Abstract
During Drosophila oogenesis, organized microtubule networks coordinate the localization of specific RNAs, the positioning of the oocyte nucleus, and ooplasmic streaming events. We used mutations in mini spindles (msps), a microtubule-associated protein, to disrupt microtubule function during mid- and late oogenesis, and show that msps is required for these microtubule-based events. Since endoplasmic reticulum (ER) organization is influenced by microtubules in other systems, we hypothesized that using msps to alter microtubule dynamics might affect the structure and organization of the ER in nurse cells and the oocyte. ER organization was monitored using GFP-tagged versions of Reticulon-like1 and protein disulfide isomerase. Analyses of living cells indicate microtubule associations mediate the movement of ER components within the oocyte. Surprisingly, the distribution and behavior of tubular ER in the oocyte differs from general ER, suggesting these two compartments of the ER interact differently with microtubules. We find that the morphology of Exu particles is msps-dependent, and that Exu is specifically associated with tubular ER in msps mutants. Our results extend previous descriptions of sponge bodies and the fusome, suggesting both are manifestations of a dynamic structure that interacts with microtubules and persists throughout oogenesis.
Keywords: mini spindles, microtubule organization, endoplasmic reticulum, reticulons, oogenesis, Exuperantia
Introduction
Early embryonic development in many organisms relies on the subcellular organization of the oocyte and requires the coordination of a variety of cellular events. Many of these processes are mediated by the cytoskeleton, and involve dynamic and often drastic transformations in cytoskeletal structure and function. The microtubule cytoskeleton of Drosophila and other animals has been implicated in a number of phenomena, including the cytoplasmic localization of developmentally important mRNAs and other macromolecules.
Mini spindles (msps) is the Drosophila member of the XMAP215/TOGp family of microtubule-associated proteins (MAPs). These proteins have been found in yeast, plants, and animals, including humans (reviewed in Ohkura et al., 2001; Kinoshita et al., 2002; Gard et al., 2004). The first member of this family, the Xenopus protein XMAP215, was identified in oocyte extracts as a MAP that influenced microtubule growth and stability (Gard and Kirschner, 1987; Vasquez et al., 1994). Initially characterized as a stabilizer of microtubules, subsequent work has demonstrated that XMAP215 function is considerably more complex than originally thought. A recent study by Brouhard and coworkers suggests XMAP215 can catalyze both polymerization and depolymerization of microtubules (Brouhard et al., 2008), and similar results have been reported for Msps using Drosophila S2 cells (Brittle and Ohkura, 2005). An emerging common feature of all msps homologs is their requirement in both mitosis and meiosis. Mutations in these genes often result in defects in spindle structure including the appearance of multi-polar and monopolar spindles. In many species, including budding and fission yeasts, C. elegans, Drosophila, Xenopus and humans, msps homologs are associated with spindle poles and centrosomes (Matthews et al., 1998; Becker and Gard, 2001; Garcia et al., 2001; Lee et al., 2001; Gergely et al., 2003).
Msps in Drosophila was originally isolated in a screen for mitotic mutants, as a mutation that causes a multispindle phenotype in cells of the larval central nervous system (Cullen et al., 1999). Subsequent studies found that msps is also required for proper structure of the female meiotic spindle, and a combination of genetic and biochemical analyses indicate that the Drosophila homolog of TACC (for transforming, acidic, coiled-coil-containing family of proteins) and the kinesin-like protein Non-claret disjunction (Ncd) are required for the localization of Msps to spindle poles (Cullen and Ohkura, 2001; Lee et al., 2001).
Drosophila oogenesis is an excellent system in which to address basic cell biology questions, and Moon and Hazelrigg (2004) demonstrated a role for msps in bicoid RNA localization during oogenesis. Oogenesis in Drosophila begins when a germ cell divides mitotically four times to produce a cyst of sixteen cells that remain connected by intercellular bridges (also called ring canals) that are the result of incomplete cytokinesis. One of the sixteen cells is specified to become the oocyte, while the other fifteen cells become nurse cells. Each cluster of 15 nurse cells, an oocyte, and a surrounding epithelium of several hundred somatic follicle cells is referred to as an egg chamber; each egg chamber produces one egg at the end of oogenesis. The growing oocyte is nurtured by the associated nurse cells, so named because they provide the oocyte with most of its cytoplasm and almost all of its organelles, proteins, and mRNA. Although the microtubule cytoskeleton of Drosophila ovaries has been intensely studied, relatively little is known about how the structure and function of microtubules is regulated during oogenesis. In this study, we used mutations in msps to investigate microtubule-based events of late oogenesis, including oocyte nuclear positioning, ooplasmic streaming, and ER organization. We find that msps is required for the proper execution of a variety of microtubule-mediated processes. Our analyses reveal a role for msps in organizing tubular ER, and present evidence that this sub-compartment of the ER may have important roles in organization of the oocyte during the last half of oogenesis.
Results
msps is required for oocyte nuclear positioning
Null alleles of msps are lethal, but the allelic combination mspsP/msps208 is viable and female sterile. As demonstrated previously, this hypomorphic combination results in a temporal loss of msps function during oogenesis; early oogenesis proceeds normally, including the initial localization of bicoid, gurken, and oskar RNAs, and the posterior localization of Kinesin (Moon and Hazelrigg, 2004 and our unpublished observations). However, previous work has shown that levels of msps RNA and protein decline beginning at stage 7 and are completely lost by stage 9/10 (Moon and Hazelrigg, 2004). This makes msps a useful tool for the examination of microtubule-based events occurring during mid-to-late oogenesis.
In order to establish the validity of using msps mutants to look at microtubule organization in the last half of oogenesis, we examined several microtubule-based processes in msps mutants. From stage 8 on, the oocyte nucleus occupies an anterior-dorsal position at the periphery of the cell, and the maintenance of this position is dependent on microtubule function. To investigate the possibility that msps is required for proper positioning of the nucleus, late stage oocytes from msps females were fixed and stained with DNA dyes (Fig. 1A-F). In wild type eggs and oocytes, the oocyte nucleus is found just below the dorsal appendages, but in msps oocytes the oocyte nucleus is mis-positioned (compare Fig. 1C and F). In most of these oocytes (>70%) the nucleus does not occupy its usual anterior-dorsal position and is instead found at random locations throughout the oocyte. To determine when these defects arise, we also looked at earlier stages of oogenesis. The movement of the oocyte nucleus to the anterior corner of the oocyte during stages 7/8 proceeds normally in msps mutants, and nuclear positioning is normal until stage 10 (Fig. 1A, D). Consistent with this observation, msps oocytes do not exhibit D-V patterning defects. However, we begin to see defects in nuclear positioning that are concomitant with the influx of nurse cell cytoplasm into the oocyte beginning at stage 10B, and mis-positioned oocyte nuclei in msps mutants are commonly observed at the boundary between entering nurse cell cytoplasm and more opaque oocyte cytoplasm. Januschke et al. (2002) have proposed a two-stage model for oocyte nuclear positioning in which the oocyte nucleus 1) establishes and maintains contact with the cell cortex, and 2) becomes positioned at the anterior-dorsal corner of the cell. We tested the possibility that mispositioned nuclei in msps oocytes might retain connections with the cortex by expressing his-GFP in msps mutants in order to visualize the entire germinal vesicle, coupled with actin staining to delineate the cell periphery. In 51 of 56 oocytes examined, all of which contained mis-positioned nuclei, the oocyte nucleus was closely associated with actin at the cortex. This suggests that in msps mutant oocytes, the nucleus retains its ability to interact with the cortical cytoskeleton, but that its anterior position cannot be maintained.
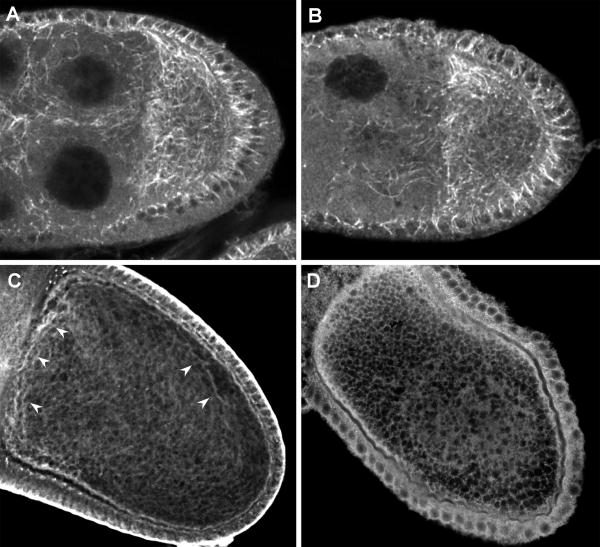
Fig. 1. Microtubule-dependent events are affected by msps mutations.
Oocytes from wild type (A-C) and mspsP/msps208 (D-F) females were fixed and stained with Sytox Green to reveal the position of the oocyte nucleus (arrowheads), and AlexaFluor-568 phalloidin to visualize actin filaments. The wild type nucleus is positioned in the anterior-dorsal corner of the oocyte, as evidenced by its position relative to the dorsal appendages. In the msps oocyte the nucleus is no longer anteriorly positioned after stage 10, although it appears to retain contact with the cell cortex (see inset in (E)).
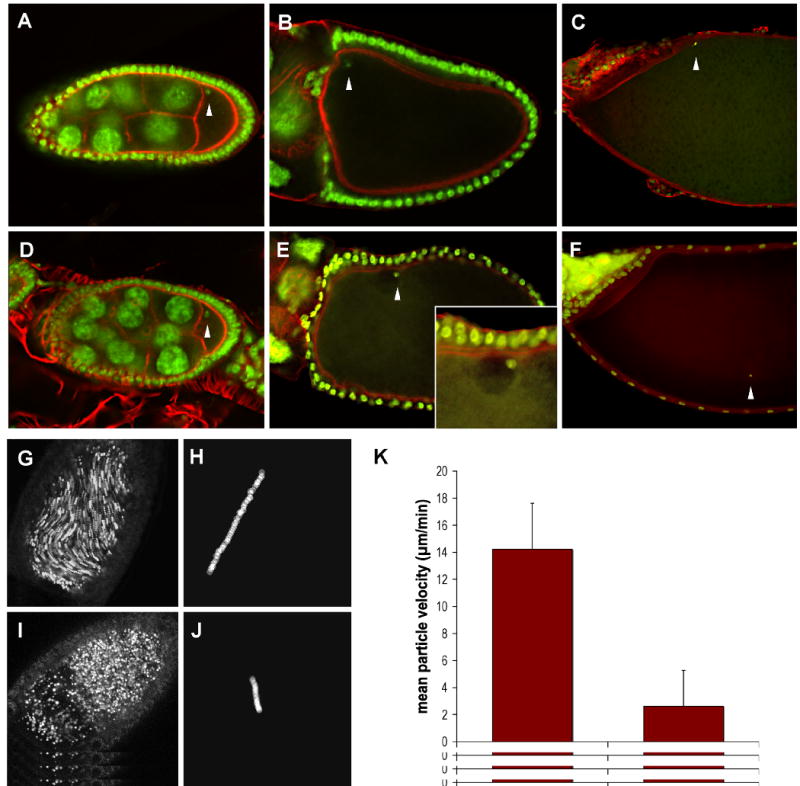
Stage 10B oocytes from wild type (G) and mspsP/msps208 (I) females were dissected into halogen oil 27 and imaged using the fluorescein channel of a Nikon confocal microscope. Five consecutive images were collected at ten-second intervals and merged. Particles whose position has changed over the time course of the experiment appear as streamers. Directional movement is evident in the wild type oocyte but not the msps oocyte, which exhibits the stratified cytoplasm commonly observed in these mutants. (H,J) The movement of a single particle was tracked over 500sec/50 frames. Individual particle tracings were used to calculate average particle velocities for wild type and mspsP/msps208 stage 10B oocytes (see text). (K) Time lapse stacks were used to calculate average particle velocities for wt and mspsP/msps208 stage 10b oocytes (n=20 for each genotype) (bottom). Bars indicate standard deviation.
Ooplasmic streaming is severely compromised in msps oocytes
Ooplasmic streaming refers to the microtubule-based directional movement of ooplasm occurring during stages 10-12 of oogenesis. During stage 10, cytoskeletal re-arrangements result in the appearance of long parallel microtubules in the oocyte that persist until stage 12 (Theurkauf et al., 1992). It is thought that during this time streaming facilitates the even incorporation of incoming nurse cell cytoplasm into the dense, yolk-laden oocyte cytoplasm as a wholesale transfer of nurse cell contents into the oocyte occurs. We monitored streaming in two ways, with the same results. In some experiments streaming was imaged using confocal microscopy, because small vesicles within the ooplasm are auto-fluorescent. Sequential time-lapse images were collected and then merged into a single composite image. Particles in motion are represented as strings, or as a blurred line, depending on their velocity (Fig. 1G, I). This technique permitted a global view of streaming and allowed detection of regional differences in particle movement rates. In other experiments, direct observations of streaming used DIC optics and time-lapse video capture techniques (see Supplemental Movies 1 and 2). Individual vesicles were then marked using ImageJ software and tracked through multiple frames of the captured video to determine particle velocities. Two examples of tracks are shown in Fig. 1 (H, J).
By tracking individual particles in st. 11 oocytes, we have been able to derive estimates of particle velocity. In our hands, wild type particle movement averages ∼14 μm/min (n=20), consistent with published values (Theurkauf, 1994a), while in msps mutants, that average is ∼2 μm/min (n=20)(Fig. 1K). It is important to note that the streaming phenotype in msps is quite variable; in some mutants, streaming velocities are reduced, but in many msps mutants little or no streaming is detectable. Occasionally, we can see streaming in one part of the oocyte, but not elsewhere. Many msps oocytes have highly stratified ooplasm, which can be distinguished from the incoming nurse cell cytoplasm by its high concentration of vesicles (Fig. 1I). In stratified oocytes, the nucleus is often found at the border between nurse cell cytoplasm and ooplasm, suggesting that the force of the incoming cytoplasm has dislodged the nucleus from its normal anterior-dorsal position. These observations coupled with the finding that nuclear positioning is defective in msps mutants support the idea that msps mutations specifically affect microtubule-based events in late-stage egg chambers.
Long microtubules are absent in msps oocytes
Since msps has been implicated in the regulation of microtubule dynamics (Brittle and Ohkura, 2005), we analyzed the distribution of microtubules in mid- to late-stage oocytes via tubulin antibody staining. In stage 8-9 egg chambers, microtubules are observed in both wild type and msps mutants. In msps mutants, microtubules appear to be partially depleted in nurse cells as reported by Moon and Hazelrigg, (2004 and Fig. 2A, B). Overall, we find that the number and length of microtubules in nurse cells decreases as oogenesis proceeds. By the time nurse cell dumping begins at stage 10B, virtually no microtubules are distinguishable in nurse cells. While nurse cells and younger oocytes are relatively permeable to antibodies, tubulin staining of mid-to-late stage oocytes is more challenging, due to the deposition of the vitelline membrane and chorion around the maturing egg. We have used two techniques to image this population of microtubules. One involves stripping the vitelline membrane and chorion off oocytes, coupled with a fluorescein “sandwich” technique (Moon and Hazelrigg, 2004). The other recently described technique includes a detergent extraction step prior to fixation (Januschke et al., 2006). Both techniques produce essentially similar results; control oocytes display the long parallel microtubules characteristic of these stages of oogenesis (Fig. 2C). In most msps oocytes, however, long microtubule arrays are completely absent, with a corresponding increase in generalized tubulin staining (Fig. 2D). The staining patterns observed are consistent with the notion that msps is responsible for mediating microtubule dynamics during oogenesis, such that a decrease in msps function inhibits the production of long parallel microtubules necessary for streaming.
Fig. 2. Microtubules are reduced or absent in the ooplasm of msps egg chambers.
Wild type (A,C) or mspsP/msps208(B,D) ovaries were fixed and stained using fluorescein-conjugated tubulin antibodies and fluorescein-conjugated 2° antibodies. During stages 8/9 (A,B), the effects of msps on microtubule organization are confined largely to the nurse cells. Cross sections through intact stage 10B oocytes. Microtubules are indicated in wild type oocytes with arrowheads, and are absent in msps oocytes.
Exu distribution is altered in msps mutants
Exuperantia (Exu) protein is required for the localization of bicoid RNA to the anterior cortex of the oocyte. Additionally, live imaging studies indicate Exu assembles into bicoid RNA-containing particles in nurse cells that undergo microtubule-dependent movement in both nurse cells and the oocyte (Theurkauf and Hazelrigg, 1998; Cha et al., 2001). Previous work has shown that msps oocytes are deficient in bicoid RNA localization (Moon and Hazelrigg, 2004), so we monitored the distribution of Exu in msps mutants, using a fully functional GFP-Exu fusion protein (Wang and Hazelrigg, 1994). In msps ovaries, GFP-Exu is present as large amorphous foci distributed throughout the cytoplasm of both the nurse cells and oocyte (Fig. 3A-D). These clusters are distinctly different from the GFP-Exu particles observed in wild type cells in both size and shape, and in observations of living msps egg chambers, Exu-containing foci are amorphous and undergo frequent changes in shape, suggesting they may be part of a dynamic membrane-bound complex (Supplemental Movie 3). The appearance of GFP-Exu clusters is limited to stages 7-10; by the end of stage 10 they have disappeared and the distribution of Exu is much more uniform. Since msps mutations interfere with microtubules, we also re-examined the distribution of GFP-Exu in two populations of colchicine-fed flies: GFP-Exu, mspsP /TM3 and GFP-Exu/TM3. We find that in the mspsP heterozygotes, colchicine treatment often leads to a phenocopy of msps defects (84%, n=31)(Fig. 3F). In contrast, large Exu foci are only found in 32% (n=34) of colchicine-treated GFP-Exu/TM3 egg chambers (Fig. 3E). One simple interpretation of these results is that the complete loss of microtubules induced by colchicine feeding is less likely to cause cluster formation in an otherwise wild type background.
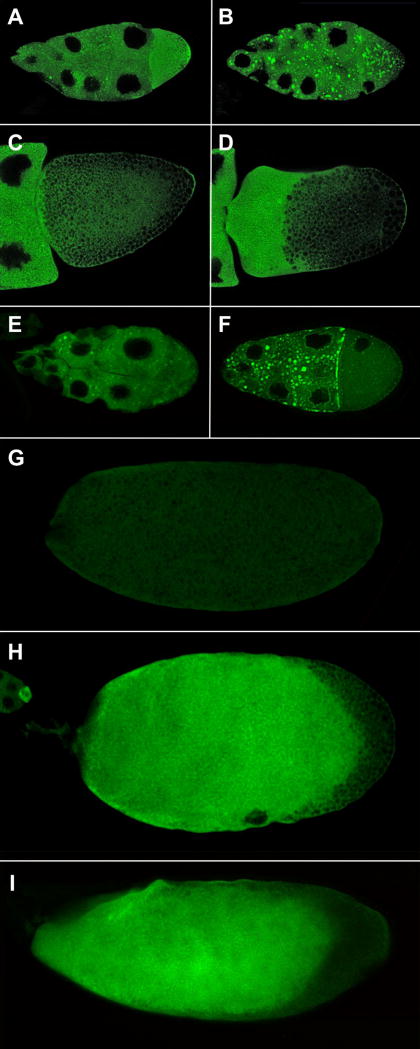
Fig. 3. Expression of GFP-Exu in wild type and msps egg chambers.
Egg chambers expressing GFP-Exu in wild type (A, C) or msps (B, D) females. Stage 8 egg chambers are shown in (A, B) and stage 10 oocytes in (C, D). The msps mutation results in the aggregation of Exu protein. Colchicine-feeding leads to similar effects more frequently in mspsP/TM3 egg chambers (F) than in control TM3 egg chambers (E). In wild type eggs (G) Exu protein is degraded by stage 14 but Exu protein often persists in msps oocytes (H) and in oocytes from females fed colchicine (I).
GFP-Exu distribution in msps egg chambers after stage 10 differs from wild type behavior of GFP-Exu in several respects. GFP-Exu accumulates to high levels in the oocyte, persists in the oocyte much longer than in wild type cells, and is often visible even in mature oocytes (Fig. 3D, H). This is in stark contrast to previous observations that Exu is absent from mature wild type oocytes, as assayed by both Western blots and immunofluorescence (Marcey et al., 1991; Wang and Hazelrigg, 1994). This suggests that there is normally some mechanism that limits the lifespan of Exu protein in the oocyte and that this system may depend on intact microtubules. The unexpected persistence of Exu protein can be explained in two ways; Exu degradation may either be msps-dependent, or it may be microtubule-dependent. To distinguish between these possibilities, we disrupted microtubules in wild type females expressing GFP-Exu using colchicine. We find that colchicine feeding often results in a persistence of GFP-Exu in late-stage oocytes, and that this phenomenon is most commonly seen in oocytes that are clearly stratified (Fig. 3I).
msps mutations have effects on ER organization
There is large body of evidence from studies in cultured cells that the microtubule cytoskeleton is intimately involved in the organization of the endoplasmic reticulum (ER) (for example, see review by (Caviston and Holzbaur, 2006)), and we were interested in the effect of msps mutations on the distribution of various ER compartments. To analyze ER organization directly, we examined several ER proteins that are available as GFP fusions generated by an exon-trapping technique (Morin et al., 2001). One such marker, protein disulfide isomerase (PDI) is a resident of the ER lumen and is considered a general ER marker (Lee and Cooley, 2007). During stages 7-10, PDI-GFP is found in nurse cells, follicle cells, and the oocyte (Fig. 4A-C). In nurse cells, it is distributed in a diffuse web-like pattern, and is concentrated in or near ring canals. Much higher concentrations of PDI-GFP are found in the oocyte, and signal is continuous from the nurse cells into the ooplasm. From stage 10 on, PDI-GFP can be detected in the ooplasm as generalized signal without a clear organization or pattern. In msps mutants, the distribution of PDI-GFP is slightly disrupted (Fig. 4D-F). Diffuse PDI-GFP clumps are evident at st. 7-9 in msps mutants, although they lack discrete borders. The texture of PDI-GFP in the oocyte of msps mutants is also uneven, and in stage 10 oocytes, a clear difference can be seen between incoming nurse cell-derived cytoplasm and ooplasm (Fig. 4F).
Fig. 4. Expression of PDI-GFP in wild type and msps egg chambers.
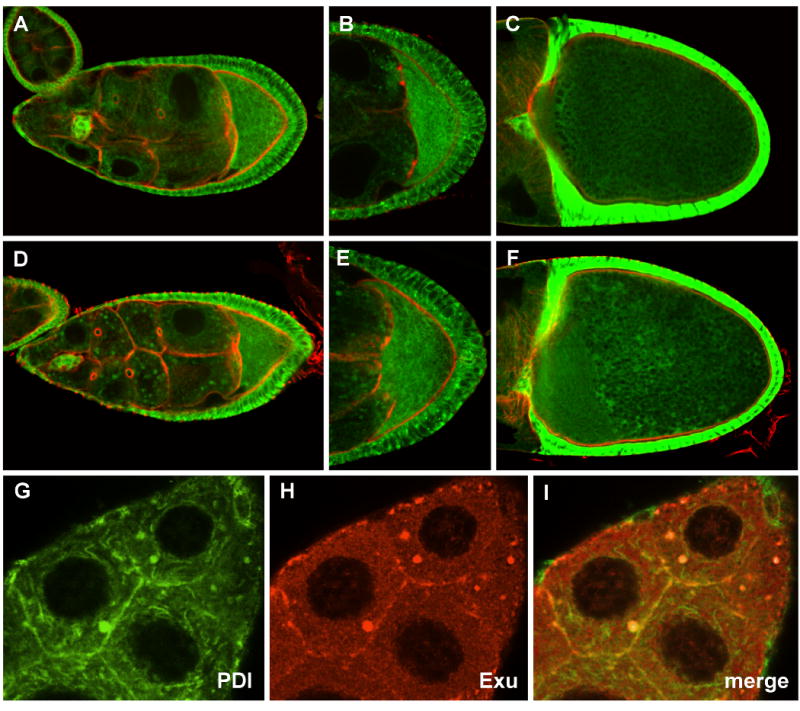
PDI-GFP distribution in wild type (A-C), or msps (D-F) egg chambers (counterstained with phalloidin (red)). During stage 9 (A, B) PDI is distributed in a mesh-like array in the nurse cells, and is present at higher concentrations in the oocyte. Msps mutations (D,E) result in minor perturbations in PDI-GFP distribution. At stage 10 PDI is uniformly distributed in wild type oocytes (C), but its distribution becomes somewhat irregular in msps mutants (F). (G-I) The distribution of PDI-GFP (G) and Exu (H) in the nurse cell cytoplasm of a stage 9 msps egg chamber were compared. Only partial overlap in signal is observed (I).
A variety of circumstantial evidence suggests that Exu associates with a subdomain of the ER. Exu has been reported to form a complex with the RNP protein Me31B (Nakamura et al, 2001). Me31B, and another protein, Tral, are components of RNPs containing silenced RNAs, and both Me31B and Tral localize to ER during earlier stages of oogenesis (Wilhelm et al., 2005; Roper, 2007). These observations prompted us to examine the distribution of Exu with respect to the ER. To test this, msps mutants expressing PDI-GFP were used in double labeling experiments with Exu antibodies to determine the precise location of Exu relative to PDI. We find that Exu overlaps with a subset of PDI-GFP (Fig. 4G-I) but that PDI-GFP staining is more extensive, suggesting Exu is not distributed throughout the ER lumen, but may be associated with a specific compartment of the ER.
Exu colocalizes with tubular ER in msps mutants
As mentioned above, Exu has been identified as a component of a complex also containing Me31B, and recently Roper demonstrated that Tral and Me31B co-localize with Rtnl-1, a component of tubular ER, in early egg chambers (Roper, 2007). Together, these data suggest that Exu could be localized to tubular ER. To test this possibility, we monitored Rtnl1-GFP, a tagged version of Drosophila reticulon, in wild type and msps egg chambers. Reticulons are integral membrane proteins that have been implicated in the formation of tubular ER in other systems (Bauer and Pelkmans, 2006; Voeltz et al., 2006). Rtnl1 has recently been characterized in Drosophila (Wakefield and Tear, 2006) and Roper identified Rtnl1 as a component of the fusome (Roper, 2007). During all stages of oogenesis we find Rtnl1-GFP in both the nurse cells and oocyte, in agreement with previous reports (Roper, 2007). However, our analysis has revealed new insights into the organization of tubular ER during mid- to late-oogenesis. In nurse cells, Rtnl1-GFP is distributed in mesh-like arrays that appear to stretch through ring canals to neighboring nurse cells or to the oocyte (Fig. 5A), and Rtnl1-GFP is often concentrated at or near ring canals. Concentrations of Rtnl1-GFP are higher in the oocyte, where it is distributed along an A-P gradient; within the oocyte, higher concentrations of Rtnl1-GFP are found at the lateral and anterior cortex (Fig. 5B, C). At this time, the distribution of Rtnl1-GFP in the oocyte mirrors that of microtubules, and during stages 10-12, Rtnl1-GFP is arrayed in long fiber-like configurations just below the cortex and surrounding the oocyte nucleus. During these stages, small amounts of Rtnl1-GFP are concentrated along the anterior cortex, and also at the posterior cortex (Fig. 5C). Because the distribution of Rtnl1-GFP in streaming oocytes is so similar to microtubules, we analyzed the movement of Rtnl1-GFP in stage 10-11 oocytes via time-lapse video capture (Fig. 6A-D and Supplemental Movie 4). We find that Rtnl1-GFP is very dynamic during streaming, and decorates long parallel fibers just below the surface of the oocyte that move with a ripple-like motion, strongly suggesting Rtnl1-GFP is associated with the parallel microtubules thought to drive streaming. Consistent with this model, tubulin staining of wild type oocytes expressing Rtnl1-GFP indicates microtubules and Rtnl1-GFP co-localize (Fig. 6E-G).
Fig. 5. Expression of Rtnl1-GFP in wild type and msps egg chambers.
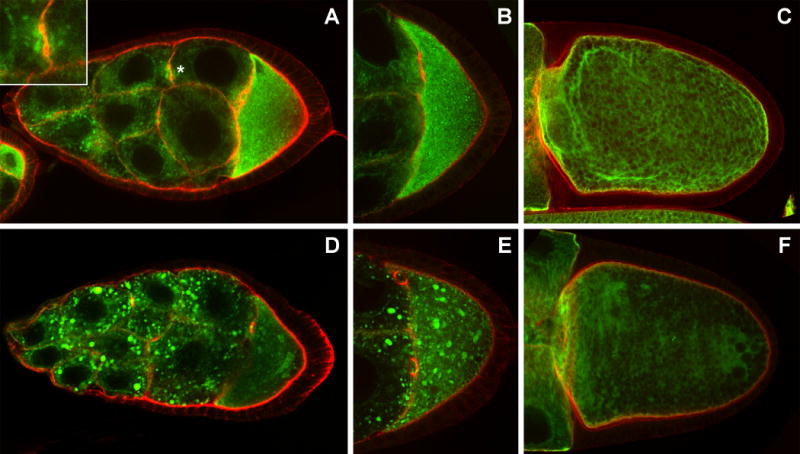
Rtnl1-GFP distribution in wild type (A-C, G), or msps (D-F) egg chambers (counterstained with phalloidin (red)). During stages 8 and 9 (A, B) Rtnl1 is distributed in a mesh-like array in the nurse cells, and is present at higher concentrations in the oocyte. The area denoted with * is a cross section through a ring canal and is enlarged in the inset. At stage 10 Rtnl1 decorates long fibers reminiscent of microtubules in the oocyte (C). Msps mutations (D-F) result in the aggregation of Rtnl1-GFP, much like Exu protein.
Fig. 6. Co-localization of Rtnl1 with microtubules and Exu.
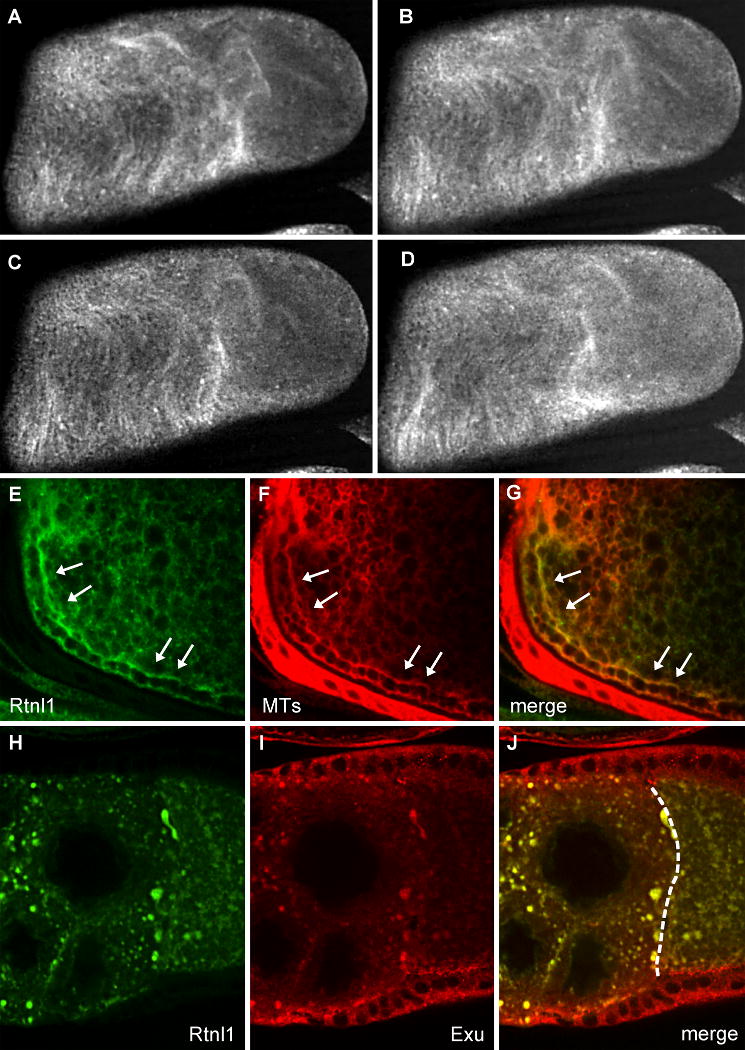
Fibers in the cytoplasm of a wild type stage 11 oocyte are decorated with Rtnl1-GFP (A-D) and move with a ripple-like motion in these live images, taken 40 seconds apart. Wild type stage 10B oocytes expressing Rtnl1-GFP (E) were stained for tubulin (F). Rtnl1 co-localizes with microtubules in streaming oocytes (G). The arrows indicate parallel subcortical microtubules typical of this stage. msps egg chambers expressing Rtnl1-GFP (H) were stained with antibodies to Exu (I). Exu colocalizes with Rtnl1 in msps mutants (J). The dashed line in (J) indicates the nurse cell-oocyte border.
The distribution of Rtnl1-GFP is strikingly different in msps mutants, where it often accumulates as large bodies in nurse cells and the oocyte during stages 7-10 (Fig. 5D-F). Additionally, the microtubule-like distribution of Rtnl1-GFP in stage 10-12 oocytes is lost in msps mutants, as are the anterior and posterior localization of Rtnl1-GFP (Fig. 5F). The Rtnl1-GFP masses observed in msps mutants are indistinguishable from those of GFP-Exu and immediately suggested the intriguing possibility that Exu is associated with tubular ER in these mutants. We analyzed the degree of congruence between Rtnl1-GFP and Exu by staining msps mutants expressing Rtnl1-GFP with antibodies to Exu. We find that virtually all of the foci characteristic of msps mutants contain both Exu and Rtnl1-GFP (Fig. 6H-J). It is unclear whether the association of Exu with tubular ER is a general phenomenon, or a condition that only arises in msps mutants. Owing to the relatively diffuse distribution of both Exu and Rtnl1 in wild type nurse cells, it was not possible to conclusively determine whether the two proteins normally co-localize (data not shown).
Discussion
The analysis of msps mutations has revealed a role for microtubules in a wide variety of cellular events occurring during mid/late oogenesis, and has uncovered associations between Exu and tubular ER. Significantly, comparisons of two ER markers in wild type egg chambers and msps mutants has provided the first evidence for differential association of ER sub-compartments with microtubules in Drosophila oocytes.
Microtubules organize tubular ER but have only minor effects on general ER
There is a substantial body of research concerned with the structure and organization of the endoplasmic reticulum, virtually all derived from work with cultured cells. How much of the data describing the ER in cultured cells are applicable to specialized cells such as the Drosophila egg chamber? This intriguing question is prompted by a number of observations; for example, microtubule organization in the oocyte varies significantly from the classic model (microtubules radiating from a centrally located MTOC) found in textbooks. The current paradigm for ER organization, based primarily on experiments using cultured cells, describes the ER as a continuous membrane compartment (Shibata et al., 2006; Vedrenne and Hauri, 2006) composed of rough ER sheets containing ribosomes, and smooth/tubular ER. While the cytoskeleton contributes to the dynamic nature of the ER (for example, see Vedrenne et al., 2005), its requirement for proper ER organization remains unclear. Several recent reports argue that tubular ER can form in the presence of reticulons and Yop1-related proteins without a need for microtubules (Voeltz et al., 2006; Hu et al., 2008). Here we show that a Drosophila reticulon, Rtnl1, is dependent on microtubules for its organization, and is tightly microtubule-associated in the oocyte. In wild type nurse cells, tubular ER is distributed as a web-like network, but in msps mutants, this network rearranges into numerous clusters that vary in size and shape. In the oocyte, the distribution of Rtnl1-GFP mirrors microtubules at all stages of oogenesis and Rtnl1-GFP appears to move with microtubules even during the forceful streaming that occurs beginning at the end of stage 10.
This portrait of tubular ER contrasts with the distribution of a general ER marker, PDI. PDI is distributed throughout the nurse cells and oocyte, as is Rtnl1-GFP, but we never observe a close correspondence of PDI with microtubules. Further, the effects of msps mutations on PDI are minor, suggesting that the two compartments of the ER interact with microtubules differentially. At the present time, it is unclear whether differences in the distribution of PDI and Rtnl1 reflect a physical independence or whether they mark two different domains of the same organelle. Strong microtubule-based cytoplasmic flows are a distinctive feature of Drosophila oogenesis, and perhaps the interaction of ER tubular membranes with microtubules may be one way of generating the displacement required to mix the oocyte cytoplasm. Since the oocyte expands considerably during this time, it may also be a convenient way to distribute membranes and/or sites of lipid synthesis throughout the periphery of the oocyte for subsequent addition into the plasma membrane. Changes in the distribution of Rtnl1-GFP and also in the luminal ER marker PDI-GFP in msps egg chambers suggest that these cells may have defects in ER trafficking. Several researchers have reported phenotypes associated with impaired ER function (Lee and Cooley, 2007), including defects in membrane addition. Consistent with this idea, we occasionally see evidence of defects in membrane addition such as unusually small oocytes, or egg chambers in which the nurse cells detach from the overlying follicle cells. However these phenotypes always make up a small portion of msps egg chambers observed (less then 5%).
Tubular ER may be a component of Exu-containing sponge bodies
Previous studies reporting that bcd RNA localization is aberrant in msps mutants prompted us to examine the behavior of Exu in msps mutants. Interestingly we have discovered that Exu is associated with tubular ER in the nurse cells of msps egg chambers. Like Rtnl1-GFP, Exu forms large, irregularly shaped clusters in msps mutants. These masses are the earliest manifestation of the msps phenotype, hinting at a process that is highly sensitive to even small changes in microtubule organization. Although we initially interpreted the clustering of GFP-Exu as aggregates, careful observation of GFP-Exu in living msps egg chambers argues against this. We analyzed the movement of the GFP-Exu bodies in living oocytes from msps females, and find that during st. 8-9 of oogenesis, the GFP-Exu foci, despite their size, undergo the same microtubule-dependent behavior previously described for wild type GFP-Exu particles (Theurkauf and Hazelrigg, 1998; Supplemental Movie 4). When following an individual cluster over multiple frames, it is clear that the morphology of the clusters is dynamic, and in general, they are rarely spherical. The irregular shape of Exu clusters immediately suggested they were part of a large membrane-bound complex, and we show here that they also contain Rtnl1. We have looked at a variety of other labeled proteins in msps mutants and do not see any evidence of aggregate formation, and so are confident that the observed structures are not a general consequence of loss of msps activity.
Although Exu and Rtnl1 co-localize in msps mutants, we were unable to determine whether they are associated in wild type nurse cells. One possible interpretation of our results is that Exu and Rtnl1 become associated in msps mutants only as a result of the reduction in microtubules that occurs (i.e. as an aggregation event). However, there is mounting evidence Exu is physiologically associated with tubular ER. First, Exu complexes in msps nurse cells do not behave like nonfunctional aggregates but rather like wild type Exu, in that they move with the same dynamics and microtubule-dependence as in wild type cells (Supplemental Movie 4 and our unpubl. observations). Second, an association of Exu with tubules is inferred from several other published studies. Wilsch-Bräuninger et al. (1997) describe membrane-containing, amorphous masses highly enriched in Exu and ER-like tubules. Roper (2007) found that Rtnl1-GFP and Me31B co-localize during early oogenesis, and as previously noted, Me31B is found in RNPs that also contain Exu (Nakamura et al., 2001). Finally, we show here that Exu and Rtnl1-GFP precisely co-localize in msps mutants.
Based on our results and the published data of others, we suggest that the movement of Exu within nurse cells may be mediated by tubular ER. In this model, ER tubules containing both Exu and Rtnl1 are transported within the nurse cells via microtubules. In msps mutants, it is possible that the drop in microtubule density in nurse cells results in stalled transport, which then leads to the fusion of transport intermediates into the large membrane-bound clusters observed. We note that it is also possible that Exu and Rtnl-1 move along microtubules independently of one another, but do not favor this alternative for the reasons outlined above. Several lines of evidence suggest that Exu-containing tubular ER, the fusome, and sponge bodies have a common origin, perhaps the smooth ER itself. Wilsch-Bräuninger et al. (1997) define sponge bodies as membrane-containing, amorphous masses highly enriched in Exu and they describe ER-like tubules in these bodies. They also report that colchicine feeding of flies results in the appearance of enlarged sponge bodies with associated Exu, a description that fits well with our observations of Exu behavior in msps egg chambers. Similarly, Roper (2007) has shown that Rtnl1 is associated with the fusome, which also contains Me31B, an RNP component that associates with Exu (Nakamura et al., 2001). Are the fusome and sponge bodies the same structure? Previous work argues against this idea; sponge bodies do not contain α-spectrin, a component of fusomes, and conversely sponge bodies contain EM-dense material, while fusomes do not (Wilsch-Bräuninger et al., 1997). However, Roper (2007) identified Rtnl1 as a component of the fusome, and the work described here suggests it is also a component of sponge bodies. Our data are consistent with a model in which the fusome and sponge bodies have a common origin – perhaps sponge bodies and the fusome originate from tubular ER that has been modified for the transport and localization of RNAs. Futher research aimed at identifying additional components of both structures may help resolve this question.
Streaming is required for Exu degradation in the oocyte
One advantage of using the msps208/mspsP genotype to investigate microtubule function is the ready availability of stratified oocytes containing separate fractions of nurse cell-derived and oocyte-derived cytoplasm in one cell. Use of this genetic tool has permitted a close scrutiny of the changes that occur as nurse cell components enter the oocyte proper, and several interesting patterns have emerged. One is the observation that GFP-Exu is much more abundant in the ooplasm of msps eggs. We find that GFP-Exu persists longer in mutant oocytes and accumulates to much higher levels than observed in wild type cells. This may be due to an increase in the stability or translation of exu mRNA, or to a change in the regulation of Exu protein stability. If the distribution of GFP-Exu in a given oocyte is compared to the pattern of stratification (as visualized with DIC optics), there is a complete correspondence, in that GFP-Exu is observed only in the nurse cell-derived cytoplasm. Treatment with colchicine produces the same effect, suggesting that microtubule function, but not msps per se, is required for this phenomenon. Based on these observations, we favor the following model: In streaming wild type ovaries, most Exu entering the oocyte is degraded by oocyte-specific factors, or by mixing of oocyte and nurse cell factors. Remaining Exu protein is cortically associated and perhaps protein-protein or protein-RNA interactions at the cortex protect this population of Exu from degradation. In msps mutants, most of the GFP-Exu entering the oocyte is never directly exposed to ooplasm and so is not targeted for proteolysis. It would be of interest to examine other proteins with a similar pattern of proteolysis during late oogenesis to determine the generality of this phenomenon.
Experimental Procedures
Fly stocks
The mspsP and msps208 alleles were used for mutant analysis. mspsP flies are homozygous lethal, and all analyses of msps phenotypes during oogenesis were performed on mspsP/msps208 females, the most severe female sterile phenotypic combination available. GFP-Exu flies are described in Wang and Hazelrigg (1994). his-GFP flies were obtained from the Bloomington Stock Center. The GFP-tagged lines G0071 (Rtnl1) and ZCL1503 (PDI) were obtained from FlyTrap (http://flytrap.med.yale.edu/).
Fluorescence microscopy
For immunostaining, ovaries were hand-dissected into PBS, fixed, and processed as described in (Theurkauf, 1994b). Fluorescently labeled tubulin antibodies (Sigma, DM1a) were used at a dilution of 1:500, followed by incubation with AlexaFluor 488 secondaries (1:2000) to enhance signal (Moon and Hazelrigg, 2004). Polyclonal Exu antibodies were used at a dilution of 1:1000. In some tubulin staining experiments an alternate method, described in Januschke et al. (Januschke et al., 2006), was used. Phalloidin staining was performed as described in (Meng and Stephenson, 2002).
Live imaging
For live imaging of streaming, fattened females were dissected in a drop of Halogen oil 27 (Sigma-Aldrich, Inc.) on a coverslip and then gently inverted onto a clean slide with additional coverslips serving as spacers. Time lapse movies were recorded by taking images of a single optical section of the oocyte every 10 seconds using the fluorescein channel of a Nikon PCM2000 confocal microscope with a 510 long pass filter. For GFP-tagged lines, stage 8-10 oocytes were prepared and imaged as described above. Streaming was also monitored using DIC optics to collect images just below the surface of the oocyte at a rate of 30 frames per minute. ImageJ software was then used to mark individual particles that were tracked over at least 30 frames. The length of each particle track was then converted into microns and used to calculate approximate velocities.
Supplementary Material
Acknowledgments
We would like to thank Tulle Hazelrigg for reagents and many stimulating conversations, Ed Stephenson for helpful comments, and an anonymous reviewer for useful feedback. We also thank Jerry Calvin for technical assistance, and the Vassar College URSI program for support of A.P.-T. This work was supported by NIH AREA grant R15- GM075997 to N.J.P.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bauer M, Pelkmans L. A new paradigm for membrane-organizing and -shaping scaffolds. FEBS Lett. 2006;580:5559–5564. doi: 10.1016/j.febslet.2006.08.077. [DOI] [PubMed] [Google Scholar]
- Becker BE, Gard DL. Antibodies to XMAP215 and XKCM1 disrupt microtubule organization and meiotic spindle assembly during oocyte maturation in Xenopus. Mol Biol Cell. 2001;12:434A–434A. [Google Scholar]
- Brittle AL, Ohkura H. Mini spindles, the XMAP215 homologue, suppresses pausing of interphase microtubules in Drosophila. EMBO J. 2005;24:1387–1396. doi: 10.1038/sj.emboj.7600629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouhard GJ, Stear JH, Noetzel TL, Al-Bassam J, Kinoshita K, Harrison SC, Howard J, Hyman AA. XMAP215 Is a Processive Microtubule Polymerase. Cell. 2008;132:79–88. doi: 10.1016/j.cell.2007.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caviston JP, Holzbaur ELF. Microtubule motors at the intersection of trafficking and transport. Trends Cell Biol. 2006;16:530–537. doi: 10.1016/j.tcb.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Cha BJ, Koppetsch BS, Theurkauf WE. In vivo analysis of Drosophila bicoid mRNA localization reveals a novel microtubule-dependent axis specification pathway. Cell. 2001;106:35–46. doi: 10.1016/s0092-8674(01)00419-6. [DOI] [PubMed] [Google Scholar]
- Cha BJ, Serbus LR, Koppetsch BS, Theurkauf WE. Kinesin I-dependent cortical exclusion restricts pole plasm to the oocyte posterior. Nat Cell Biol. 2002;4:592–598. doi: 10.1038/ncb832. [DOI] [PubMed] [Google Scholar]
- Cullen CF, Ohkura H. Msps protein is localized to acentrosomal poles to ensure bipolarity of Drosophila meiotic spindles. Nat Cell Biol. 2001;3:637–642. doi: 10.1038/35083025. [DOI] [PubMed] [Google Scholar]
- Cullen CF, Deak P, Glover DM, Ohkura H. mini spindles: A gene encoding a conserved microtubule-associated protein required for the integrity of the mitotic spindle in Drosophila. J Cell Biol. 1999;146:1005–1018. doi: 10.1083/jcb.146.5.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia MA, Vardy L, Koonrugsa N, Toda T. Fission yeast ch-TOG/XMAP215 homologue Alp14 connects mitotic spindles with the kinetochore and is a component of the Mad2-dependent spindle checkpoint. EMBO J. 2001;20:3389–3401. doi: 10.1093/emboj/20.13.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard DL, Kirschner MW. A microtubule-associated protein from Xenopus eggs that specifically promotes assembly at the plus-end. J Cell Biol. 1987;105:2203–2215. doi: 10.1083/jcb.105.5.2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard DL, Becker BE, Josh Romney S. MAPping the eukaryotic tree of life: structure, function, and evolution of the MAP215/Dis1 family of microtubule-associated proteins. Int Rev Cytol. 2004;239:179–272. doi: 10.1016/S0074-7696(04)39004-2. [DOI] [PubMed] [Google Scholar]
- Gergely F, Draviam VM, Raff JW. The ch-TOG/XMAP215 protein is essential for spindle pole organization in human somatic cells. Genes Dev. 2003;17:336–341. doi: 10.1101/gad.245603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Shibata Y, Voss C, Shemesh T, Li Z, Coughlin M, Kozlov MM, Rapoport TA, Prinz WA. Membrane proteins of the endoplasmic reticulum induce high-curvature tubules. Science. 2008;319:1247–50. doi: 10.1126/science.1153634. [DOI] [PubMed] [Google Scholar]
- Januschke J, Gervais L, Gillet L, Keryer G, Bornens M, Guichet A. The centrosome-nucieus complex and microtubule organization in the Drosophila oocyte. Dev. 2006;133:129–139. doi: 10.1242/dev.02179. [DOI] [PubMed] [Google Scholar]
- Januschke J, Gervais L, Dass S, Kaltschmidt JA, Lopez-Schier H, St Johnston D, Brand AH, Roth S, Guichet A. Polar transport in the Drosophilia oocyte requires Dynein and Kinesin I cooperation. Curr Biol. 2002;12:1971–1981. doi: 10.1016/s0960-9822(02)01302-7. [DOI] [PubMed] [Google Scholar]
- Kinoshita K, Habermann B, Hyman AA. XMAP215: a key component of the dynamic microtubule cytoskeleton. Trends Cell Biol. 2002;12:267–273. doi: 10.1016/s0962-8924(02)02295-x. [DOI] [PubMed] [Google Scholar]
- Lee MJ, Gergely F, Jeffers K, Peak-Chew SY, Raff JW. Msps/XMAP215 interacts with the centrosomal protein D-TACC to regulate microtubule behaviour. Nat Cell Biol. 2001;3:643–649. doi: 10.1038/35083033. [DOI] [PubMed] [Google Scholar]
- Lee S, Cooley L. Jagunal is required for reorganizing the endoplasmic reticulum during Drosophila oogenesis. J Cell Biol. 2007;176:941–952. doi: 10.1083/jcb.200701048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcey D, Watkins WS, Hazelrigg T. The Temporal and Spatial-Distribution Pattern of Maternal Exuperantia Protein - Evidence for a Role in Establishment but Not Maintenance of Bicoid Messenger-Rna Localization. EMBO J. 1991;10:4259–4266. doi: 10.1002/j.1460-2075.1991.tb05004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews LR, Carter P, Thierry-Mieg D, Kemphues K. ZYG-9, a Caenorhabditis elegans protein required for microtubule organization and function, is a component of meiotic and mitotic spindle poles. J Cell Biol. 1998;141:1159–1168. doi: 10.1083/jcb.141.5.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng J, Stephenson EC. Oocyte and embryonic cytoskeletal defects caused by mutations in the Drosophila swallow gene. Dev Genes Evol. 2002;212:239–247. doi: 10.1007/s00427-002-0238-z. [DOI] [PubMed] [Google Scholar]
- Moon W, Hazelrigg T. The Drosophila microtubule-associated protein mini spindles is required for cytoplasmic microtubules in oogenesis. Curr Biol. 2004;14:1957–1961. doi: 10.1016/j.cub.2004.10.023. [DOI] [PubMed] [Google Scholar]
- Morin X, Daneman R, Zavortink M, Chia W. A protein trap strategy to detect GFP-tagged proteins expressed from their endogenous loci in Drosophila. Proc Natl Acad Sci U S A. 2001;98:15050–15055. doi: 10.1073/pnas.261408198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura A, Amikura R, Hanyu K, Kobayashi S. Me31B silences translation of oocyte-localizing RNAs through the formation of cytoplasmic RNP complex during Drosophila oogenesis. Dev. 2001;128:3233–3242. doi: 10.1242/dev.128.17.3233. [DOI] [PubMed] [Google Scholar]
- Ohkura H, Garcia MA, Toda T. Dis1/TOG universal microtubule adaptors - one MAP for all? J Cell Sci. 2001;114:3805–3812. doi: 10.1242/jcs.114.21.3805. [DOI] [PubMed] [Google Scholar]
- Roper K. Rtnl1 is enriched in a specialized germline ER that associates with ribonucleoprotein granule components. J Cell Sci. 2007;120:1081–1092. doi: 10.1242/jcs.03407. [DOI] [PubMed] [Google Scholar]
- Shibata Y, Voeltz GK, Rapoport TA. Rough sheets and smooth tubules. Cell. 2006;126:435–439. doi: 10.1016/j.cell.2006.07.019. [DOI] [PubMed] [Google Scholar]
- Theurkauf WE. Premature Microtubule-Dependent Cytoplasmic Streaming in Cappuccino and Spire Mutant Oocytes. Science. 1994a;265:2093–2096. doi: 10.1126/science.8091233. [DOI] [PubMed] [Google Scholar]
- Theurkauf WE. Immunofluorescence analysis of the cytoskeleton during oogenesis and early embryogenesis. Methods Cell Biol. 1994b;44:489–505. doi: 10.1016/s0091-679x(08)60928-0. [DOI] [PubMed] [Google Scholar]
- Theurkauf WE, Hazelrigg TI. In vivo analyses of cytoplasmic transport and cytoskeletal organization during Drosophila oogenesis: characterization of a multi-step anterior localization pathway. Dev. 1998;125:3655–3666. doi: 10.1242/dev.125.18.3655. [DOI] [PubMed] [Google Scholar]
- Theurkauf WE, Smiley S, Wong ML, Alberts BM. Reorganization of the Cytoskeleton During Drosophila Oogenesis - Implications for Axis Specification and Intercellular Transport. Dev. 1992;115:923–936. doi: 10.1242/dev.115.4.923. [DOI] [PubMed] [Google Scholar]
- Vasquez RJ, Gard DL, Cassimeris L. Xmap from Xenopus Eggs Promotes Rapid Plus End Assembly of Microtubules and Rapid Microtubule Polymer Turnover. J Cell Biol. 1994;127:985–993. doi: 10.1083/jcb.127.4.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vedrenne C, Hauri HP. Morphogenesis of the endoplasmic reticulum: Beyond active membrane expansion. Traffic. 2006;7:639–646. doi: 10.1111/j.1600-0854.2006.00419.x. [DOI] [PubMed] [Google Scholar]
- Vedrenne C, Klopfenstein DR, Hauri HP. Phosphorylation controls CLIMP-63-mediated anchoring of the endoplasmic reticulum to microtubules. Mol Biol Cell. 2005;16:1928–37. doi: 10.1091/mbc.E04-07-0554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voeltz GK, Prinz WA, Shibata Y, Rist JM, Rapoport TA. A class of membrane proteins shaping the tubular endoplasmic reticulum. Cell. 2006;124:573–586. doi: 10.1016/j.cell.2005.11.047. [DOI] [PubMed] [Google Scholar]
- Wakefield S, Tear G. The Drosophila reticulon, Rtnl-1, has multiple differentially expressed isoforms that are associated with a sub-compartment of the endoplasmic reticulum. Cell Mol Life Sci. 2006;63:2027–2038. doi: 10.1007/s00018-006-6142-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SX, Hazelrigg T. Implications for Bcd Messenger-Rna Localization from Spatial-Distribution of Exu Protein in Drosophila Oogenesis. Nature. 1994;369:400–403. doi: 10.1038/369400a0. [DOI] [PubMed] [Google Scholar]
- Wilhelm JE, Buszczak M, Sayles S. Efficient protein trafficking requires trailer hitch, a component of a ribonucleoprotein complex localized to the ER in Drosophila. Dev Cell. 2005;9:675–685. doi: 10.1016/j.devcel.2005.09.015. [DOI] [PubMed] [Google Scholar]
- Wilhelm JE, Mansfield J, Hom-Booher N, Wang S, Turck CW, Hazelrigg T, Vale RD. Isolation of a ribonucleoprotein complex involved in mRNA localization in Drosophila oocytes. J Cell Biol. 2000;148:427–440. doi: 10.1083/jcb.148.3.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilsch-Bräuninger M, Schwarz H, Nüsslein-Volhard C. A sponge-like structure involved in the association and transport of maternal products during Drosophila oogenesis. J Cell Biol. 1997;139:817–829. doi: 10.1083/jcb.139.3.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.