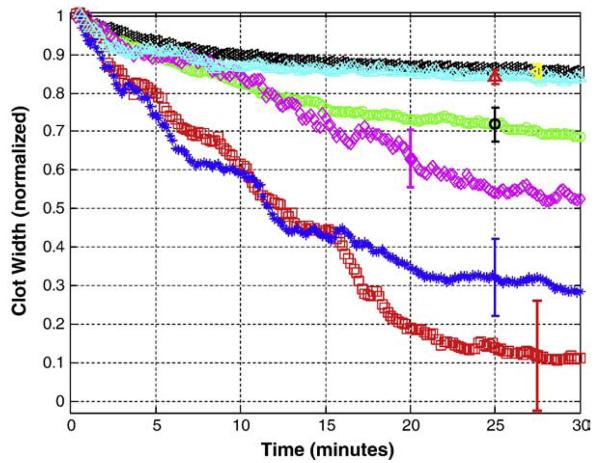
Fig. 1.
Average CWNC(t) as a function of time for clots exposed to tPA, t-ELIP, and ultrasound. Normalized average clot width is shown for control(-US) (◁), control(+US) (△), tPA(-US) (○), t-ELIP(-US) (◇), tPA-treated(+US) (*), and t-ELIP(+US) (□) trials. The vertical bars show representative standard deviations for the data.
The 120 kHz ultrasound exposures used an unfocused transducer (Sonic Concepts, Inc., Woodburn, WA) to provide the acoustic exposures. The transducer was mounted at one end of the tank at a 30° angle to the tank bottom allowing ultrasound exposure of the sample clot. The 120 kHz ultrasound parameters used were a pressure of 0.35 MPap-p, a pulse repetition frequency (PRF) of 1667 Hz, and a duty cycle of 50%, and a time-averaged acoustic intensity of 0.5 W/cm2. These ultrasound parameters yielded substantial clot lysis in previous work [12,32,39,40].
Experimental protocol
Clots were exposed to one of three treatment regimens, with (+US) or without (−US) 120 kHz ultrasound: (1) hFFP alone (control); (2) hFFP and tPA ([tPA]=3.15 μg/ml) (tPA); or (3) hFFP and t-ELIP ([t-ELIP]=3.15 μg/ml) (t-ELIP). The tPA concentration was chosen to be well within the therapeutic dosage range in humans; the t-ELIP concentration was chosen to allow comparison between results [38,41,42]. For t-ELIP treatments, the concentration of tPA in each aliquot of t-ELIP was approximately 400 μg/ml. A small volume (78.75 μl) of the reconstituted t-ELIP was used to attain the desired tPA concentration of 3.15 μg/ml.
Individual trials began by introducing 1 ml of hFFP (control trials) or 1 ml of hFFP containing tPA or t-ELIP (all other trials) into the sample holder. Typically this was performed by injection; for trials involving t-ELIP, a gravity feed was used to protect the structural integrity of the ELIP at the beginning of the experiment. At time t equal to zero, the solution was in contact with the clot. The ends of the sample holder were exposed to atmospheric pressure, and the clot surface to a static fluid column. Clots were exposed to a specific treatment regimen for 30 minutes at 37 C; previous studies have shown that the majority of thrombolysis occurs within a 30 minute window [5]. Each regimen used at least 12 clots (average number 36, range 12-85), from at least four donors (average number 9, range 4-20).
Determination of the normalized corrected clot width
The clot width data were subsequently corrected for the finite suture size used in these experiments. This yields the expression
| (1) |
where CWCorrected (t) is the value of the total clot width at time t adjusted for the average suture width, SW. SW was found to be 95±15 μm, based on measurements of 252 samples of the 7-0 suture; this value was used with the CW(t) data to calculate CWCorrected(t) for each trial, using Eq.(1). The CWCorrected (t) data were then normalized to the initial value of CWCorrected at time equal to zero, yielding the expression
| (2) |
where CWNC (t) is the normalized (N) and corrected (C) clot width. Note that CWNC(t) is equal to 1 at time t equal to zero, and becomes equal to zero if the clot is totally lysed leaving only the suture contributing to the sample width. This parameter is therefore used to compare the clot data among the various treatment groups, and averaged for all clots in a given treatment group. This treatment of the data is a modification of that used in previous work, where the normalized clot width did not take into account the finite suture diameter [32,33,38].
Statistical analysis
The effects of the treatment protocol on lytic rate and lytic efficacy at 30 minutes were evaluated using a mixed-model analysis of variance. An estimate of the fixed effects of tPA, t-ELIP, and ultrasound was determined and the covariance structure was modeled arising from the within-subject design. CWNC(t) data are presented as mean values with standard deviations at each point in time. Parameter estimates, standard errors and/or 95% confidence intervals of the estimates are used to report the effects of ultrasound, t-ELIP, and tPA and their combinations. These calculations were performed using SAS v8.02 (SAS Institute, Cary, NC) and a p value less than 0.05 was considered significant.
Results
Normalized clot width versus time
Fig. 1 illustrates the average CWNC(t) versus time for the treatment groups. The time course of the clot width for control(+US) trials is similar to that of control(−US) trials. The clot width decreases to a greater extent for tPA(−US), t-ELIP(−US), tPA(+US), and t-ELIP(+US) trials.
The average CWNC(t) as a function of time t was well described by the equation;
| (3) |
for all treatment groups. Here, CWNC,Fit (t) is the normalized average clot width (dimensionless), B is a parameter of the fit (dimensionless), and k is a rate constant (min−1) [32]. The quality of the fits overall was quite good (2 ≥ 0.93 for all trials). The details of these fits are not shown in the work presented here; relevant examples and details of the fitting procedure can be found in Meunier et al [32] and Shaw et al [31,38].
In some cases, the clot was completely lysed by a given treatment leaving only the suture remaining. This occurred for 4 trials or for 27% of the clots (n = 15) in the tPA(+US) group and in 1 trial or 8% of sample clots (n = 12) for the t-ELIP(+US) group. Although this is a qualitative observation, it does imply that ultrasound and tPA, either as the free enzyme or bound in ELIP, is capable of completely lysing the clot.
Fractional clot loss
The total decrease of the average CWNC(t) following thirty minutes of exposure is defined as the fractional clot loss (FCL), which can be expressed as,
| (4) |
The FCL values for each treatment group are shown in Fig. (2). In this figure, the values are plotted as means with standard errors. Note that the greater the clot loss, the smaller average CWNC becomes, thereby increasing FCL. If on average, the clot is completely lysed, FCL is 1. As is evident from Fig. 2, clot lysis is maximized when 120 kHz ultrasound is applied during tPA or t-ELIP exposure. FCL was substantially larger in the tPA(+US) (71%; 95% Confidence Interval 56-86%) and t-ELIP(+US) (89%, CI 76-100%) groups than in the tPA(−US) (31%, CI 26-37%) or t-ELIP(−US) (48%, CI 31-64%) trials. The FCL values for the control group were 15% (CI 12-17%) and 16% (CI 9-24%) for the control (−US) and control (+US) groups respectively. Interestingly, there was a trend towards larger values of FCL for t-ELIP than those for tPA exposure alone regardless of ultrasound treatment, although the confidence intervals overlap. Both tPA and t-ELIP treatment groups exhibited larger FCL values than the control group.
Fig. 2.
Fractional clot loss for each treatment group. The FCL values are shown as mean+standard deviation, in units of % of normalized clot width. Note that the largest average FCL is found for the t-ELIP(+US) treated clots. Ultrasound treatment significantly increased FCL for the tPA and t-ELIP treated clots.
Initial Lytic Rate
Qualitatively, the initial decrease in clot diameter was greater in the tPA(+US) and t-ELIP(+US) groups compared to the other groups during early lytic exposure. This initial decrease can be quantified by fitting the data to CWNC,Fit (Eq. (3)) and obtaining values for B and k for a given treatment group. This allows one to define the initial lytic rate LR as
| (5) |
The derivation of this expression is discussed in detail in previous work [32,38].
The values for the initial lytic rates for each treatment group are shown in Fig. (3). Note that the LR value obtained for t-ELIP (−US) (1.9%/min, 95% Confidence interval -0.17-4.05), and t-ELIP(+US) (5.0%/ min, CI 3.82-6.20) are somewhat smaller on average than those obtained for tPA(-US) (2.5%/min, CI 2.26-2.77) and tPA(+US) (6.6%/ min, CI 5.61-7.61) treated clots respectively. For purposes of comparison the LR values for control(−US) and control(+US) treated clots are 1.8%/min (CI 1.64-1.96) and 2.9%/min (CI 2.23-3.66) respectively. There was substantial variation in the degree of clot lysis in the first few minutes of lytic exposure for the t-ELIP exposed clots. This is reflected in the large confidence limits for LR in this group. Overall, the LR values for ultrasound treated clots were larger than those not exposed to ultrasound.
Fig. 3.
Initial lytic rate for each treatment group. The initial lysis rates shown for each trial group are determined from Eq. (5). Values for the lytic rate are given as mean+standard error, in units of % of normalized clot width per minute.
Discussion
The trend towards increased efficacy of t-ELIP suggests some advantage to encapsulating tPA. t-ELIP has a greater affinity for fibrin than tPA alone [7]. J. Heeremans et al [43] demonstrated greater efficacy of tPA containing liposomes compared with tPA in an in-vivo murine model. In addition, encapsulation did not increase systemic activation of α2-antiplasmin and plasminogen over that of tPA. Similarly, Tiukinhoy-Laing et al [30] found increased lysis in their t-ELIP (+US) group compared with the t-ELIP(−US) in an in-vitro porcine clot model. We speculate that encapsulation of tPA may protect the drug from plasminogen activator inhibitor 1 (PAI-1) and other tPA inhibitors. In order to demonstrate this, it would be necessary to quantitatively measure levels of the various fibrinolytic enzymes and inhibitory proteins associated with them. For example, quantifying the levels of free tPA, PAI-1 and tPA-PAI-1 complex would be needed to demonstrate liposome “shielding” of tPA from PAI-1. Similarly, demonstrating increase fibrin specificity of t-ELIP would require measurements of t-ELIP-fibrin and tPA-fibrin complexes in-vitro. It is a limitation of the current study that a definitive statement as to the mechanism of t-ELIP lytic efficacy can not be made.
The addition of 120 kHz ultrasound increases tPA and t-ELIP thrombolysis (Figs. 2 and 3). A possible mechanism is cavitation or the creation of small microbubbles by the acoustic field. In inertial cavitation [44], these bubbles are created and destroyed over very short times; approximately the duration of a single ultrasound pulse. In stable cavitation, the microbubbles persist for long periods of time, oscillating in size in response to the time varying acoustic pressure. Recently [19,20], S Datta et al demonstrated a correlation between the presence of stable cavitation and UET. They observed that stable cavitation was present at pressure amplitudes greater than 0.2±0.2 MPa; comparable to the 0.18 MPa value used here. Inertial cavitation was observed at a higher threshold of 0.27± 0.06 MPa [19]. Lytic efficacy of rt-PA was greatest for pressure amplitudes between these two threshold values [19]. Similar results were observed by Prokop et al [21] in in-vitro human clot. Overall, it is plausible that a cavitation mechanism could be partially responsible for the increased lytic efficacy of rt-PA and t-ELIP with ultrasound.
There are several notable limitations of the work presented here. First, this work is performed in-vitro, and does not replicate the complex in-vivo or clinical scenario of lytic therapy. In addition, the clots were manufactured from the blood of healthy donors, thus reducing the applicability of these results to individuals on anticoagulants or other medications, or those suffering from significant medical problems. Finally, it is difficult to distinguish between the tPA (+US) and t-ELIP(+US) treated groups as the clots are rather small. Both of these treatments exhibit substantial lytic efficacy as shown here, thus measuring differences in efficacy between the two is limited by the small amount of substrate clot available for lysis. Despite these limitations, this study illustrates some of the capabilities of combination t-ELIP and ultrasound therapy in human clots.
In conclusion, this study has shown that the thrombolytic efficacy of tPA incorporation into echogenic liposomes is comparable to that of tPA alone in an in-vitro human clot model that directly measures clot size during lytic treatment. The addition of 120 kHz ultrasound with t-ELIP substantially increases the rate of lysis compared with either tPA or t-ELIP alone. The combination has the potential to improve the treatment of ischemic stroke and other thrombotic disorders.
Acknowledgements
The authors gratefully acknowledge support from The Distinguished Chair for Clinical Research in Emergency Medicine Foundation, NIH/NINDS (K02-NSO56253, 1R01 NS047603), and (7R01HL074002-04, 2R56HL059586-08). The authors also gratefully acknowledge the helpful assistance of Denise Smith and Jonathan Kopechek in preparing and handling the echogenic liposomes in solution.
Footnotes
Conflict of interest
Details of nature of conflict of interest: No conflicts.
References
- 1.Adams HP, Jr., Brott TG, Furlan AJ, Gomez CR, Grotta J, Helgason CM, Kwiatkowski T, Lyden PD, Marler JR, Torner J, Feinberg W, Mayberg M, Thies W. Guidelines for Thrombolytic Therapy for Acute Stroke: a Supplement to the Guidelines for the Management of Patients with Acute Ischemic Stroke. A statement for healthcare professionals from a Special Writing Group of the Stroke Council, American Heart Association. Stroke; a journal of cerebral circulation. 1996;27:1711–8. [PubMed] [Google Scholar]
- 2.Grotta JC, Burgin S, El-Mitwalli A, Long M, Campbell M, Morgenstern LB, Malkoff M, Alexandrov AV. tissue-type plasminogen activator for ischemic stroke: Houston experience 1996 to 2000. Arch Neurol. 2001:58. doi: 10.1001/archneur.58.12.2009. [DOI] [PubMed] [Google Scholar]
- 3.Tanne D, Bates VE, Verro P, Kasner SE, Binder JR, Patel SC, Mansbac HH, Daley S, Schultz LR, Scott P, Dayno JM, Verecskey-Porter K, Benesch C, Book D, Coplin WM, Dulli D, Levine SR. Initial clinical experience with IV tissue plasminogen activator for acute ischemic stroke: a multicenter survey. The t-PA Stroke Survey Group. Neurology. 1999;53:424–7. doi: 10.1212/wnl.53.2.424. [DOI] [PubMed] [Google Scholar]
- 4.Hammer M, Krieger D. Hypothermia for acute ischemic stroke: Not just another neuroprotectant. Neurologist. 2003;9:280–9. doi: 10.1097/01.nrl.0000094628.29312.2b. [DOI] [PubMed] [Google Scholar]
- 5.Alexandrov AV, Molina CA, Grotta JC, Garami Z, Ford SR, Alvarez-Sabin J, Montaner J, Saqqur M, Demchuk AM, Moye LA, Hill MD, Wojner AW. Ultrasound-enhanced systemic thrombolysis for acute ischemic stroke. N Engl J Med. 2004;351:2170–8. doi: 10.1056/NEJMoa041175. [DOI] [PubMed] [Google Scholar]
- 6.McPherson DD, Huang S, Holland CK. Echogenic liposomes for molecular targeted therapeutic delivery. J Acoust Soc Am. 2008;123:3216. [Google Scholar]
- 7.Tiukinhoy-Laing SD, Buchanan K, Parikh D, Huang S, Macdonald RC, McPherson DD, Klegerman ME. Fibrin targeting of tissue plasminogen activator-loaded echogenic liposomes. J Drug Target. 2007;15:109–14. doi: 10.1080/10611860601140673. [DOI] [PubMed] [Google Scholar]
- 8.Alexandrov AV, Mikulik R, Ribo M, Sharma VK, Lao AY, Tsivgoulis G, Sugg RM, Barreto A, Sierzenski P, Malkoff MD, Grotta JC. A pilot randomized clinical safety study of sonothrombolysis augmentation with ultrasound-activated perflutrenlipid microspheres for acute ischemic stroke. Stroke; a journal of cerebral circulation. 2008;39:1464–9. doi: 10.1161/STROKEAHA.107.505727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daffertshofer M, Huang Z, Fatar M, Popolo M, Schroeck H, Kuschinsky W, Moskowitz MA, Hennerici MG. Efficacy of sonothrombolysis in a rat model of embolic ischemic stroke. Neurosci Lett. 2004;361:115–9. doi: 10.1016/j.neulet.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 10.Suchkova V, Siddiqi FN, Carstensen EL, Dalecki D, Child S, Francis CW. Enhancement of fibrinolysis with 40-kHz ultrasound. Circulation. 1998;98:1030–5. doi: 10.1161/01.cir.98.10.1030. [DOI] [PubMed] [Google Scholar]
- 11.Behrens S, Daffertshofer M, Spiegel D, Hennerici M. Low-frequency, low-intensity ultrasound accelerates thrombolysis through the skull. Ultrasound Med Biol. 1999;25:269–73. doi: 10.1016/s0301-5629(98)00158-6. [DOI] [PubMed] [Google Scholar]
- 12.Cheng JY, Shaw GJ, Holland CK. In vitro microscopic imaging of enhanced thrombolysis with 120-kHz ultrasound in a human clot model. Acoust Res Lett Online. 2005;6:25–9. doi: 10.1121/1.1815039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suchkova VN, Matsunaga TO, Schumann P, Francis CW. Enhancement of ultrasound-mediated fibrinolysis by platelet-targeted microbubble contrast agents. Blood. 2001;98 [Google Scholar]
- 14.Eggers J, Koch B, Meyer K, Konig I, Seidel G. Effect of ultrasound on thrombolysis of middle cerebral artery occlusion. Ann Neurol. 2003;53:797–800. doi: 10.1002/ana.10590. [DOI] [PubMed] [Google Scholar]
- 15.Daffertshofer M, Gass A, Ringleb P, Sitzer M, Sliwka U, Els T, Sedlaczek O, Koroshetz WJ, Hennerici MG. Transcranial low-frequency ultrasound-mediated thrombolysis in brain ischemia: Increased risk of hemorrhage with combined ultrasound and tissue plasminogen activator - Results of a phase II clinical trial. Stroke; a journal of cerebral circulation. 2005;36:1441–6. doi: 10.1161/01.STR.0000170707.86793.1a. [DOI] [PubMed] [Google Scholar]
- 16.Sakharov DV, Hekkenberg RT, Rijken DC. Acceleration of fibrinolysis by high-frequency ultrasound: The contribution of acoustic streaming and temperature rise. Thromb Res. 2000;100:333–40. doi: 10.1016/s0049-3848(00)00319-4. [DOI] [PubMed] [Google Scholar]
- 17.Francis CW, Blinc A, Lee S, Cox C. Ultrasound accelerates transport of recombinant tissue plasminogen activator into clots. Ultrasound Med Biol. 1995;21:419–24. doi: 10.1016/0301-5629(94)00119-x. [DOI] [PubMed] [Google Scholar]
- 18.Everbach EC, Charles WF. Cavitational mechanisms in ultrasound-accelerated thrombolysis at 1 MHz. Ultrasound Med Biol. 2000;26:1153–60. doi: 10.1016/s0301-5629(00)00250-7. [DOI] [PubMed] [Google Scholar]
- 19.Datta S, Coussios CC, McAdory LE, Tan J, Porter T, De C ourten-Myers G, Holland CK. Correlation of cavitation with ultrasound enhancement of thrombolysis. Ultrasound Med Biol. 2006;32:1257–67. doi: 10.1016/j.ultrasmedbio.2006.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Datta S, Coussios CC, Ammi AY, Mast TD, de Courten-Myers GM, Holland CK. Ultrasound-enhanced thrombolysis using Definity as a cavitation nucleation agent. Ultrasound Med Biol. 2008;34:1421–33. doi: 10.1016/j.ultrasmedbio.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prokop AF, Soltani A, Roy RA. Cavitational Mechanisms in Ultrasound-Accelerated Fibrinolysis. Ultrasound Med Biol. 2007;33:924–33. doi: 10.1016/j.ultrasmedbio.2006.11.022. [DOI] [PubMed] [Google Scholar]
- 22.Molina CA, Ribo M, Rubiera M, Montaner J, Santamarina E, Delgado-Mederos R, Arenillas JF, Huertas R, Purroy F, Delgado P, Alvarez-Sabi?n J. Microbubble administration accelerates clot lysis during continuous 2-MHz ultrasound monitoring in stroke patients treated with intravenous tissue plasminogen activator. Stroke; a journal of cerebral circulation. 2006;37:425–9. doi: 10.1161/01.STR.0000199064.94588.39. [DOI] [PubMed] [Google Scholar]
- 23.Perren F, Loulidi J, Poglia D, Landis T, Sztajzel R. Microbubble potentiated transcranial duplex ultrasound enhances IV thrombolysis in acute stroke. J Thromb Thrombolysis. 2008;25:219–23. doi: 10.1007/s11239-007-0044-6. [DOI] [PubMed] [Google Scholar]
- 24.Coussios CC, Holland CK, Jakubowska L, Huang SL, MacDonald RC, Nagaraj A, McPherson DD. In vitro characterization of liposomes and Optison by acoustic scattering at 3.5 MHz. Ultrasound Med Biol. 2004;30:181–90. doi: 10.1016/j.ultrasmedbio.2003.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith DAB, Porter TM, Martinez J, Huang S, MacDonald RC, McPherson DD, Holland CK. Destruction Thresholds of Echogenic Liposomes with Clinical Diagnostic Ultrasound. Ultrasound Med Biol. 2007;33:797–809. doi: 10.1016/j.ultrasmedbio.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 26.Heeremans JL, Prevost R, Feitsma H, Kluft C, Crommelin DJ. Clot accumulation characteristics of plasminogen-bearing liposomes in a flow-system. Groningen Utrecht Institute for Drug Exploration. Thromb Haemost. 1998;79:144–9. [PubMed] [Google Scholar]
- 27.Leach JK, Patterson E, O'Rear EA. Distributed intraclot thrombolysis: mechanism of accelerated thrombolysis with encapsulated plasminogen activators. J Thromb Haemost. 2004;2:1548–55. doi: 10.1111/j.1538-7836.2004.00884.x. [DOI] [PubMed] [Google Scholar]
- 28.Gore JM, Sloan M, Price TR, Young Randall AM, Bovill E, Collen D, Forman S, Knatterud GL, Sopko G, Terrin ML. Intracerebral hemorrhage, cerebral infarction, and subdural hematoma after acute myocardial infarction and thrombolytic therapy in the thrombolysis in myocardial infarction study. Thrombolysis in myocardial infarction, Phase II, pilot and clinical trial. Circulation. 1991;83:448–59. doi: 10.1161/01.cir.83.2.448. [DOI] [PubMed] [Google Scholar]
- 29.Smith DAB, Vaidya S, Kopechek JA, Hitchcock KE, Huang SL, McPherson DD, Holland CK. Echogenic liposomes loaded with recombinant tissue-type plasminogen activatory (rt-PA) for image-guided, ultrasound-triggered drug release. J Acoust Soc Am. 2007;122:3007. [Google Scholar]
- 30.Tiukinhoy-Laing SD, Huang S, Klegerman M, Holland CK, McPherson DD. Ultrasound-facilitated thrombolysis using tissue-plasminogen activator-loaded echogenic liposomes. Thromb Res. 2007;119:777–84. doi: 10.1016/j.thromres.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shaw GJ, Meunier JM, Lindsell CJ, Holland CK. Tissue plasminogen activator concentration dependence of 120 kHz ultrasound-enhanced thrombolysis. Ultrasound Med Biol. 2008;34:1783–92. doi: 10.1016/j.ultrasmedbio.2008.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meunier JM, Holland CK, Lindsell CJ, Shaw GJ. Duty Cycle Dependence of Ultrasound Enhanced Thrombolysis in a Human Clot Model. Ultrasound Med Biol. 2007;33:576–83. doi: 10.1016/j.ultrasmedbio.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meunier JM, Holland CK, Pancioli AM, Lindsell CJ, Shaw GJ. Effect of low frequency ultrasound on combined rt-PA and eptifibatide thrombolysis in human clots. Thromb Res. 2008 doi: 10.1016/j.thromres.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jaffe GJ, Green GD, Abrams GW. Stability of recombinant tissue plasminogen activator. Am J Ophthalmol. 1989;108:90–1. doi: 10.1016/s0002-9394(14)73272-6. [DOI] [PubMed] [Google Scholar]
- 35.Huang SL, Hamilton AJ, Nagaraj A, Tiukinhoy SD, Klegerman ME, McPherson DD, Macdonald RC. Improving ultrasound reflectivity and stability of echogenic liposomal dispersions for use as targeted ultrasound contrast agents. J Pharm Sci. 2001;90:1917–26. doi: 10.1002/jps.1142. [DOI] [PubMed] [Google Scholar]
- 36.Heeremans JL, Gerritsen HR, Meusen SP, Mijnheer FW, Gangaram Panday RS, Prevost R, Kluft C, Crommelin DJ. The preparation of tissue-type Plasminogen Activator (t-PA) containing liposomes: entrapment efficiency and ultracentrifugation damage. J Drug Target. 1995;3:301–10. doi: 10.3109/10611869509015959. [DOI] [PubMed] [Google Scholar]
- 37.Marinkovic SV, Miliasavljevic MM, Kovacevic MS, Stevia ZD. Perforating branches of the middle cerebral artery: Microanatomy and clinical significance of their intracerebral segments. Stroke; a journal of cerebral circulation. 1958;15:1022–9. doi: 10.1161/01.str.16.6.1022. [DOI] [PubMed] [Google Scholar]
- 38.Shaw GJ, Meunier JM, Lindsell CJ, Holland CK. Tissue Plasminogen Activator Concentration Dependence of 120 kHz Ultrasound-Enhanced Thrombolysis. Ultrasound Med Biol. 2008 doi: 10.1016/j.ultrasmedbio.2008.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shaw GJ, Bavani N, Dhamija A, Lindsell CJ. Effect of mild hypothermia on the thrombolytic efficacy of 120 kHz ultrasound enhanced thrombolysis in an in-vitro human clot model. Thromb Res. 2006;117:603–8. doi: 10.1016/j.thromres.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 40.Meunier JM, Smith DAB, Holland CK, Huang S, McPherson DD, Shaw GJ. 120 kHz pulsed ultrasound enhanced thrombolysis with tissue plasminogen activator-loaded echogenic liposomes. J Acoust Soc Am. 2007;122:3052. [Google Scholar]
- 41.Tanswell P, Seifried E, Stang E, Krause J. Pharmacokinetics and hepatic catabolism of tissue-type plasminogen activator. Arzneim-Forsch. 1991;41:1310–9. [PubMed] [Google Scholar]
- 42.Seifried E, Tanswell P, Ellbruck D, Haerer W, Schmidt A. Pharmacokinetics and haemostatic status during consecutive infusions of recombinant tissue-type plasminogen activator in patients with acute myocardial infarction. Thromb Haemost. 1989;61:497–501. [PubMed] [Google Scholar]
- 43.Heeremans JL, Prevost R, Bekkers ME, Los P, Emeis JJ, Kluft C, Crommelin DJ. Thrombolytic treatment with tissue-type plasminogen activator (t-PA) containing liposomes in rabbits: a comparison with free t-PA. Thromb Haemost. 1995;73:488–94. [PubMed] [Google Scholar]
- 44.Holland CK, Apfel RE. Thresholds for transient cavitation produced by pulsed ultrasound in a controlled nuclei environment. J Acoust Soc Am. 1990;88:2059–69. doi: 10.1121/1.400102. [DOI] [PubMed] [Google Scholar]