Abstract
Autism spectrum disorders (ASD) impact social functioning and communication, and individuals with these disorders often have restrictive and repetitive behaviors. Accumulating data indicate that ASD is associated with alterations of neural circuitry. Functional MRI (FMRI) studies have focused on connectivity in the context of psychological tasks. However, even in the absence of a task, the brain exhibits a high degree of functional connectivity, known as intrinsic or resting connectivity. Notably, the default network, which includes the posterior cingulate cortex, retro-splenial, lateral parietal cortex/angular gyrus, medial prefrontal cortex, superior frontal gyrus, temporal lobe, and parahippocampal gyrus, is strongly active when there is no task. Altered intrinsic connectivity within the default network may underlie offline processing that may actuate ASD impairments. Using FMRI, we sought to evaluate intrinsic connectivity within the default network in ASD. Relative to controls, the ASD group showed weaker connectivity between the posterior cingulate cortex and superior frontal gyrus and stronger connectivity between the posterior cingulate cortex and both the right temporal lobe and right parahippocampal gyrus. Moreover, poorer social functioning in the ASD group was correlated with weaker connectivity between the posterior cingulate cortex and the superior frontal gyrus. In addition, more severe restricted and repetitive behaviors in ASD were correlated with stronger connectivity between the posterior cingulate cortex and right parahippocampal gyrus. These findings indicate that ASD subjects show altered intrinsic connectivity within the default network, and connectivity between these structures is associated with specific ASD symptoms.
Autism spectrum disorders (ASD), a set of debilitating neurodevelopmental conditions that impact social functioning, communication and are associated with restricted and repetitive interests and activities (APA, 1994), are often described as disorders of altered brain connectivity (Belmonte et al., 2004; Courchesne et al., 2007; Just et al., 2007; Koshino et al., 2008). Whereas many functional MRI (FMRI) studies reported that ASD is associated with weaker connectivity between various structures (Villalobos et al., 2005; Welchew et al., 2005; Kana et al., 2006; Just et al., 2007; Kleinhans et al., 2008; Koshino et al., 2008), other studies documented that ASD subjects have regions of stronger connectivity (Mizuno et al., 2006; Turner et al., 2006). Thus, the neural circuitry of ASD appears to manifest as both weaker and stronger connectivity. However, it is unclear what situations give rise to these different forms of altered functioning.
Although almost all functional neuroimaging investigations of ASD have focused on the examination of the brain response to specific cognitive or affective events (e.g., the presentation of faces or words), it is well established that the brain generates patterns of brain activation independent of specific external stimuli (Buckner and Vincent, 2007; Raichle and Snyder, 2007). These patterns of brain activation are called intrinsic, spontaneous or resting activation. Over the last decade, investigations of intrinsic activation have primarily focused on the default network, a set of neural structures that are highly active in the absence of a task (Gusnard et al., 2001; Raichle et al., 2001). Structures of the default mode include the posterior cingulate cortex, retro-splenial, lateral parietal cortex/angular gyrus, medial prefrontal cortex, superior frontal gyrus, temporal lobe, and the parahippocampal gyrus (Shulman et al., 1997; Greicius et al., 2003; Fox et al., 2005).
Activation of the default mode is evident not only when subjects are alert, but also during sleep and under anesthesia (Vincent et al., 2007; Horovitz et al., 2008). Moreover, intrinsic activation consumes much more energy than what is documented in response to tasks (Raichle and Mintun, 2006). For these reasons, it has been proposed that intrinsic activation is not limited to spontaneous thought processes (Raichle and Snyder, 2007). Instead, intrinsic activation may relate more to fundamental aspects of the central nervous system, such as maintaining balance between excitatory and inhibitory inputs, mentalizing about hypothetical events or low-level monitoring the external environment (Raichle and Snyder, 2007; Buckner, Andrews-Hanna and Schacter, 2008).
Little work has been done to examine intrinsic connectivity and the default network in ASD. Understanding how intrinsic connectivity differs in ASD will help to provide a more complete picture of the brain correlates of the disorder. Specifically, altered connectivity may emerge exclusively during tasks in which ASD subjects are impaired (e.g., face recognition). By examining intrinsic connectivity, it is possible to determine whether ASD subjects show altered brain function more generally (i.e., even in the absence of a task). In addition, linking alterations in the default mode to specific impairments may help to delineate the functions of this network. To date, two published studies have examined intrinsic connectivity in ASD in the absence of a task. The first study conducted a connectivity analysis of a large cohort of subjects during brief rest periods between blocks of tasks (Cherkassky et al., 2006). They found that both the control and ASD groups activated the default network, but the ASD group showed weaker connectivity throughout the brain. Recently, using a procedure that is more typically used for examining the default network in which subjects lay in the MRI without performing a task for a prolonged period, Kennedy and Courchesne found that ASD subjects showed less connectivity with two areas of the default network, the medial prefrontal cortex and the angular gyrus (Kennedy and Courchesne, 2008). Other regions were equivalent between groups.
In the present study, our goal was to further evaluate the default network in ASD. The procedures we used were similar to investigations of nonimpaired individuals (Greicius et al., 2003; Fox et al., 2005). Specifically, we monitored the default network for 10 minutes as subjects lay in the MRI while not performing a task. In addition, unlike the one other study that examined the default network over a sustained period of time (Kennedy and Courchesne, 2008), the present study used a single seed for evaluating the default network rather than averaging multiple seeds. Based on seminal studies of the default mode, we used a seed in the posterior cingulate cortex (Shulman et al., 1997; Fox et al., 2005). This seed was found to most effectively reveal connectivity in the default network (Fox et al., 2005). By using a single seed, it is possible to more precisely determine where subjects with ASD show altered connectivity. In addition, our secondary goal was to examine how the severity of the specific symptoms of ASD (social impairment, language/communication deficits, and restricted and repetitive behaviors) maps onto components of the default network. Since ASD is a heterogeneous set of disorders (Happe, Ronald and Plomin, 2006), correlating specific symptoms to brain function may help to establish a more precise connection between behavior and brain in this population.
In order to investigate our primary hypothesis, we followed the findings of prior ASD connectivity studies and predicted that relative to the control group, the ASD group would show altered connectivity within the default network and that the altered connectivity would manifest as both weaker and stronger coupling between the posterior cingulate cortex and other regions in the default network. We also tested an exploratory hypothesis in which we predicted that specific core features of ASD (social impairment, restricted and repetitive behaviors, and communication) would be associated with altered connectivity between specific structures of the default network.
METHODS
Participants
Twelve adults with ASD and 12 comparison adults participated in the study. Table 1 provides details about the subject characteristics. Participants were recruited for the study through the University of Michigan Autism and Communication Disorders Center and through posted flyers. An ASD diagnosis was determined based on the Autism Diagnostic Observation Schedule (Lord et al., 2000), the Autism Diagnostic Interview-Revised (ADI-R) (Lord, Rutter and Le Couteur, 1994) and clinical consensus (Lord et al., 2006). The University of Michigan Institutional Review Board approved the procedures and all participants signed consents. Seven participants were diagnosed with autism, 2 with Asperger’s and 3 with pervasive developmental disorder not otherwise specified. All participants were given the Peabody Picture Vocabulary Test (Dunn and Dunn, 1997) and the Ravens Progressive Matrices (Raven, 1960) to evaluate cognitive functioning. Exclusionary criteria were nonverbal cognitive functioning below 85, another neurological disorder that existed, or the subject had braces. There were no significant group differences in age, verbal/nonverbal measures of cognitive function, gender, or handedness. Eleven ASD subjects received psychotropic medications (five on selective serotonin reuptake inhibitors, four on stimulants, two on neuroeleptics, one on a tricyclic, and one on a benzodiazepine). As described in the results, the medications were not found to influence the findings. None of the comparison subjects were on psychotropic medications.
Table 1.
Subject characteristics.
| ASD | Control | |||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Age in years | 26 | 5.93 | 27 | 6.1 |
| Cognitive function (verbal) | 117 | 13.67 | 110 | 18.00 |
| Cognitive function (nonverbal) | 119 | 13.68 | 118 | 13.41 |
| Male: female | 11:1 | 10:2 | ||
| Handedness left:right | 0:12 | 1:11 | ||
FMRI Data Acquisition
FMRI data were collected from a 3 Tesla GE Signa scanner at the University of Michigan. A reverse spiral sequence (Glover and Law, 2001) (TR=2000 ms, TE=30 ms, flip angle=90°, FOV=22 cm, 64×64 matrix, 40 contiguous axial 3 mm slices) was used to collect 300 T2*-weighted BOLD images. Slices were prescribed parallel to the AC-PC line. For the structural images, a 3D T1 axial overlay (TR=8.9, TE=1.8, flip angle=15°, FOV=26 cm, slice thickness=1.4 mm, 124 slices; matrix=256 ×160) was acquired for anatomical localization. To facilitate normalization, a 110 sliced (sagittal) inversion-prepped T1-weighted anatomical image using spoiled gradient-recalled acquisition in steady state (SPGR) imaging (flip angle=15°, FOV=26 cm, 1.4 mm slice thickness) was acquired.
FMRI Procedures
A visual fixation cross was presented to the subject using a rear projection visual display. Participants were instructed to keep their eyes centered on the cross and to not think about anything in particular. Duration of data collection was 10 minutes. A pressure belt was placed around the abdomen of each subject to monitor respiratory signal. A pulse oximeter was placed on the subject’s finger to monitor cardiac signal. The respiratory, cardiac and fMRI data collection were synchronized.
FMRI Data Analysis
The acquired functional MRI data were preprocessed as part of the standard processing stream at the University of Michigan. First, k-space outliers in the raw data greater than two standard deviations from the mean were replaced with the average of their temporal neighbors. Second, images were reconstructed using field map correction to remove distortions from magnetic field inhomogeneity. Third, physiological variations in the data from the cardiac and respiratory rhythms were removed using a regression analysis (Glover, Li and Ress, 2000). This approach removed the effects of the first and second order harmonics of the externally collected physiological waveforms. Fourth, slice timing differences were then corrected using local sinc interpolation (Oppenheim, Schafer and Buck, 1999). Finally, we used MCFLIRT in FMRIB Software Library (Jenkinson et al., 2002) to perform motion correction (using the 10th image volume as the reference). For all subjects, motion was less than 3 mm in the x y or z direction. There were no group differences in motion.
The preprocessed data were then normalized to MNI space using SPM2 (Wellcome Department of Cognitive Neurology, London, UK; http://www.fil.ion.ucl.ac.uk), by registering the high-resolution T1 anatomical images to the functional images, then finding the transformation to align the T1 to the MNI template, and finally applying this transformation to the functional data.
The following procedures were used to generate functional connectivity images (low frequency timecourse correlation maps). First, the data were low-pass filtered with a 0.08 Hz cutoff frequency in order to examine the frequency band of interest, and to exclude higher frequency sources of noise (Biswal et al., 1995). Second, following previous work (Shulman et al., 1997; Fox et al., 2005), the seed region of the posterior cingulate cortex was centered at −5 −49 40 Talairach-Tournoux space and converted to MNI space with coordinates of −5 −53 41. A four voxel square on the axial plane was placed around it for the seed. Third, the timecourse of the seed was unit normalized to remove differences in variance between subjects. Fourth, the average seed region timecourse in the filtered data was correlated with all other low-pass filtered pixels to form functional connectivity maps for each subject.
Z scores from each subject were carried forward to the group-level random effects analysis, which was carried out using SPM2. Summary images contrasting the difference between the two groups were then generated. Regions of interest were defined using PickAtlas (Maldjian et al., 2002).
We performed the following steps to protect against inflating the risk of a type 1 error due to multiple comparisons. First, we evaluated the default network in the control and ASD groups separately using a threshold of P < .05 family-wise error correction. Specifically, using the posterior cingulate cortex, we examined resting connectivity with the 11 other regions of the default network (right retro-splenial, lateral parietal cortex/angular gyrus [left/right], medial prefrontal cortex [left/right], superior frontal gyrus [left/right], temporal lobe [left/right], and the parahippocampal gyrus [left/right]). Second, to establish whether a given region within the default network showed significant group differences in activation, we performed a correction by dividing the .05 error by 11 (the number of regions in the default network) yielding a p value of .0045.
For the analysis of symptom severity within the ASD group, only default network regions where significant group differences were found were considered. Thus, to control for multiple comparisons, the P value of .05 was divided by the number of regions found to be significant in the analysis of group differences.
RESULTS
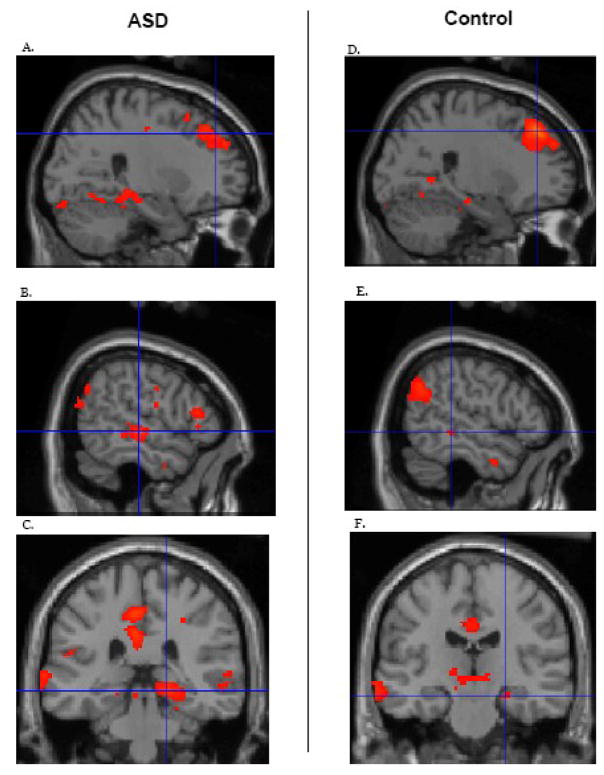
Both the ASD and control groups separately showed functional connectivity that is comparable to what was reported in prior investigations of the default network (Shulman et al., 1997; Greicius et al., 2003; Fox et al., 2005) (Figure 1). Using a conservative threshold of P = .05 family-wise error (FWE) correction, connectivity in both groups was evident in the posterior cingulate cortex, retro-splenial, lateral parietal cortex (bilateral), medial prefrontal cortex (bilateral), superior frontal cortex (bilateral), temporal lobe (bilateral), and the parahippocampal gyrus (bilateral).
Figure 1.
Functional connectivity within specific regions of the default network for the ASD (left) and control (right) groups separately. All other regions of the default network also showed significant connectivity. Statistics are provided in Table 2. Figure 1A shows superior frontal gyrus activation in the ASD group, xyz coordinates 26 42 40. Figure 1B depicts temporal lobe activation in the ASD group xyz coordinates, 56 −22 −2. Figure 1C illustrates parahippocampal gyrus activation in the ASD group, xyz coordinates, 18 −32 −8. Figure 1D shows superior frontal gyrus activation in the control group, xyz coordinates 24 38 44. Figure 1E illustrates temporal lobe activation in the control group, xyz coordinates, 54 −36 −4. Finally, figure 1F shows parahippocampal gyrus activation in the control group, xyz coordinates, 24 −20 −16. The threshold was set at p < .05 FWE. For this and subsequent figures, the brain images are presented in neurological space.
To test the primary hypothesis that the ASD relative to the control group would show altered intrinsic connectivity within the default network, we performed t-tests between groups using the threshold described in the methods. Relative to the ASD group, the control group showed stronger connectivity between the posterior cingulate cortex (the seed) and the right superior frontal gyrus (Figure 2; Table 2). Relative to the control group, the ASD group showed stronger connectivity between the posterior cingulate cortex and the right temporal lobe and the posterior cingulate cortex and the right parahippocampal gyrus (Figure 3; Table 2).
Figure 2.
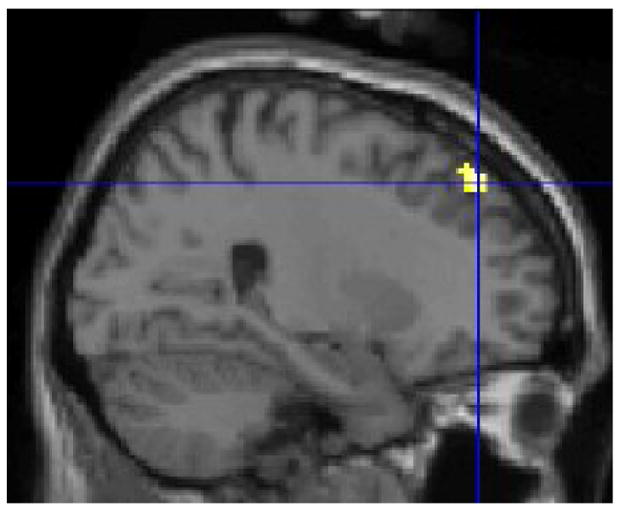
The control group relative to the ASD group showed stronger positive functional connectivity in the right superior frontal gyrus, t(22) = 3.36 p = .001, xyz 26 42 46. For the illustration, the threshold was set at p = .01 with a minimum cluster size of 50 voxels. The figure depicts voxels showing significant correlation with the seed and the voxels showing a significant difference in correlation between subject groups.
Table 2.
Functional connectivity in the default network in regions where group differences were found. Threshold was p < .05 FWE within subject groups and p < .0045 based on the correction for between subject groups. Minimum cluster size reported was 10 when p value set at .05 for group differences.
| Region | Comparison | x y z | t | Cluster size | Brodmann Area |
|---|---|---|---|---|---|
| Right superior frontal gyrus | ASD | 4 60 −8 | 9.36 | 19 | 10 |
| 26 42 40 | 8.41 | 194 | 9 | ||
| 2 32 50 | 8.39 | 94 | 8 | ||
| 4 54 28 | 8.17 | 26 | 9 | ||
| 30 18 58 | 7.88 | 32 | 8 | ||
| Control | 24 38 44 | 12.35 | 700 | 8 | |
| 4 60 2 | 8.25 | 20 | 10 | ||
| 4 28 56 | 8.12 | 80 | 8 | ||
| 30 20 56 | 7.11 | 27 | 8 | ||
| ASD-Control | ns | ||||
| Controls-ASD | 26 42 46 | 3.36 | 114 | 8 | |
| Right temporal lobe | ASD | 56 −22 −2 | 9.10 | 308 | 21 |
| 48 −68 22 | 8.11 | 140 | 39 | ||
| 46 −56 16 | 6.79 | 20 | 22 | ||
| Control | 50 −56 28 | 9.40 | 186 | 39 | |
| 48 4 −30 | 8.14 | 63 | 21 | ||
| 54 −36 −4 | 7.37 | 13 | 21 | ||
| ASD-Control | 54 −20−2 | 3.56 | 265 | 41 | |
| Controls-ASD | ns | ||||
| Right parahippocampal gyrus | ASD | 18 −32 −8 | 9.52 | 316 | 30 |
| Control | 12 −46 2 | 7.71 | 118 | 30 | |
| 24 −20 −16 | 7.49 | 14 | 28 | ||
| 18 −50 −10 | 7.29 | 13 | 19 | ||
| ASD-Control | 18 −28 −12 | 3.66 | 62 | 35 | |
| Control-ASD | ns |
Figure 3.
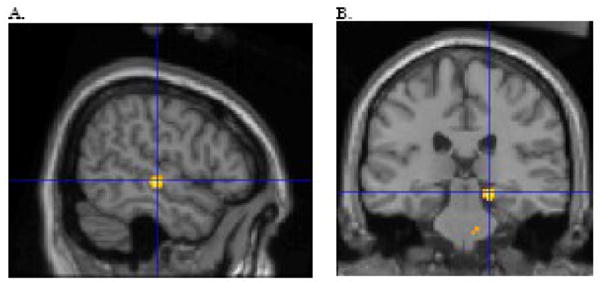
The ASD relative to the control group showed stronger functional connectivity in the right temporal lobe, t(22) = 3.56 p = .001, xyz 54 −20 −2 (A) and the right parahippocampal gyrus, t(22) = 3.66 p = .001, xyz 18 −28 −12 (B). For the illustration, the threshold was set at p = .01 with a minimum cluster size of 50 voxels. The figure depicts voxels showing significant correlation with the seed and the voxels showing a significant difference in correlation between subject groups.
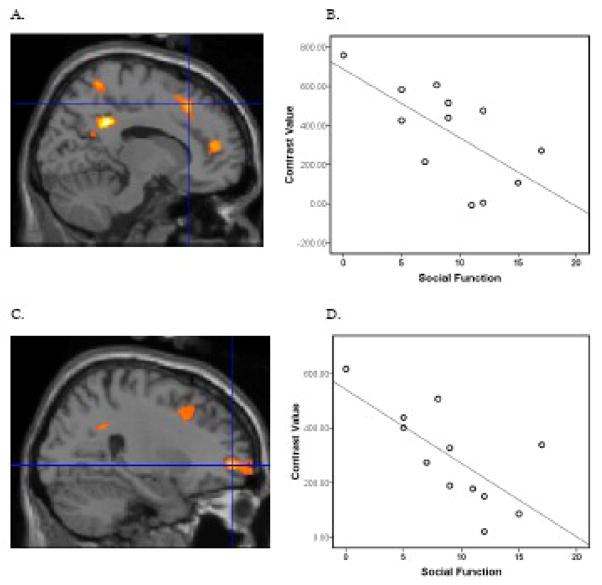
To test the exploratory hypothesis that altered intrinsic connectivity in the ASD group would correlate with symptoms, we performed regression analyses of symptom scores and the structures where group differences were found (right superior frontal gyrus, right temporal lobe and the right parahippocampal gyrus). As described above, we controlled for multiple comparisons by dividing the P value of .05 by the number of regions found to be significant in the analysis of group differences (3), yielding a p value of .017. Social function, as evaluated with the ADI-R measure of total reciprocal social interaction (current), was negatively associated with intrinsic connectivity between the posterior cingulate cortex and the right superior frontal gyrus, t(10)=3.02, p = .006, xyz coordinates 14 26 48 (Figure 4) and t(10)=3.37, p = .004, xyz coordinates 26 54 −2 (Figure 4). More severely compromised social function was associated with weaker connectivity between these structures. Severity of restricted and repetitive behaviors based on the ADI-R measure of total restricted, repetitive and stereotyped patterns of behavior was positively associated with connectivity of the posterior cingulate cortex and the right parahippocampal gyrus, t(10)=3.92, p = .001, xyz coordinates 26, −10 −28 (Figure 5). The more extreme the restricted and repetitive behaviors score the stronger the connectivity between these structures. None of the measures of ASD symptoms (social impairment, communication deficits (verbal and nonverbal), and restricted/repetitive behaviors) was associated connectivity between the posterior cingulate cortex and the right temporal lobe.
Figure 4.
Within the ASD group, social functioning based on the ADI-R measure of total reciprocal social interaction (current), was negatively correlated with functional connectivity with two areas of the superior frontal gyrus, t(10)=3.02, p = .006, xyz coordinates 14 26 48 (A) and t(10)=3.37, p = .004, xyz coordinates 26 54 −2 (C). A higher score in the ADI-R measure indicates worse social function. For Figure 4A and 4C, the threshold was set at p = .05 with a minimum cluster size of 50 voxels. To illustrate this association, contrast values were extracted from a 4 mm sphere around the peak activation and plotted with the ADI-R measure of social function, Pearson r = −.66, P = .019 for xyz coordinates 14 26 48 (B) and Pearson r= −.707 P = .008 and for xyz coordinates 26 54 −2 (D). Although other areas correlated with social function, analyses focused on areas of the default network where group differences were found.
Figure 5.
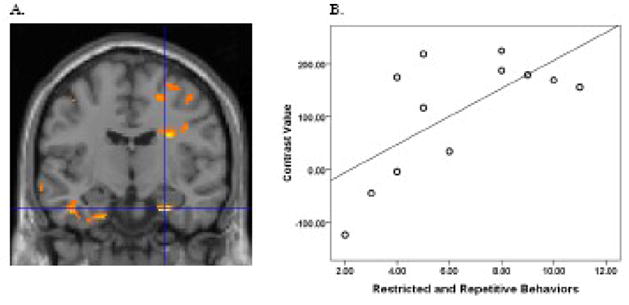
For subjects with ASD, severity of restricted and repetitive behaviors based on the ADI-R measure of total restricted, repetitive and stereotyped patterns of behavior positively correlated with functional connectivity in the right parahippocampal gyrus, t(10)=3.92, p = .001, xyz coordinates 26 54 −2 (A). A higher score in the ADI-R measure indicates worse restricted and repetitive behaviors. For Figure 5A, the threshold was set at p = .05 and a minimum cluster size of 20 voxels. To illustrate this association, contrast values were extracted from a 4 mm sphere around the peak activation and plotted with the ADI-R measure of restricted and repetitive behaviors, Pearson r = .67, P = .016 for xyz coordinates 26 54 −2 (B). As in Figure 4, analyses focused on areas of the default network where group differences were found.
Examination of medication
To determine whether specific classes of medications influenced the results, ASD subjects who received each class were removed and the remaining ASD subjects were compared to controls to evaluate whether the connectivity effects were still present. First, when the 5 ASD subjects who received selective serotonin reuptake inhibitors were removed, the finding that the control relative to the ASD group showed stronger connectivity in the right superior frontal gyrus remained, t(17) = 3.25, p = .002 and the ASD relative to the controls still showed stronger connectivity in the right temporal lobe, t(17) = 3.16, p = .003 and the right parahippocampal gyrus, t(17) = 4.08, p < .001.
Second, when the four ASD subjects who received stimulants were removed from the analysis, the finding that the control relative to the ASD group showed stronger connectivity in the right superior frontal gyrus remained, t(18) = 2.73, p = .008. In addition, the ASD relative to the control group continued to show stronger connectivity in the right temporal lobe, t(18) = 2.63, p = .008 and the right parahippocampal gyrus, t(18) = 3.08, p = .004.
Third, when the two ASD subjects taking neuroleptics were removed and the group differences remained. Specifically, relative to the ASD group, controls showed stronger connectivity between the posterior cingulate cortex and right superior frontal gyrus, t(20) = 3.47, p = .001. Moreover, the ASD relative to the control group still showed stronger connectivity between the posterior cingulate cortex and the right temporal lobe t(20) = 3.40, p = .001, and the posterior cingulate cortex and right parahippocampal gyrus, t(20) = 3.96, p < .001.
Fourth, the two ASD subjects on bupropion were removed and group differences were still evident. Relative to the ASD group, the control group showed stronger connectivity between the posterior cingulate cortex and right superior frontal gyrus, t(20) = 3.05, p = .003. In addition, compared to the controls, subjects with ASD showed stronger connectivity between the posterior cingulate cortex and the right temporal lobe t(20) = 3.31, p = .002, and the posterior cingulate cortex and right parahippocampal gyrus, t(20) = 3.68, p = .001.
Fifth, when the one ASD subject on a tricyclic was removed, the group differences remained. Relative to the ASD group, the control group showed stronger connectivity between the posterior cingulate cortex and right superior frontal gyrus, t(21) = 3.07, p = .003. In addition, compared to the controls, subjects with ASD showed stronger connectivity between the posterior cingulate cortex and the right temporal lobe t(21) = 3.27, p = .002, and the posterior cingulate cortex and right parahippocampal gyrus, t(21) = 3.69, p = .001.
Finally, when the one ASD subject who was receiving a benzodiazepine was removed, the group differences were still apparent. Compared to the ASD group, the control group showed stronger connectivity between the posterior cingulate cortex and right superior frontal gyrus, t(21) = 3.52, p = .001. In addition, compared to the controls, subjects with ASD showed stronger connectivity between the posterior cingulate cortex and the right temporal lobe t(21) = 3.59, p = .001, and the posterior cingulate cortex and right parahippocampal gyrus, t(21) = 3.80, p = .001.
DISCUSSION
The present study examined differences between the ASD and control groups in default network intrinsic functional connectivity. Both groups showed robust connectivity throughout the default network. However, relative to controls, the ASD subjects showed alterations in functional connectivity. Specifically, the ASD group showed weaker connectivity than controls between the posterior cingulate cortex and the right superior frontal gyrus. In addition, the ASD group showed stronger connectivity relative to the control group between the posterior cingulate cortex and two areas: the right temporal lobe and the right parahippocampal gyrus. Moreover, in an exploratory analysis, the extent of altered connectivity within the ASD group was associated with core ASD symptoms. Within the ASD group, poorer social functioning was associated with weaker connectivity between the posterior cingulate cortex and the right superior frontal gyrus. Finally, more severe restricted and repetitive behaviors were associated with stronger connectivity between the posterior cingulate cortex and the right parahippocampal gyrus.
Unlike the present work that examined functional connectivity at rest, there are a growing number of studies that documented functional connectivity as ASD subjects performed specific tasks. Many of these task-driven FMRI studies found that subjects with ASD had weaker connectivity relative to controls (Villalobos et al., 2005; Welchew et al., 2005; Kana et al., 2006; Just et al., 2007; Kleinhans et al., 2008; Koshino et al., 2008), but others showed that ASD is associated with stronger connectivity between regions (Mizuno et al., 2006; Turner et al., 2006). The present study extends these findings by showing that ASD subjects demonstrate both weaker and stronger connectivity during rest.
Two other published studies examined functional connectivity during rest in an ASD sample. The first study evaluated a large sample of individuals (57 with ASD and 57 controls) during multiple 24 second rest periods between blocks of tasks (Cherkassky et al., 2006). Relative to the control group, the ASD group showed weaker connectivity between the ventral anterior cingulate cortex and the posterior cingulate and the ventral anterior cingulate cortex and the precuneus. Moreover, when connectivity between the parahippocampal gyrus and the other structures of the default network was averaged together, the ASD group showed overall weaker connectivity. The discrepancy in findings between the present study and Cherkassky et al (2006) may be due to differences in the procedures. Specifically, whereas the present study acquired resting connectivity data over a sustained period of time (10 minutes), Cherkassky et al examined connectivity during multiple 24 second periods of rest between blocks of a task. Further work is necessary to understand the functioning of the default network over long versus short periods of time.
In the second study that examined ASD functional connectivity during rest, Kennedy and Courchesne (2008) acquired FMRI data over a sustained period of time (7 min, 10 s). In the analysis, activation from three seed ROIs (posterior cingulate cortex, medial prefrontal cortex, and angular gyrus) were averaged together and group differences in connectivity between this averaged activation and the default network were examined. Relative to controls, the ASD subjects showed weaker connectivity selectively in the medial prefrontal cortex and the left angular gyrus. Although the analytic procedures differ from the present work, the findings are somewhat complementary. Kennedy and Courchesne (2008) found selective areas of weaker connectivity when multiple seed regions were combined. In the present study, we found a selective area of weaker connectivity and two selective areas of stronger connectivity when only the posterior cingulate cortex was used as the seed. Task driven FMRI studies have found that subjects with ASD have areas of weaker connectivity (Just et al., 2007; Koshino et al., 2008) and stronger connectivity (Mizuno et al., 2006; Turner et al., 2006). Kennedy and Courchesne (2008) shows that ASD is related to selective areas of weaker connectivity. The present study builds on this finding and indicates that ASD is associated with specific areas of weaker connectivity and also specific areas of stronger connectivity.
Task-driven FMRI studies show that social functioning relies on multiple neural structures, including the amygdala, fusiform gyrus, anterior cingulate and ventral prefrontal cortex (Winston et al., 2002; Eisenberger, Lieberman and Williams, 2003; Iacoboni et al., 2004). Moreover, in ASD, social impairment is associated with altered functional connectivity, activation and morphology within these same areas (Ohnishi et al., 2000; Dapretto et al., 2006; Munson et al., 2006; Nacewicz et al., 2006; Kleinhans et al., 2008). The present findings suggest that components of the default network, notably the posterior cingulate cortex and superior frontal gyrus, also may play a role in social function in ASD. Since these results were found in the absence of a task, it is possible that connectivity between the posterior cingulate cortex and superior frontal gyrus may relate to some aspect of offline processing of social function, such as memory consolidation involving social interactions, or maintenance that supports social processing.
Relative to social function, little work has examined brain correlates of restricted and repetitive behaviors in ASD. One functional imaging study reported a negative correlation between left superior temporal sulcus activation in response to task of biological motion and the repetitive behavior score (Freitag et al., 2008). In addition, two studies linked disturbances in anterior cingulate function and restricted and repetitive behaviors (Shafritz et al., 2008; Thakkar et al., 2008). Turning to the present study, further investigation is required to understand the finding that connectivity between the posterior cingulate cortex and parahippocampal gyrus correlated positively with restricted and repetitive behaviors. The hyper-connectivity may give rise to these behavioral abnormalities. On the other hand, the connectivity patterns may be the result of the restricted and repetitive behaviors; for example, increased connectivity may be a neural manifestation of a constant effort to control these behaviors.
There are at least four limitations to the present work. First, the procedures used in the present study for examining resting connectivity make it impossible to know precisely what processes underlie the group differences in the default network. It has been persuasively argued that the default network during rest is primarily involved in intrinsic processes that are independent of awareness (Fox and Raichle, 2007; Raichle and Snyder, 2007; Buckner et al., 2008). Nevertheless, it is possible that there may be some systematic differences between groups in stimulus-independent thoughts that underlie our present findings. Second, as in other fMRI studies, the degree of connectivity merely indexes correlations in activation between regions. Thus, these results do not demonstrate that there are group differences in how brain structures communicate with one another. Moreover, motion, physiology, and activation of the seed may contribute to the group differences in connectivity. However, there were no group differences in motion; physiological variations were regressed out of the time series data; and the time series data from the seed were normalized. Thus, these potential influences are unlikely to be factors in the present study. Third, the sample size in the study was small. Therefore, these findings should be considered preliminary until replicated. And, fourth, most subjects with ASD received psychotropic medication. Use of medication is exceptionally high in ASD (Oswald and Sonenklar, 2007). Thus, excluding these subjects would lead to an unrepresentative sample, potentially with a very different symptom profile. Since subjects used different classes of medications, it was possible to evaluate whether each class influenced the results. Specifically, as documented in the results section, ASD subjects using particular classes of medications were removed from the analyses and the same results remained, indicating that the medication did not underlie the results.
Future directions for this line of research include the following. First, using diffusion tensor imaging (DTI), it would be possible to examine the integrity of white matter tracts within the default network in ASD. To date, multiple DTI studies have reported white matter alterations in participants with ASD (Barnea-Goraly et al., 2004; Alexander et al., 2007; Ben Bashat et al., 2007; Keller, Kana and Just, 2007; Lee et al., 2007; Sundaram et al., 2008; Thakkar et al., 2008). Moreover, recently, two groups used DTI and showed that white matter tracts mapped onto specific connections of the default network (Mandl et al., 2008; Greicius et al., 2009). Combining DTI and resting connectivity with an ASD sample would help to determine whether the altered functional connectivity in ASD is instantiated anatomically. Second, the present study used a predefined seed in the posterior cingulate cortex that was based on investigations of healthy adults. It is possible that individuals with ASD may have default network connectivity that is most pronounced in a different area. Future research may wish to use self-organizing map algorithms to evaluate differences in connectivity without a predefined model (Ngan and Hu, 1999; Peltier, Polk and Noll, 2003). Third, since these procedures do not involve a cognitive task, they can be replicated on a sample of low functioning individuals. From this, it would be possible to associate functional connectivity of the default network to level of adaptive functioning. Fourth, age and developmental level could also be correlated with connectivity. In particular, these procedures are suitable for very young children and so early risk and manifestations of ASD could be related to functional connectivity. And fifth, following the approach which recently showed that polymorphisms of the serotonin transporter gene are associated with gray matter volume in ASD (Wassink et al., 2007), it may be fruitful to link ASD-relevant polymorphisms to alterations in intrinsic functional connectivity.
Despite evidence that the default network at rest commands greater metabolic activity than cognitive or affective tasks (Raichle and Mintun, 2006), it is unclear what function(s) the default network subserves. Recently, studies reported altered functioning of the default network in multiple clinical populations, including depression (Greicius et al., 2009; Grimm et al., 2009; Sheline et al., 2009), attention deficit/hyperactivity disorder (Castellanos et al., 2008), schizophrenia (Garrity et al., 2007; Zhou et al., 2007), and mild cognitive impairment (Sorg et al., 2007). Moreover, developmental studies have documented that children and older adults, too, show differences in default network activation relative to young adults (Andrews-Hanna et al., 2007; Fair et al., 2008; Thomason et al., 2008). Combined with the present findings and the studies reported above on ASD, the default network appears to be altered in many different populations. Thus, it appears likely that the default network does not subserve a narrow, circumscribed function. Instead, the default network may support many different functions or it may be involved in a fundamental capacity of the central nervous system.
Acknowledgments
This research was supported in part by the National Institutes Health (K22 MH068017 to C.S.M, U19 HD35482 to C.L. and MH066496 to C.L.). Drs. Lord and Risi receive royalties from a publisher of diagnostic instruments described in this paper. They give all profits generated by the University of Michigan Autism and Communication Disorders Center (UMACC) in regards to this paper and all other UMACC projects to a charity.
We thank the families who participated. We also thank Dr. D. Noll for methodological advice, and H.M.C. Louro, K. Newnham and C. Hammond for technical support.
Footnotes
Data from this study were presented at the International Meeting for Autism Research, London, UK, May 2008.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander AL, Lee JE, Lazar M, Boudos R, DuBray MB, Oakes TR, Miller JN, Lu J, Jeong EK, McMahon WM, Bigler ED, Lainhart JE. Diffusion tensor imaging of the corpus callosum in Autism. Neuroimage. 2007;34:61–73. doi: 10.1016/j.neuroimage.2006.08.032. [DOI] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Snyder AZ, Vincent JL, Lustig C, Head D, Raichle ME, Buckner RL. Disruption of large-scale brain systems in advanced aging. Neuron. 2007;56:924–35. doi: 10.1016/j.neuron.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- APA. Diagnostic and Statistical Manual of Mental Disorders. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- Barnea-Goraly N, Kwon H, Menon V, Eliez S, Lotspeich L, Reiss AL. White matter structure in autism: preliminary evidence from diffusion tensor imaging. Biol Psychiatry. 2004;55:323–6. doi: 10.1016/j.biopsych.2003.10.022. [DOI] [PubMed] [Google Scholar]
- Belmonte MK, Allen G, Beckel-Mitchener A, Boulanger LM, Carper RA, Webb SJ. Autism and abnormal development of brain connectivity. J Neurosci. 2004;24:9228–31. doi: 10.1523/JNEUROSCI.3340-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Bashat D, Kronfeld-Duenias V, Zachor DA, Ekstein PM, Hendler T, Tarrasch R, Even A, Levy Y, Ben Sira L. Accelerated maturation of white matter in young children with autism: a high b value DWI study. Neuroimage. 2007;37:40–7. doi: 10.1016/j.neuroimage.2007.04.060. [DOI] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–41. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Vincent JL. Unrest at rest: default activity and spontaneous network correlations. Neuroimage. 2007;37:1091–6. doi: 10.1016/j.neuroimage.2007.01.010. discussion 1097–9. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Margulies DS, Kelly C, Uddin LQ, Ghaffari M, Kirsch A, Shaw D, Shehzad Z, Di Martino A, Biswal B, Sonuga-Barke EJ, Rotrosen J, et al. Cingulate-precuneus interactions: a new locus of dysfunction in adult attention-deficit/hyperactivity disorder. Biol Psychiatry. 2008;63:332–7. doi: 10.1016/j.biopsych.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherkassky VL, Kana RK, Keller TA, Just MA. Functional connectivity in a baseline resting-state network in autism. Neuroreport. 2006;17:1687–90. doi: 10.1097/01.wnr.0000239956.45448.4c. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Pierce K, Schumann CM, Redcay E, Buckwalter JA, Kennedy DP, Morgan J. Mapping early brain development in autism. Neuron. 2007;56:399–413. doi: 10.1016/j.neuron.2007.10.016. [DOI] [PubMed] [Google Scholar]
- Dapretto M, Davies MS, Pfeifer JH, Scott AA, Sigman M, Bookheimer SY, Iacoboni M. Understanding emotions in others: mirror neuron dysfunction in children with autism spectrum disorders. Nat Neurosci. 2006;9:28–30. doi: 10.1038/nn1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn LM, Dunn LM. Peabody Picture Vocabulary Test. Circle Pines, MN: American Guidance Services; 1997. [Google Scholar]
- Eisenberger NI, Lieberman MD, Williams KD. Does rejection hurt? An FMRI study of social exclusion. Science. 2003;302:290–2. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- Fair DA, Cohen AL, Dosenbach NU, Church JA, Miezin FM, Barch DM, Raichle ME, Petersen SE, Schlaggar BL. The maturing architecture of the brain’s default network. Proc Natl Acad Sci U S A. 2008;105:4028–32. doi: 10.1073/pnas.0800376105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–11. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102:9673–8. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitag CM, Konrad C, Haberlen M, Kleser C, von Gontard A, Reith W, Troje NF, Krick C. Perception of biological motion in autism spectrum disorders. Neuropsychologia. 2008;46:1480–94. doi: 10.1016/j.neuropsychologia.2007.12.025. [DOI] [PubMed] [Google Scholar]
- Garrity AG, Pearlson GD, McKiernan K, Lloyd D, Kiehl KA, Calhoun VD. Aberrant “default mode” functional connectivity in schizophrenia. Am J Psychiatry. 2007;164:450–7. doi: 10.1176/ajp.2007.164.3.450. [DOI] [PubMed] [Google Scholar]
- Glover GH, Li TQ, Ress D. Image-based method for retrospective correction of physiological motion effects in fMRI: RETROICOR. Magn Reson Med. 2000;44:162–7. doi: 10.1002/1522-2594(200007)44:1<162::aid-mrm23>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003;100:253–8. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Supekar K, Menon V, Dougherty RF. Resting-state functional connectivity reflects structural connectivity in the default mode network. Cereb Cortex. 2009;19:72–8. doi: 10.1093/cercor/bhn059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm S, Boesiger P, Beck J, Schuepbach D, Bermpohl F, Walter M, Ernst J, Hell D, Boeker H, Northoff G. Altered negative BOLD responses in the default-mode network during emotion processing in depressed subjects. Neuropsychopharmacology. 2009;34:932–843. doi: 10.1038/npp.2008.81. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:4259–64. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Happe F, Ronald A, Plomin R. Time to give up on a single explanation for autism. Nat Neurosci. 2006;9:1218–20. doi: 10.1038/nn1770. [DOI] [PubMed] [Google Scholar]
- Horovitz SG, Fukunaga M, de Zwart JA, van Gelderen P, Fulton SC, Balkin TJ, Duyn JH. Low frequency BOLD fluctuations during resting wakefulness and light sleep: a simultaneous EEG-fMRI study. Hum Brain Mapp. 2008;29:671–82. doi: 10.1002/hbm.20428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacoboni M, Lieberman MD, Knowlton BJ, Molnar-Szakacs I, Moritz M, Throop CJ, Fiske AP. Watching social interactions produces dorsomedial prefrontal and medial parietal BOLD fMRI signal increases compared to a resting baseline. Neuroimage. 2004;21:1167–73. doi: 10.1016/j.neuroimage.2003.11.013. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–41. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Just MA, Cherkassky VL, Keller TA, Kana RK, Minshew NJ. Functional and anatomical cortical underconnectivity in autism: evidence from an FMRI study of an executive function task and corpus callosum morphometry. Cereb Cortex. 2007;17:951–61. doi: 10.1093/cercor/bhl006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kana RK, Keller TA, Cherkassky VL, Minshew NJ, Just MA. Sentence comprehension in autism: thinking in pictures with decreased functional connectivity. Brain. 2006;129:2484–93. doi: 10.1093/brain/awl164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller TA, Kana RK, Just MA. A developmental study of the structural integrity of white matter in autism. Neuroreport. 2007;18:23–7. doi: 10.1097/01.wnr.0000239965.21685.99. [DOI] [PubMed] [Google Scholar]
- Kennedy DP, Courchesne E. The intrinsic functional organization of the brain is altered in autism. Neuroimage. 2008;39:1877–85. doi: 10.1016/j.neuroimage.2007.10.052. [DOI] [PubMed] [Google Scholar]
- Kleinhans NM, Richards T, Sterling L, Stegbauer KC, Mahurin R, Johnson LC, Greenson J, Dawson G, Aylward E. Abnormal functional connectivity in autism spectrum disorders during face processing. Brain. 2008;131:1000–12. doi: 10.1093/brain/awm334. [DOI] [PubMed] [Google Scholar]
- Koshino H, Kana RK, Keller TA, Cherkassky VL, Minshew NJ, Just MA. fMRI investigation of working memory for faces in autism: visual coding and underconnectivity with frontal areas. Cereb Cortex. 2008;18:289–300. doi: 10.1093/cercor/bhm054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JE, Bigler ED, Alexander AL, Lazar M, DuBray MB, Chung MK, Johnson M, Morgan J, Miller JN, McMahon WM, Lu J, Jeong EK, et al. Diffusion tensor imaging of white matter in the superior temporal gyrus and temporal stem in autism. Neurosci Lett. 2007;424:127–32. doi: 10.1016/j.neulet.2007.07.042. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, DiLavore PS, Shulman C, Thurm A, Pickles A. Autism from 2 to 9 years of age. Arch Gen Psychiatry. 2006;63:694–701. doi: 10.1001/archpsyc.63.6.694. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Jr, Leventhal BL, DiLavore PC, Pickles A, Rutter M. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30:205–23. [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24:659–85. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Maldjian J, Laurienti P, Burdette J, Kraft R. An Automated Method for Neuroanatomic and Cytoarchitectonic Atlas-based Interrogation of fMRI Data Sets. NeuroImage. 2002;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Mandl RC, Schnack HG, Luigjes J, van den Heuvel MP, Cahn W, Kahn RS, Hulshoff Pol HE. Tract-based Analysis of Magnetization Transfer Ratio and Diffusion Tensor Imaging of the Frontal and Frontotemporal Connections in Schizophrenia. Schizophr Bull. 2008 doi: 10.1093/schbul/sbn161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno A, Villalobos ME, Davies MM, Dahl BC, Muller RA. Partially enhanced thalamocortical functional connectivity in autism. Brain Res. 2006;1104:160–74. doi: 10.1016/j.brainres.2006.05.064. [DOI] [PubMed] [Google Scholar]
- Munson J, Dawson G, Abbott R, Faja S, Webb SJ, Friedman SD, Shaw D, Artru A, Dager SR. Amygdalar volume and behavioral development in autism. Arch Gen Psychiatry. 2006;63:686–93. doi: 10.1001/archpsyc.63.6.686. [DOI] [PubMed] [Google Scholar]
- Nacewicz BM, Dalton KM, Johnstone T, Long MT, McAuliff EM, Oakes TR, Alexander AL, Davidson RJ. Amygdala volume and nonverbal social impairment in adolescent and adult males with autism. Arch Gen Psychiatry. 2006;63:1417–28. doi: 10.1001/archpsyc.63.12.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngan SC, Hu X. Analysis of functional magnetic resonance imaging data using self-organizing mapping with spatial connectivity. Magn Reson Med. 1999;41:939–46. doi: 10.1002/(sici)1522-2594(199905)41:5<939::aid-mrm13>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Ohnishi T, Matsuda H, Hashimoto T, Kunihiro T, Nishikawa M, Uema T, Sasaki M. Abnormal regional cerebral blood flow in childhood autism. Brain. 2000;123 (Pt 9):1838–44. doi: 10.1093/brain/123.9.1838. [DOI] [PubMed] [Google Scholar]
- Oppenheim A, Schafer R, Buck J. Discrete-time signal processing. Upper Saddle River, NJ: Prentice Hall; 1999. [Google Scholar]
- Oswald DP, Sonenklar NA. Medication use among children with autism spectrum disorders. J Child Adolesc Psychopharmacol. 2007;17:348–55. doi: 10.1089/cap.2006.17303. [DOI] [PubMed] [Google Scholar]
- Peltier SJ, Polk TA, Noll DC. Detecting low-frequency functional connectivity in fMRI using a self-organizing map (SOM) algorithm. Hum Brain Mapp. 2003;20:220–6. doi: 10.1002/hbm.10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–82. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, Mintun MA. Brain work and brain imaging. Annu Rev Neurosci. 2006;29:449–76. doi: 10.1146/annurev.neuro.29.051605.112819. [DOI] [PubMed] [Google Scholar]
- Raichle ME, Snyder AZ. A default mode of brain function: a brief history of an evolving idea. Neuroimage. 2007;37:1083–90. doi: 10.1016/j.neuroimage.2007.02.041. discussion 1097–9. [DOI] [PubMed] [Google Scholar]
- Raven JC. Guide to using the Standard Progressive Matrices. London, UK: Lewis; 1960. [Google Scholar]
- Shafritz KM, Dichter GS, Baranek GT, Belger A. The neural circuitry mediating shifts in behavioral response and cognitive set in autism. Biol Psychiatry. 2008;63:974–80. doi: 10.1016/j.biopsych.2007.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Barch DM, Price JL, Rundle MM, Vaishnavi SN, Snyder AZ, Mintun MA, Wang S, Coalson RS, Raichle ME. The default mode network and self-referential processes in depression. Proc Natl Acad Sci U S A. 2009;106:1942–7. doi: 10.1073/pnas.0812686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman GL, Fiez JA, Corbetta M, Buckner RL, Miezin FM, Raichle ME, Petersen SE. Common blood flow changes across visual tasks: II. Decreases in cerebral cortex. Journal of Cognitive Neuroscience. 1997;9:648–663. doi: 10.1162/jocn.1997.9.5.648. [DOI] [PubMed] [Google Scholar]
- Sorg C, Riedl V, Muhlau M, Calhoun VD, Eichele T, Laer L, Drzezga A, Forstl H, Kurz A, Zimmer C, Wohlschlager AM. Selective changes of resting-state networks in individuals at risk for Alzheimer’s disease. Proc Natl Acad Sci U S A. 2007;104:18760–5. doi: 10.1073/pnas.0708803104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaram SK, Kumar A, Makki MI, Behen ME, Chugani HT, Chugani DC. Diffusion tensor imaging of frontal lobe in autism spectrum disorder. Cereb Cortex. 2008;18:2659–65. doi: 10.1093/cercor/bhn031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakkar KN, Polli FE, Joseph RM, Tuch DS, Hadjikhani N, Barton JJ, Manoach DS. Response monitoring, repetitive behaviour and anterior cingulate abnormalities in autism spectrum disorders (ASD) Brain. 2008;131:2464–78. doi: 10.1093/brain/awn099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomason ME, Chang CE, Glover GH, Gabrieli JD, Greicius MD, Gotlib IH. Default-mode function and task-induced deactivation have overlapping brain substrates in children. Neuroimage. 2008;41:1493–503. doi: 10.1016/j.neuroimage.2008.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner KC, Frost L, Linsenbardt D, McIlroy JR, Muller RA. Atypically diffuse functional connectivity between caudate nuclei and cerebral cortex in autism. Behav Brain Funct. 2006;2:34. doi: 10.1186/1744-9081-2-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalobos ME, Mizuno A, Dahl BC, Kemmotsu N, Muller RA. Reduced functional connectivity between V1 and inferior frontal cortex associated with visuomotor performance in autism. Neuroimage. 2005;25:916–25. doi: 10.1016/j.neuroimage.2004.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Patel GH, Fox MD, Snyder AZ, Baker JT, Van Essen DC, Zempel JM, Snyder LH, Corbetta M, Raichle ME. Intrinsic functional architecture in the anaesthetized monkey brain. Nature. 2007;447:83–6. doi: 10.1038/nature05758. [DOI] [PubMed] [Google Scholar]
- Wassink TH, Hazlett HC, Epping EA, Arndt S, Dager SR, Schellenberg GD, Dawson G, Piven J. Cerebral cortical gray matter overgrowth and functional variation of the serotonin transporter gene in autism. Arch Gen Psychiatry. 2007;64:709–17. doi: 10.1001/archpsyc.64.6.709. [DOI] [PubMed] [Google Scholar]
- Welchew DE, Ashwin C, Berkouk K, Salvador R, Suckling J, Baron-Cohen S, Bullmore E. Functional disconnectivity of the medial temporal lobe in Asperger’s syndrome. Biol Psychiatry. 2005;57:991–8. doi: 10.1016/j.biopsych.2005.01.028. [DOI] [PubMed] [Google Scholar]
- Winston JS, Strange BA, O’Doherty J, Dolan RJ. Automatic and intentional brain responses during evaluation of trustworthiness of faces. Nat Neurosci. 2002;5:277–83. doi: 10.1038/nn816. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Liang M, Tian L, Wang K, Hao Y, Liu H, Liu Z, Jiang T. Functional disintegration in paranoid schizophrenia using resting-state fMRI. Schizophr Res. 2007;97:194–205. doi: 10.1016/j.schres.2007.05.029. [DOI] [PubMed] [Google Scholar]