Abstract
Aims
This study examined whether voucher-based reinforcement therapy (VBRT) contingent on smoking abstinence during pregnancy is an effective method for decreasing maternal smoking during pregnancy and improving fetal growth.
Design
A two-condition, parallel groups, randomized controlled trial was conducted.
Setting
The trial was conducted in a university-based research clinic.
Participants
A total of 82 smokers entering prenatal care participated in the trial.
Intervention
Participants were randomly assigned to either contingent or non-contingent voucher conditions. Vouchers exchangeable for retail items were available during pregnancy and for 12 weeks postpartum. In the contingent condition, vouchers were earned for biochemically-verified smoking abstinence; in the non-contingent condition, vouchers were earned independent of smoking status.
Measurements
Smoking outcomes were evaluated using urine-toxicology testing and self-report. Fetal growth outcomes were evaluated using serial ultrasound examinations performed during the third trimester.
Findings
Contingent vouchers significantly increased point-prevalence abstinence at the end-of-pregnancy (41% vs. 10%) and at the 12-week postpartum assessment (24% vs. 3%). Serial ultrasound examinations indicated significantly greater growth in terms of estimated fetal weight, femur length, and abdominal circumference in the contingent compared to the non-contingent conditions.
Conclusions
These results provide further evidence that VBRT has a substantive contribution to make to efforts to decrease maternal smoking during pregnancy and provide new evidence on positive effects on fetal health.
Keywords: vouchers, contingency management, pregnant women, smoking cessation, fetal growth
Introduction
Maternal smoking is a leading preventable cause of poor pregnancy outcomes and infant morbidity and mortality 1,2. Effective interventions exist for promoting smoking cessation during pregnancy, but cessation rates are often low (< 20%), especially among women who smoke more (> 10 cigarettes/day) and are less educated 3–5.
A small but growing literature suggests that voucher-based reinforcement therapy (VBRT) in which women earn vouchers exchangeable for retail items contingent on biochemically-verified abstinence may have particular promise for promoting smoking cessation during pregnancy and postpartum. VBRT is a form of contingency management that began as a novel intervention for outpatient treatment of cocaine dependence 6. Results of a recent meta-analysis of 30 controlled studies support the efficacy of VBRT for treatment of a wide range of substance use disorders, including cigarette smokers 7,8.
The seminal report on the use of VBRT with pregnant and postpartum smokers was a randomized trial involving low-income pregnant smokers assigned to VBRT or usual-care control conditions 9. In the VBRT condition, pregnant smokers received a monthly $50 voucher contingent on biochemically-verified smoking abstinence throughout pregnancy and for two months postpartum. Biochemically-confirmed smoking cessation rates at end-of-pregnancy were 32% vs. 9% in the VBRT and control conditions, respectively, and 21% vs. 6% at the 2-month postpartum assessment. The magnitude of these treatment effects exceeded results from several decades of research on this topic and thus warranted further investigation.
In our efforts to further examine the use of VBRT to treat pregnant smokers, a pilot study was conducted with low-income women who were still smoking upon entering prenatal care 10. Our research indicates that an initial period of sustained abstinence is important to sustaining abstinence longer term 11–13. To establish initial abstinence with pregnant smokers, abstinence monitoring in our voucher-based intervention begins at a relatively high frequency that gradually tapers downward during the antepartum period. The value of the voucher-based incentives escalates with successive drug-negative specimens (e.g., $6.25 for the first negative sample, $7.50 for the second, etc.) and includes a reset contingency wherein drug use following a period of abstinence resets the monetary value of the voucher back to the initial low level 14. To protect against relapse postpartum 15, the frequency of abstinence monitoring increases again during the initial postpartum weeks followed by a second tapering downward across 12 weeks of postpartum treatment.
In the initial pilot study by our group, participants were initially assigned to either contingent or non-contingent voucher conditions as consecutive admissions and later randomly. Vouchers were available antepartum and for the first 12 weeks postpartum and were earned for biochemically-verified smoking abstinence in the contingent condition and independent of smoking status in the non-contingent condition. Biochemically-verified, point-prevalence abstinence was significantly greater in the contingent than the non-contingent conditions at the end-of-pregnancy (37% vs. 9%), 12-week postpartum (33% vs. 0%), and 24-week postpartum (27% vs. 0%) assessments. The results provided further evidence of the efficacy of vouchers for promoting abstinence in pregnant smokers and suggested that those effects may continue following discontinuation of VBRT postpartum.
While these results were encouraging, they were tempered by the fact that the majority of participants were assigned as consecutive admissions rather than randomly. The current report presents the outcomes of a completely randomized clinical trial. In addition to a more rigorous experimental design, we were also interested in examining effects on fetal growth 16,17 assessed using serial ultrasound examinations.
Method
Participants
Participants were recruited from one of four large group obstetric practices and the Women, Infants, and Children (WIC) program in the greater Burlington, VT area. Study inclusion criteria were: self-report smoking (even a puff in the past 7 days) at a prenatal visit; self-report an estimated gestational age of ≤20 weeks; reside within the county in which the study clinic is located; plan to remain in the geographical area for 6 months following delivery; and speaks English. Exclusion criteria were: incarceration; having previously participated in the study; or currently residing with someone who participated in the study. All women receiving prenatal care at these clinics completed a brief questionnaire regarding basic sociodemographics and smoking status, including age, race, years of education, estimated gestational age (EGA), and smoking frequency in the past 7 days. Those who endorsed smoking in the past 7 days were subsequently contacted by study staff regarding study participation.
A total of 182 women were deemed study eligible and could be contacted. Eighty-two (45%) of these women agreed to participate and were enrolled; 43 (24%) expressed interest, but failed to complete the enrollment process; 57 (31%) were contacted and refused participation. When the characteristics noted above were compared between those who did and did not enroll in the study, only one significant difference was noted. Mean (±SEM) gestational age was 9.1 ± 0.4 among study participants vs. 10.4 ± 0.5 weeks among non-participants. Of the 82 women who agreed to participate, 40 were randomly assigned to the contingent vouchers condition and 42 to the non-contingent condition. Randomization was stratified based on the clinic where participants received their prenatal care. The only criterion for withdrawing a participant from the trial following treatment assignment was pregnancy termination/fetal demise; 5 women (3 contingent and 2 non-contingent) were withdrawn from the study based on that criterion, leaving 77 women whose results were used in analyses of study outcome. The study was approved by the University of Vermont Institutional Review Board and all participants provided written informed consent.
Assessments
Participants completed questionnaires examining socio-demographics, current smoking status/history, smoking environment, and motivation, confidence and intentions to quit smoking, and provided breath and urine specimens at the study-intake assessment. Appropriately modified versions of this battery were completed one month after the study-intake assessment, at the end of pregnancy (≥ 28 weeks gestation), and at 2, 4, 8, 12, and 24 weeks postpartum. At these assessments and throughout the abstinence-monitoring period (see below), breath specimens were analyzed using Micro Smokerlyzer carbon monoxide (CO) monitors (Bedfont Scientific Ltd., Kent, UK). An onsite enzyme immunoassay test (EMIT; Microgenics Corp., Fremont, CA) run on a Roche Cobas Miras analyzer (distributed by Dade Behring, Inc., Deerfield, IL) was used to determine urine-cotinine levels. Gas chromatography performed by an outside laboratory (LabStat, Kitchener, Ontario, Canada) confirmed urine-cotinine levels in urine specimens collected at the end-of-pregnancy, 12- (end of VBRT) and 24-week postpartum (end-of-study) assessments.
Treatment Interventions
Abstinence-monitoring schedule
Study participants chose one of the next two Mondays as their “quit” date. Beginning on the quit date, participants reported to the study clinic or were met by clinic staff at a site convenient for them to provide breath and urine samples for abstinence monitoring and to complete a timeline follow-back procedure to assess smoking frequency since their last visit 18. The monitoring schedule was the same for participants in the contingent and non-contingent vouchers conditions. In both conditions, abstinence monitoring was daily for the initial 5 days (Monday-Friday) of the cessation effort; beginning in the 2nd week monitoring decreased to twice weekly (Mondays, Thursdays) for the next 7 weeks, then once a week (Wednesdays) for 4 weeks and then every other Wednesday until delivery. During the postpartum period, monitoring was increased to once a week (Wednesdays) for the initial 4 weeks, and then biweekly (every other Wednesday) for the next 8 weeks, with abstinence monitoring ending at the end of week 12. There was no monitoring between the end of the intervention at week 12 postpartum and the final 24-week postpartum assessment.
Contingent-voucher condition
Vouchers redeemable for retail items were earned contingent on submitting breath CO specimens ≤ 6 ppm 19 during the initial five days of the cessation effort in order to detect and reinforce initial cessation efforts. Beginning in week 2 of abstinence monitoring, vouchers were delivered contingent on urine-cotinine levels ≤ 80 ng/ml 19, a criterion that required a longer duration of smoking abstinence than breath CO. Voucher delivery was independent of self-reported smoking status and based exclusively on meeting the biochemical-verification criterion. Vouchers began at $6.25, and escalated by $1.25 per consecutive negative specimen to a maximum of $45.00, where they remained barring positive test results or missed abstinence monitoring visits. Positive test results or missed visits reset the voucher value back to the original low value, but two consecutive negative tests restored the value to the pre-reset level. No cash was ever given to participants and all voucher purchases were made by clinic staff.
Non-contingent-voucher condition
In this condition, vouchers were delivered independent of smoking status. Voucher values were $15.00 per visit antepartum and $20.00 per visit postpartum, which were estimated to be sufficient to sustain participation in abstinence monitoring and to result in payment amounts that were comparable to the average earnings in the contingent condition. All else was the same as in the contingent-voucher condition.
Other services
In addition to the monitoring and voucher-based incentives mentioned above, participants received usual care for smoking cessation provided through their obstetric clinics, which typically involved provider inquiry regarding smoking status and a discussion of the advantages of quitting during pregnancy. Study staff did not attempt to influence those clinic practices. As part of the study intervention, study staff reviewed a smoking cessation pamphlet with all participants at the initial intake assessment that was designed for pregnant women 20. Those abstinent at the end-of-pregnancy assessment received a pamphlet highlighting reasons to remain abstinent postpartum 21. Participants were also informed that use of nicotine replacement products might cause urine samples to test positive for cotinine.
Ultrasound Examinations and Infant Outcomes at Delivery
Two ultrasound examinations were performed at approximately 30 and 34 weeks gestation for the purpose of estimating fetal growth. Fifty-seven women (29 contingent, 28 non-contingent) completed both examinations. Measures of fetal growth included estimates of fetal biparietal diameter, head circumference, abdominal circumference, and femur length obtained using standardized techniques by a sonographer who was blind to the participants’ treatment condition and smoking status. Head circumference, abdominal circumference and femur length were combined according to the method of Hadlock 22 to calculate estimated fetal weight. Estimates were also made of lean body mass accretion in the fetal thigh employing previously reported techniques 23. All fetal measurements were performed in triplicate and the mean value was assigned as the best estimate of the specific parameter. Serial ultrasound assessment of fetal size was obtained to generate individualized estimates of fetal growth in the mid third trimester that were compared between treatment conditions. To examine whether factors known to influence fetal growth were significantly different between the randomized conditions, we recorded maternal age, pre-pregnancy body mass index (BMI), maternal weight gain in pregnancy, 1-hour glucose screening results, illicit drug exposure, parity, infant gender, infant birth weight and gestational age at delivery from the obstetrical chart. In utero fetal growth, particularly fetal femur length and lean body mass, have been shown to be sensitive markers of the effects of maternal smoking 24–28. More generally, analysis of repeated measures, such as those generated by serial ultrasounds, provides greater statistical power relative to related measures collected at a single time point (e.g., birth weight). Much of the subject-to-subject variability in estimated growth parameters is accounted for by the initial ultrasound, providing a more sensitive analysis of growth and allowing significant differences to emerge in smaller samples.
Infant outcome measures obtained from the maternal medical record included gestational age at delivery, birth weight, and nursery admission (newborn vs. neonatal intensive care unit, NICU).
Statistical Methods
The primary analysis of smoking abstinence was based on all participants randomized with the exception of the five women who were withdrawn due to pregnancy termination/fetal demise (n = 77). Contingent and non-contingent treatment conditions were compared on participant characteristics using chi square tests for categorical measures and t-tests for continuous measures. Fisher’s Exact tests were used to compare conditions on assessment compliance at the end-of-pregnancy, 12- and 24-week postpartum assessments. The two treatment conditions were compared on both point prevalence and continuous abstinence from smoking, with missing specimens treated as smoking-positive. Point prevalence abstinence was compared between conditions at the end of pregnancy and at 12- and 24-weeks postpartum using Fisher’s Exact tests. To be declared abstinent at a given assessment, a participant had to 1) self-report no smoking, not even a puff, in the past 7 days and 2) have a urine-cotinine level of ≤ 80 ng/ml. T-tests were used to compare the two conditions on mean weeks of continuous abstinence, defined as self-report of no smoking between clinic visits and negative biochemical confirmation test results. The percentage of participants in each condition who were continuously abstinent through the entire third trimester was compared using Fisher’s Exact test. Treatment conditions were compared on fetal growth parameters for the subset of participants in which serial ultrasounds were obtained (n = 57). Growth rates were computed based on the number of days between ultrasounds and converted to weekly growth rates. Analyses of covariance were used to evaluate treatment differences in growth rates controlling for participants’ pre-pregnancy BMI. This variable was significantly different between treatment conditions and also a significant predictor of growth outcomes. Analyses of covariance were used in a similar manner to compare fetal growth between abstainers (n = 15) and smokers (n = 42) independent of treatment conditions. While the study was not powered to detect differences between treatment conditions on infant outcomes at delivery, contingent and non-contingent treatment conditions were compared using chi square tests for categorical measures and t-tests for continuous measures. Analysis of covariance was used for treatment condition comparisons of birth weight in order to adjust for the group differences in participants’ pre-pregnancy BMI. Data from two sets of twins born to participants in the contingent condition and one set of twins born to a participant in the non-contingent condition were excluded from these analyses because multiple gestation is confounded with these outcomes; additionally, data from the infant of one participant in the contingent was missing, leaving 34 and 39 infants born to participants in the contingent and non-contingent conditions, respectively. All statistical analyses were performed using SAS version 9.1 (SAS Institute, Cary, NC). Statistical significance was determined based on α =.05.
Results
Participant Characteristics
No significant differences in socio-demographics or smoking characteristics were noted between women randomized to the contingent and non-contingent voucher conditions at the study-intake assessment (Table 1).
Table 1.
Participant Characteristics
| Characteristics | Contingenta (n = 37) | Non-contingenta (n = 40) | p-value |
|---|---|---|---|
| Demographics: | |||
| Age (years) | 25.3 ± 6.1 | 23.4 ± 4.1 | .13 |
| Education (years) | 11.9 ± 2.6 | 11.8 ± 1.9 | .87 |
| % Caucasian | 89 | 98 | .14 |
| % Married | 14 | 23 | .31 |
| % Private insurance | 19 | 13 | .44 |
| % 1st pregnancy | 54 | 45 | .43 |
| Weeks pregnant at intake | 8.9 ± 2.7 | 9.5 ± 3.6 | .41 |
| Smoking Characteristics: | |||
| Cigs/day prepregnancy | 18.7 ± 8.9 | 18.4 ± 6.5 | .86 |
| Cigs/day in past 7 days | 7.9 ± 5.6 | 9.5 ± 5.9 | .24 |
| Age started smoking (years) | 13.9 ± 2.4 | 14.0 ± 2.8 | .79 |
| Intake CO (ppm) | 10.1 ± 5.6 | 11.9 ± 6.6 | .20 |
| Intake urinary cotinine (ng/ml) | 943.4 ± 562.3 | 1000.5 ± 590.4 | .67 |
| % living with other smoker(s) | 73 | 85 | .19 |
| Smoking Attitudes: | |||
| Amount want to quitb | 3.9 ± 0.4 | 3.8 ± 0.6 | .57 |
| Confidence to quitb | 3.3 ± 0.7 | 3.2 ± 0.7 | .87 |
| Intend to quit while pregnantc | 4.8 ± 0.4 | 4.8 ± 0.5 | .55 |
Note: Values represent mean ± SD, unless otherwise specified.
Treatment conditions are described in the text
Assessed by a four-point scale: 1 = none, 4 = a lot
Assessed by a five-point scale: 1 = definitely not, 5 = definitely
Assessment Compliance
Compliance with periodic assessments was relatively high (83%–95%) and not significantly different between the two treatment conditions at any assessment point.
Smoking Abstinence
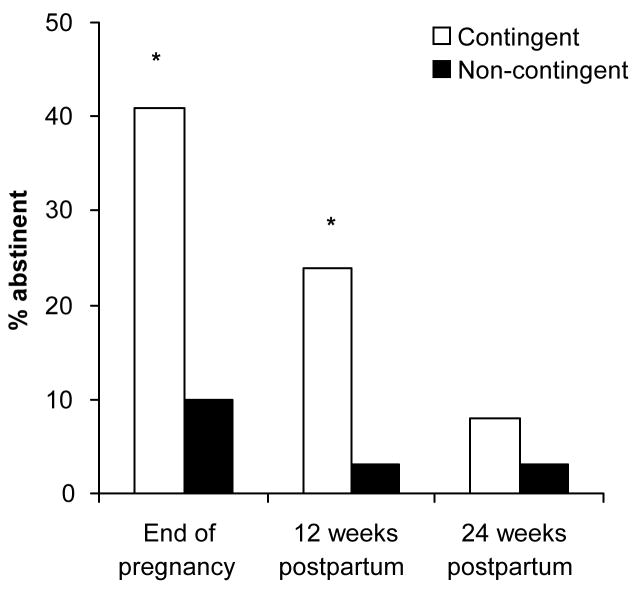
Biochemically-confirmed point-prevalence abstinence levels were significantly greater among women in the contingent than the non-contingent condition at the end of pregnancy assessment (41% vs. 10%; Fisher’s Exact test, p = .003) and at the 12-week postpartum assessment (24% vs. 3%; Fisher’s Exact test, p = .006) (Figure 1). Point-prevalence abstinence at the 24-week postpartum assessment, conducted three months after the discontinuation of the voucher program, was not significantly different (8% vs. 3%).
Figure 1.
Point-prevalence abstinence at the end of pregnancy, 12, and 24 weeks postpartum. Women in the contingent condition (n=37) received voucher-based reinforcement therapy contingent on biochemically-verified smoking abstinence, and those in the non-contingent condition (n=40), received vouchers independent of smoking status. * indicates a significant difference between conditions (p < .05).
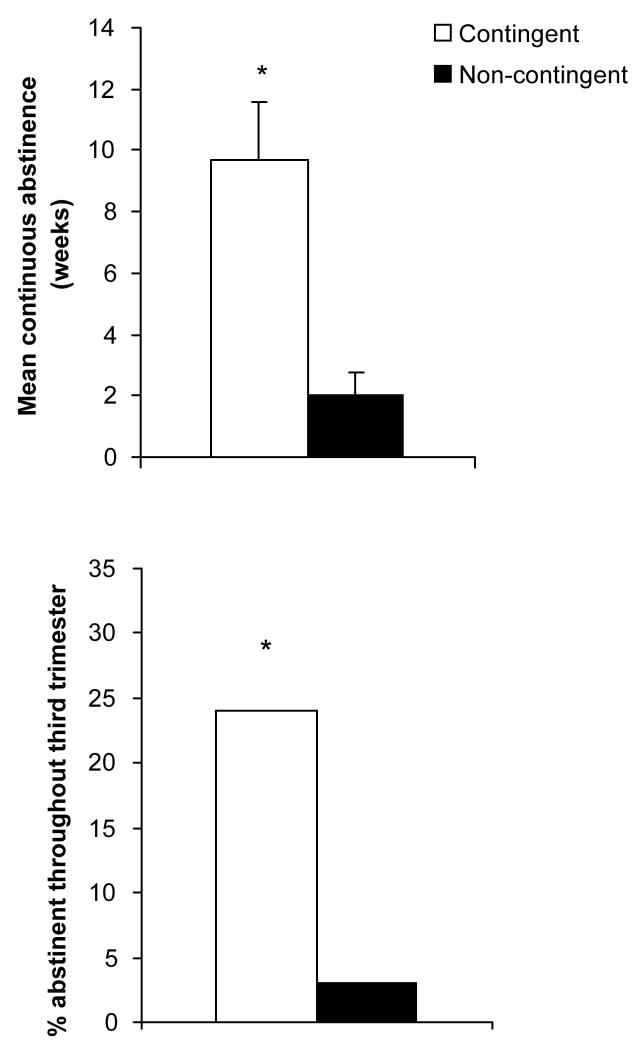
Mean weeks (±SEM) of continuous abstinence during the antepartum period was significantly greater in the contingent than the non-contingent conditions, with those in the contingent condition achieving 9.7 ± 1.9 weeks compared to 2.0 ± .8 weeks in the non-contingent condition (t(75)=3.90, p <.001; Figure 2, top panel). Additionally, a greater percentage of women in the contingent compared to the non-contingent conditions sustained abstinence throughout the third trimester, with 24% of the contingent condition maintaining abstinence through the third trimester compared to 3% in the non-contingent condition (Fisher’s Exact test, p=.005; Figure 2, bottom panel).
Figure 2.
Mean (±SEM) weeks of continuous abstinence antepartum (top panel) and percentage of participants abstinent throughout the third trimester (bottom panel) in the contingent and non-contingent conditions. See Figure 1 for description of conditions. * indicates a significant difference between conditions (p < .05).
There was no evidence that contingent participants who continued to smoke reduced their level of smoking. Mean decreases in urine cotinine levels from the intake assessment to the end-of-pregnancy assessment were 39 and 33 ng/ml in the contingent and non-contingent conditions, respectively.
Voucher earnings through 12 weeks postpartum, at which time the voucher program ended, was not significantly different between the two treatment conditions. Total mean (±SD) voucher earnings were $461 ± 456 (range: $0 – $1,180) and $413 ± 163 (range: $75 $670) in the contingent and non-contingent conditions, respectively.
The only report of the use of nicotine replacement products during the study was by one participant in the contingent condition who reported two days of gum use in the first week of the study when abstinence was determined by CO level.
Fetal Growth and Infant Outcomes at Delivery
Demographic characteristics and smoking status at the end-of-pregnancy assessment were compared between those participants who did (n=57) and did not (n = 20) complete both ultrasound examinations and no significant differences were noted. Among those who completed both ultrasound examinations, the percentage of women in each treatment condition abstinent at the end-of-pregnancy assessment was strikingly consistent with the abstinence differences observed in the total sample. In the contingent condition, 12/29 (41%) of the subset were abstinent compared to 15/37 (41%) in the total sample of women treated in that condition. In the non-contingent condition, 3/28 (11%) of the subset were abstinent compared to 4/40 (10%) in the total sample of women treated in that condition.
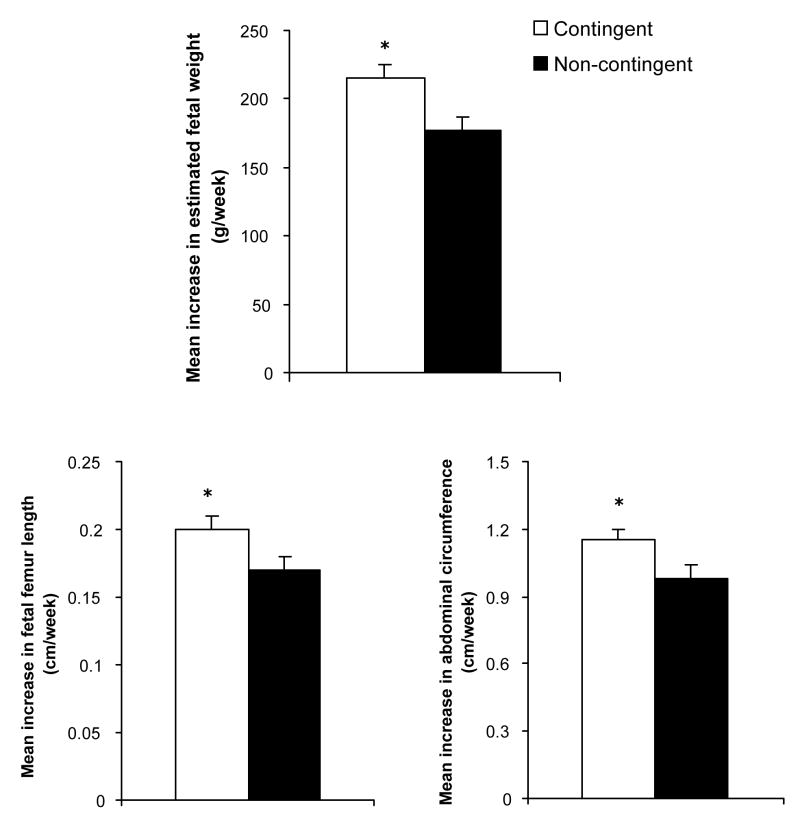
There was a significantly greater increase in estimated fetal weight in the contingent compared to the non-contingent conditions (F(1,55) = 8.4, p = .006; Table 2 & Figure 3, top panel). In addition, estimated growth rates of two of the three individual parameters used to compute estimated fetal weight, fetal femur length and fetal abdominal circumference, were also significantly greater in the contingent compared to the non-contingent conditions (F(1,54) = 4.4 and 4.1, respectively, ps ≤ .05, Table 2 & Figure 3, bottom panels). A trend towards greater growth in the contingent vs. the non-contingent condition was noted for lean thigh area (Table 2), but was not statistically different. There were no differences in head circumference or biparietal diameter (Table 2).
Table 2.
Change in Estimated Fetal Growth Measures between Serial Ultrasound Examinations
| Measure | Contingent (n = 29) | Non-contingent (n = 28) | p-value |
|---|---|---|---|
| Fetal weight (g/week) | 215.6 ± 9.1 | 177.2 ± 9.7 | .006 |
| Head circumference (cm/week) | 0.69 ± 0.04 | 0.67 ± 0.05 | .76 |
| Abdominal circumference (cm/week) | 1.15 ± 0.05 | 0.98 ± 0.06 | .05 |
| Femur length (cm/week) | 0.20 ± 0.01 | 0.17 ± 0.01 | .04 |
| Biparietal diameter (cm/week) | 0.20 ± 0.01 | 0.20 ± 0.01 | .97 |
| Lean thigh area (cm2/week) | 0.77 ± 0.05 | 0.68 ± 0.05 | .24 |
Note: Values represent least square mean ± SEM, significance based on analysis of covariance.
Figure 3.
Mean (±SEM) rates of growth in estimated fetal weight (top panel), fetal femur length (bottom left panel), and fetal abdominal circumference (bottom right panel) between ultrasound assessments conducted during the third trimester. See Figure 1 for description of conditions. * indicates a significant difference between conditions (p < .05).
When fetal growth measures were compared between abstainers and smokers independent of treatment condition, there was a significantly greater increase in estimated fetal weight (214.9 ± 13.0 vs. 190.3 ± 8.2; F(1,54) = 6.54, p = .01). A trend towards greater growth in abstainers vs. smokers was noted for abdominal circumference, but was not statistically different. There were no differences in head circumference, femur length, lean thigh area, or biparietal diameter.
Though mean birth weight was not significantly different between the two treatment conditions (Table 3), the mean difference (253 g) was greater than that estimated at either of the ultrasound examinations, supporting the pattern of divergent growth rates between conditions. Trends towards better outcomes in the contingent as compared to the non-contingent condition were also noted for the percentages of low birth weight infants and preterm births as well as mean gestational age at delivery (Table 3), but as expected, were not statistically different. There were no differences in the percentage of NICU admissions.
Table 3.
Infant Outcomes at Delivery
| Measure | Contingent (n = 34) a,b | Non-contingent (n = 39) a | p-value |
|---|---|---|---|
| Birth weight (grams) | 3355 ± 96 | 3102 ± 89 | .06c |
| % Low birth weight | 9 | 21 | .16 |
| Gestational age at delivery (weeks) | 39.1 ± 0.4 | 38.5 ± 0.3 | .27 |
| % Preterm birth | 9 | 23 | .10 |
| % NICU admissions | 12 | 15 | .74 |
Note: Values represent mean ± SEM, unless otherwise specified.
Twin data from two sets of contingent and one set of non-contingent infants were removed from these analyses.
Values for the infant of one contingent participant who delivered out-of-state and could not be contacted postpartum are missing.
Significance based on analysis of covariance.
Discussion
The antepartum abstinence levels observed in the contingent condition in the present study were more than double the abstinence rates typically reported when using efficacious interventions with lower-income pregnant smokers 3–5. These results, coupled with those from prior trials 9,10 provide compelling evidence supporting the efficacy of VBRT for promoting smoking cessation among pregnant women.
Contingent incentives were effective in establishing biochemically-verified periods of continuous smoking abstinence during the antepartum period that averaged nearly 10 weeks in duration compared to 2 weeks in the non-contingent condition. The timing of these abstinence differences are also important in that 24% of the women treated in the contingent condition abstained throughout the third trimester compared to 3% of those treated in the non-contingent condition. Previous research by us and others underscores the importance of smoking status during the third trimester to fetal growth 16,17,29.
Consistent with the results on smoking abstinence, results from the present study documented greater fetal growth in the third trimester among women in the contingent compared to the non-contingent condition. The growth effects observed in the present study are consistent with both the overall effects of smoking on fetal growth as well as evidence that smoking disproportionately affects femur length 26–28. These results also underscore the benefits of using measures of fetal growth collected by serial ultrasound examination as outcome measures in this type of research. As noted earlier, the opportunity for repeated measures afforded by the ultrasound examinations provides greater statistical power with smaller samples sizes. While we were not powered to detect differences in outcomes measured at single time points, most of the infant outcomes at delivery, especially birth weight, showed trends toward better outcomes in the contingent as compared to the non-contingent condition, but were not statistically different.
While there is relatively extensive correlational evidence showing that smoking abstinence has significant benefits on fetal growth and neonatal birth weight, there is limited experimental evidence. In a seminal experimental study on smoking cessation among pregnant women, Sexton and Hebel 30 reported significantly greater maternal smoking abstinence and neonatal birth weight in the experimental compared to the control condition. In that study, biochemically-confirmed smoking abstinence was 43% vs. 20% at the end of pregnancy and neonatal birth weight was 3,278 g vs. 3,186 g. This 92-g mean difference is consistent with the 253-g mean difference observed in the present study. We are aware of two other experimental reports in the literature examining effects of smoking cessation on neonatal birth weight that reported 71 g 31 and 218 g 32 increases in the experimental condition. Future studies using VBRT to more thoroughly examine effects on fetal growth and neonatal birth weight may be informative in terms of examining the replicability of the present findings and for gaining further understanding of how quitting smoking impacts these important outcomes.
Smoking abstinence continued at a high level into the postpartum period and the magnitude of the effect was again consistent when compared to results from our prior non-randomized trial 10. Both studies further examined abstinence after discontinuation of the voucher program (24 weeks postpartum). Smoking abstinence rates were significantly greater in the contingent compared to the non-contingent condition in the non-randomized trial, but not in the present study. While outcomes pointed to greater abstinence in the contingent condition in both studies, further investigation with a larger sample appears necessary to better estimate the magnitude of the effect after the discontinuation of incentives. Nevertheless, the results from the antepartum period are quite promising regarding the potential utility of this type of intervention. Given the substantive improvements in fetal growth observed in the present study, even short-term interventions directed at smoking cessation for the duration of the pregnancy may be very beneficial.
Two potential limitations of this study warrant comment. First, our rate of enrollment in the study (45%) is lower than that reported with other interventions in this literature 9,33,34. It is possible that the enrollment rate in the present study is an artifact of our method of recruitment. In order to enroll enough participants to meet the goals of our research protocol, we recruit in multiple clinics. Doing so introduces delay, as most potential participants are first contacted a day or two after their prenatal care appointment, and by a health care professional who is not a member of the prenatal care practice. Ideally, recruitment would occur as part of the prenatal visit and by a member of the practice. Thus, the enrollment rates observed in the present study may not be representative of the rate that could be achieved if a program were implemented by a single practice. Despite the lower enrollment rate in the present study, comparison of demographics, smoking characteristics and smoking attitudes with other reports in this literature suggest that our sample is quite comparable 9,33,34. Second, cost is an obvious practical issue with regard to incentive-based interventions. We have not yet performed a formal cost analysis. Nevertheless, the cost of incentives (an average of $334 during the antepartum period) appears reasonable when considered against the cost of caring for neonates adversely affected by cigarette smoke exposure. A recent study in the U.S. 35, for example, estimated that maternal smoking results in additional medical costs during the year after birth ranging between $1,142 and $1,358 for every woman who smokes during pregnancy. Such costs dramatically underscore the seriousness of this public health problem. The general lack of progress in promoting abstinence rates above 20% using low-cost interventions with relatively broad reach suggests that two treatment approaches may be necessary. To address this recalcitrant behavior, we may need both interventions of lower intensity and cost that have greater reach, but also interventions of higher intensity and cost with perhaps less reach such as the voucher-based intervention investigated in the present study. The latter approach is the typical approach for addressing illicit drug abuse among pregnant women and it may be more efficacious among subsets of pregnant women smokers who have typically not fared well with the lower-intensity approach. Overall, the present results are encouraging regarding the potential utility of voucher-based incentives for increasing smoking abstinence and improving fetal growth among pregnant women.
Acknowledgments
We thank Alissa Dumeer, Paula Glasmann, Natalie Jacuzzi, Luke McHale, Adrien Preston, Ed Reimann, Lauren Shapiro, Lindsay Simpson, Rachel Vitale, and Jin Yoon for assistance with data collection and entry. This research was supported by research grants R01DA14028 (STH) and GCRC MO1RR109.
References
- 1.Cnattingius S. The epidemiology of smoking during pregnancy: Smoking prevalence, maternal characteristics, and pregnancy outcomes. Nicotine Tob Res. 2004;6(Suppl 2):S125–S140. doi: 10.1080/14622200410001669187. [DOI] [PubMed] [Google Scholar]
- 2.U.S. Department of Health and Human Services. Women and smoking. A report of the Surgeon General. U.S. Department of Health and Human Services, Public Health Service, Office of the Surgeon General; Rockville, MD: 2001. [Google Scholar]
- 3.Ershoff DH, Ashford TH, Goldenberg RL. Helping pregnant women quit smoking: An overview. Nicotine Tob Res. 2004;6(Suppl 2):S101–S105. doi: 10.1080/14622200410001669204. [DOI] [PubMed] [Google Scholar]
- 4.Melvin CL, Gaffney CA. Treating nicotine use and dependence of pregnant and parenting smokers: An update. Nicotine Tob Res. 2004;6(Suppl 2):S107–S124. doi: 10.1080/14622200410001669231. [DOI] [PubMed] [Google Scholar]
- 5.Windsor R. Smoking cessation or reduction in pregnancy treatment methods: A meta- evaluation of the impact of dissemination. Am J Med Sci. 2003;326:216–222. doi: 10.1097/00000441-200310000-00013. [DOI] [PubMed] [Google Scholar]
- 6.Higgins ST, Delaney DD, Budney AJ, Bickel WK, Hughes JR, Foerg F, et al. A behavioral approach to achieving initial cocaine abstinence . Am J Psychiatry. 1991;148:1218–1224. doi: 10.1176/ajp.148.9.1218. [DOI] [PubMed] [Google Scholar]
- 7.Lussier JP, Heil SH, Mongeon JA, Badger GJ, Higgins ST. A meta-analysis of voucher-based reinforcement therapy for substance use disorders. Addiction. 2006;101:192–203. doi: 10.1111/j.1360-0443.2006.01311.x. [DOI] [PubMed] [Google Scholar]
- 8.Shoptaw S, Rotheram-Fuller E, Yang X, Frosch D, Nahom D, Jarvik ME, et al. Smoking cessation in methadone maintenance. Addiction. 2002;97:1317–1328. doi: 10.1046/j.1360-0443.2002.00221.x. [DOI] [PubMed] [Google Scholar]
- 9.Donatelle RJ, Prows SL, Champeau D, Hudson D. Randomized controlled trial using social support and financial incentives for high risk pregnant smokers: Significant other support (SOS) program. Tob Control. 2000;9:iii67–iii69. doi: 10.1136/tc.9.suppl_3.iii67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Higgins ST, Heil SH, Solomon L, Plebani Lussier J, Abel R, Lynch ML, et al. A pilot study on voucher-based incentives to promote abstinence from cigarette smoking during pregnancy and postpartum. Nicotine Tob Res. 2004;6:1015–1020. doi: 10.1080/14622200412331324910. [DOI] [PubMed] [Google Scholar]
- 11.Higgins ST, Badger GJ, Budney AJ. Initial abstinence and success in achieving longer-term cocaine abstinence. Exp Clin Psychopharmacol. 2000;8:377–386. doi: 10.1037//1064-1297.8.3.377. [DOI] [PubMed] [Google Scholar]
- 12.Higgins ST, Heil SH, Dumeer AM, Thomas CS, Solomon LJ, Bernstein IM. Smoking status in the initial weeks of quitting as a predictor of smoking-cessation outcomes in pregnant women. Drug Alcohol Depend. 2006;85:138–141. doi: 10.1016/j.drugalcdep.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 13.Higgins ST, Wong CJ, Badger GJ, Ogden DE, Dantona RL. Contingent reinforcement increases cocaine abstinence during outpatient treatment and 1 year of follow-up. J Consult Clin Psychol. 2000;68:64–72. doi: 10.1037//0022-006x.68.1.64. [DOI] [PubMed] [Google Scholar]
- 14.Roll JM, Higgins ST. A within-subject comparison of three different schedules of reinforcement of drug abstinence using cigarette smoking as an exemplar. Drug Alcohol Depend. 2000;58:103–109. doi: 10.1016/s0376-8716(99)00073-3. [DOI] [PubMed] [Google Scholar]
- 15.Mullen PD. How can more smoking suspension during pregnancy become lifelong abstinence? Lessons learned about predictors, interventions, and gaps in our accumulated knowledge. Nicotine Tob Res. 2004;6(Suppl 2):S217–S238. doi: 10.1080/14622200410001669150. [DOI] [PubMed] [Google Scholar]
- 16.Bernstein IM, Mongeon MA, Badger GJ, Solomon L, Heil SH, Higgins ST. Maternal smoking and its association with birth weight. Obstet Gynecol. 2005;106:986–991. doi: 10.1097/01.AOG.0000182580.78402.d2. [DOI] [PubMed] [Google Scholar]
- 17.MacArthur C, Knox EG. Smoking in pregnancy: Effects of stopping at different stages. Br J Obstet Gynaecol. 1988;95:551–555. doi: 10.1111/j.1471-0528.1988.tb09481.x. [DOI] [PubMed] [Google Scholar]
- 18.Brown RA, Burgess ES, Sales SD, Whitley JA, Evans DM, Miller IW. Reliability and validity of a smoking timeline follow-back interview. Psychol Addict Behav. 1998;12:101–112. [Google Scholar]
- 19.Higgins ST, Heil SH, Badger GJ, Mongeon J, Solomon L, McHale L, et al. Biochemical verification of smoking status in pregnant and recently postpartum women . Exp Clin Psychopharmacol. 2007;15:58–66. doi: 10.1037/1064-1297.15.1.58. [DOI] [PubMed] [Google Scholar]
- 20.Secker-Walker RH, Solomon LJ, Flynn BS, Skelly JM, Mead PB. Reducing smoking during pregnancy and postpartum: Physician’s advice supported by individual counseling. Prev Med. 1998a;27:422–430. doi: 10.1006/pmed.1998.0287. [DOI] [PubMed] [Google Scholar]
- 21.Secker-Walker RH, Solomon LJ, Flynn BS, Skelly JM, Mead PB. Smoking relapse prevention during pregnancy: A trail of coordinated advice from physicians and individual counseling. Am J Prev Med. 1998b;15:25–31. doi: 10.1016/s0749-3797(98)00029-4. [DOI] [PubMed] [Google Scholar]
- 22.Hadlock FP, Harrist R, Sharman RS, Deter RL, Park SK. Estimation of fetal weight with the use of head, body, and femur measurements a prospective study. Am J Obstet Gynecol. 1985;151:333–337. doi: 10.1016/0002-9378(85)90298-4. [DOI] [PubMed] [Google Scholar]
- 23.Bernstein IM, Goran MI, Amini S, Catalano PM. Differential growth of fetal tissues in the second half of gestation. Am J Obstet Gynecol. 1997;176:28–32. doi: 10.1016/s0002-9378(97)80006-3. [DOI] [PubMed] [Google Scholar]
- 24.Bernstein IM, Plociennik K, Stahle S, Badger GJ, Secker-Walker R. The impact of maternal smoking on fetal growth and body composition. Am J Obstet Gynecol. 2000;183:883–7. doi: 10.1067/mob.2000.109103. [DOI] [PubMed] [Google Scholar]
- 25.Lindsay CA, Thomas AJ, Catalano PM. The effect of smoking tobacco on neonatal body composition. Am J Obstet Gynecol. 1997;177:1124–8. doi: 10.1016/s0002-9378(97)70027-9. [DOI] [PubMed] [Google Scholar]
- 26.D’Souza SW, Black P, Richards B. Smoking in pregnancy: associations with skinfold thickness, maternal weight gain, and fetal size at birth. Brit Med J. 1981;282:1661–3. doi: 10.1136/bmj.282.6277.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harrison GG, Branson RS, Vaucher YE. Association of maternal smoking with body composition of the newborn. Am J Clin Nutr. 1983;38:757–62. doi: 10.1093/ajcn/38.5.757. [DOI] [PubMed] [Google Scholar]
- 28.Luciano A, Bolognani M, Biondani P, Ghizzi C, Zoppi G, Signori E. The influence of maternal passive and light active smoking on intrauterine growth and body composition of the newborn. Eur J Clin Nutr. 1998;52:760–3. doi: 10.1038/sj.ejcn.1600643. [DOI] [PubMed] [Google Scholar]
- 29.Lieberman E, Gremy I, Lang JM, Cohen AP. Low birthweight at term and the timing of fetal exposure to maternal smoking. Am J Public Health. 1994;84:1127–1131. doi: 10.2105/ajph.84.7.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sexton M, Hebel JR. A clinical trial of change in maternal smoking and its effect on birth weight. JAMA. 1984;251:911–915. [PubMed] [Google Scholar]
- 31.Hjalmarson AIM, Hahn L, Svanberg B. Stopping smoking in pregnancy: Effect of a self-help manual in controlled trial. Br J Obstet Gynaecol. 1991;98:260–264. doi: 10.1111/j.1471-0528.1991.tb13390.x. [DOI] [PubMed] [Google Scholar]
- 32.Ershoff DH, Aaronson NK, Danaher BG, Wasserman FW. Behavioral, health, and cost outcomes of an HMO-based prenatal health education program. Public Health Rep. 1983;98:536–547. [PMC free article] [PubMed] [Google Scholar]
- 33.Emmons KM, Sorenson G, Klar N, Digianni L, Barclay G, Schmidt K, et al. Healthy Baby Second-Hand Smoke Study: Project brief. Tob Control. 2000;9:iii58–iii60. doi: 10.1136/tc.9.suppl_3.iii58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Solomon LJ, Secker-Walker RH, Flynn BS, Skelly JM, Capeless EL. Proactive telephone peer support to help pregnant women stop smoking . Tob Control. 2000;9:iii72–iii74. doi: 10.1136/tc.9.suppl_3.iii72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miller DP, Villa KF, Hogue SL, Sivapathasundaram D. Birth and first-year costs for mothers and infants attributable to maternal smoking. Nicotine Tob Res. 2001;3:25–35. doi: 10.1080/14622200020032079. [DOI] [PubMed] [Google Scholar]