Abstract
Many metazoan cells can take up exogenous double-stranded (ds) RNA and use it to initiate an RNA silencing response, however, the mechanism for this uptake is ill-defined. Here, we identify the pathway for dsRNA uptake in Drosophila melanogaster S2 cells. Biochemical and cell biological analyses, and a genome-wide screen for components of the dsRNA-uptake machinery, indicated that dsRNA is taken up by an active process involving receptor-mediated endocytosis. Pharmacological inhibition of endocytic pathways disrupted exogenous dsRNA entry and the induction of gene silencing. This dsRNA uptake mechanism seems to be evolutionarily conserved, as knockdown of orthologues in Caenorhabditis elegans inactivated the RNA interference response in worms. Thus, this entry pathway is required for systemic RNA silencing in whole organisms. In Drosophila cells, pharmacological evidence suggests that dsRNA entry is mediated by pattern-recognition receptors. The possible role of these receptors in dsRNA entry may link RNA interference (RNAi) silencing to other innate immune responses.
RNAi is a highly conserved dsRNA-guided mechanism that mediates sequence-specific gene silencing1. A number of animal cells can naturally take up exogenous dsRNA and use it to initiate RNAi silencing2,3. In some organisms, such as Drosophila, certain cells can efficiently take up dsRNA but seem to be unable to transmit this dsRNA to other cells in the body4. dsRNA uptake without further transmission to other cells has also been reported for some mammalian cell types5–7. Other organisms (such as C. elegans or juvenile grasshoppers) can both take up dsRNA and spread it systemically to elicit an RNAi response throughout the entire animal8,9. The mechanisms of uptake and spread of dsRNA are poorly understood. It is unclear whether dsRNA enters cells through passive, non-specific mechanisms, or whether there is an active mechanism that controls entry. Genetic analysis to identify genes involved in systemic spread of dsRNA in C. elegans isolated several mutants unable to distribute an ingested dsRNA signal from the gut throughout the body8,10,11. One of these, SID-1 (also known as RSD-8) is a putative transmembrane protein required for systemic spread8. When expressed ectopically in Drosophila cells, SID-1 enhanced the RNAi response observed at low dsRNA concentrations12, raising the possibility that SID-1 may function as a channel on the cell surface for uptake of dsRNA. However, endogenous sid-1 homologues have not been found in the Drosophila genome, yet Drosophila S2 cells effectively take up dsRNA and use it to induce gene silencing. As it is difficult to distinguish between uptake and systemic spread mechanisms in whole animal screens, we decided to directly examine the dsRNA-uptake mechanism using cultured Drosophila cells. An added advantage of this approach is that it allowed the detection of essential genes and processes that would be lethal in the context of a whole organism. Here, we report the identification of the dsRNA-uptake pathway in Drosophila using a combination of biochemical, cell biological and genomic approaches. Taken together, our results indicate that dsRNA entry and initiation of an RNAi silencing response requires receptor-mediated endocytosis in Drosophila S2 cells. A genome-wide RNAi screen implicated numerous components of endocytosis and vesicle-mediated trafficking in dsRNA uptake and, importantly, RNAi silencing. Furthermore, orthologues of these genes are also critical for the RNAi response in C. elegans, pointing to conserved evolution of this entry pathway.
RESULTS
Initiation of efficient gene silencing depends on exogenous dsRNA length
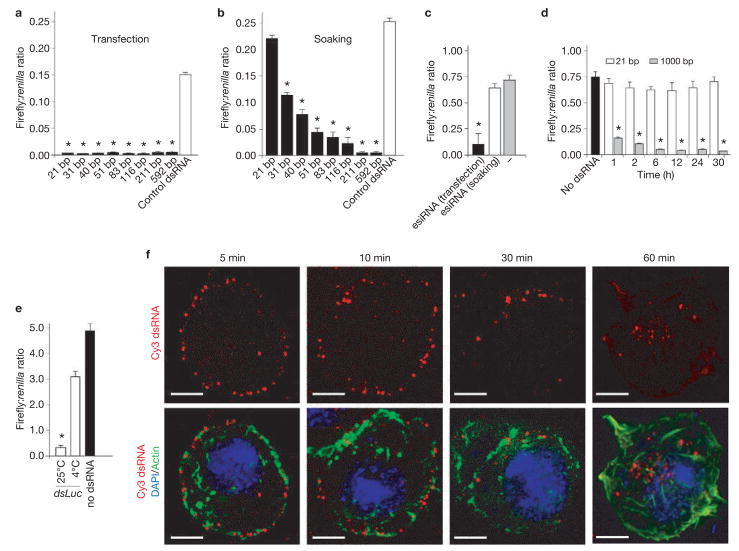
Drosophila S2 cells can efficiently take up dsRNA over a wide range of concentrations (see Supplementary Information, Fig. S1a) and use it to initiate an RNAi response. To explore the properties of dsRNA entry pathway, we initially determined whether there was an optimal dsRNA length for efficient entry through the natural uptake machinery of Drosophila S2 cells. dsRNAs targeting firefly luciferase, and ranging from 21–592 base pairs (bp) in length, were either added to the culture supernatant (‘soaking’) or forcibly introduced into cells by transfection, as a control (Fig. 1a, b). As expected, transfection of dsRNA resulted in effective firefly luciferase silencing irrespective of the dsRNA length (Fig. 1a). In contrast, uptake of dsRNAs added to the medium was clearly length-dependent (Fig. 1b). It was possible that uptake of dsRNA could exhibit sequence specificity, as this could influence efficient uptake of short dsRNA to a greater extent than longer dsRNAs. Therefore, the RNase Dicer was used to derive a mixed pool of 21-mer siRNAs from a 1000 bp dsRNA fragment. Importantly, this diverse pool of short siRNAs (enzymatically generated siRNA; esiRNA) contained a mix of all the sequences used in Fig. 1a and b, yet failed to enter S2 cells, even though it effectively induced silencing after transfection (Fig. 1c).
Figure 1.
RNAi in Drosophila S2 cells is dependent on the length of the dsRNA. (a, b). Silencing of luciferase expression after exposure of S2 cells to dsRNA by transfection (a) or by adding dsRNA in the culture supernatant (soaking; b). S2 cells were cotransfected with expression plasmids encoding firefly and Renilla luciferase. Specific dsRNA targeting firefly luciferase was either transfected into the cell in conjunction with the expression plasmids or was added to the culture supernatant one day after transfection. Luciferase activity was monitored after 48 h and it is expressed as firefly:Renilla ratio. Luciferase activity after treatment with specific dsRNA of different sizes was compared with treatment with a non-specific dsRNA (control dsRNA). Results represent averages and s.d. from four independent experiments. The asterisk indicates P<0.01 with respect to untreated control (−). (c) Silencing of firefly luciferase expression after transfection or soaking pools of specific 21 bp siRNAs generated by cleavage of long dsRNA by recombinant Dicer (esiRNA) compared with luciferase activity in the absence of dsRNA (−). Results represent averages and s.d. from three independent experiments. The asterisk indicates P<0.01 with respect to untreated control (−). (d) Time course comparing silencing of luciferase after soaking of S2 cells in dsRNA of 21 or 1000 bp. The experiment was performed as described in a and b but dsRNA was washed away after the indicated incubation times. Results represent averages and s.d. from three independent experiments. The asterisk indicates P<0.01 with respect to no dsRNA control, (e) Silencing of luciferase expression after soaking cells with specific dsRNA at 4 °C or 25 °C. S2 cells were transfected with expression plasmids encoding firefly and Renilla luciferase and exposed to dsRNA at 4 °C or 25 °C. After 1 h incubation, the cells were washed and further cultured at 25 °C for 48 h, and luciferase activity was monitored. Results represent averages and s.d. from three independent experiments. The asterisk indicates P<0.01 with respect to no dsRNA control, (f) Cellular localization of Cy3-labelled dsRNA over time. Shortly after incubation, dsRNA seemed to bind in a punctate pattern to the cell surface (5 min). Over the course of 60 min the dsRNA was internalized but remained in small punctate structures. Optical sections were deconvolved and flattened into a two-dimensional projection for presentation. The 60 min time point corresponds to an independent experiment. The scale bar in f represents 2 μM.
The kinetics of long and short dsRNA uptake by S2 cells were then compared. Cells were pulsed by incubation with dsRNAs targeting firefly luciferase for different times, washed extensively to remove free dsRNA and cultured for 48 h to allow subsequent transfer of the dsRNA into the RNAi machinery and cleavage of the target RNA. dsRNA of 1,000 bp rapidly became tightly associated with cells — a 1 h pulse already yielded significant firefly luciferase silencing compared with untreated control (Fig. 1d). Similar kinetics were observed using dsRNA of 200 bp (data not shown). In contrast, exposure of S2 cells to 21 bp siRNA did not result in any significant silencing, even after prolonged incubation times of up to 30 h (Fig. 1d).
Whether internalization of functional dsRNA by the endogenous S2 cell uptake machinery is temperature dependent was also examined (Fig. 1e). RNAi silencing of firefly luciferase was inefficient when cells were pulsed with dsRNA for 60 min at 4 °C instead of 25 °C (Fig. 1e). This temperature dependence indicates that natural uptake of dsRNA into Drosophila S2 cells is an active process.
The kinetics of uptake were also examined by fluorescence microscopy to determine the subcellular distribution of dsRNA during the early phases of uptake. Cells were incubated with cy3-labelled dsRNA followed by extensive washing and monitoring by fluorescence microscopy (Fig 1f). Importantly, the labelled Cy3 dsRNA was fully active in a functional assay (see Supplementary Information, Fig. S1b, c). Shortly after incubation (5 min), dsRNA seemed to bind in a punctate pattern to the cell surface (Fig. 1f). Over the course of 60 min at 25 °C, the dsRNA was internalized but remained in punctate structures (Fig. 1f). Thus, S2 cells have an active mechanism for uptake and subcellular localization of long dsRNA.
The uptake pathway discriminates between dsRNA and DNA
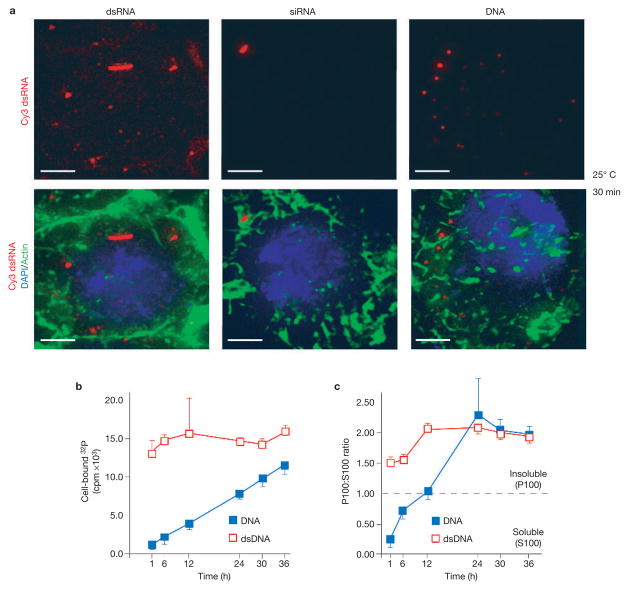
Fluorescence microscopy revealed distinctions in the interaction of dsRNA, DNA or siRNAs with S2 cells. Long dsRNA bound to cells and was localized in large puncta in the cell interior (Fig. 2a). Low-level binding and no obvious internalization of siRNA was observed (Fig. 2a). DNA bound less efficiently than long dsRNA and, while seemingly internalized, it was localized to peripheral puncta that were smaller than those seen for dsRNA (Fig. 2a).
Figure 2.
An active mechanism for uptake of long dsRNA in Drosophila S2 cells. (a) Subcellular localization of Cy3-labelled 500 bp dsRNA, siRNA and 500 bp DNA. (b) Association of radiolabelled dsRNA or DNA with S2 cells over time. S2 cells were incubated with radiolabelled dsRNA or DNA of the same size and sequence and cell-bound radioactivity was determined in a scintillation counter. Results represent averages and s.d. from two independent experiments. (c) Subcellular localization of radiolabelled dsRNA or DNA with S2 cells over time. S2 cells were incubated with dsRNA or DNA for the indicated times, lysed and fractionated by ultracentrifugation. The data are expressed as the ratio of radioactivity in pellet to supernatant. Results represent averages and s.d. from two independent experiments. The scale bar in a represents 2 μm.
Consistent with the fluorescence microscopy analysis, the time course of uptake and localization of dsRNA and DNA was substantially different. Incubation of S2 cells with 32P-radiolabelled dsRNA or DNA of the same sequence and length, followed by measurement of cell-associated radioactivity (32P), showed that 1000 bp dsRNA rapidly associated with the cells (Fig. 2b). This measurement is in good agreement with the rapid uptake of dsRNA observed using the RNAi functional assay (Fig. 1d). In contrast, DNA association with cells was less efficient and slower (Fig. 2b).
The subcellular distribution of dsRNA during uptake was also analysed using a biochemical approach and compared with the uptake of DNA. Cells were incubated with 32P-labelled DNA or dsRNA for the indicated times, washed to remove unbound material, lysed and separated into soluble (S100) and insoluble (P100) fractions. Whereas the S100 fraction contained a marker for soluble cytoplasmic components (tubulin), P100 contained markers corresponding to membranous organelles, including the plasma membrane, Golgi, endosomes and lysosomes (see Supplementary Information, Fig. S1d). The amount of 32P-labelled DNA or dsRNA in each fraction was then estimated and expressed as the ratio of P100:S100 (Fig. 2c). Strikingly, as early as 1 h after incubation, dsRNA was enriched in the pellet fraction. In contrast, DNA was initially observed in the soluble fraction and was only found in the pellet fraction at later times.
These data show distinctions in the binding and uptake of short dsRNA, longer dsRNA and DNA with S2 cells and indicate that long dsRNA is rapidly bound on the cell surface and seems to accumulate in intracellular structures or bodies.
Genome-wide screen for genes involved in dsRNA uptake
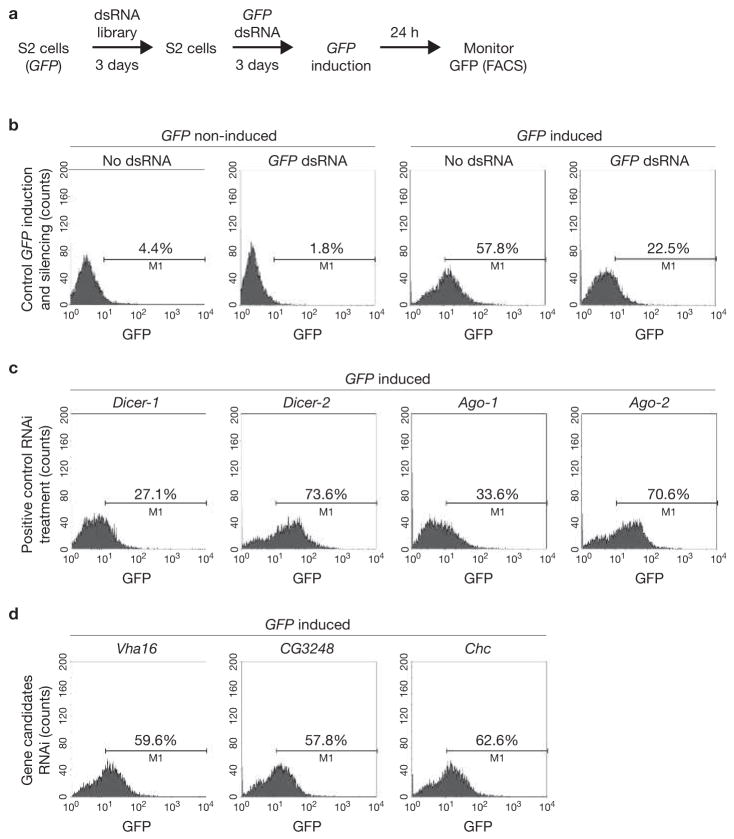
To identify components required for RNAi silencing triggered by exogenously added dsRNA, we undertook a genome-wide functional screen. An ‘RNAi of RNAi’ approach13,14 was used to downregulate cellular genes required for RNAi silencing of an inducible GFP reporter (Fig. 3a, b). We used a dsRNA library that targets the 7,216 Drosophila genes that have known homologues in C. elegans and mammals and corresponds to approximately 50% of the Drosophila genome15. Positive controls for RNAi-mediated downregulation of the GFP reporter included the core components of the RNAi machinery, Dicer 2 and Argonaute 2 (Ago-2, Fig. 3c). Fluorescence activated cell sorting (FACS) analysis indicated that targeting Dicer-2 or Ago-2 reduced the level of silencing and thus increased the GFP signal (Fig. 3c). In contrast, targeting their close homologues Dicer-1 and Ago-1, which do not function in processing of long dsRNA but instead in the microRNA pathway, inhibited GFP silencing to a lesser extent (Fig. 3c). The initial screen identified 66 genes required for RNAi silencing (Fig. 3d and data not shown). Three secondary screens using different RNAi reporters yielded a subset of 23 genes required for RNAi silencing of all reporters (Fig. 3d and Table 1). Importantly, downregulation of the genes identified in this screen did not affect microRNA production or function (see Supplementary Information, Fig. S2). Thus, the genes identified in our screen are specifically involved in exogenous dsRNA uptake and processing.
Figure 3.
RNAi screen for genes involved in the RNAi pathway. (a) Schematic representation of the screening approach. S2 cells stably transfected with GFP under the inducible metallothionein promoter are treated with dsRNA from the RNAi library for three days. The cells are then split and refed with dsRNA from the RNAi library and with dsRNA targeting GFP. After a further three day incubation, GFP expression is induced by the addition of CuSO4 to the culture supernatant and GFP expression is monitored. (b) Induction of GFP expression and RNAi in the reporter cell line. GFP expression is monitored on a FACS Calibur flow cytometer and analysed using Cellquest software. GFP expression is induced on addition of CuSO4 in the culture medium. (c) Validation of the RNAi of RNAi approach. S2–GFP cells were pretreated with dsRNA targeting the RNAi-associated genes Dicer-1, Dicer-2, Ago-1 and Ago-2, followed by RNAi of the GFP marker gene.(d) Examples of three genes identified in the RNAi screen. Knockdown of V-H-ATPase subunit {Vha l6), CG3248 anti Clathrin heavy chain (chc) inhibits subsequent RNAi of GFP, as evident by a high GFP expression.
Table 1.
Identification of genes involved in RNAi function in Drosophila S2 cells
| Readoutb |
||||||
|---|---|---|---|---|---|---|
| Groupa | Gene ID | Gene name | Relish–GFP | LAMP GFP | dlAP1 | Luciferase |
| Proton transport | CG3161 | Vhal6 | +++ | +++ | ++ | +++ |
| CG17332 | VhaSFD | ++ | + | + | ++ | |
|
| ||||||
| Vesicle mediated transport | CG9012 | Clathrin he | +++ | +++ | ++ | +++ |
| CG7057 | AP-50 | ++ | ++ | ++ | ++ | |
| CG5915 | Rab7 | ++ | ++ | ++ | ++ | |
| CG6025 | Arf72A | + | + | ++ | +++ | |
|
| ||||||
| Intracellular transport | CG54125 | ninaC | + | + | ++ | ++ |
| CG6177 | IdlCp | +++ | ++ | + | + | |
| CG3248 | +++ | ++ | ++ | ++ | ||
| CG3911 | + | ++ | + | ++ | ||
| CG18028 | light | + | + | + | + | |
|
| ||||||
| Lipid metabolism | CG3495 | Gmer | + | + | ++ | +++ |
| CG5373 | Pi3K59F | + | + | ++ | +++ | |
| CG12070 | Saposin-r | ++ | +++ | +++ | +++ | |
|
| ||||||
| Proteolysis and peptidolysis | CG4572 | +++ | +++ | +++ | +++ | |
| CG5053 | + | + | ++ | ++ | ||
| CG8184 | + | + | ++ | ++ | ||
| CG8773 | + | + | ++ | ++ | ||
|
| ||||||
| Other | CG9659 | egghead | +++ | + | ++ | ++ |
|
| ||||||
| Unknown | CG5161 | ++ | + | ++ | + | |
| CG5382 | ++ | + | ++ | ++ | ||
| CG5434 | +++ | +++ | ++ | ++ | ||
| CG8671 | + | + | ++ | ++ | ||
|
| ||||||
| RNAi function | CG6493 | Dicer 2 | +++ | ++ | + | ++ |
| CG4792 | Dicer 1 | + | ++ | ++ | ++ | |
| CG7439 | Argonaute 2 | +++ | +++ | +++ | +++ | |
|
| ||||||
| Control | − | − | − | − | ||
Genes were classified according to their biological function as annotated in Flybase (http://flybase.bio.indiana.edu/annot/).
Inhibition of RNAi function was assayed using four different secondary RNAi assays. Score: +++, expression of reporter gene equal to control in which expression of marker genes is not suppressed by RNAi; ++ and +, intermediate levels of expression of the reporter gene; −, expression of the reporter gene was silenced to the same level as control that only received marker gene RNAi treatment.
Strikingly, components of the endocytic pathway were strongly represented in the screen, including genes for clathrin heavy chain and its adaptor AP-50, which mediate early endocytic uptake, as well as rab7, Arf72A (ARF-like 1 orthologue), light (vacuolar protein sorting Vsp41 orthologue) and vacuolar H+-ATPase (V-H-ATPase), involved in controlling endocytic vesicle trafficking and protein sorting (Table 1). The screen also identified members of the conserved oligomeric Golgi complex (COG) family: IdlCp and CG3248, (Cog2 and Cog3 orthologues respectively), the gene CG3911 (transport protein particle –TRAPP- component 3 orthologue) and genes involved in cyoskeleton organization and protein transport (ninaC). Therefore, it seems that exogenous dsRNA enter the RNAi pathway through the intracellular vesicle network. This conclusion is further supported by the identification of two genes involved in lipid metabolism and modification, Pi3K and Saposin–r. In addition to these relatively well-annotated genes, the screen also identified genes of unknown function. Taken together with the conclusions of our biochemical analysis, this genome-wide screen indicates that the pathway of dsRNA uptake relies on receptor-mediated endocytosis.
Pharmacological inhibitors of the dsRNA-uptake machinery
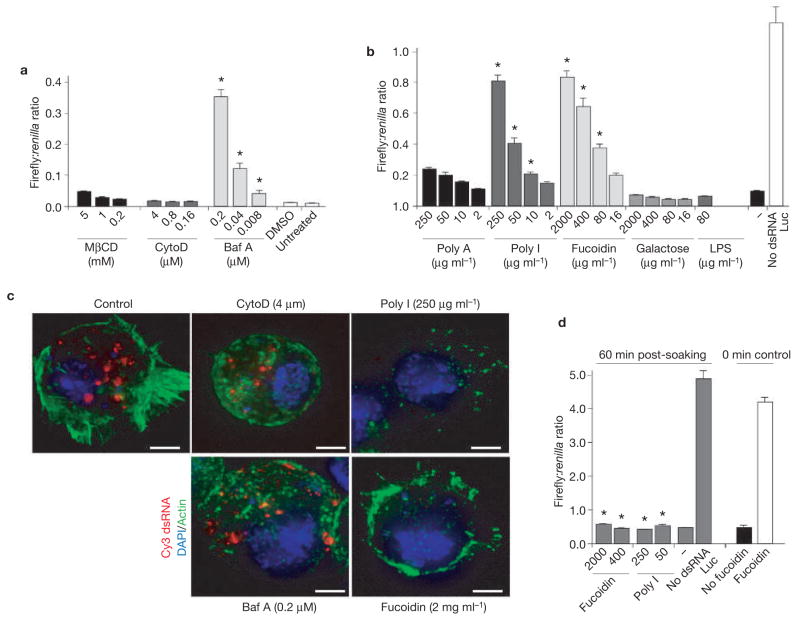
The role of the endocytic pathway in dsRNA entry was then explored using a pharmacological approach that tested the effect of inhibitors of cellular uptake mechanisms on RNAi silencing. Bafilomycin-A1 (Baf A), a specific inhibitor of V-H-ATPase, strongly inhibited silencing of the reporter gene (Fig. 4a). In contrast, methyl-β-cyclodextran and cytochalasin-D, inhibitors of caveolae mediated endocytosis or phagocytosis16,17, respectively, did not affect RNA silencing (Fig. 4a). Therefore, it seems that V-H-ATPase, a component of the endosomal-lysosomal acidification process18, is required for dsRNA entry and RNA silencing in S2 cells, but not caveolae or phagocytosis. These conclusions are consistent with our finding that downregulation of subunits of V-H-ATPase inhibit silencing that is mediated by exogenous dsRNA (Table 1). In good agreement with the role of V-H-ATPase at early stages of the endocytic pathway19–21, the exogenous dsRNA still accumulated in vesicles on treatment with Baf A (Fig. 4c).
Figure 4.
Endocytic uptake of dsRNA into Drosophila S2 cells is mediated by scavenger receptors, (a) Silencing of luciferase expression in the presence of inhibitors of the endocytic pathway. S2 cells were transfected with expression plasmids encoding firefly and Renilla luciferase. One day after transfection, the cells were exposed to different concentrations of methylβ-cyclodextran (MβCD), Cytochalasin D (CytoD) or Bafilomycin A (Baf A) for 30 min and dsRNA was added in the presence of these inhibitors. Controls include incubations of dsRNA in the presence of the solvent dimethylsulfoxide (DMSO) and in the absence of inhibitor (untreated). Results represent averages and s.d. from three independent experiments. The asterisk indicates P<0.01 with respect to untreated control.(b) Silencing of luciferase expression in the presence of competitive inhibitors of scavenger receptors. The experiment was performed as described in a, using the inhibitors of scavenger receptors poly-inosinic acid (Poly I) and Fucoidin. Controls include incubation of S2 cells with dsRNA in the presence of compounds that are not known to inhibit scavenger receptors, poly-adenosinic acid, galactose and lipopolysaccharide (LPS) and in the absence of inhibitors (−). Results represent averages and s.d. from three independent experiments. The asterisk indicates P<0.01 with respect to untreated control (−). (c) Subcellular localization of Cy3-labelled dsRNA after incubation in the presence of inhibitors of the endocytic pathway and of scavenger receptors. (d) Inhibitors of scavenger receptors do not affect RNAi when added after incubation of S2 cells with dsRNA. S2 cells were transfected with plasmids encoding firefly and Renilla luciferase. Cells were then incubated with dsRNA for 1 h, washed and fucoidin or poly I was added to the culture. The bars labelled ‘0 min control’ indicate incubations of Fucoidin in the presence of dsRNA and confirm the data in Fig. 4b. Results represent averages and s.d. from three independent experiments. No significant statistical difference was observed (asterisk), P>0.05, with respect to untreated control (−). The scale bar in c represents 2 μm.
dsRNA uptake is blocked by inhibitors of pattern-recognition receptors
Although our biochemical experiments hinted at the existence of a surface receptor for entry of long dsRNAs, the screen did not identify any putative candidates for this function. This would be expected if these genes are not represented in the initial dsRNA library, which only targeted conserved genes. Alternatively, dsRNA uptake could be mediated by several related receptors with overlapping function. As dsRNA is a long polymer with a relatively regular structure, we reasoned that dsRNA recognition may be mediated by receptors that recognize repetitive patterns in biological macromolecules. There are two major classes of these so-called ‘patternrecognition receptors’: the Toll receptors and the scavenger receptors22,23. A candidate approach was used to systematically examine the contribution of different classes of receptors to dsRNA uptake and silencing. RNAi-mediated downregulation was carried out for all annotated pattern-recognition receptors and the efficiency of the RNAi treatment was monitored by semi-quantitative RT–PCR (see Supplementary information, Fig. S3d, e). Downregulation of eight Toll receptors had no effect on dsRNA uptake or silencing (see Supplementary information, Fig. S3c). Similarly, individual downregulation of nineteen annotated genes coding for scavenger receptors in Drosophila did not result in significant inhibition of RNAi silencing (see Supplementary Information, Fig. S3a and Table S1), even under conditions where downregulation of scavenger receptor class C, type I (Sr-CI) dramatically reduced bacterial uptake (see Supplementary Information, Fig. S3b).
The putative role of pattern-recognition receptors in dsRNA uptake and silencing was then evaluated using a pharmacological approach. We examined whether macromolecules known to interact with scavenger receptors competed for binding of the dsRNA, and thus inhibited RNAi silencing. PolyI and fucoidin, well known ligands of the scavenger-receptor family24, strongly inhibited both dsRNA binding and uptake, as assessed by fluorescent dsRNA (Fig. 4c), and dsRNA-initiated silencing (Fig. 4b). In contrast, chemically related molecules that interact with other receptors, but do not inhibit scavenger receptors (such as the polysaccharide LPS and the nucleic acid polyA, or the monosaccharide galactose), did not affect dsRNA-initiated silencing (Fig. 4b). Importantly, polyI and fucoidin inhibited silencing only if added early during dsRNA uptake (Fig. 4d), suggesting that binding and uptake of dsRNA is mediated by members of the scavenger-receptor family. Although RNAi downregulation of individual scavenger receptors did not result in inhibition of RNAi function, the strong inhibition observed by the pharmacological treatments may indicate that multiple scavenger receptors with overlapping functions participate in dsRNA uptake to induce RNAi. To explore this possibility, mixed dsRNAs targeting each known receptor were transfected into S2 cells. None of these mixtures produced a significant reduction in RNAi silencing (data not shown). Consistent with these results, a recent report showed that fluorescent dsRNA is internalized by Sr-CI and Eater receptor-mediated endocytosis in S2 cells25. However, this study was also unable to demonstrate that downregulation of scavenger receptors impairs RNAi function. In principle, it is possible that the simultaneous targeting of multiple genes by RNAi is not effective enough to impair the dsRNA binding to the remaining receptors. It is also possible that novel, unidentified members of this diverse family are responsible for this uptake function.
Conservation of entry pathways between Drosophila and C. elegans
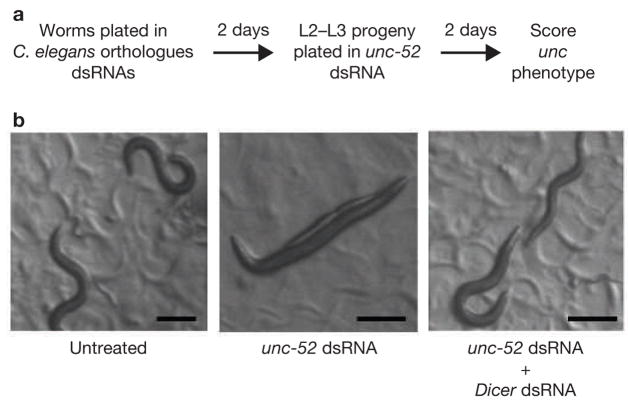
Many components of the core RNAi machinery are highly conserved through evolution. However, organisms exhibit substantial differences in their ability to take up and spread dsRNA for silencing26. To determine whether the genes identified in the Drosophila ‘RNAi of RNAi’ screen also participate in RNAi function in other organisms, we studied the effect of knocking down Drosophila orthologues in the nematode C. elegans. Worms were fed Escherichia coli expressing dsRNAs targeting the C. elegans orthologues of the Drosophila genes required for dsRNA uptake. Two days later the progeny were challenged with a second dsRNA targeting unc-52, which is essential for muscle development in both embryos and larvae (Fig. 5). Knockdown of unc-52 causes severe defects in myofilament assembly and leads to paralysis27,28; therefore, inhibition of RNAi silencing should alleviate the phenotype normally associated with RNAi knockdown of unc-52. As expected, knockdown of Dicer suppressed the phenotypic consequences of the treatment with unc-52 dsRNA (Fig. 5 and Table 2). Strikingly, worms treated with dsRNAs corresponding to several of the genes identified in the Drosophila screen inactivated the systemic spread of RNAi silencing in worms (Table 2, normal). Our experiments indicated that, similar to Drosophila S2 cells, several components of C. elegans intracellular vesicle transport (F22G12.5, C06G3.10, ZK1098.5 and ZK1098.5), as well as lipid modifying enzymes (R01H2.5 and Pi3K) are required for systemic RNAi. Notably, many of the core components of the endocytic pathway involved in dsRNA uptake in Drosophila were essential for viability of the worms and could not be tested (Table 2). In addition, the orthologues of several Drosophila genes with unknown function were also required for systemic RNAi in C. elegans (B0464.4, W05H7.3, Y45G12B.2 and C54H2.1). Thus, the basic machinery that mediates dsRNA upake in Drosophila is also required for systemic spread of the RNAi signal in C. elegans, suggesting that the dsRNA entry pathway is evolutionarily conserved and functionally relevant in intact organisms.
Figure 5.
Orthologues of the Drosophila genes confer an RNAi phenotype in C. elegans. (a) Schematic representation of the experimental design. Worms were grown on bacteria expressing dsRNA targeting specific genes. L2–L3 progeny was subsequently plated on bacteria expressing dsRNA specific for unc-52 and unc phenotype was monitored after 48 hours.
(b) C. elegans grown on bacteria expressing dsRNA control targeting Dicer are incapable of processing unc-52 dsRNA and do not display the unc52 RNAi phenotype. The scale bars represent 0.2 mm.
Table 2.
dsRNA uptake pathway is conserved among organisms: Drosophila orthologues affect RNAi in C. elegans
| Group | C. elegans gene ID | C. elegans gene name | Drosophila orthologue | IPTGa | |
|---|---|---|---|---|---|
| 0.2 μM | 2.0 μM | ||||
| Proton transport | R10E11.2 | vha-2 | Vha16 | Non-viable | Non-viable |
| T14F9.1 | vha-15 | VhaSFD | Non-viable | Non-viable | |
|
| |||||
| Vesicle mediated transport | T20G5.1 | chc-1 | Clathrin hc | Non-viable | Non-viable |
| R160.1 | dpy-23 | AP-50 | Unc | Unc | |
| W03C9.3 | rab-7 | Rab7 | Unc | Non-viable | |
| F54C9.10 | arl-1 | Arf72A | ND | Normal | |
|
| |||||
| Intracellular transport | F22G12.5 | ninaC.5 | Normal | Normal | |
| C06G3.10 | cgo-2 | IdlCp | Normal | Unc | |
| ZK1098.5 | CG3911 | Normal | Normal | ||
| F32A6.3 | vps-41 | light | Normal | Normal | |
|
| |||||
| Lipid metabolism | R01H2.5 | Gmer | Normal | Normal | |
| B0025.1 | vps-34 | Pi3K | Normal | Unc | |
|
| |||||
| Proteolysis and peptidolysis | F41C3.5 | CG4572 | Unc | Unc | |
| K05B2.2 | CG5053 | Unc | Unc | ||
| T07F10.1 | CG8773 | Unc | Unc | ||
|
| |||||
| Other | B0464.4 | bre-3 | egghead | Normal | Unc |
|
| |||||
| Unknown | W05H7.3 | sedl-1 | CG5161 | Normal | Normal |
| Y45G12B.2 | CG5382 | Normal | Unc | ||
| F08D12.1 | CG5434 | Unc | Non-viable | ||
| C54H2.1 | sym-3 | CG8671 | Normal | Normal | |
|
| |||||
| RNAi function | K12H4.8 | dcr-1 | Dicer 2 | Normal | Normal |
|
| |||||
| Control | Vector Control | Unc | Unc | ||
Expression of dsRNA in the bacteria used for the primary RNAi treatment was induced with two different concentrations of IPTG. ND, non-determined. Unc, unc-52 RNAi phenotype.
DISCUSSION
The phenomenon of RNAi silencing is widely conserved among all higher eukaryotes. Exploiting this process is becoming increasingly important as an experimental tool, as well as for therapeutic applications. Although most cells possess the basic RNAi core machinery, some cell types have the intriguing ability to naturally take up exogenous dsRNA and use it to initiate RNAi silencing2,3,5–7. Furthermore, some organisms, such as plants, C. elegans and planaria (Girardia tigrina) can transmit the RNA silencing signal from cell to cell, resulting in the systemic spread of the RNAi response8,26,29,30. It is currently believed that insects lack a pathway for the systemic spreading of RNAi. Nevertheless, injected dsRNA elicits cell non-autonomous RNAi in adult Drosophila, juvenile grasshopper, Tribolium castaneum (flour beetle) and Anopheles gambiae9,31–33. In plants, it seems that systemic spread relies on the plasmodesmal channel system, which connects all the cells in the plant34,35. However, this system is absent from animal organisms. Despite the importance of RNAi processes, little is known about the machineries that mediate either dsRNA uptake or systemic spread of the RNAi signal in animal cells. A number of genetic screens using C. elegans have identified components required for systemic spread of an RNAi signal. Because systemic RNA silencing is a multistep process that requires uptake, amplification and spread of the silencing signal, the specific functions of these components within this complex process have not been precisely defined. Here, we sought to specifically identify the machinery that mediates uptake of exogenous dsRNA to induce an RNAi response using a less complex model system. As Drosophila S2 cells can efficiently take up exogenous dsRNA they provided with a well-defined system to identify the mechanism and components of dsRNA entry. Using biochemical, genomic and pharmacological approaches we found that dsRNA enters the RNAi pathway through an active and specific pathway that involves clathrin-mediated endocytosis. Furthermore, biochemical and pharmacological analyses implicate scavenger-like pattern-recognition receptors in dsRNA entry. We also examined whether C. elegans homologues of components of the Drosophila dsRNA entry pathway function in systemic spread of an ingested dsRNA signal. Whereas downregulation of core endocytosis components (such as clathrin and V-H-ATPase) was lethal in C. elegans, downregulation of several components of vesicular intracellular transport and lipid metabolism blocked systemic spread of the RNAi signal. It thus seems that RNAi spread is an active process that involves vesicle-mediated intracellular trafficking and depends on lipid modifications and cytoskeleton guidance. Based on these experiments, we hypothesize that the dsRNA entry pathway we have identified in Drosophila is conserved in other animal cells. The severity of the phenotype observed for downregulation of the endocytic pathway may account for the inability to detect this pathway of entry in screens carried out in whole C. elegans animals.
The identification of components of the endocytic pathway required for dsRNA entry to initiate an RNAi response raises a number of interesting questions. Several lines of evidence, including the requirements of clathrin, ARF72A, V-H-ATPase and Rab 7 for exogenous dsRNA-initiated silencing (Table 1 and Fig. 4a), suggest that endocytic vesicles are critical in the entry pathway. However, the RNAi uptake pathway would need to deviate from standard endocytic uptake at some point if it is to deliver dsRNA to the cytoplasm. It is tempting to speculate that the RNAi signal may be directly translocated, perhaps through SID-1-like channels, from specialized entry vesicles to the RNAi machinery. Intriguingly, several components of the RNAi machinery, including dicer and ago-2, are membrane associated or have membrane-anchoring domains (Saleh, M.C., University of California San Francisco and Joachimiak, M., University of California Berkley; unpublished observations and ref. 36). Our observation that in cells defective for V-H-ATPase, dsRNA still accumulates in vesicles (Fig. 4c) but does not initiate an RNAi response (Table 1 and Fig. 4a) suggests that the V-H-ATPase activity controls progression of the dsRNA through the RNAi entry pathway. Future studies should determine the mechanisms by which dsRNA is loaded onto the RNAi apparatus. This, in turn, may explain why some cells are uniquely able to take up exogenous dsRNA to initiate an RNAi response.
The observation that members of the scavenger-receptor family act as receptors of dsRNA may provide insight into the physiological role of this pathway, as these proteins have well-known roles in the ancestral innate immune response24,37. For example, scavenger receptors participate in the uptake of bacterial pathogens and have also been implicated in the uptake of chaperone-peptide complexes38. It is thought that the chaperone-bound peptide is translocated from vesicles to the cytosol to enter the antigen-presentation pathway in a process that bears some similarities to dsRNA uptake39,40. The pathway for dsRNA uptake may thus serve a protective role to prevent the spread of viral infections by uptake of viral replicative intermediate dsRNAs that are released on cell lysis.
RNAi has tremendous potential for specific and effective therapeutic applications but the main obstacle to achieving in vivo therapies by RNAi technologies is delivery. Our observations that the pathway of dsRNA entry utilizes components of the endocytic machinery may provide a starting point to develop novel strategies for RNAi delivery. The identification and exploitation of this natural RNAi entry pathway may provide more effective and non-toxic strategies of dsRNA delivery.
METHODS
Cells, plasmids and reagents
Drosophila S2 cells (Invitrogen, Carlsbad, CA) were cultured at 25 °C in Schneider’s Drosophila medium (GIBCO-Invitrogen, Carlsbad, CA), supplemented with 10% heat inactivated fetal calf serum, 2 mM L-glutamine, 100 U ml−1 penicillin and 100 mg ml−1 streptomycin. Stable S2 cell lines were cultured in the same medium, additionally supplemented with 300 μg ml−1 hygromycin B. S2 cells stably expressing GFP–Relish protein have been previously described15. S2 Lamp–GFP cells were kindly provided by the laboratory of R. Vale at University of Calfornia San Francisco. Firefly and Renilla luciferase sequences from the plasmids pGL3 and pRL-CMV (Promega, Madison, WI) were cloned into pMT/V5-HisB (Invitrogen) allowing copper-inducible expression from a metallothionein promoter. A luciferase construct that can be targeted by the endogenous miRNA, miR2b, was generated by inserting two copies of the mature miR2b sequence in sense and antisense orientation of the 3′UTR of pMT-GL3. Transfections were performed using Effectene transfection reagent (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Firefly and Renilla luciferase expression was analysed using the Dual-Luciferase reporter assay system (Promega) and analysed on a Tecan Ultra-evolution plate reader. To evaluate the significance of the differences in firefly luciferase counts a student’s t-test was used. Pharmacological inhibitors of the endocytic pathway and competitive inhibitors of scavenger receptors were purchased from Sigma Aldrich (St Louis, MO).
RNAi methods
dsRNA was generated by in vitro transcription using T7 RNA-polymerase and RNAi experiments were performed as previously described15. Synthetic siRNA targeting firefly luciferase mRNA was obtained from Dharmacon, (Lafayette, CO). A pool of esiRNA targeting firefly luciferase mRNA was generated by cleavage of 1000 bp dsRNA with recombinant human Dicer (Stratagene, La Jolla, CA), according to the manufacturers’ instructions. The residual uncleaved dsRNA was removed from the esiRNA preparation using Microcon 100 microconcentrators (Millipore, Billerica, MA).
The Drosophila RNAi library has been previously described15. Genes involved in RNAi were identified using an RNAi of RNAi approach13,14. dsRNA from the RNAi library was used to knockdown specific Drosophila mRNAs (primary RNAi). RNAi function was assessed using secondary RNAi of a GFP–Relish fusion. For the genome-wide RNAi screen, approximately 2 μg of library dsRNA were aliquoted in 100 μl of S2 medium and 4 × 104 S2 GFP–Relish cells were added in an additional 100 μl of medium to 96-well microplates. At day 4, the cells were split at the same approximate initial density and 2 μg of library dsRNA and 2 μg of dsRNA targeting the GFP–Relish mRNA were added into glass-bottomed 96-well microplates (BD Biosciences Pharmingen, San Diego, CA). At day 7 GFP–Relish expression was induced by the addition of 500 μM CuSO4. At day 8, the cells were washed, fixed with 3.7% formaldehyde and mounted in Fluoromount-G (Southern Biotechnology Associates, Birmingham, AL). GFP expression was evaluated visually under a Leica IMRB microscope. Positive candidates in the initial screen were confirmed using the same approach, but GFP expression was evaluated using a FACSCalibur flow cytometer and CellQuest software (Becton-Dickinson, Franklin Lakes, NJ).
To confirm the identity of the dsRNA in the RNAi library, the templates for in vitro transcription were cloned and sequenced in pCRII-Topo vectors (Invitrogen). These plasmids were used as templates for in vitro transcription and the assay was repeated with similar results (data not shown). Positive candidates were further confirmed with the same approach using three additional secondary RNAi assays: RNAi against a LAMP–GFP fusion in S2 cells stably expressing this fusion protein; RNAi against the anti-apoptotic factor dIAP1 in wild-type S2 cells using cell viability as a readout; and RNAi against firefly luciferase after transient transfection of wild-type S2 cells with firefly and Renilla luciferase expression vectors.
RNAi in C. elegans
All C. elegans experiments were performed with wild-type N2 worms at 20 °C. RNAi was induced by feeding the nematodes with bacteria expressing dsRNA. The RNAi constructs were obtained from the Ahringer RNAi library41. HT115 bacteria transformed with RNAi vectors expressing dsRNA of the genes of interest were grown at 37 °C in LB with 10 μg mL−1 tetracycline and 50 μg mL−1 carbenicillin, then seeded onto nematode growth media-carbenicillin plates and supplemented with two different concentrations of IPTG (0.2 and 2 μM). The presence of the insert of each clone of interest from the RNAi library was verified by PCR analysis with T7 primer.
Feeding of RNAi bacteria to worms was carried out as previously described42,43. Briefly, young adult worms were transferred to candidate RNAi bacteria in two different concentrations of IPTG. L2–L3 progeny were recovered and plated in unc-52 RNAi bacteria plates with 2.0 μM IPTG. Two days later, the unc phenotype was scored.
Immunofluoresence microscopy
dsRNA and DNA were fluorescently labelled using the Silencer siRNA labelling kit Cy3 (Ambion, Austin, TX). Unincorporated dye was removed using HS200 gel filtration columns (Pharmacia, Piscataway, NJ). Labelling of dsRNA was verified by a decreased electrophoretic mobility on agarose gel of the labelled dsRNA as compared with unlabelled dsRNA (see Supplementary Information, Fig. S1b). S2 cells were incubated for the indicated times with labelded dsRNA, washed with PBS and deposited on Superfrost Plus Gold slides (Fisher Scientific, Pittsbury, PA) for immunofluorescence microscopy. Cells were fixed for 10 min in 4% formaldehyde (Sigma). Actin was visualized with oregon green 488-coupled phalloidin (Molecular Probes, Eugene, OR). Cells were mounted using Vectashield with DAPI (Vector, Burlingame, CA) as a nuclear counterstain. Images were captured on an Olympus IX70 microscope driven by DeltaVision software (Applied Precision, Issaquah, WA). Optical sections were deconvolved using the same software and flattened into a two-dimensional projection for presentation. All the images were then imported to and processed in Adobe Photoshop and Adobe Illustrator.
Incorporation of radiolabelled nucleic acids
Uniformly labelled dsRNA was generated by in vitro transcription in the presence of α-32P-UTP. DNA was terminally labelled using polynucleotide kinase and γ-32P-ATP. The same amount of counts of radiolabelled dsRNA or DNA were added to S2 cells after serum starvation for 1 h. At different times, the cells were washed with PBS and lysed in hypotonic buffer (10 mM HEPES at pH 7.3, 6 mM β-mercaptoethanol, complete protease inhibitor (Roche, Indianapolis, IN) and 0.5 units ml−1 RNasin (Promega). After ultracentrifugation at 100.000g for 60 min, the amount of radioactivity in the soluble and insoluble fraction was measured in a scintillation counter (Beckman Coulter, Fullerton, CA). The pellet was resuspended in cold PBS and sonicated at low level for 5 sec.
The efficiency of separation of membrane bound and cytoplasmic fractions over pellet and supernatant was assessed by western blotting using antibodies directed against Syntaxin as marker for plasma membrane, Lava 1 (Golgi), Rab5 (early endosomes), cathepsin-L (lysosome) and tubulin (cytoplasm). Antibodies were kindly provided by the laboratory of G. Davis at University of California San Francisco.
Supplementary Material
Acknowledgments
We are grateful to members of the Andino and O’Farrell labs for support and discussions; G. Yudowski, F. Martin and M. Strandh for help with equipment and reagents; C. Murphy and M. Van Gilst for discussions and advice on C. elegans experiments; M. Von Zastrow for advice on immunofluorescence microscopy; and J. Frydman for critical reading of the manuscript. We thank the Davis, Kenyon, Kornberg and Vale laboratories at UCSF for materials and advice on a number of experiments. J. Ahringer kindly provided RNAi clones for the C. elegans experiments. This work was supported by a European Molecular Biology Organization (EMBO) fellowship to R.P.V.R. and a National Institutes of Health (NIH) grant AI40085 to R.A.
Footnotes
Note: Supplementary Information is available on the Nature Cell Biology website.
COMPETING FINANCIAL INTERESTS
The authors declare that they have no competing financial interests.
References
- 1.Meister G, Tuschl T. Mechanisms of gene silencing by double-stranded RNA. Nature. 2004;431:343–349. doi: 10.1038/nature02873. [DOI] [PubMed] [Google Scholar]
- 2.Clemens JC, et al. Use of double-stranded RNA interference in Drosophila cell lines to dissect signal transduction pathways. Proc Natl Acad Sci USA. 2000;97:6499–6503. doi: 10.1073/pnas.110149597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Worby CA, Simonson-Leff N, Dixon JE. RNA interference of gene expression (RNAi) in cultured Drosophila cells. Sci STKE. 2001;95:PL1. doi: 10.1126/stke.2001.95.pl1. [DOI] [PubMed] [Google Scholar]
- 4.Roignant JY, et al. Absence of transitive and systemic pathways allows cell-specific and isoform-specific RNAi in Drosophila. RNA. 2003;9:299–308. doi: 10.1261/rna.2154103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lingor P, Michel U, Scholl U, Bahr M, Kugler S. Transfection of ‘naked’ siRNA results in endosomal uptake and metabolic impairment in cultured neurons. Biochem Biophys Res Commun. 2004;315:1126–1133. doi: 10.1016/j.bbrc.2004.01.170. [DOI] [PubMed] [Google Scholar]
- 6.Bitko V, Musiyenko A, Shulyayeva O, Barik S. Inhibition of respiratory viruses by nasally administered siRNA. Nature Med. 2005;11:50–55. doi: 10.1038/nm1164. [DOI] [PubMed] [Google Scholar]
- 7.Duxbury MS, Ashley SW, Whang EE. RNA interference: a mammalian SID-1 homologue enhances siRNA uptake and gene silencing efficacy in human cells. Biochem Biophys Res Commun. 2005;331:459–463. doi: 10.1016/j.bbrc.2005.03.199. [DOI] [PubMed] [Google Scholar]
- 8.Winston WM, Molodowitch C, Hunter CP. Systemic RNAi in C. elegans requires the putative transmembrane protein SID-1. Science. 2002;295:2456–2459. doi: 10.1126/science.1068836. [DOI] [PubMed] [Google Scholar]
- 9.Dong Y, Friedrich M. Nymphal RNAi: systemic RNAi mediated gene knockdown in juvenile grasshopper . BMC Biotechnol. 2005;5:25. doi: 10.1186/1472-6750-5-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tijsterman M, May RC, Simmer F, Okihara KL, Plasterk RH. Genes required for systemic RNA interference in Caenorhabditis elegans. Curr Biol. 2004;14:111–116. doi: 10.1016/j.cub.2003.12.029. [DOI] [PubMed] [Google Scholar]
- 11.Timmons L, Tabara H, Mello CC, Fire AZ. Inducible systemic RNA silencing in Caenorhabditis elegans. Mol Biol Cell. 2003;14:2972–2983. doi: 10.1091/mbc.E03-01-0858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feinberg EH, Hunter CP. Transport of dsRNA into cells by the transmembrane protein SID-1. Science. 2003;301:1545–1547. doi: 10.1126/science.1087117. [DOI] [PubMed] [Google Scholar]
- 13.Hammond SM, Boettcher S, Caudy AA, Kobayashi R, Hannon G. J Argonaute2, a link between genetic and biochemical analyses of RNAi. Science. 2001;293:1146–1150. doi: 10.1126/science.1064023. [DOI] [PubMed] [Google Scholar]
- 14.Bernstein E, Caudy AA, Hammond SM, Hannon GJ. Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature. 2001;409:363–366. doi: 10.1038/35053110. [DOI] [PubMed] [Google Scholar]
- 15.Foley E, O’Farrell PH. Functional dissection of an innate immune response by a genome-wide RNAi screen. PLoS Biol. 2004;2:E203. doi: 10.1371/journal.pbio.0020203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sieczkarski SB, Whittaker GR. Dissecting virus entry via endocytosis. J Gen Virol. 2002;83:1535–1545. doi: 10.1099/0022-1317-83-7-1535. [DOI] [PubMed] [Google Scholar]
- 17.Conner SD, Schmid SL. Regulated portals of entry into the cell. Nature. 2003;422:37–44. doi: 10.1038/nature01451. [DOI] [PubMed] [Google Scholar]
- 18.Hurtado-Lorenzo A, et al. V-ATPase interacts with ARNO and Arf6 in early endosomes and regulates the protein degradative pathway. Nature Cell Biol. 2006;8:124–136. doi: 10.1038/ncb1348. [DOI] [PubMed] [Google Scholar]
- 19.Sun-Wada GH, Wada Y, Futai M. Diverse and essential roles of mammalian vacuolar-type proton pump ATPase: toward the physiological understanding of inside acidic compartments. Biochim Biophys Acta. 2004;1658:106–114. doi: 10.1016/j.bbabio.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 20.Sun-Wada GH, Wada Y, Futai M. Lysosome and lysosome-related organelles responsible for specialized functions in higher organisms, with special emphasis on vacuolar-type proton ATPase. Cell Struct Funct. 2003;28:455–463. doi: 10.1247/csf.28.455. [DOI] [PubMed] [Google Scholar]
- 21.Nishi T, Forgac M. The vacuolar (H+)-ATPases — nature’s most versatile proton pumps. Nature Rev Mol Cell Biol. 2002;3:94–103. doi: 10.1038/nrm729. [DOI] [PubMed] [Google Scholar]
- 22.Medzhitov R, Janeway CA., Jr Self-defense: the fruit fly style. Proc Natl Acad Sci USA. 1998;95:429–430. doi: 10.1073/pnas.95.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 24.Peiser L, Mukhopadhyay S, Gordon S. Scavenger receptors in innate immunity. Curr Opin Immunol. 2002;14:123–128. doi: 10.1016/s0952-7915(01)00307-7. [DOI] [PubMed] [Google Scholar]
- 25.Ulvila J, et al. Double-stranded RNA is internalized by scavenger receptor-mediated endocytosis in Drosophila S2 cells. J Biol Chem. 2006;281:14370–14375. doi: 10.1074/jbc.M513868200. [DOI] [PubMed] [Google Scholar]
- 26.Voinnet O. Non-cell autonomous RNA silencing. FEBS Lett. 2005;579:5858–5871. doi: 10.1016/j.febslet.2005.09.039. [DOI] [PubMed] [Google Scholar]
- 27.Hresko MC, Williams BD, Waterston RH. Assembly of body wall muscle and muscle cell attachment structures in Caenorhabditis elegans. J Cell Biol. 1994;124:491–506. doi: 10.1083/jcb.124.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Williams BD, Waterston RH. Genes critical for muscle development and function in Caenorhabditis elegans identified through lethal mutations. J Cell Biol. 1994;124:475–490. doi: 10.1083/jcb.124.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Newmark PA, Reddien PW, Cebria F, Sanchez Alvarado A. Ingestion of bacterially expressed double-stranded RNA inhibits gene expression in planarians. Proc Natl Acad Sci USA. 2003;100(Suppl 1):11861–11865. doi: 10.1073/pnas.1834205100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pineda D, et al. Searching for the prototypic eye genetic network: Sine oculis is essential for eye regeneration in planarians. Proc Natl Acad Sci USA. 2000;97:4525–4529. doi: 10.1073/pnas.97.9.4525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dzitoyeva S, Dimitrijevic N, Manev H. γ-aminobutyric acid B receptor 1 mediates behavior-impairing actions of alcohol in Drosophila: adult RNA interference and pharmacological evidence. Proc Natl Acad Sci USA. 2003;100:5485–5490. doi: 10.1073/pnas.0830111100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bucher G, Scholten J, Klingler M. Parental RNAi in Tribolium (Coleoptera) Curr Biol. 2002;12:R85–R86. doi: 10.1016/s0960-9822(02)00666-8. [DOI] [PubMed] [Google Scholar]
- 33.Blandin S, et al. Reverse genetics in the mosquito Anopheles gambiae: targeted disruption of the Defensin gene. EMBO Rep. 2002;3:852–856. doi: 10.1093/embo-reports/kvf180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yoo BC, et al. A systemic small RNA signaling system in plants. Plant Cell. 2004;16:1979–2000. doi: 10.1105/tpc.104.023614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baulcombe D. RNA silencing in plants. Nature. 2004;431:356–363. doi: 10.1038/nature02874. [DOI] [PubMed] [Google Scholar]
- 36.Cikaluk DE, et al. GERp95, a membrane-associated protein that belongs to a family of proteins involved in stem cell differentiation. Mol Biol Cell. 1999;10:3357–3372. doi: 10.1091/mbc.10.10.3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kocks C, et al. Eater, a transmembrane protein mediating phagocytosis of bacterial pathogens in Drosophila. Cell. 2005;123:335–346. doi: 10.1016/j.cell.2005.08.034. [DOI] [PubMed] [Google Scholar]
- 38.Reed RC, Nicchitta CV. Chaperone-mediated cross-priming: a hitchhiker’s guide to vesicle transport (review) Int J Mol Med. 2000;6:259–264. doi: 10.3892/ijmm.6.3.259. [DOI] [PubMed] [Google Scholar]
- 39.Castellino F, et al. Receptor-mediated uptake of antigen/heat shock protein complexes results in major histocompatibility complex class I antigen presentation via two distinct processing pathways. J Exp Med. 2000;191:1957–1964. doi: 10.1084/jem.191.11.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Castellino F, Zappacosta F, Coligan JE, Germain RN. Large protein fragments as substrates for endocytic antigen capture by MHC class II molecules. J Immunol. 1998;161:4048–4057. [PubMed] [Google Scholar]
- 41.Kamath RS, et al. Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature. 2003;421:231–237. doi: 10.1038/nature01278. [DOI] [PubMed] [Google Scholar]
- 42.Dillin A, Crawford DK, Kenyon C. Timing requirements for insulin/I GF-1 signaling in C. elegans. Science. 2002;298:830–834. doi: 10.1126/science.1074240. [DOI] [PubMed] [Google Scholar]
- 43.Fraser AG, et al. Functional genomic analysis of C. elegans chromosome I by systematic RNA interference. Nature. 2000;408:325–330. doi: 10.1038/35042517. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.