Abstract
Objective
To evaluate whether adding comorbid conditions to a risk model can help predict in-hospital outcome and long-term mortality after percutaneous coronary intervention (PCI).
Design
Retrospective chart review
Setting
Academic medical center.
Patients
7,659 patients who had 9,032 PCIs.
Interventions
PCI performed at Mayo Clinic between January 1, 1999, and June 30, 2004.
Main Outcome Measures
The Mayo Clinic Risk Score (MCRS) and the coronary artery disease (CAD)-specific index for determination of comorbid conditions in all patients.
Results
The mean MCRS score was 6.5±2.9. The CAD-specific index was 0 or 1 in 46%, 2 or 3 in 30%, and 4 or higher in 23%. The rate of in-hospital major adverse cardiovascular events (MACE) increased with higher MCRS and CAD-specific index (Cochran-Armitage test, P<.001 for both models). The c-statistic for the MCRS for in-hospital MACE was 0.78; adding the CAD-specific index did not improve its discriminatory ability for in-hospital MACE (c-statistic=0.78; likelihood ratio test, P=.29). A total of 707 postdismissal deaths occurred after 7,253 successful procedures. The c-statistic for all-cause mortality was 0.69 for the MCRS model alone and 0.75 for the MCRS and CAD-specific indices together (likelihood ratio test, P<.001), indicating significant improvement in the discriminatory ability.
Conclusions
Addition of comorbid conditions to the MCRS adds significant prognostic information for postdismissal mortality but adds little prognostic information about in-hospital complications after PCI. Such health-status measures should be included in future risk stratification models that predict long-term mortality after PCI.
Keywords: angioplasty, comorbid conditions, complications, mortality, prognosis, risk factors
Introduction
A third of the population in the United States aged 65 to 79 years and 70% of the population aged 80 years and older have at least 1 comorbid condition (1). Comorbid conditions increase the risk of disability and mortality over and above the risk from individual diseases (2,3). Because patients frequently present with multiple comorbid conditions at the time of percutaneous coronary intervention (PCI) and because technical advances in PCI now allow sicker patients to be treated, it is likely that patients' comorbid conditions will have greater influence on their long-term outcomes after PCI. As the burden of coronary artery disease (CAD) and revascularization shifts toward older segments of the population, the assessment of comorbid conditions must be prioritized in the risk assessment models developed for PCI.
Earlier attempts to incorporate this important factor into risk assessment models have been hampered by the lack of uniform definitions of comorbid conditions. The recent development of constructs to define comorbid conditions relevant to patients with CAD may better illuminate the prognostic importance of their presence and severity on the patients' PCI outcomes (4). At present, however, most risk-prediction models that are developed to determine outcome after angioplasty focus on in-hospital complications after PCI (5-10). Lack of inclusion of comorbid illnesses is likely to be a substantial barrier to the further evolution of prognostic models of follow-up events and may limit our ability to inform patients of the expected outcomes after treatment.
There is a critical need for development of models to assess long-term adverse outcomes. Most current models restrict the prediction to either in-hospital or short-term outcomes. We previously developed and validated the Mayo Clinic Risk Score (MCRS), which accurately predicts in-hospital major adverse cardiovascular events (MACE), and contains several variables that are linked to long-term outcomes (8,11). Its value in prediction of long-term outcome has not yet been evaluated. We previously demonstrated significant improvement in the discriminatory ability of the existing prediction models for myocardial infarction (MI) with the addition of comorbid illnesses (12). The aim of the present study was to evaluate the value of the MCRS in prediction of long-term events and whether the addition of comorbid conditions to the MCRS provides additional information to predict in-hospital and long-term mortality in patients undergoing PCI.
Methods
Study Population
Since 1979, patients undergoing PCI at Mayo Clinic have been prospectively enrolled in a longitudinal follow-up registry. Patients in this registry have undergone follow-up evaluations by trained study coordinators at 6 months, 1 year, and then annually since their PCI. For the present study, we reviewed 9,259 consecutive PCI procedures performed in 7,847 patients at Mayo Clinic Rochester between January 1, 1999, and June 30, 2004. One hundred eighty-eight patients with 227 procedures did not allow research use of their records and were excluded, leaving 9,032 observations for analysis. The Mayo Clinic Institutional Review Board approved the study.
Quantifying Comorbid Conditions
Information on comorbid conditions was collected at the time of the index PCI and was abstracted from the patients' medical records for this study. The method for developing the CAD-specific index has been previously described (4). Briefly, the CAD-specific index was developed, to assess and determine the prognostic influence of comorbid conditions, from a cohort of 1,471 patients with CAD who underwent coronary angiography between 1985 and 1989 and were followed up through 2000 in the Duke Databank for Cardiovascular Diseases. After the final model was determined, a weight for each comorbid condition was derived using the log hazard ratios from the model (Table 1). A weight of 2 was assigned to diabetes mellitus, and weights for the other conditions were calculated relative to diabetes and rounded to the nearest integer. Weight 1 was given to current smoker, hypertension, or history of stroke or transient ischemic attack (TIA); weight 2 corresponds with diabetes mellitus, chronic obstructive pulmonary disease, peripheral vascular disease, tumor, lymphoma, or leukemia. Weight 3 was given to patients with diabetes mellitus with sequelae, 5 to metastatic solid tumor, and 7 to patients with moderate to severe renal disease. In the present study, we separated the patients into 3 groups: those with a CAD-specific index score of 0 or 1; those with a score of 2 or 3, and those with a score of 4 or higher (maximum score observed is 21).
Table 1.
Mayo Clinic Risk Score and CAD-Specific Index
| Mayo Clinic Risk Score | CAD-specific index | ||
|---|---|---|---|
| Risk factor | Integer coefficient | Risk factor | Integer coefficient |
| Age* | 1 | Current smoker | 1 |
| Urgent/emergency PCI | 2 | Hypertension | 1 |
| CHF, NYHA class ≥III | 2 | History of CVA/TIA | 1 |
| Multivessel disease | 2 | DM | 2 |
| Thrombus in any lesion | 2 | DM with sequelae | 3 |
| Moderate to severe renal disease | 3 | COPD | 2 |
| Pre-PCI shock | 5 | PVD | 2 |
| LMCA with ≥70% stenosis | 5 | Tumor/lymphoma/leukemia | 2 |
| Moderate to severe renal disease | 7 | ||
| Metastatic cancer | 5 | ||
CAD, coronary artery disease; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; CVA, cerebrovascular accident; DM, diabetes mellitus; LMCA, left main coronary artery; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; PVD, peripheral vascular disease; TIA, transient ischemic attack.
Scored as 1 point for every decade older than 30 years (eg, a 90-year-old person would have a score of 6 for age).
Mayo Clinic Risk Score
The MCRS model (Table 1) incorporates 5 clinical variables (older age, nonelective intervention, renal disease, cardiogenic shock, and New York Heart Association class III or greater heart failure symptoms) and 3 angiographic variables (angiographic thrombus, left main disease, or multivessel disease) that accurately predict in-hospital complications (8). The weighted integers (range, 0-27) group the patients undergoing PCI into different risk categories: 0 to 5 (very low risk), 6 to 8 (low risk), 9 to 11 (moderate risk), 12 to 14 (high risk), and 15 or greater (very high risk).
Clinical Outcomes
In-hospital MACE included death, Q wave MI, stroke or TIA, or need for emergent coronary artery bypass grafting surgery (CABG). TIA was defined as loss of neurologic function caused by ischemia with abrupt onset persisting for 24 hours or less and clearing without residual signs. Stroke was defined as a loss of neurologic function caused by an ischemic event persisting longer than 24 hours or leaving residual signs. Diabetes mellitus with sequelae was defined as renal disease, retinopathy, peripheral neuropathy, gastroparesis, or peripheral circulatory disease documented in the medical history. Multivessel disease was defined as stenosis of more than 70% in more than 1 major epicardial coronary artery or 1 of their major branches. The only follow-up event documented was time to all-cause mortality, as was used in the original CAD-specific index for comorbid conditions. The follow-up data were censored after the point of last contact. If the presence of a component risk factor was unknown, it was treated as not present.
Patient outcome data were stratified into short-term (in-hospital) and long-term outcomes. We evaluated the influence of comorbid conditions (CAD-specific index) on the ability of MCRS to predict short-term outcome. Furthermore, we investigated 1) whether known determinants of short-term outcome (ie, MCRS) can also provide prognostic information for long-term mortality and 2) whether information about comorbid conditions can provide incremental long-term prognostic information over and above that determined by the factors that predict in-hospital outcome (MCRS).
Statistical Analysis
Continuous variables are summarized as means±SD. Discrete variables are presented as frequencies. Because data were missing for some variables, percentages were calculated based on the number of PCIs with information available. Kaplan-Meier methods were used to estimate survival rates during follow-up. The Spearman rank correlation was used to estimate the association between the MCRS and the CAD-specific index. The Cochran-Armitage test for trend was used to evaluate increasing event rates across the risk score indices. Logistic regression models were used to assess associations with in-hospital MACE.
Cox proportional hazards regression models were used to analyze time to all-cause mortality on long-term follow-up among patients with a successful PCI (defined as <50% residual stenosis in at least 1 treated lesion, with no in-hospital death, Q wave MI, or CABG). Successful PCIs were used because poor long-term outcome is already expected for those with poor short-term outcome—ie, unsuccessful PCI. If patients had multiple successful PCIs within the study period, only the earliest was used for the follow-up analysis of all-cause mortality. The additional prognostic value of the CAD-specific index, when the MCRS was already accounted for, was tested by comparing a model with both scores to the model with the MCRS alone.
Results
The mean age of 7,659 patients undergoing 9,032 PCIs was 67±12 years and 71% were men (Table 2). Eighty-two percent had a history of hyperlipidemia, 73% had hypertension, 25% had diabetes mellitus (32% of these with sequelae), 3% had renal disease, 11% had congestive heart failure on presentation, and 11% had peripheral vascular disease. Sixty-six percent of PCIs were either urgent or emergent, and 51% were multivessel interventions (Table 2). All characteristics were similar for the subset of patients with successful PCI.
Table 2.
Patient Baseline and Angiographic Characteristics During All PCIs and Successful PCIs*
| Value† | ||
|---|---|---|
| Characteristic | All PCIs | Successful PCIs |
| Age, y | 66.9±12.0 | 66.6±12.0 |
| Men | 6,370/9,032 (71) | 5,094/7,253 (70) |
| Hypertension | 6,244/8,615 (73) | 4,873/6,896 (71) |
| History of cholesterol ≥240 | 6,767/8,247 (82) | 5,272/6,559 (80) |
| Body mass index | 29.7±5.7 (n=9,022) | 29.7±5.7 (n=7,246) |
| Smoking status | ||
| Never | 3,140/8,899 (35) | 2,554/7,135 (36) |
| Former | 4,238/8,899 (48) | 3,306/7,135 (46) |
| Current | 1,521/8,899 (17) | 1,275/7,135 (18) |
| DM | 2,279/8,976 (25) | 1,763/7,201 (24) |
| With sequelae | 683/2,160 (32) | 514/1,668 (31) |
| Moderate or severe renal disease | 312/8,948 (3) | 244/7,190 (3) |
| CHF on presentation | 1,019/9,032 (11) | 760/7,253 (10) |
| NYHA class ≥III | 553/8,324 (7) | 395/6,742 (6) |
| LV ejection fraction | ||
| >40% | 4,352/9,032 (48) | 3,644/7,253 (50) |
| NA | 3,691/9,032 (41) | 2,840/7,253 (39) |
| ≤40% | 989/9,032 (11) | 769/7,253 (11) |
| CVA/TIA | 1,103/8,748 (13) | 840/7,032 (12) |
| Peripheral vascular disease | 943/8,663 (11) | 706/6,975 (10) |
| Tumor, lymphoma, or leukemia | 1,041/8,934 (12) | 833/7,169 (12) |
| Metastatic cancer | 76/8,986 (1) | 60/7,214 (1) |
| COPD | 1,030/8,732 (12) | 803/7,023 (11) |
| MI, day of PCI or in ≤24 h | 1,569/8,876 (18) | 1,298/7,117 (18) |
| Preprocedural shock | 337/8,995 (4) | 213/7,225 (3) |
| PCI urgency | ||
| Elective | 3,029/9,029 (34) | 2,480/7,250 (34) |
| Urgent | 4,282/9,029 (47) | 3,392/7,250 (47) |
| Emergent | 1,718/9,029 (19) | 1,378/7,250 (19) |
| LMCA ≥70% stenosed | 138/9,032 (2) | 88/7,253 (1) |
| Multivessel disease | 4,365/8,505 (51) | 3,513/6,893 (51) |
| Thrombus in any lesion | 2,417/8,421 (29) | 2,039/6,835 (30) |
CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; CVA/TIA, cerebrovascular accident/transient ischemic attack; DM, diabetes mellitus; LMCA, left main coronary artery; LV, left ventricle; MI, myocardial infarction; NA, not available; NYHA, New York Heart Association; PCI, percutaneous coronary intervention.
A total of 9,032 PCI procedures in 7,659 patients and 7,253 successful PCI procedures in unique patients.
Mean±SD or no. of patients with characteristic/no. of patients with available data (%).
The MCRS and the CAD-specific index scores (Table 1) were determined for each patient at the time of each PCI procedure (N=9,032). The mean MCRS score was 6.5±2.9. Thirty-eight percent of the patients were in the very low MCRS group, 42% were in the low-risk group, 15% had moderate risk, 3% had high risk, and 2% had very high risk. Forty-six percent had a CAD-specific index of 0 or 1, 30% had a score of 2 or 3, and 24% had a score of 4 or higher.
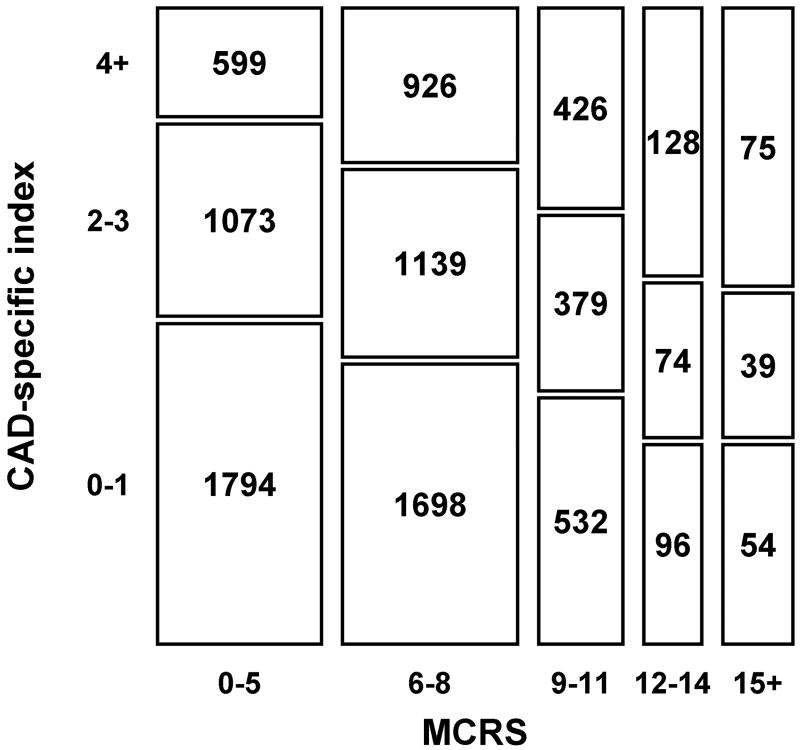
Figure 1 depicts the association of the MCRS and the CAD-specific index. More than half of the patients with a lower MCRS (0-5) had a CAD-specific score of 0 or 1, which indicates few associated comorbid conditions. Only 17% of patients with an MCRS of 0 to 5 had a CAD-specific index score of 4 or higher. In contrast, 44% of patients in the high and very high risk MCRS subgroups had a CAD-specific index of 4 or greater, which indicates a higher prevalence of comorbid conditions in the high-risk groups. Patients who were high risk for PCI but with few comorbid conditions had more in-hospital MACE (11/122 [9%]) than did patients who were low risk for PCI with several comorbid conditions (0/69 [0%]) (P=.01). However, long-term survival is similar between these 2 groups; 2-year mortality was 6.9% for those high risk for PCI with few comorbid conditions and 5.4% for those low risk for PCI with several comorbid conditions (P=.4). Although the prevalence of comorbid conditions was greater with a higher MCRS, the Spearman rank correlation coefficient between the MCRS and the CAD-specific index was low (rs=0.142), which indicates weak association between the MCRS and the CAD-specific index.
Fig. 1.
Mosaic plot indicating the correlation between Mayo Clinic Risk Score (MCRS) and coronary artery disease (CAD)-specific index. The frequency of PCIs for each cross-tabulation cell is shown within a rectangle that is proportional in size to the frequency.
In the cohort, 330 patients (3.7%) had in-hospital MACE. The incidence of MACE increased with higher MCRS and higher CAD-specific index (Cochran-Armitage test for trend, P<.001 for both models). The c-statistic for the MCRS for in-hospital events was 0.78 (95% confidence interval [CI], 0.75-0.81). The addition of the CAD-specific index to the MCRS did not improve its discriminatory ability for in-hospital MACE (c-statistic=0.78, [95% CI, 0.75-0.81]; likelihood ratio test, P=.29).
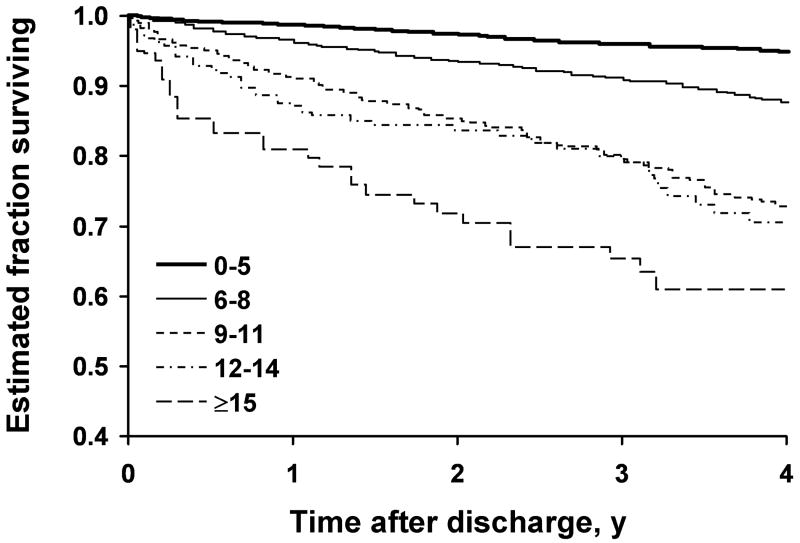
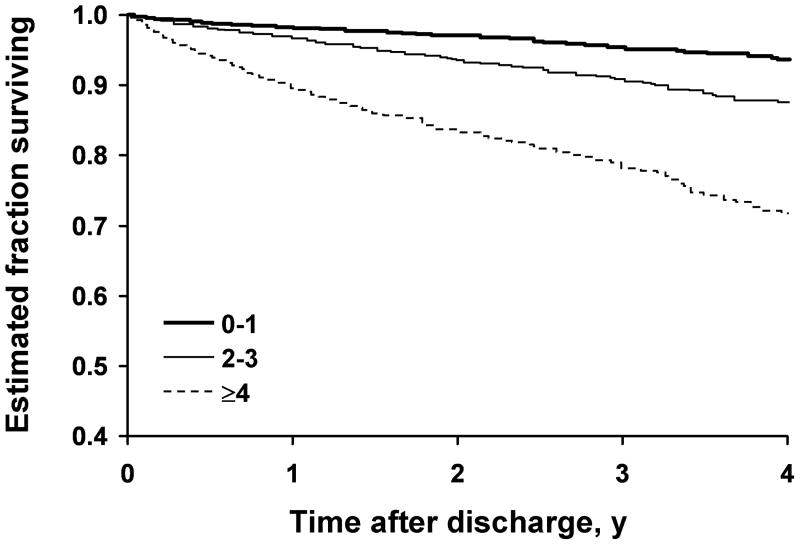
A total of 707 postdismissal deaths occurred in 7,253 patients after 7,253 successful procedures. The median length of follow-up for these procedures was 3.0 years; 75% of the patients had at least 13 months of follow-up. The Kaplan-Meier survival curves for the MCRS groups (Fig. 2 A) and the CAD-specific index groups (Fig. 2 B) indicated worse survival in patients with higher MCRS at the time of PCI and in patients with higher CAD-specific comorbid condition index scores. Cox regression models indicated that the hazard ratios for follow-up mortality had a quadratic (curved) relationship with the MCRS and CAD-specific index. With higher index scores, subsequent increases did not produce as large an increase in the hazard ratio (Table 3).
Fig. 2.
Kaplan-Meier curves estimating survival among patients. A, Patients grouped by Mayo Clinic Risk Score. B, Patients grouped by coronary artery disease (CAD)-specific index of comorbid conditions.
Table 3.
Hazard Ratios for Follow-up Mortality by the MCRS and CAD-Specific Index
| Score | Hazard ratio (CI) | |
|---|---|---|
| MCRS | CAD-specific index | |
| 0 | 0.08 (0.04-0.15) | 0.54 (0.49-0.59) |
| 2 | 0.20 (0.14-0.29) | 1.00 (Reference)* |
| 4 | 0.47 (0.39-0.55) | 1.71 (1.60-1.82) |
| 6 | 1.00 (Reference)* | 2.68 (2.41-2.97) |
| 8 | 1.97 (1.73-2.26) | 3.86 (3.39-4.39) |
| 10 | 3.60 (2.85-4.55) | 5.12 (4.39-5.97) |
| 15 | 11.4 (8.07-16.0) | 7.17 (5.16-9.96) |
CAD, coronary artery disease; CI, confidence interval; MCRS, Mayo Clinic Risk Score.
Reference levels are the index sample medians.
The addition of the CAD-specific index to the MCRS significantly increased the ability to predict death after successful PCI. The c-statistic for all-cause mortality was 0.69 (95% CI, 0.67-0.71) for the MCRS model alone (including linear and quadratic parameters) and was 0.75 (95% CI, 0.73-0.77) for the model with both the MCRS and the CAD-specific index (both linear and quadratic). The likelihood ratio test for the significance of adding the CAD-specific index terms to the model was χ2=43.1 with 2 df (P<.001), indicating significant improvement in the discriminatory ability of the model.
Discussion
The present study demonstrates the significant incremental prognostic information obtained from adding comorbid conditions to an existing risk model for patients undergoing PCI, particularly for long-term survival. The comorbid condition measurement, however, did not significantly improve the predictive ability for in-hospital complications after PCI.
Comorbid Conditions and Outcome in Patients With Cardiovascular Disease
Comorbid conditions are defined as the concurrent presence of 2 or more medically diagnosed diseases in the same person. The prevalence of comorbid conditions increases with age, and their presence is associated with higher health care utilization, disability, and mortality (2,3,13). The Charlson index, comprising 12 chronic conditions, and more recently the CAD-specific index are common examples of the instruments available for more systematic and comprehensive inclusion of comorbid conditions into risk prediction models. Both models provide powerful tools to predict all-cause mortality (14). For this study, we chose the latter because it was developed and designed from data on patients with documented CAD (4,15). Both models have been shown to predict long-term survival in a spectrum of disease patterns with CAD. Comorbid conditions routinely serve as prognostic determinants for CAD patients presenting with chronic stable angina or acute coronary syndrome (12,16-18). The important role of comorbid conditions in predicting post-MI outcomes was underscored by the report of the Cardiovascular Cooperative Project, which focused on Medicare beneficiaries (17). We recently evaluated the TIMI (Thrombolysis in MI) and PREDICT (Predicting Risk of Death in Cardiac Disease Tool) scores in a geographically defined cohort of patients with MI from Olmsted County, Minnesota, and demonstrated the important incremental value of comorbid conditions and ejection fraction for risk stratification (12).
CAD is increasingly becoming a disease of the elderly, such that comorbid conditions are expected to affect outcome after PCI. However, comorbid conditions are not routinely included in current risk-outcome analyses in patients with CAD. Comorbid conditions are less prevalent in younger persons and are less likely to be included in any of the scores derived from clinical trials, which typically include younger persons (19). In addition, the similarity in clinical presentation in patients with comorbid conditions and patients with frailty and disability blurs the prognostic distinction derived from these diagnoses (20-22). The current models for prediction of outcome in patients undergoing PCI or presenting with acute coronary syndromes use considerable weighting of clinical presentation, measures of left ventricular function, and angiographic disease, such that these models are likely to be more predictive of early hazard.
Comorbid Conditions and Risk Stratification Models for PCI
Current risk prediction models for PCI were developed without consideration or systematic inclusion of comorbid conditions (5,6,8-10,23,24). In the present study, we gained no additional prognostic information for in-hospital complications after PCI with the addition of comorbid conditions; the in-hospital events were largely determined by acuity of presentation, left ventricular dysfunction, age, and angiographic variables. During follow-up, the significant improvement in the discriminant accuracy of the MCRS with the addition of the CAD-specific index underscores the importance of comorbid conditions in determining long-term survival after the index PCI.
Although the prevalence of comorbid conditions was greater with a higher MCRS, the association between the MCRS and the CAD-specific index was weak. Thus, both models appear to provide complementary long-term prognostic information. Additionally, the relationship between outcome and the index of interest is quadratic (nonlinear), indicating that there may be a threshold of concomitant risks at which additional risks have little effect on outcome. Identification of factors associated with postdismissal mortality after successful PCI may encourage more frequent surveillance, more intensive medical management, or consideration of alternative strategies.
Limitations
This study is limited by its retrospective design. Only those comorbid illnesses defined by the CAD-specific index were included in the analysis. New comorbid conditions (eg, diabetes mellitus) may have developed in some patients on follow-up, further complicating the analysis. The c-statistic of 0.75 after inclusion of CAD-specific comorbid conditions indicates good discrimination by the MCRS model. However, other unmeasured variables may further improve the discriminatory ability of the current model. All-cause mortality was the only follow-up event measured, and from the present study we cannot extrapolate our results to cardiovascular mortality or other MACE.
Conclusions
In conclusion, this study showed that the addition of comorbid conditions to the MCRS adds significant prognostic information for postdismissal all-cause mortality but adds little prognostic information for in-hospital complications after PCI. Patients' comorbid conditions must be included in future risk stratification models that predict long-term mortality after PCI.
Abbreviations
- CABG
coronary artery bypass grafting surgery
- CAD
coronary artery disease
- CI
confidence interval
- MACE
major adverse cardiovascular events
- MCRS
Mayo Clinic Risk Score
- MI
myocardial infarction
- PCI
percutaneous coronary intervention
- TIA
transient ischemic attack
Footnotes
The Corresponding Author has the right to grant on behalf of all authors and does grant on behalf of all authors, an exclusive license (or non exclusive for government employees) on a worldwide basis to the BMJ Publishing Group Ltd and its licencees, to permit this article (if accepted) to be published in Heart and any other BMJ Group products and to exploit all subsidiary rights, as set out in our licence (http://heart.bmjjournals.com//ifora/licence.pdf).
Mayo Foundation for Medical Education and Research is the author under the work-for-hire provision of the copyright law. The signature of the authorized agent transfers the copyright to BMJ Publishing Group Ltd that applies to Mayo Foundation authors (M Singh, CS Rihal, VL Roger, RJ Lennon, A Jahangir, and DR Holmes Jr). By: Mrs. Roberta Schwartz, Agent for Mayo Foundation.
References
- 1.Guralnik JM, LaCroix AZ, Everett DF, Kovar MG. Aging in the eighties: the prevalence of comorbidity and its association with disability Advance data from vital and health statistics; no 170. Hyattsville, MD: United States Department of Health and Human Services, Public Health Service, Centers for Disease Control, National Center for Health Statistics; 1989. [Google Scholar]
- 2.Fried LP, Kronmal RA, Newman AB, Bild DE, Mittelmark MB, Polak JF, Robbins JA, Gardin JM. Risk factors for 5-year mortality in older adults: the Cardiovascular Health Study. JAMA. 1998;279:585–92. doi: 10.1001/jama.279.8.585. [DOI] [PubMed] [Google Scholar]
- 3.Fried LP, Bandeen-Roche K, Kasper JD, Guralnik JM. Association of comorbidity with disability in older women: the Women's Health and Aging Study. J Clin Epidemiol. 1999;52:27–37. doi: 10.1016/s0895-4356(98)00124-3. [DOI] [PubMed] [Google Scholar]
- 4.Sachdev M, Sun JL, Tsiatis AA, Nelson CL, Mark DB, Jollis JG. The prognostic importance of comorbidity for mortality in patients with stable coronary artery disease. J Am Coll Cardiol. 2004;43:576–82. doi: 10.1016/j.jacc.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 5.Moscucci M, Kline-Rogers E, Share D, O'Donnell M, Maxwell-Eward A, Meengs WL, Kraft P, DeFranco AC, Chambers JL, Patel K, McGinnity JG, Eagle KA. Simple bedside additive tool for prediction of in-hospital mortality after percutaneous coronary interventions. Circulation. 2001;104:263–8. doi: 10.1161/01.cir.104.3.263. [DOI] [PubMed] [Google Scholar]
- 6.Qureshi MA, Safian RD, Grines CL, Goldstein JA, Westveer DC, Glazier S, Balasubramanian M, O'Neill WW. Simplified scoring system for predicting mortality after percutaneous coronary intervention. J Am Coll Cardiol. 2003;42:1890–5. doi: 10.1016/j.jacc.2003.06.014. [DOI] [PubMed] [Google Scholar]
- 7.Rihal CS, Grill DE, Bell MR, Berger PB, Garratt KN, Holmes DR., Jr Prediction of death after percutaneous coronary interventional procedures. Am Heart J. 2000;139:1032–8. doi: 10.1067/mhj.2000.105299. [DOI] [PubMed] [Google Scholar]
- 8.Singh M, Lennon RJ, Holmes DR, Jr, Bell MR, Rihal CS. Correlates of procedural complications and a simple integer risk score for percutaneous coronary intervention. J Am Coll Cardiol. 2002;40:387–93. doi: 10.1016/s0735-1097(02)01980-0. [DOI] [PubMed] [Google Scholar]
- 9.Shaw RE, Anderson HV, Brindis RG, Krone RJ, Klein LW, McKay CR, Block PC, Shaw LJ, Hewitt K, Weintraub WS. Development of a risk adjustment mortality model using the American College of Cardiology-National Cardiovascular Data Registry (ACC-NCDR) experience: 1998-2000. J Am Coll Cardiol. 2002;39:1104–12. doi: 10.1016/s0735-1097(02)01731-x. [DOI] [PubMed] [Google Scholar]
- 10.Resnic FS, Ohno-Machado L, Selwyn A, Simon DI, Popma JJ. Simplified risk score models accurately predict the risk of major in-hospital complications following percutaneous coronary intervention. Am J Cardiol. 2001;88:5–9. doi: 10.1016/s0002-9149(01)01576-4. [DOI] [PubMed] [Google Scholar]
- 11.Singh M, Rihal CS, Selzer F, Kip KE, Detre K, Holmes DR. Validation of Mayo Clinic risk adjustment model for in-hospital complications after percutaneous coronary interventions, using the National Heart, Lung, and Blood Institute dynamic registry. J Am Coll Cardiol. 2003;42:1722–8. doi: 10.1016/j.jacc.2003.05.007. [DOI] [PubMed] [Google Scholar]
- 12.Singh M, Reeder GS, Jacobsen SJ, Weston S, Killian J, Roger VL. Scores for post-myocardial infarction risk stratification in the community. Circulation. 2002;106:2309–14. doi: 10.1161/01.cir.0000036598.12888.de. [DOI] [PubMed] [Google Scholar]
- 13.Wolff JL, Starfield B, Anderson G. Prevalence, expenditures, and complications of multiple chronic conditions in the elderly. Arch Intern Med. 2002;162:2269–76. doi: 10.1001/archinte.162.20.2269. [DOI] [PubMed] [Google Scholar]
- 14.Chirinos JA, Veerani A, Zambrano JP, Schob A, Perez G, Mendez AJ, Chakko S. Evaluation of comorbidity scores to predict all-cause mortality in patients with established coronary artery disease. Int J Cardiol. 2007 Apr 12;117:97–102. doi: 10.1016/j.ijcard.2006.06.005. Epub 2006 Jul 12. [DOI] [PubMed] [Google Scholar]
- 15.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 16.Jacobs DR, Jr, Kroenke C, Crow R, Deshpande M, Gu DF, Gatewood L, Blackburn H. PREDICT: a simple risk score for clinical severity and long-term prognosis after hospitalization for acute myocardial infarction or unstable angina: the Minnesota heart survey. Circulation. 1999;100:599–607. doi: 10.1161/01.cir.100.6.599. [DOI] [PubMed] [Google Scholar]
- 17.Krumholz HM, Chen J, Chen YT, Wang Y, Radford MJ. Predicting one-year mortality among elderly survivors of hospitalization for an acute myocardial infarction: results from the Cooperative Cardiovascular Project. J Am Coll Cardiol. 2001;38:453–9. doi: 10.1016/s0735-1097(01)01395-x. [DOI] [PubMed] [Google Scholar]
- 18.Lichtman JH, Krumholz HM, Wang Y, Radford MJ, Brass LM. Risk and predictors of stroke after myocardial infarction among the elderly: results from the Cooperative Cardiovascular Project. Circulation. 2002;105:1082–7. doi: 10.1161/hc0902.104708. [DOI] [PubMed] [Google Scholar]
- 19.Morrow DA, Antman EM, Charlesworth A, Cairns R, Murphy SA, de Lemos JA, Giugliano RP, McCabe CH, Braunwald E. TIMI risk score for ST-elevation myocardial infarction: a convenient, bedside, clinical score for risk assessment at presentation: an intravenous nPA for treatment of infarcting myocardium early II trial substudy. Circulation. 2000;102:2031–7. doi: 10.1161/01.cir.102.17.2031. [DOI] [PubMed] [Google Scholar]
- 20.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA. Fraility in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–56. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 21.Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G. Untangling the concepts of disability, fraility, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci. 2004;59:255–63. doi: 10.1093/gerona/59.3.m255. [DOI] [PubMed] [Google Scholar]
- 22.Pressley JC, Patrick CH. Fraility bias in comorbidity risk adjustments of community-dwelling elderly populations. J Clin Epidemiol. 1999;52:753–60. doi: 10.1016/s0895-4356(99)00056-6. [DOI] [PubMed] [Google Scholar]
- 23.Ellis SG, Weintraub W, Holmes D, Shaw R, Block PC, King SB., III Relation of operator volume and experience to procedural outcome of percutaneous coronary revascularization at hospitals with high interventional volumes. Circulation. 1997;95:2479–84. doi: 10.1161/01.cir.95.11.2479. [DOI] [PubMed] [Google Scholar]
- 24.Holmes DR, Jr, Berger PB, Garratt KN, Mathew V, Bell MR, Barsness GW, Higano ST, Grill DE, Hammers LN, Rihal CS. Applicaton of the New York State PTCA mortality model in patients undergoing stent implantation. Circulation. 2000;102:517–22. doi: 10.1161/01.cir.102.5.517. [DOI] [PubMed] [Google Scholar]