Abstract
Purpose
To investigate the safety and pharmacokinetics of R(+)XK469, a quinoxaline analogue, in patients with advanced refractory solid tumours. Preclinical studies suggested that efficacy was independent of schedule but that toxicity was decreased by dividing the dose.
Methods
R(+)XK469 was initially administered as a 30 min intravenous infusion on days 1–5 of a 21-d cycle. Based on the demonstration of a long half-life, the dosing schedule was subsequently amended to infusion on days 1, 3 and 5 of a 21-d cycle. An alternate single-dose schedule of once every 21 d was also explored. Blood samples were collected for pharmaco-kinetic studies.
Results
Dose-limiting toxicity (DLT) was neutropaenia. There was significant interindividual variability in clearance as evidenced by a coefficient of variation of 46%. A flat-dosing scheme (not based on body surface area) was justified by the absence of correlation between clearance and body surface area. A partial response was observed in a patient with nasopharyngeal carcinoma.
Conclusions
The recommended phase II doses are 850–1100 mg/d on days 1, 3 and 5 of a 21-d cycle and 2500 mg on day 1 of a 21-d cycle. The observed interpatient pharmacokinetic variability should prompt investigation into the presence of genetic polymorphism in relevant metabolizing enzymes.
Keywords: Pharmacokinetics, Phase I, Quinoxaline, R(+)XK469, XK469
1. Introduction
XK469 (2-[4-[(7-chloro-2-quinoxalinyl)oxy]phenoxy]-propanoic acid) is a member of the quinoxaline family of antitumour agents, which are analogues of the herbicide quizalofop-ethyl. The parent drug, XB947, was discovered after finding that moving the chloride molecule in the quizalofop-ethyl structure from the 6-position to the 7-position results in antitumour activity.1 However, this agent was poorly soluble and difficult to administer by routes other than intraperitoneally in murine models. As a result, XK469 was developed and found to be both water-soluble and more active than XB947. Both the S(−) and R(+) free acid enantiomers have been confirmed by the National Cancer Institute (NCI) as having in vivo efficacy in mice. Pharmacology studies showed that the S(−)XK469 is rapidly and almost completely converted to R(+)XK469 in vivo in mice, rats and dogs.2–4 Moreover, toxicology studies suggested that the R(+) enantiomer was the less toxic of the two. Therefore, further development in animals and humans has been performed with R(+)XK469 (Fig. 1).
Fig. 1.
The chemical structure of R(+)XK469.
The exact mechanism of action of XK469 is unknown. COMPARE analysis of cytotoxicity data from the NCI-60 cell line screen showed that its mechanism of action is unique in that it was not similar to that of any known cytotoxic agent in the NCI database. Whilst not definitive, the available data suggest that XK469 acts as a selective topoisomerase IIβ inhibitor.5–9 Other proposed mechanisms include XK469-induced inhibition of cyclin B1 ubiquitination10 and apoptosis via binding of the peripheral benzodiazepine receptor.11
Preclinical studies of XK469 demonstrated significant antitumour activity in a variety of in vitro and in vivo tumour models including murine pancreatic ductal, colon, breast, prostate and small and non-small cell lung cancer models.1,12 Pharmacokinetic studies demonstrated a mean elimination half-life of 13.5 h in rats13 and 13.2 h in dogs14 after intravenous administration. Preclinical studies of XK469 in mice also indicated that host recovery from drug-related toxicity was significantly worsened at high daily doses but that antitumour activity was related to total dose and not adversely impacted by dividing the dose over several days.15
A phase I study of the R(+) free acid enantiomer of XK469 was initiated to define the toxicity profile and maximum tolerated dose (MTD) in adults with advanced solid malignancies. Based upon the preclinical data supporting an improved toxicity profile with a divided-dose as compared to a single-dose schedule, an initial regimen of intravenous infusion daily on days 1–5 of a 21-d cycle was used. As ongoing pharmacokinetic and toxicity analysis during the conduct of the study showed a longer than expected half-life of R(+)XK469 in humans and a favourable toxicity profile, the regimen was changed to daily on days 1, 3 and 5 of a 21-d cycle. After the establishment of the phase II recommended dose on this schedule, a single-dose schedule of once every 21 d was investigated.
2. Patients and methods
2.1. Patient selection
Patients with a histologically confirmed solid tumour or lymphoma who were refractory to known effective therapy or for whom no proven effective antitumour treatment existed were eligible for enrolment. Patients had to be ≥18 years of age, have a Karnofsky performance status ≥70% with a life expectancy of >3 months, be ≥4 weeks out from most recent chemotherapy and radiotherapy (≥6 weeks for nitrosureas and mitomycin C) and have adequate end organ function as defined by a white blood cell count ≥3000/μl, absolute neutrophil count (ANC) ≥1500/μl, platelet count ≥100,000/μl, total bilirubin within normal limits and serum creatinine ≤1.5 × upper limit of normal. Patients with active central nervous system (CNS) disease or previously treated CNS metastasis requiring continued corticosteroid or anticonvulsant therapy, with other serious intercurrent medical illness, or who were pregnant were excluded. All patients provided written informed consent prior to enrolment. The study was conducted in accordance with the Declaration of Helsinki and with the approval of the Institutional Review Board of the University of Chicago.
2.2. Treatment plan and dose escalation
R(+)XK469 was supplied by the National Cancer Institute in a 250 mg vial. The clear, colourless solution contained 5 mg of R(+)XK469 per ml with Dibasic Phosphate USP, phosphoric acid NF and sodium hydroxide NF. The drug was diluted before infusion with 0.9% sodium chloride USP to yield a final concentration no lower than 0.05 mg/ml. R(+)XK469 was administered as a continuous intravenous infusion over 30 min by means of a regulated infusion pump for individual doses of up to 2500 mg. For doses higher than 2500 mg, a 60 min infusion was used due to the volume required.
The treatment schedules used are listed in Table 1. The trial was initiated with Schedule A at a dose that was 1/10 of the MTD in rats, but was changed to Schedule B when preliminary pharmacokinetic analysis demonstrated a longer than expected half-life for the drug. Once the phase II recommended dose for Schedule B was determined, the single-dose scheme of Schedule C was investigated with a starting dose that was 50% of the total cycle dose at the phase II recommended dose level of Schedule B. Dosing was not based on body surface area (i.e. flat dose).
Table 1.
Treatment schedules
| Schedules | Treatment day(s) | Cycle length (d) | Infusion length (min) | Starting dose (mg/d) |
|---|---|---|---|---|
| A | 1, 2, 3, 4, 5 | 21 | 30 | 15 |
| B | 1, 3, 5 | 21 | 30 | 360 |
| C | 1 | 21 | 30 or 60 | 1600 |
Dose escalation in Schedules A and B followed an accelerated titration scheme. Initial dose escalations were of 100% (i.e. doubling of the dose) in cohorts of up to two assessable patients. Upon development of any grade ≥2 toxicity, according to the Common Toxicity Criteria, version 2.0, that was either probably or definitely therapy-related or any grade ≥3 toxicity that was possibly, probably or definitely therapy-related, dose increments were decreased to 50% and cohort sizes were increased to three. Once DLT was observed, the cohort at that dose level and all subsequent dose levels was increased to 6 patients. Dose escalation then continued with 25% dose increments provided no more than one of 6 patients in a cohort experienced DLT. For Schedule C, dose escalations were limited to 25% in patient cohorts of 3. Upon observation of DLT, cohorts were expanded to 6 patients.
The MTD was defined as the highest dose studied for which the incidence of DLT was <33%. Cohort expansion to a maximum of 20 and 12 patients was planned at the MTD for Schedules B and C, respectively, to increase the experience at those doses. For Schedules A and B, intrapatient dose escalation was permitted in 50% increments in the absence of progressive disease or grade ≥2 toxicity and provided at least 1 patient had completed one cycle at that dose or higher.
For all three schedules, DLT was defined as any of the following occurring during the first cycle of therapy and deemed attributable to the drug: grade ≥3 non-haematologic toxicity except fatigue, alopaecia, nausea or vomiting; grade 4 anaemia or thrombocytopaenia and fever associated with grade ≥3 neutropaenia. For Schedules A and B, grade ≥3 neutropaenia on a scheduled treatment day or failure to administer R(+)XK469 on time due to toxicity during the first cycle were also DLTs. For schedule C, DLT was further defined by the failure to recover absolute neutrophil count to grade ≤2 by day 29 (or 7 days beyond the planned first day of cycle two).
2.3. Pretreatment and follow-up studies
Prior to initiation of therapy, a complete history and physical examination were performed; a complete blood count (CBC), comprehensive metabolic panel and serum pregnancy test (when indicated) were obtained and appropriate radiologic assessment studies (CT and/or MRI scans) and multi-gated acquisition (MUGA) scan were completed. History, physical examination, toxicity assessment and CBC were done weekly for the duration of time on study. A complete metabolic panel was obtained weekly during the first cycle and then on day 1 of all subsequent cycles. Radiologic assessment and follow-up MUGA scans were performed every two cycles. Response and progression were objectively evaluated using the international criteria proposed by the Response Evaluation Criteria in Solid Tumours Committee.16
2.4. Pharmacokinetic sampling and methods
Blood samples for pharmacokinetic analysis were obtained during the first week of treatment. For Schedule A, blood was collected into sodium heparinised plastic vacutainers (Becton Dickinson, Franklin Lakes, NJ) before drug infusion; at 15 and 30 min during infusion and at 15 and 30 min and 1, 2, 4, 6, 8 and 24 h after the end of infusion on day 1. For Schedule B, blood was similarly collected on day 1. On day 3, blood samples were collected prior to infusion; at 30 min during the infusion and at 2, 4 and 24 h after the end of the infusion. On day 5, blood samples were collected prior to infusion. For Schedule C, at doses ≤2500 mg (30 min infusion), blood was collected at the same time points on day 1 as was done in Schedules A and B. For doses greater than 2500 mg (60 min infusion), sampling time points were similar except that during the infusion, samples were taken at 30 and 60 min. For all doses in Schedule C, additional samples were collected at 24 and 48 h after the end of the infusion. Plasma was separated by centrifugation and stored at −80 °C until analysed.
Plasma samples were analysed for R(+)XK469 concentrations using high-performance liquid chromatography (HPLC). Briefly, plasma aliquots (200 μl) were vortexed and centrifuged (14,000 rpm, 10 min, room temperature) after the addition of 600 μl of internal standard (70 μg/ml paclitaxel in methanol). The supernatants were combined with 2 ml of 0.2 M ammonium acetate (pH 5) and vortexed for 10 s. Solid phase extraction was performed using Oasis® HLB 3 cc/60 mg solid phase extraction cartridges (Waters Corporation, Milford, MA) conditioned with 3 ml of methanol followed by 3 ml of 0.2 M ammonium acetate (pH 5). After the addition of samples, the cartridges were washed with 3 ml of deionised water. The eluents were collected using 1 ml of methanol and evaporated to dryness using nitrogen gas (37 °C). Samples were reconstituted in 300 μl of methanol, vortexed for 10 s and 30 μl aliquots were injected into the HPLC (Waters Corporation, Milford, MA). R(+)XK469 concentrations were measured using a Luna Phenyl–Hexyl analytical column (50 × 3 mm, 5 μm, Phenomenex, Torrance, CA) and a Phenyl–Propyl Securityguard™ cartridge (4 × 2.00 mm, Phenomenex, Torrance, CA). The mobile phase consisted of 5 M tetrabutyl ammonium dihydrogen phosphate (1 l combined with 1 ml glacial acetic acid and 0.5 ml ammonium hydroxide) (TBAP) and methanol. The following gradient was applied using a flow rate of 0.5 ml/min: 0–2 min 85% TBAP and 15% methanol; 2.1–18 min, go from 85% TBAP and 15% methanol to 30% TBAP and 70% methanol; 18.1–23 min 30% TBAP and 70% methanol, 23.1–25 min, go from 30% TBAP and 70% methanol to 85% TBAP and 15% methanol and 25.1–35 min, 85% TBAP and 15% methanol. The eluate was monitored using UV detection at 244 nm (for R(+)XK469) and 230 nm (for paclitaxel). Retention times for R(+)XK469 and paclitaxel were 25.7 and 29.3 min, respectively. R(+)XK469 concentrations were calculated using a standard curve (range: 0.114–197 μg/ml). Samples with concentrations that fell outside of the range of the standard curve were reanalysed by diluting with blank plasma prior to extraction, and the final concentration values were determined after applying the appropriate dilution factor. Intra-assay reproducibility (%CV = 0.7–2.2) and accuracy (range: 97.2–101.5) were determined by performing three measurements of the same six standards on the same day. Inter-assay reproducibility (%CV = 1.7–3.6) and accuracy (range: 99.8–100.9) were determined by assays of the same six standards in triplicate on three days.
2.5. Pharmacokinetic analysis
Estimates of pharmacokinetic parameters for R(+)XK469 were derived from individual concentration–time data sets by non-compartmental analyses.17 The values of the maximum plasma concentration (Cmax) were directly recorded from experimental observations. The area under the plasma concentration versus time curve from time 0 to the last measurable concentration T (AUC0→T) was calculated using log trapezoidal summation. The apparent first order elimination rate constant (λ) in the terminal phase was estimated by log-linear regression using a weighting factor of x−2. The absolute value of λwas used to estimate the half-life (t½) using the formula t½ = ln(2)/λ. The last measurable concentration and λ were used to extrapolate the AUC0→T to estimate the area under the curve from time 0 to infinity (AUC0→∞). Total body clearance (Cl) was calculated by dividing dose by AUC0→∞. The volume of distribution at steady state (Vss) was determined using standard non-compartmental methods.
3. Results
3.1. Patient demographics
Between December 2001 and March 2007, a total of 81 patients were enroled in the study – 11, 42 and 28 patients, respectively, on Schedules A, B and C. Patient characteristics are shown in Table 2. Seventy-seven patients were assessable for toxicity and 76 were assessable for response. Four patients were dose-escalated after cycle 1. Patients were withdrawn from the study for progressive disease (n = 50), intercurrent medical illness or clinical deterioration (n = 11), palliative surgery or radiation (n = 2), withdrawal of consent (n = 2), drug-related toxicity (n = 1) and death (n = 4).
Table 2.
Patient characteristics
| Characteristic | Number of patients |
|---|---|
| Patients enroled | |
| Schedule A | 11 |
| Schedule B | 42 |
| Schedule C | 28 |
| Median age (range) | 60 (22–82) |
| Median no. of cycles (range) | 2 (1–10) |
| Gender | |
| Male | 44 (54%) |
| Female | 37 (46%) |
| Karnofsky performance status | |
| 100% | 30 (37%) |
| 90% | 34 (42%) |
| 80% | 18 (22%) |
| 70% | 2 (2%) |
| Tumour types | |
| Bladder | 10 (12%) |
| Breast | 2 (2%) |
| Colorectal | 32 (40%) |
| Esophageal | 3 (4%) |
| Head and neck | 9 (11%) |
| Kidney | 3 (4%) |
| Liver | 3 (4%) |
| Lung | 6 (7%) |
| Mesothelioma | 2 (2%) |
| Pancreas | 2 (2%) |
| Sarcoma | 2 (2%) |
| Other | 8 (10%) |
| Prior therapy | |
| Chemotherapy | 79 (98%) |
| Immunotherapy | 3 (4%) |
| Radiation | 28 (35%) |
In Schedule A, the 240 mg dose level was expanded, and subsequent dose escalations decreased to 50% after a patient enroled at 120 mg and escalated to 180 mg in cycle 2 experienced a transient ischaemic attack. No similar toxicity was observed in any other patients. In Schedule B, the 540 mg dose level was expanded to 6 patients and subsequent dose escalations decreased to 25% in the interest of patient safety after the third patient enroled at that dose developed atrial fibrillation with a rapid ventricular response. The patient had a previous history of atrial fibrillation and congestive heart failure, was effectively managed with diuretics and rate-controlling medications, and remained on study at the same dose. No similar toxicity was observed in any other patients, and the arrhythmia was felt to be unrelated to R(+)XK469.
In Schedule B, further enrolment to the 1400 mg dose level was discontinued after the first patient treated developed severe toxicity. Instead, enrolment continued at the 1100 mg level, and the dose level was ultimately expanded to a total of 20 patients as the presumed phase II recommended dose. In Schedule C, the 2500 mg dose level was expanded to 12 patients as the MTD.
3.2. Haematologic toxicity
Table 3 details the observed haematological toxicity in cycle 1 for all treatment schedules and dose levels. For both Schedules B and C, myelosuppression was the principal toxicity encountered and neutropaenia was the DLT. For Schedule B, DLT was observed at the 850 mg dose level in 1 patient in the form of prolonged neutropaenia with grade 4 ANC on the scheduled day 1 of cycle 2 (day 22 of treatment). At the expanded 1100 mg dose level, 7 patients (35%) experienced DLT: 2 patients had grade 3 neutropaenia on day 22 that resolved to grade 0 by day 29; 3 patients had grade 4 neutropaenia on day 22 with improvement of two of those to grade 2 by day 29 and the third to grade 2 on day 36; 1 patient had fever in the setting of grade 4 neutropaenia during week 3 and was administered filgastrim and 1 patient with history of bladder cancer and recurrent urinary tract infections developed pyelonephritis in the setting of an ANC of 0 μl−1. The only patient treated at the 1400 mg dose level had a nadir ANC of 0 μl−1 that did not resolve to grade 1 until day 36.
Table 3.
Haematologic toxicity in cycle 1
| Dose level (mg) | N | Anaemia |
Neutropaenia |
Thrombocytopaenia |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Grades 1–2 | Grade 3 | Grade 4 | Grades 1–2 | Grade 3 | Grade 4 | Grades 1–2 | Grade 3 | Grade 4 | ||
| Schedule A (mg) | ||||||||||
| 15 | 2 | 1 | ||||||||
| 30 | 2 | 1 | ||||||||
| 60 | 2 | 2 | ||||||||
| 120 | 1 | |||||||||
| 240 | 4 | 1 | ||||||||
| Schedule B (mg) | ||||||||||
| 360 | 3 | 3 | ||||||||
| 540 | 6 | 3 | 1 | 1 | ||||||
| 675 | 5 | 2 | 1 | |||||||
| 850 | 7 | 3 | 1 | 2 | 1 | 2 | ||||
| 1100 | 20 | 11 | 1 | 6 | 5 | 8 | 3 | 1 | ||
| 1400 | 1 | 1 | 1 | 1 | ||||||
| Schedule C (mg) | ||||||||||
| 1600 | 5 | 1 | 2 | 1 | ||||||
| 2000 | 3 | 3 | ||||||||
| 2500 | 14 | 3 | 2 | 3 | 5 | 5 | 3 | 1 | ||
| 3200 | 6 | 1 | 1 | 2 | 4 | 1 | 1 | |||
For Schedule C, DLT due to fever in the setting of grade 4 neutropaenia was observed in 2 patients at the 3200 mg dose level. As a result, the 2500 mg dose level was expanded to 12 patients. At that dose level DLT was seen in 1 patient (8%) in the form of death due to sepsis in the setting of grade 4 neutropaenia.
Whilst routine administration of growth factor was prohibited, there were several instances in which the treating physician deemed it necessary to administered filgastrim for grade 4 neutropaenia. In every instance, except the death at the 2500 mg dose level, patients quickly responded with elevation of the neutrophil count.
3.3. Non-haematologic toxicity
The non-haematologic side-effects of R(+)XK469 were mostly mild to moderate in severity. Table 3 details the observed non-haematologic toxicities in cycle 1 across all dose levels in all three schedules. The single most common toxicity was fatigue. Chills, fever, dysgeusia, hypocalcaemia, hypokalemia, hypomagnesemia, hyponatremia, peripheral sensory neuropathy, rash and elevated transaminases occurred in less than 4% of the patients and only with grade 1 severity. Because of observed myocardial dysfunction in animal toxicology studies, MUGA scans were obtained at baseline and every two cycles thereafter, but no significant decreases in left ventricular ejection fraction were noted. Several patients who were on chronic and stable warfarin doses experienced elevations of the INR after beginning R(+)XK469 therapy. No non-haematologic toxicities were dose limiting (shown in Table 4).
Table 4.
Non-haematologic toxicity in cycle 1
| Dose level | N | Alopecia |
Anorexia |
Constipation |
Diarrhoea |
Fatigue |
Mucositis |
Nausea/Vomiting |
|||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Grade |
Grade |
Grade |
Grade |
Grade |
Grade |
Grade |
|||||||||||||||||||||||
| 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | ||
| Schedule A (mg) | |||||||||||||||||||||||||||||
| 15 | 2 | ||||||||||||||||||||||||||||
| 30 | 2 | ||||||||||||||||||||||||||||
| 60 | 2 | ||||||||||||||||||||||||||||
| 120 | 1 | 1 | 1 | ||||||||||||||||||||||||||
| 240 | 4 | ||||||||||||||||||||||||||||
| Schedule B (mg) | |||||||||||||||||||||||||||||
| 360 | 3 | 2 | |||||||||||||||||||||||||||
| 540 | 6 | 2 | 4 | 1 | |||||||||||||||||||||||||
| 675 | 5 | 1 | 2 | 3 | 1 | ||||||||||||||||||||||||
| 850 | 7 | 1 | 1 | 1 | 2 | 3 | 1 | 1 | 1 | 1 | |||||||||||||||||||
| 1100 | 20 | 9 | 3 | 3 | 1 | 3 | 8 | 2 | 1 | 2 | 5 | 2 | |||||||||||||||||
| 1400 | 1 | 1 | 1 | ||||||||||||||||||||||||||
| Schedule C (mg) | |||||||||||||||||||||||||||||
| 1600 | 5 | 2 | 1 | 1 | 1 | 1 | |||||||||||||||||||||||
| 2000 | 3 | 1 | 1 | 1 | 1 | ||||||||||||||||||||||||
| 2500 | 14 | 1 | 1 | 1 | 2 | 2 | 1 | ||||||||||||||||||||||
| 3200 | 6 | 1 | 1 | 3 | 3 | 1 | |||||||||||||||||||||||
3.4. Pharmacokinetic and pharmacodynamic evaluation
Plasma samples were obtained as per the protocol from 80 of the 81 patients enroled. Pharmacokinetic parameters are reported for day 1 for patients treated on Schedule A and C. For patients on Schedule B, parameters were calculated for both days 1 and 3.
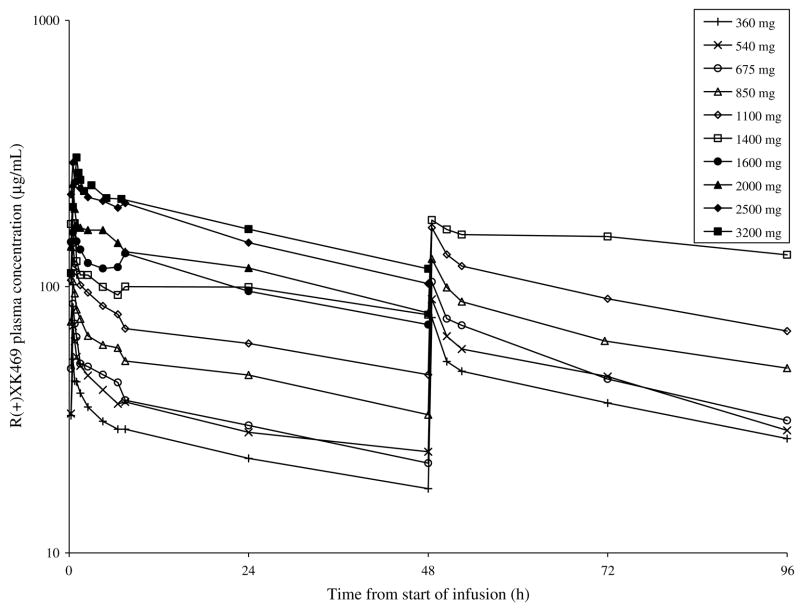
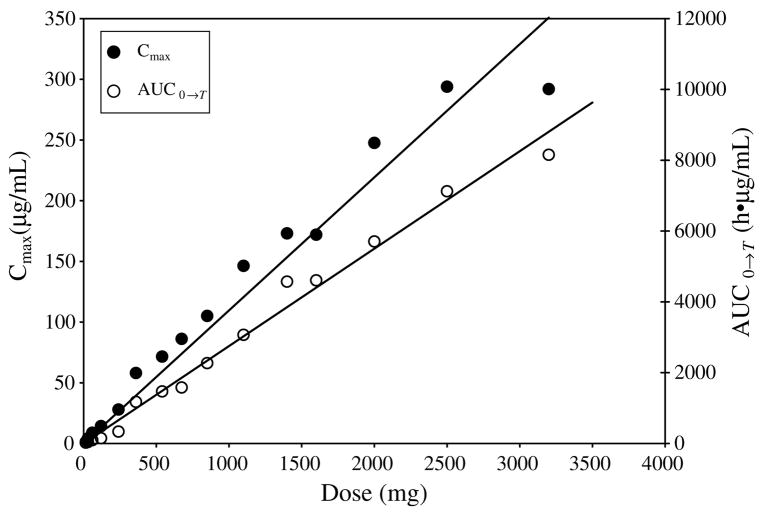
The time course of plasma concentrations of R(+)XK469 following drug infusion are shown in Fig. 2. The calculated pharmacokinetic parameters are summarised in Table 5. The mean apparent half-life over all dose levels was 63 h. There was no significant correlation between clearance and dose (r = −0.03) or body surface area (r = 0.12). The mean clearance over all dose levels for day 1 was 192 ml/h. There was accumulation of drug as evidenced by a mean accumulation ratio (AUC0→∞ on Day 3: Day 1) of 1.5. Fig. 3 displays mean Cmax and AUC0→T versus dose. There was no evidence of non-linearity over the R(+)XK469 doses investigated, with peak plasma concentrations and systemic exposure proportionally increasing with dose.
Fig. 2.
Mean R(+)XK469 plasma concentrations by dose level for cycle 1.
Table 5.
Mean (coefficient of variation) pharmacokinetic parameters for cycle 1 of R(+)XK469
| Dose level | N | Cmax (μg/ml) | AUC0→T (h μg/ml) | AUC0→∞ (h μg/ml) | t½ (h) | Cl (ml/h) | Vss (L) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Day 1 | Day 3 | Day 1 | Day 3 | Day 1 | Day 3 | Day 1 | Day 1 | Day 1 | ||
| Schedule A (mg) | ||||||||||
| 15 | 2 | 1.2 (0.03) | 24 (0.10) | 106 (0.72) | 66 (0.75) | 191 (0.72) | 13 (0.50) | |||
| 30 | 2 | 4.1 (0.29) | 41 (0.18) | 162 (0.30) | 57 (0.11) | 194 (0.30) | 15 (0.19) | |||
| 60 | 2 | 8.8 (0.02) | 92 (0.19) | 223 (0.44) | 31 (0.33) | 297 (0.44) | 12 (0.10) | |||
| 120 | 1 | 14.3 | 148 | 347 | 30 | 345 | 15 | |||
| 240 | 4 | 27.9 (0.14) | 335 (0.10) | 1.285 (0.54) | 56 (0.60) | 244 (0.64) | 14 (0.16) | |||
| Schedule B (mg) | ||||||||||
| 360 | 3 | 58.0 (0.31) | 79.9 (0.18) | 1.177 (0.14) | 1.885 (0.24) | 2.577 (0.25) | 4.091 (0.37) | 54 (0.36) | 147 (0.29) | 11 (0.22) |
| 540 | 6 | 71.5 (0.20) | 89.6 (0.32) | 1.469 (0.20) | 2.010 (0.28) | 5.002 (0.77) | 5.564 (0.74) | 90 (0.85) | 152 (0.51) | 14 (0.20) |
| 675 | 5 | 86.2 (0.18) | 104.2 (0.14) | 1.581 (0.12) | 2.195 (0.22) | 3.033 (0.18) | 4.335 (0.30) | 61 (0.71) | 220 (0.23) | 13 (0.24) |
| 850 | 7 | 105.1 (0.17) | 127.1 (0.15) | 2.270 (0.24) | 3.307 (0.29) | 5.161 (0.59) | 7.748 (0.53) | 43 (0.55) | 216 (0.56) | 13 (0.14) |
| 1100 | 20 | 146.3 (0.22) | 166.5 (0.21) | 3.067 (0.26) | 4.489 (0.28) | 7.903 (0.49) | 10.531 (0.43) | 55 (0.41) | 169 (0.45) | 14 (0.31) |
| 1400 | 1 | 173.1 | 177.7 | 4.571 | 6.718 | 16.473 | 35.930 | 107 | 84 | 13 |
| Schedule C (mg) | ||||||||||
| 1600 | 5 | 172.0 (0.21) | 4.607 (0.28) | 13.785 (0.55) | 75 (0.47) | 144 (0.51) | 14 (0.32) | |||
| 2000 | 3 | 247.6 (0.22) | 5.704 (0.12) | 11.204 (0.19) | 47 (0.10) | 183 (0.18) | 12 (0.09) | |||
| 2500 | 14 | 293.9 (0.19) | 7.125 (0.30) | 13.905 (0.52) | 42 (0.33) | 215 (0.39) | 11 (0.16) | |||
| 3200 | 5a | 292.3 (0.20) | 8.155 (0.21) | 16.445 (0.21) | 49 (0.08) | 193 (0.24) | 13 (0.22) | |||
One patient had blood levels drawn at significantly insufficient time points.
Fig. 3.
Mean Cmax and AUC0→T versus dose.
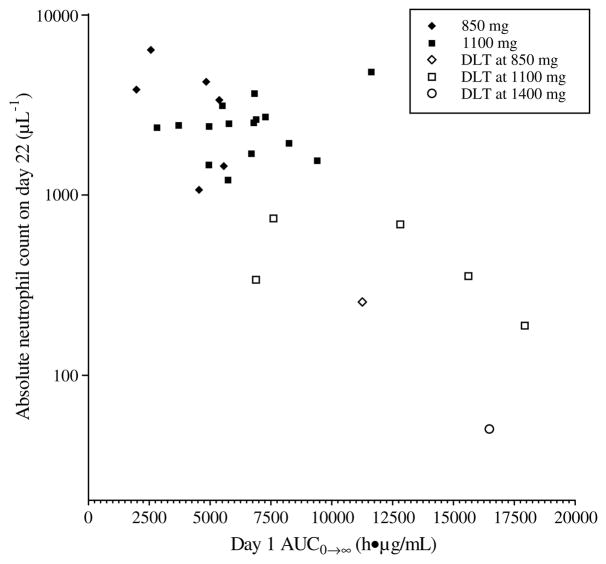
As evidenced in Table 5, there was considerable interindividual variability in clearance with a coefficient of variation of 46% over all doses. This variability translated into interpatient differences in toxicity. Fig. 4 shows bone marrow toxicity as a function of drug exposure in Schedule B which had the largest number of patients and greatest incidence of neutropaenia. There was a statistically significant inverse relationship between AUC0→∞ and neutropaenia (ln(ANC) versus AUC0→∞: r = −0.75, p < 0.01). The dose levels represented in the graph (850, 1100 and 1400 mg) are those at which DLTwas observed in Schedule B. The majority of those patients who failed to return to an ANC of at least 1500 μl−1 (grade 1) by day 22 had lower than average clearance. Of note, all the 7 patients who experienced DLT based upon grade ≥3 neutropaenia on day 22 had lower clearance values than the mean clearance value and 5 of those 7 patients (71%) had a clearance value that was more than one standard deviation below the mean.
Fig. 4.
Absolute neutrophil count on day 22, the scheduled first day of cycle 2, versus AUC0→∞ on day 1 for Schedule B: dose levels 850, 1100 and 1400 mg; r = −0.75, p < 0.01.
3.5. Antitumour activity
No complete responses were observed. One heavily pre-treated patient with metastatic nasopharyngeal carcinoma had a partial response after two treatment cycles. Upon re-imaging after cycle four, he had disease progression requiring removal from study. A second patient with metastatic squamous cell head and neck cancer remained on treatment for ten cycles before developing progressive disease. Both patients were enroled at the 3200 mg dose level of Schedule C, though for the second patient it was reduced to 2500 mg in cycle two due to toxicity.
4. Discussion
This phase I dose-finding and pharmacokinetic study of R(+)XK469 was originally designed to explore a schedule of daily × 5 repeated every 21 d based on preclinical data that indicated less toxicity and the lack of adverse impact on efficacy when the drug is administered via divided the doses in contrast to a single large dose. However, pharmacokinetic analysis revealed a much longer half-life in humans than expected and it has been hypothesised that the drug is sequestered (i.e. in bile) and recirculated resulting in an apparent long terminal phase.18 It was the long half-life that prompted the modification of the schedule to infusion on days 1, 3 and 5 of a 21-d cycle. With the observation that humans tolerated higher doses than predicted by the preclinical data and that severe toxicity was essentially limited to myelosuppression, a single-dose strategy of once every 21 d was then investigated. The dose-limiting toxicity of R(+)XK469 in both the divided-dose and single-dose schedules was neutropaenia. The lack of correlation between clearance and body surface area justified the flat-dosing scheme used.
With cohort expansion of the 1100 mg dose level (total dose = 3300 mg) to 20 patients in Schedule B, the final incidence of DLT was 35% at what was presumed to be the phase II recommended dose based upon the severe toxicity observed at 1400 mg and the absence of DLT in the first 6 patients enroled at 1100 mg. Many investigators would consider this rate of DLT unacceptable for phase II investigation. Therefore, the recommended dose for phase II investigation on Schedule B is 850–1100 mg/d (total dose = 2550–3300 mg). For Schedule C, the phase II recommended dose is 2500 mg. The similarity in the total doses between the two schedules challenges the preclinical data that suggested that a divided-dose schedule is required for R(+)XK469.
It is an interesting observation that the overall incidence of DLT at the 1100 mg dose level in Schedule B was ≥33%. By definition this dose is too high to be the MTD. However, if expansion was limited to 6 patients, as is typically done at the MTD in phase I studies, we would have reported a complete absence of DLT at this dose. This example illustrates the usefulness of larger patient cohorts at the MTD in phase I studies of agents with large interpatient pharmacokinetic variability (as is the case for R(+)XK469) so as not to underestimate toxicity. In the absence of cohort expansion, greater consideration should be given to obtaining pharmacokinetic data in phase II studies for agents with marked pharmacokinetic variability to better correlate drug pharmacokinetics and toxicity.
As described in Table 5, there was significant interpatient variability in drug clearance. There was correlation between toxicity and clearance with those patients having clearance values more than one standard deviation below the mean at higher risk of prolonged severe neutropaenia. One can implicate interindividual genetic variability, and the observation of such pharmacokinetic variability can prompt the discovery of relevant genetic polymorphisms.19 R(+)XK469 is metabolised primarily by aldehyde oxidase.20 Whilst no functional polymorphisms of the gene encoding this enzyme have been reported to date,21 the results of this study should prompt further genetic investigation of aldehyde oxidase.
Myelosuppression was dose-limiting. Whilst growth factor was not routinely administered in this trial, it should be noted that in those instances where the treating physician felt that filgastrim was clinically warranted, patients quickly responded. It is reasonable to assume that with growth factor support, the incidence of prolonged severe neutropaenia would be lower than observed in this study and that higher doses may safely be administered. This is particularly salient as antitumour activity was documented at 3200 mg, a dose greater than the MTD. The partial response observed in the patient with nasopharyngeal carcinoma and the prolonged stable disease in a patient with laryngeal carcinoma point to head and neck cancer as at least one area in which to consider further development of R(+)XK469. In a separate study, R(+)XK469 has shown activity in leukaemia, including a complete remission22 and further studies in haematological malignancies are warranted.
Acknowledgments
This study was supported by the Phase I Clinical Trials of Anticancer Agents Grant (NIH/NCI U01 CA69852) and the UCCRC Cancer Center Support Grant (P30 CA14599). Preliminary data from this study were presented at the 39th Annual Meeting of the American Society of Clinical Oncology, Chicago, Illinois, May 31 to June 3, 2003 and the 40th Annual Meeting of the American Society of Clinical Oncology, New Orleans, Louisiana, June 5 to 8, 2004.
Footnotes
Conflict of interest statement
H.L. Kindler serves as a consultant and M.J. Ratain serves as an investigator for Bristol-Myers Squibb.
References
- 1.LoRusso PM, Parchment R, Demchik L, et al. Preclinical antitumor activity of XK469 (NSC 656889) Invest New Drugs. 1999;16:287–96. doi: 10.1023/a:1006206814025. [DOI] [PubMed] [Google Scholar]
- 2.Wiegand RA, Doyle TD, Grieshaber CK, et al. Normal-phase separation of XK469 enantiomers (R:NSC-698215, S:NSC-698216) applied to preclinical pharmacology. Proceedings of the AACR-NCI-EORTC international conference on molecular targets and cancer therapeutics; 1999; Washington, DC. [Abstract 542] [Google Scholar]
- 3.Zheng H, Covey JM, Tosca PJ, Turner N, Chan KK. Chiral high-performance liquid chromatographic analysis of the enantiomers of XK469, a new antitumor agent, in plasma and urine. J Pharm Biomed Anal. 2002;28:287–94. doi: 10.1016/s0731-7085(01)00566-0. [DOI] [PubMed] [Google Scholar]
- 4.Zheng H, Jiang C, Chiu MH, Covey JM, Chan KK. Chiral pharmacokinetics and inversion of enantiomers of a new quinoxaline topoisomerase IIβ poison in the rat. Drug Metab Dispos. 2002;30:344–8. doi: 10.1124/dmd.30.3.344. [DOI] [PubMed] [Google Scholar]
- 5.Gao H, Huang KC, Yamasaki EF, Chan KK, Chohan L, Snapka RM. XK469, a selective topoisomerase IIβ poison. Proc Natl Acad Sci USA. 1999;96:12168–73. doi: 10.1073/pnas.96.21.12168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Snapka RM, Gao H, Grabowski DR, et al. Cytotoxic mechanism of XK469: resistance of topoisomerase IIβ knockout cells and inhibition of topoisomerase I. Biochem Biophys Res Commun. 2001;280:1155–60. doi: 10.1006/bbrc.2001.4249. [DOI] [PubMed] [Google Scholar]
- 7.Mensah-Osman EJ, Al-Katib AM, Dandashi MH, Mohammad RM. 2-[4-(7-chloro-2-quinoxalinyloxy)phenoxy]-propionic acid (XK469) inhibition of topoisomerase IIβ is not sufficient for therapeutic response in human Waldenstrom’s macroglobulinemia xenograft model. Mol Cancer Ther. 2002;1:1315–20. [PubMed] [Google Scholar]
- 8.Gao H, Yamasaki EF, Chan KK, et al. DNA sequence specificity for topoisomerase II poisoning by the quinoxaline anticancer drugs XK469 and CQS. Mol Pharmacol. 2003;63:1382–8. doi: 10.1124/mol.63.6.1382. [DOI] [PubMed] [Google Scholar]
- 9.Ding Z, Parchment RE, LoRusso PM, et al. The investigational new drug XK469 induces G2 – M cell cycle arrest by p53-dependent and -independent pathways. Clin Cancer Res. 2001;7:3336–42. [PubMed] [Google Scholar]
- 10.Lin H, Liu XY, Subramanian B, Nakeff A, Valeriote F, Chen BD. Mitotic arrest induced by XK469, a novel antitumor agent, is correlated with the inhibition of cyclin B1 ubiquitination. Int J Cancer. 2002;97:121–8. doi: 10.1002/ijc.1570. [DOI] [PubMed] [Google Scholar]
- 11.Kessel D, Horwitz JP. Pro-apoptotic interactions between XK469 and the peripheral benzodiazepine receptor. Cancer Lett. 2001;168:141–4. doi: 10.1016/s0304-3835(01)00518-3. [DOI] [PubMed] [Google Scholar]
- 12.Corbett TH, LoRusso P, Demchick L, et al. Preclinical antitumor efficacy of analogs of XK469 sodium-(2-[4-(7-chloro-2-quinoxalinyloxy)phenoxy]propionate. Invest New Drugs. 1998;16:129–9. doi: 10.1023/a:1006174622061. [DOI] [PubMed] [Google Scholar]
- 13.Boinpally RR, Zhou SL, LoRusso PM, Parchment RE. Bioavailability and pharmacokinetics of the investigational anticancer agent XK469 (NSC 698215) in rats following oral and intravenous administration. Cancer Chemother Pharmacol. 2005;55:404–7. doi: 10.1007/s00280-004-0862-6. [DOI] [PubMed] [Google Scholar]
- 14.Tosca PJ, Turner NA, Hassler CR, et al. Toxicity of R(+)XK469 (NSC-698215) and S(−)XK469 (NSC-698216) in rodents and beagle dogs. Proc Am Assoc Cancer Res. 2000;41:705. [Abstract 4480] [Google Scholar]
- 15.Polin L, White K, Kushner J, et al. Preclinical efficacy evaluations of XK-469 dose schedule, route and cross-resistance behavior in tumor bearing mice. Invest New Drugs. 2002;20:13–22. doi: 10.1023/a:1014469828729. [DOI] [PubMed] [Google Scholar]
- 16.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 17.Gibaldi M, Perrier D. Pharmacokinetics. 2. New York, NY: Marcel-Dekker; 1982. Noncompartmental analysis based on statistical moment theory. [Google Scholar]
- 18.Alousi AM, Boinpally R, Wiegand R, et al. A phase 1 trial of XK469: toxicity profile of a selective topoisomerase IIβ inhibitor. Invest New Drugs. 2007;25:147–54. doi: 10.1007/s10637-006-9024-5. [DOI] [PubMed] [Google Scholar]
- 19.Undevia SD, Gomez-Abuin G, Ratain MJ. Pharmacokinetic variability of anticancer agents. Nat Rev Cancer. 2005;5:447–58. doi: 10.1038/nrc1629. [DOI] [PubMed] [Google Scholar]
- 20.Anderson LW, Collins JM, Klecker RW, et al. Metabolic profile of XK469 (2(R)-[4-(7-chloro-2-quinoxalinyl)oxyphenoxy]-propionic acid; NSC698215) in patients and in vitro: low potential for active or toxic metabolites or for drug–drug interactions. Cancer Chemother Pharmacol. 2005;56:351–7. doi: 10.1007/s00280-004-0962-3. [DOI] [PubMed] [Google Scholar]
- 21.Kim TW, Liu W, Undevia SD, et al. A pharmacogenetic study of aldehyde oxidase in patients treated with R(+)XK69. The Annual Meeting of the Korean Cancer Association; Korea: Seoul. 2005. [Google Scholar]
- 22.Stock W, Undevia SD, Faderl S, et al. Phase I study of XK469R (NSC 698215), a quinoxaline phenoxypropionic acid derivative, in patients with refractory hematological malignancies. Blood (ASH Annual Abstracts) 2006;108 doi: 10.1007/s10637-008-9129-0. [Abstract 1952] [DOI] [PMC free article] [PubMed] [Google Scholar]