Abstract
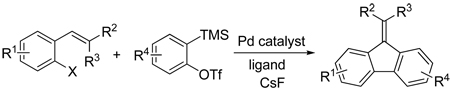
The palladium-catalyzed annulation of arynes by substituted ortho-halostyrenes produces substituted 9- fluorenylidenes in good yields. This methodology provides this important carbocyclic ring system in a single step, which involves the generation of two new carbon-carbon bonds, occurs under relatively mild reaction conditions and tolerates a variety of functional groups, including cyano, ester, aldehyde and ketone groups.
9-Fluorenylidenes are key structural units found in many compounds possessing biological activity. Derivatives of 9H-fluoren-9-ylidenes, commonly known as 9-fluorenylidenes, are pharmaceutically and cosmetically significant. The 9-fluorenylidene derivative Paranylene is used in dispersible formulations of anti- inflammatory agents,1 while the 9-fluorenylidene derivative Lumefantrine is used in dermatological and photostable cosmetic compositions.2 3-Fluoren-9- ylidene-2′-hydroxy-3-phenylpropiophenone exhibits thermochromic properties.3 2,4,7-Trinitro-9- fluorenylmethacrylate (TNFMN) has been used to study the donor-acceptor interactions of poly(FIMA’s) with different tacticities.4
Due to the pharmaceutical and biological importance of these compounds, the synthesis of 9-fluorenylidenes is important. In the literature, 9-fluorenylidenes are mainly synthesized, either from 9H-fluoren-9-one derivatives using a Wittig reaction5 or from 9H-fluorene derivatives.6
Transition metal-catalyzed annulation reactions are very valuable from a synthetic point of view.7 The Pd- catalyzed annulation of alkynes by substituted aryl and vinylic halides has been employed for the synthesis of a variety of carbocycles and heterocycles,8 and some of these reactions have also been recently extended to arynes. The major difficulty in employing arynes is the high reactivity of arynes9 compared to alkynes, and the harsh reaction conditions often needed to generate arynes in situ. A common problem associated with the high reactivity of arynes is their Pd-catalyzed cyclotrimerization10 to form polycyclic aromatic hydrocarbons. Also, the harsh reaction conditions often required to obtain arynes severely limit the functional group compatibility of the chemistry. It has been reported that the silylaryl triflate 2a in the presence of CsF generates benzyne under very mild reaction conditions.11 This method of aryne generation has been used in our research laboratories and reported in the literature for a variety of electrophilic and nucleophilic reactions,12 Pd- catalyzed annulation reactions,13 cycloaddition reactions14 and insertion reactions.15
The palladium-catalyzed alkyne annulation of methyl 3-(2-iodophenyl)acrylate has been reported in the literature.16 To the best of our knowledge, analogous palladium-catalyzed aryne insertions and subsequent cyclization of ortho-halostyrenes, have not been reported previously. We report herein an efficient approach to 9- fluorenylidenes, which proceeds in good yields from starting materials that are readily available or easy to synthesize, and involves a palladium-catalyzed annulation of arynes.
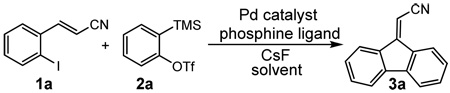 |
(1) |
(E)-3-(2-Iodophenyl)acrylonitrile (1a) was used as a model system for optimization of the reaction conditions using 2-(trimethylsilyl)phenyl trifluoromethanesulfonate (2a) as the aryne precursor. We optimized the reaction conditions with respect to different ratios of the acetonitrile/toluene solvent system; the phosphine ligands P(o-tolyl)3, tris(2,4,6-trimethoxyphenyl)phosphine, tris(2,6-dimethoxyphenyl)phosphine, tri(2- methoxyphenyl)phosphine, tri-(2-furyl)phosphine, [(CH3)3P·AgI]4, tri(t-butyl)phosphine, 2-(di-tert- butylphosphino)biphenyl, xantphos, dppp, dppf, and dppm; the temperatures (85 °C, 100 °C, 110 °C and, 120 °C) at which the reaction is run; the amount of the aryne precursor; the amount of CsF; and various Pd(dba)2 catalyst loadings (5-20 mol %) with different ratios of Pd(dba)2 catalyst to dppm ligand. Our “optimal” procedure involves heating 0.3 mmol of 1a, 1.5 equiv of 2a, 10 mol % of Pd(dba)2, 20 mol % of dppm, and 3.0 equiv of CsF in 5 ml of 1:1 acetonitrile/toluene at 110 °C in a sealed vial for 24 h.
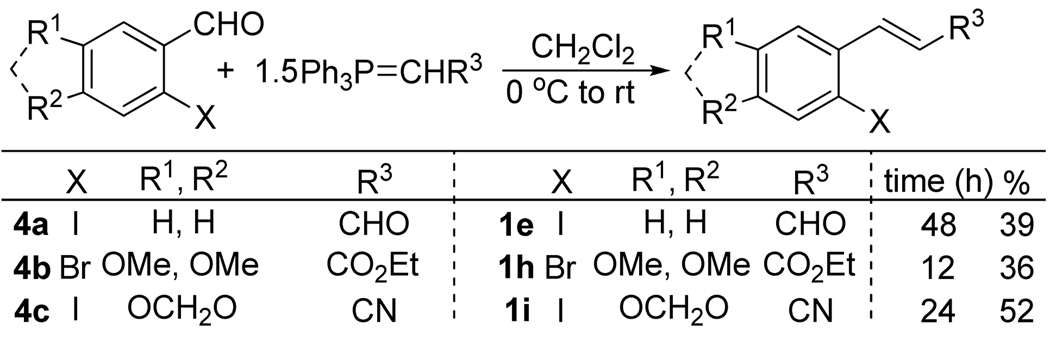
After obtaining our best reaction conditions for the aryne annulation, we examined the scope of this reaction on various substrates. Aryl halides 1a and 1g were obtained from commercial sources. Aryl halides 1e, 1h, and 1i were prepared by standard Wittig chemistry (Scheme 1), using commercially available aldehydes 4a and 4b, while 4c was prepared according to a literature procedure.17 Aryl halides 1b,18 1c,18 1d,19 1f,20 were prepared according to literature procedures. The benzyne precursor 2a is commercially available, while the aryne precursor 2b14 has been prepared according to a literature procedure.
Scheme 1.
Starting Material Preparation
The reaction of aryl iodide 1a under our optimized conditions with 2-(trimethylsilyl)phenyl trifluoromethanesulfonate (2a) as the benzyne precursor gave an 91% isolated yield of the desired product 3a (Table 1, entry 1). Compound 3a was obtained in a slightly lower 79% yield, when the reaction was carried out using the corresponding aryl bromide, (E)-3-(2- bromophenyl)acrylonitrile (1b). The improved yield from the aryl iodide is no doubt a direct result of the more facile oxidative addition of aryl iodides over aryl bromides. The cis isomer (Z)-3-(2- bromophenyl)acrylonitrile (1c) gave a slightly lower yield of 72% than the corresponding trans isomer, (E) 3-(2- bromophenyl)acrylonitrile (1b) (compare entries 2 and 3). With an ethyl ester as the electron-withdrawing group (EWG) on the double bond of the ortho-halostyrene 1d, the yield dropped to 75% under our optimized conditions (compare entries 2 and 4). With an aldehyde as the EWG on the ortho-halostyrene 1e, a 76% yield of the desired product 3c was obtained (entry 5). The yield is comparable to that obtained with an ester group present on the double bond of the ortho-halostyrene. With a ketone present in the ortho-halostyrene 1f, the yield dropped to 61% (entry 6). When a stronger electron- withdrawing nitro group was placed on the ortho- halostyrene, the reaction failed to give the desired product 3e (entry 7). Instead, we observed the formation of a polymeric residue. We believe 2-(2- nitrovinyl)iodobenzene (1g) undergoes polymerization under our reaction conditions.
Table 1.
Synthesis of 9-Fluorenylidenes by the Palladium-Catalyzed Aryne Annulation of ortho-Halostyrenes.a
| entry | Ortho-halostyrene | benzyne precursor | product(s) | % isolated yield |
|---|---|---|---|---|
| 1 |  |
 |
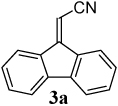 |
91 |
| 2 |  |
2a | 3a | 79 |
| 3 | 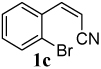 |
2a | 3a | 72 |
| 4 |  |
2a |  |
75 |
| 5 |  |
2a |  |
76 |
| 6 |  |
2a | 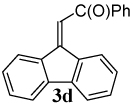 |
61 |
| 7 |  |
2a |  |
0 |
| 8 |  |
2a |  |
<5b |
| 9 |  |
2a |  |
73c |
| 10 | 1a |  |
 |
82d [1:11] |
Representative procedure: ortho-halostyrene 1a-i (0.3 mmol), silylaryl triflate 2a,b (1.5 equiv), 10 mol % of Pd(dba)2, 20 mol % of dppm, CsF (3 equiv) and 1:1 CH3CN/PhCH3 (5 mL) were placed in a 4 dram vial. The vial was sealed with a screw cap. The reaction was then stirred at 110 °C for 24 h.
GC yields.
Eight percent of a minor isomer is observed by GC analysis.
The ratio was determined by H1 NMR spectroscopy.
When electron-donating methoxy groups were placed on the aryl bromide 1h, we obtained an extremely poor yield (<5%), presumably because oxidative addition to palladium is unfavorable in such electron-rich aryl bromides (compare entries 4 and 8). On the other hand, the electron-rich substrate 1i with a carbon-iodine, instead of a carbon-bromine bond, gave the desired product in a 73% yield (entry 9). The stereochemistry of the major product has not been rigorously established, but is assumed to be the less hindered E-isomer. Eight percent of a minor isomer has also been observed.
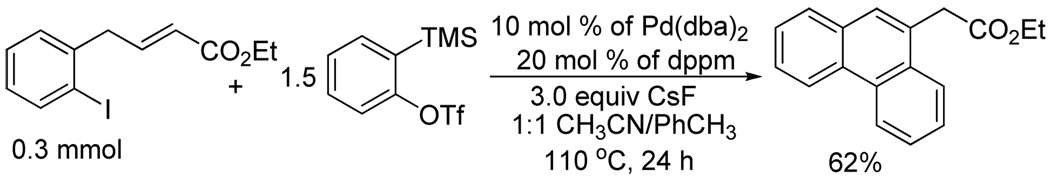
We have also studied the scope of the reaction using a different aryne precursor. The aryl iodide 1a upon reaction with the aryne precursor 2b gave the desired compounds 3i and 3j as a 1:11 mixture of inseparable isomers in an 82% overall yield (entry 10). It is unclear as to which stereoisomer is the major product. The ortho- halo allylicbenzene, ethyl (E)-4-(2-iodophenyl)but-2- enoate on reaction with 2a gave a modest 62% yield of the ethyl (phenanthren-9-yl)acetate (Fig. 1). The reactions to obtain the phenanthrene nucleus are under further investigation.
Figure 1.
Synthesis of ethyl (phenanthren-9-yl)acetate
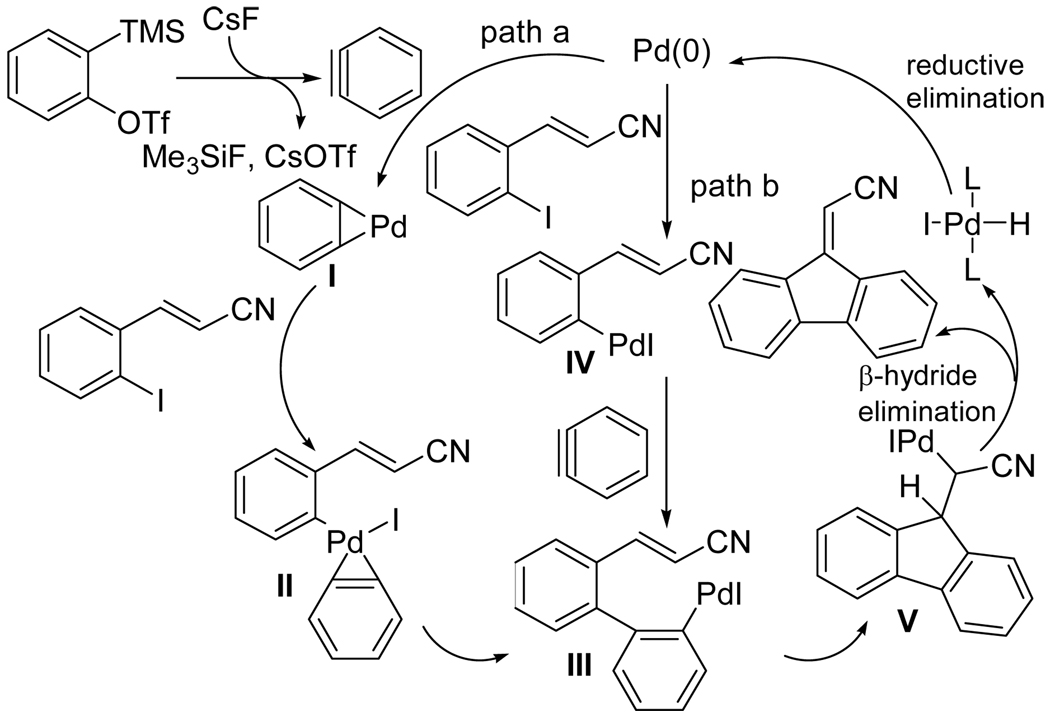
We propose the following possible mechanisms for these reactions based on the known reactions of organopalladium compounds with alkynes (Scheme 2).8 The reaction can follow two pathways, path a or path b. In path a, the aryne generated from the triflate in the presence of the fluoride source coordinates with Pd(0), affording palladacycle I.21 Oxidative addition of the aryl halide to I might generate an arylpalladium(IV) complex II. Upon reductive elimination, complex II could afford a new arylpalladium intermediate III. Alternatively, according to path b, Pd(0) might add oxidatively to the aryl halide to afford the arylpalladium(II) intermediate IV, which in turn might add to the aryne22 to afford arylpalladium intermediate III. Regardless of how intermediate III is generated, the palladium-carbon bond in this intermediate can then add across the neighboring carbon-carbon double bond to afford intermediate V, which directly affords the fluorenylidene product by β- hydride elimination.
Scheme 2.
Tentative Mechanisms
Our investigation has shown that a range of fluorenylidenes can be obtained from simple starting materials that are readily available or easily synthesized, using a one step palladium-catalyzed aryne insertion of o- halostyrenes. The arynes are obtained in situ under mild reaction conditions from the corresponding 2- (trimethylsilyl)aryl trifluoromethanesulfonates and CsF. Our methodology is tolerant of a variety of functional groups, including cyano, ester, aldehyde, ketone, and methoxy groups, which can provide a handle for further organic transformations. This methodology provides a very convenient and general approach to this important class of aromatic hydrocarbons.
Supplementary Material
General experimental procedures and spectral data for all previously unreported starting materials and products. This material is available free of charge via the Internet at http://pubs.acs.org.
Acknowledgements
We thank the National Institute of General Medical Sciences (GM070620), the National Institutes of Health Kansas University Chemical Methodologies and Library Development Center of Excellence (P50 GM069663), and the National Science Foundation for support of this research, and Johnson Matthey, Inc., and Kawaken Fine Chemicals Co., Ltd., for donations of palladium catalysts.
References
- 1.Britten NJ, Burns JW, Hallberg JW, Waldron NA, Watts JL. 2004. Dispersible formulations of an anti-inflammatory agent. PCT Int. Appl. WO 2004082588. [Google Scholar]
- 2.Mueller S, Herzog B, Quass K. 2006. Photostable cosmetic or dermatological compositions containing sunscreens. PCT Int. Appl. WO 2006034968. [Google Scholar]
- 3.Schoenberg A, Singer E. Ber. 1961;94:248. [Google Scholar]
- 4.Ishizawa H, Nakano T, Yade T, Tsuji M, Nakagawa O, Yamaguchi T. J. Polym. Sci., Part A: Polym. Chem. 2004;42:4656. [Google Scholar]
- 5.(a) Trippett S, Walker DM. J. Chem. Soc. 1961:2130. [Google Scholar]; (b) Kendurkar PS, Tewari RS. Ind. J. Chem., Sect. B. 1977;15B:290. [Google Scholar]; (c) Bestmann HJ, Schlosser W. Synthesis. 1979:201. [Google Scholar]; (d) Wittig G, Schoch-Gruebler U. J. Liebigs Ann. Chem. 1978;2:362. [Google Scholar]; (e) Abdel-Malek HA. Egypt. J. Chem. 2005;48:129. [Google Scholar]
- 6.(a) Ye J, Ge J, Chen X, Zhao Z, Lu P. Tetrahedron. 2007;63:11040. [Google Scholar]; (b) Cadierno V, Diez J, Garcia-Garrido SE, Gimeno J, Nebra N. Adv. Synth. Catal. 2006;348:2125. [Google Scholar]; (c) Hudgens DP, Taylor C, Batts TW, Patel MK, Brown ML. Bioorg. Med. Chem. 2006;14:8366. doi: 10.1016/j.bmc.2006.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Lukes V, Vegh D, Hrdlovic P, Stefko M, Matuszna K, Laurinc V. Synth. Met. 2005;148:179. [Google Scholar]
- 7.(a) Ojima I, Tzamarioudaki M, Li Z, Donovan RJ. Chem. Rev. 1996;96:635. doi: 10.1021/cr950065y. [DOI] [PubMed] [Google Scholar]; (b) Rubin M, Sromek AW, Gevorgyan V. Synlett. 2003:2265. [Google Scholar]
- 8.(a) Larock RC. Pure Appl. Chem. 1999;71:1435. [Google Scholar]; (b) Larock RC. Topics in Organometallic Chemistry. Berlin, Germany: Springer; 2005. p. 147. [Google Scholar]
- 9.For reviews on the use of arynes in organic synthesis, see: Kessar SV. In: Comprehensive Organic Synthesis. Trost BM, Fleming I, editors. Vol. 4. New York, NY: Pergamon; 1991. p. 483. Pellissier H, Santelli M. Tetrahedron. 2003;59:701.
- 10.(a) Peña D, Escudero S, Pérez D, Guitián E, Castedo L. Angew. Chem., Int. Ed. 1998;37:2659. doi: 10.1002/(SICI)1521-3773(19981016)37:19<2659::AID-ANIE2659>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]; (b) Peña D, Pérez D, Guitián E, Castedo L. Org. Lett. 1999;1:1555. doi: 10.1021/ol005916p. [DOI] [PubMed] [Google Scholar]; (c) Peña D, Cobas A, Pérez D, Guitián E, Castedo L. Org. Lett. 2000;2:1629. doi: 10.1021/ol005916p. [DOI] [PubMed] [Google Scholar]
- 11.Himeshima Y, Sonoda T, Kobayashi H. Chem. Lett. 1983:1211. [Google Scholar]
- 12.(a) Yoshida H, Honda Y, Shirakawa E, Hiyama T. Chem. Commun. 2001:1880. doi: 10.1039/b103745p. [DOI] [PubMed] [Google Scholar]; (b) Yoshida H, Shirakawa E, Honda Y, Hiyama T. Angew. Chem., Int. Ed. 2002;41:3247. doi: 10.1002/1521-3773(20020902)41:17<3247::AID-ANIE3247>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]; (c) Liu Z, Larock RC. Org. Lett. 2003;5:4673. doi: 10.1021/ol0358612. [DOI] [PubMed] [Google Scholar]; (d) Liu Z, Larock RC. Org. Lett. 2004;6:99. doi: 10.1021/ol0361406. [DOI] [PubMed] [Google Scholar]; (e) Jeganmohan M, Cheng C-H. Synthesis. 2005:1693. [Google Scholar]; (f) Bhuvaneswari S, Jeganmohan M, Yang M-C, Cheng C-H. Chem. Commun. 2008:2158. doi: 10.1039/b800118a. [DOI] [PubMed] [Google Scholar]
- 13.(a) Liu Z, Zhang X, Larock RC. J. Am. Chem. Soc. 2005;127:15716. doi: 10.1021/ja055781o. [DOI] [PubMed] [Google Scholar]; (b) Zhang X, Larock RC. Org. Lett. 2005;7:3973. doi: 10.1021/ol0514597. [DOI] [PubMed] [Google Scholar]; (c) Liu Z, Larock RC. Org. Lett. 2004;6:3739. doi: 10.1021/ol048564l. [DOI] [PubMed] [Google Scholar]; (d) Liu Z, Larock RC. Tetrahedron. 2007;63:347. doi: 10.1016/j.tet.2006.10.071. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Henderson JL, Edwards AS, Greaney MF. J. Am. Chem. Soc. 2006;128:7426. doi: 10.1021/ja0615526. [DOI] [PubMed] [Google Scholar]; (f) Jayanth TT, Cheng C-H. Chem. Commun. 2006:894. doi: 10.1039/b515846j. [DOI] [PubMed] [Google Scholar]
- 14.(a) Liu Z, Shi F, Martinez PDG, Raminelli C, Larock RC. J. Org. Chem. 2008;73:219. doi: 10.1021/jo702062n. [DOI] [PubMed] [Google Scholar]; (b) Raminelli C, Liu Z, Larock RC. J. Org. Chem. 2006;71:4689. doi: 10.1021/jo060523a. [DOI] [PubMed] [Google Scholar]; (c) Shi F, Waldo JP, Chen Y, Larock RC. Org. Lett. 2008;10:2409. doi: 10.1021/ol800675u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.(a) Liu Z, Larock RC. J. Am. Chem. Soc. 2005;127:13112. doi: 10.1021/ja054079p. [DOI] [PubMed] [Google Scholar]; (b) Tambar UK, Stoltz BM. J. Am. Chem. Soc. 2005;127:5340. doi: 10.1021/ja050859m. [DOI] [PubMed] [Google Scholar]
- 16.Grigg R, Kennewell P, Teasdale A, Sridharan V. Tetrahedron Lett. 1993;34:153. [Google Scholar]
- 17.Matveenko M, Kokas OJ, Banwell MG, Willis AC. Org. Lett. 2007;9:3683. doi: 10.1021/ol701552r. [DOI] [PubMed] [Google Scholar]
- 18.Solodenko W, Kunz U, Jas G, Kirschning A. Bioorg. Med. Chem. Lett. 2002;12:1833. doi: 10.1016/s0960-894x(02)00265-2. [DOI] [PubMed] [Google Scholar]
- 19.Métay E, Léonel E, Sulpice-Gaillet C, Nédélec JY. Synthesis. 2005:1682. [Google Scholar]
- 20.Davey W, Gwilt JR. J. Am. Chem. Soc. 1957;79:1015. [Google Scholar]
- 21.(a) Yoshida H, Ikadai J, Shudo M, Ohshita J, Kunai A. Organometallics. 2005;24:156. [Google Scholar]; (b) Yoshida H, Tanino K, Ohshita J, Kunai A. Angew. Chem., Int. Ed. 2004;43:5052. doi: 10.1002/anie.200460189. [DOI] [PubMed] [Google Scholar]
- 22.Henderson JL, Edwards AS, Greaney MF. Org. Lett. 2007;9:5589. doi: 10.1021/ol702584t. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
General experimental procedures and spectral data for all previously unreported starting materials and products. This material is available free of charge via the Internet at http://pubs.acs.org.