Abstract
Background:
We evaluated the cross-sectional relationship of blood pressure (BP) components with cognitive impairment after adjusting for potential confounders.
Methods:
Reasons for Geographic and Racial Differences in Stroke (REGARDS) is a national, longitudinal population cohort evaluating stroke risk in 30,228 black and white men and women ≥45 years old. During the in-home visit, BP measurements were taken as the average of 2 measurements using a standard aneroid sphygmomanometer. Excluding participants with prior stroke or TIA, the present analysis included 19,836 participants (enrolled from December 2003 to March 2007) with complete baseline physical and cognitive evaluations. Incremental logistic models examined baseline relationships between BP components (systolic blood pressure [SBP], diastolic blood pressure [DBP], and pulse pressure [PP]) and impaired cognitive status (score of ≤4 on 6-Item Screener) after adjusting for demographic and environmental characteristics, cardiovascular risk factors, depressive symptoms, and current use of any antihypertensive medication.
Results:
Higher DBP levels were associated with impaired cognitive status after adjusting for demographic and environmental characteristics, risk factors, depressive symptoms, and antihypertensive medications. An increment of 10 mm Hg in DBP was associated with a 7% (95% confidence interval [CI] 1%–14%, p = 0.0275) higher odds of cognitive impairment. No independent association was identified between impaired cognitive status and SBP (odds ratio [OR] 1.02, 95% CI 0.99–1.06) or PP (OR 0.99, 95% CI 0.95–1.04). There was no evidence of nonlinear relationships between any of the BP components and impaired cognitive status. There was no interaction between age and the relationship of impaired cognitive status with SBP (p = 0.827), DBP (p = 0.133), or PP (p = 0.827) levels.
Conclusions:
Higher diastolic blood pressure was cross-sectionally and independently associated with impaired cognitive status in this large, geographically dispersed, race- and sex-balanced sample of stroke-free individuals.
GLOSSARY
- AD
= Alzheimer disease;
- ARIC
= Atherosclerosis Risk in Community;
- BMI
= body mass index;
- BP
= blood pressure;
- CES-D-4
= Center for Epidemiologic Studies-Depression–4-item version;
- CI
= confidence interval;
- DBP
= diastolic blood pressure;
- IQR
= interquartile range;
- OR
= odds ratio;
- PP
= pulse pressure;
- REGARDS
= Reasons for Geographic and Racial Differences in Stroke;
- SBP
= systolic blood pressure.
Hypertension and dementia are common disorders in elderly individuals. Among people aged 65 years and older, the prevalence of dementia is estimated to be approximately 8%, and the prevalence of hypertension is estimated to be 65%.1,2 The relation of blood pressure (BP) with cognitive function and dementia has, in recent years, received much attention from epidemiologic research.3–14 However, the findings of cross-sectional studies of BP and cognitive function have varied greatly. Some studies found higher rates of cognitive impairment associated with elevated BP,3–6 others with low BP,7 and other reports documented a U-shaped relationship8,9 or lack of any association.10 Similarly, the results of longitudinal population-based studies investigating the relation between BP and cognitive status are inconsistent with some studies reporting no significant effect of BP on cognition,11 others documenting an association of hypertension with cognitive decline,12,13 and recent reports showing a U-shaped relationship between BP and cognitive function.7,14 Moreover, interest has recently focused on the potential association of the pulsatile component of BP (pulse pressure [PP]) with impaired cognitive function, with inconsistent results being reported across different studies.15–17
This study aims to extend previous research by capitalizing on the large, geographically dispersed, race- and sex-balanced sample of the Reasons for Geographic and Racial Differences in Stroke (REGARDS) Study. The study offers the opportunity to adjust for numerous potential confounders of the relationship between BP and cognitive function and also to evaluate the potential interaction of BP and age on cognitive function.
METHODS
Study design.
REGARDS is a national, population-based, longitudinal cohort study with oversampling from the Stroke Belt region of the United States, an area that has stroke mortality rates higher than the rest of the country.18 Beginning in February 2003, a total of 30,000 individuals aged 45 years or older were being enrolled with the goal of equal representation of whites and blacks and men and women.18 The goal was to select 20% (achieved 21%) of the sample from the “buckle” of the Stroke Belt (coastal plain region of North Carolina, South Carolina, and Georgia), 30% (achieved 35%) from the Stroke Belt states (remainder of North Carolina, South Carolina, and Georgia, plus Alabama, Mississippi, Tennessee, Arkansas, and Louisiana), and the remaining 50% (achieved 44%) from the other 40 contiguous states. Methodologic details are available elsewhere.18 Cross-sectional analyses reported in this article are drawn from baseline data of subjects, who 1) were enrolled between December 18, 2003, and March 1, 2007; 2) reported no history of stroke, ministroke, or TIA; 3) had completed the baseline home physical; and 4) were administered the Center for Epidemiologic Studies-Depression–4-item version (CES-D-4) and the 6-Item Screener for assessment of cognitive status. The 6-Item Screener was added to the baseline evaluation in December 2003, 11 months after enrollment began (February 2003).
The present analysis was performed on the June 2007 data freeze. At that time, data were available in 27,731 individuals (92% of the eventually recruited 30,228 participants). Of these, 2,833 (10%) were not included in the present analyses because of history of prior stroke or TIA, resulting in 24,898 remaining participants. Of these, cognitive assessments were not available on 5,062 (20%) individuals. The vast majority of the participants with unavailable cognitive assessments were recruited before December 2003, when the 6-Item Screener was added in the baseline evaluation. The remaining 19,836 individuals were included in the present analyses.
Standard protocol approvals, registration, and consents.
The study methods have been reviewed and approved by the institutional review boards of all participating institutions. Written informed consent was obtained from all patients involved in the present study.
Data collection and definitions.
Defined according to previously recommended standards, the cooperation rate (participation rate among known eligible participants) was more than 60%, and the response rate (participation rate among known and imputed eligible participants) was between 25% and 40% depending on the assumptions made regarding eligibility among households where no contact was made.19
Demographic variables included in the present analyses were age, sex, race, and region of residence (categorized as Stroke Belt, Stroke Buckle, or other regions). Definitions of behavioral variables (smoking history, alcohol use, exercise habits, and educational level) and vascular risk factors (diabetes mellitus, hypercholesterolemia, and obesity) have been previously described.18
BP measurements.
BP was taken as an average of 2 measurements taken after the participant was seated for 5 minutes, and was measured by a trained technician using a standard protocol and regularly tested aneroid sphygmomanometer. All BP measurements were taken during the home visits on Monday through Thursday mornings (morning visits conducted because of the requirement for fasting).18 BP quality control was monitored by central examination of digit preference, and retraining of technicians took place as necessary.
Assessment of cognitive status.
Designed for either in-person or telephone administration, the 6-Item Screener is a test of global cognitive function derived from the widely used Mini-Mental State Examination.20 The 6-Item Screener has been validated against the Mini-Mental State Examination, other cognitive measures, and diagnoses of dementia and cognitive impairment in the absence of dementia in 2 populations: a community-based survey of 344 black adults with a second-stage formal diagnostic evaluation and a clinical sample of 651 adults (16.1% black) with the same diagnostic evaluation.20 Items assess recall and temporal orientation. Scores range from 0 to 6; a score of 4 or fewer correct indicates cognitive impairment.21
Assessment of depressive symptoms.
The CES-D-4 was used to evaluate depressive symptoms. Scores range from 0 to 12; a score ≥4 indicates an elevated level of psychological distress.22
Statistical analyses.
The relationship between BP variables (systolic blood pressure [SBP], diastolic blood pressure [DBP], and PP) and cognitive impairment was evaluated in a set of incremental logistic regression models. All BP variables were entered in the logistic regression models as continuous variables. First, crude associations were estimated (model I) followed by subsequent adjustment for 1) demographic factors (age, sex, race, and region) (model II); 2) further adjustment for environmental factors (smoking, alcohol use, exercise habits, and educational level) (model III); (3) additional adjustment for vascular risk factors (diabetes mellitus, hypercholesterolemia, and obesity) (model IV); (4) additional adjustment for depressive symptoms (model V); and (5) final adjustment for current use of any antihypertensive medication (model VI). Unadjusted and adjusted odds ratios (ORs) with 95% confidence intervals (95% CIs) were estimated. Because an interaction between age and BP components on risk for cognitive impairment and dementia has been suggested,23 we also investigated the presence of interactions between SBP, DBP, or PP with age on the likelihood of cognitive impairment.
RESULTS
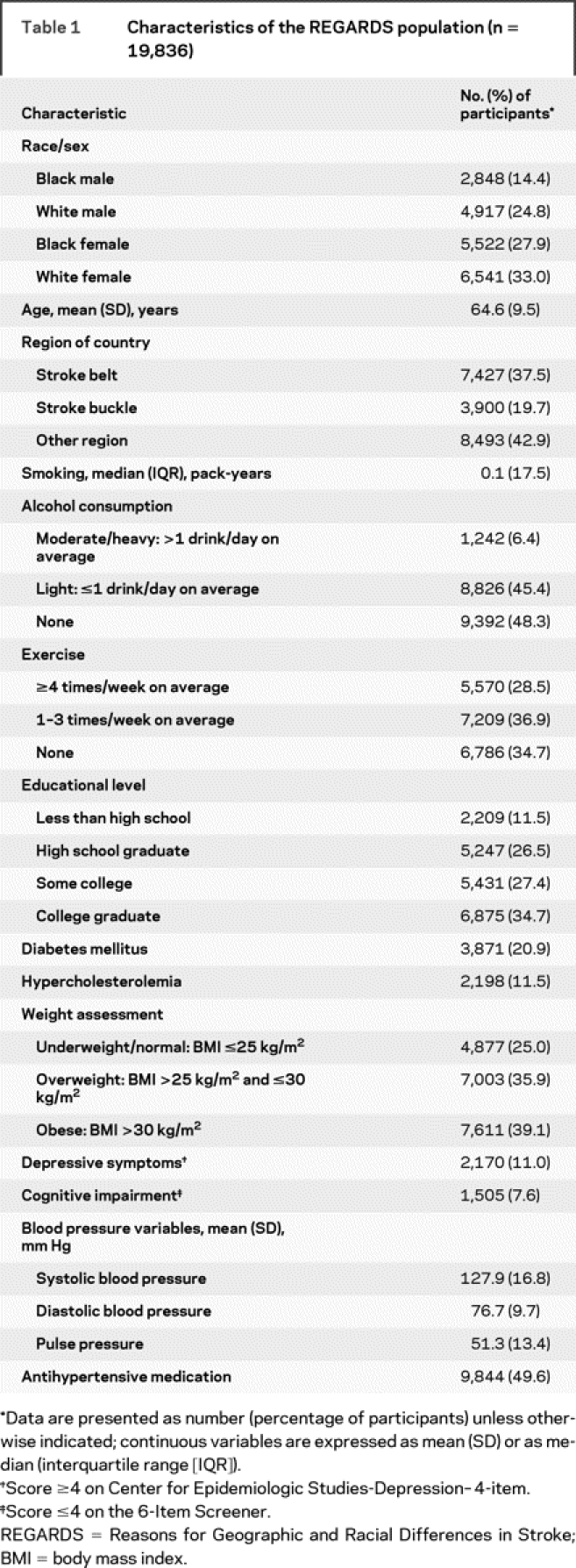
The sample of 19,836 individuals included in the present analyses had a mean age of 64.6 years (SD 9.5 years) and was 42.3% black, 39.2% male, and 57.2% from the Stroke Belt (table 1). Demographic characteristics, environmental and stroke risk factors, and prevalence of depressive symptoms and cognitive impairment are also presented in detail in table 1. Impaired cognitive status was identified in 1,505 individuals (7.6%). A total of 9,844 (49.6%) individuals were under antihypertensive medications. After comparing baseline characteristics and BP variables between individuals enrolled in the present analyses and individuals excluded because of missing cognitive assessments or prior history of stroke or TIA, we documented higher SBP (130 ± 17 vs 127 ± 17 mm Hg, p < 0.001), DBP (77.1 ± 10 vs 76.7 ± 10 mm Hg, p = 0.002), and PP (53 ± 14 vs 51 ± 13 mm Hg, p < 0.001) levels in the group of patients who were excluded from the analyses. Also, patients who were excluded from the analyses were older (68 ± 9 vs 64 ± 10 years, p < 0.001) and had a higher prevalence of diabetes mellitus (26% vs 21%, p < 0.001) compared with patients who were included in the analyses. In addition, they were more frequently using antihypertensive medications (57.3% vs 49.6%, p < 0.001).
Table 1 Characteristics of the REGARDS population (n = 19,836)
Results of incremental logistic regression models estimating ORs for impaired cognitive status appear in table 2. Higher SBP (OR 1.13, 95% CI 1.10–1.17), DBP (OR 1.08, 95% CI 1.02–1.14), and PP (OR 1.17, 95% CI 1.12–1.21) levels were associated with cognitive impairment in unadjusted logistic regression models. In multivariable analysis, higher DBP levels were associated with impaired cognitive status after adjusting for demographic (model II) and environmental (model III) characteristics, vascular risk factors (model IV), depressive symptoms (model V), and current use of any antihypertensive medication (model VI). An increment of 10 mm Hg in DBP was associated with a 7% (95% CI 1%–14%, p = 0.0275) higher odds of cognitive impairment in the final multivariable logistic regression model. No independent association was identified between impaired cognitive status and SBP (OR 1.02, 95% CI 0.99–1.06) or PP (OR 0.99, 95% CI 0.95–1.04) after adjusting for demographics, vascular risk factors, environmental behaviors, depressive symptoms, and antihypertensive medications.
Table 2 Blood pressure variables and ORs (95% CI) for cognitive impairment
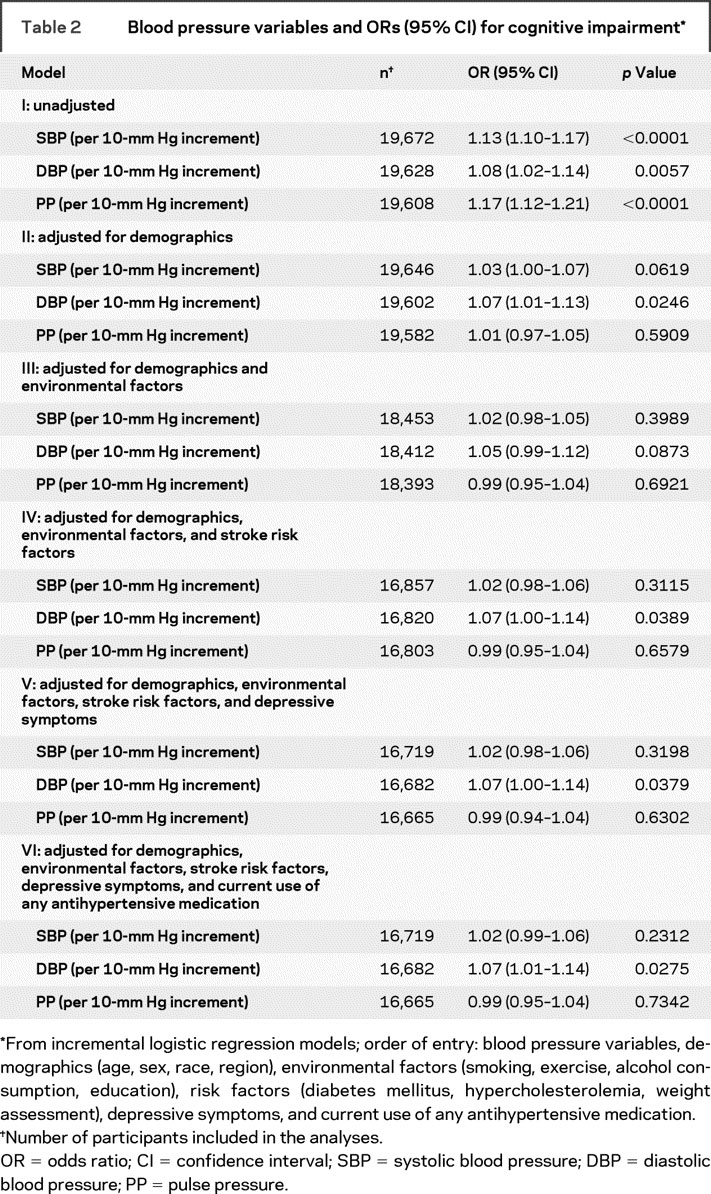
There was no significant evidence of nonlinear relationships between any of the BP components and impaired cognitive status (p > 0.05). There was no evidence of interaction between age and the relationship of impaired cognitive status with SBP (p = 0.827), DBP (p = 0.133), or PP (p = 0.827) levels. Also no interaction was identified between race and the relationship of impaired cognitive status with SBP (p = 0.899), DBP (p = 0.966), or PP (p = 0.858) levels.
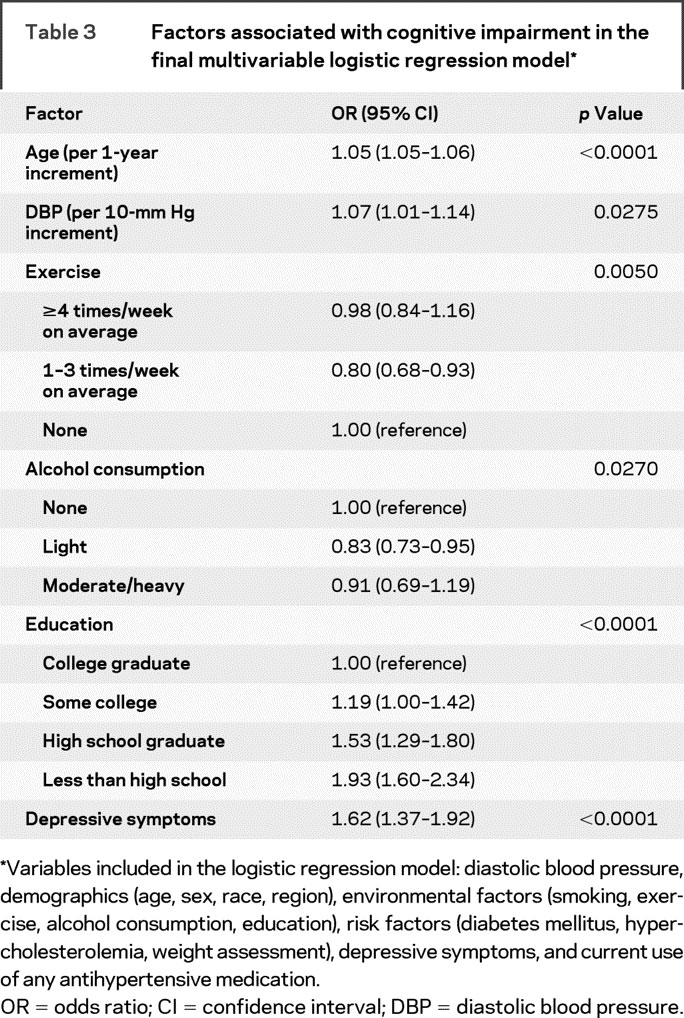
The following factors were independently associated with a higher likelihood of cognitive impairment in multivariate analysis (table 3): higher age, lower educational level, depressive symptoms, and higher DBP values. Individuals with light alcohol consumption and moderate exercise habits had lower odds of cognitive impairment compared with patients with no alcohol consumption and no exercise habits. In the final multivariable analyses, we also evaluated the relationship of hypertension with impaired cognitive status (BP components were substituted by hypertension in the incremental logistic regression models). Hypertension (defined as SBP ≥140 mm Hg or DBP ≥90 mm Hg or currently taking antihypertensive medication) was not related to impaired cognitive status after adjustment for potential confounders (OR 0.91, 95% CI 0.79–1.05, p = 0.1981). Moreover, no interaction was identified between antihypertensive medication and the relationship of impaired cognitive status with SBP (p = 0.138), DBP (p = 0.133), or PP (p = 0.225) levels. Finally, we evaluated the relationship of BP variables with impaired cognitive status after including patients with prior stroke or TIA in our analyses. An increment of 10 mm Hg in DBP was associated with an 8% (95% CI 2%–15%, p = 0.008) higher odds of cognitive impairment in the final multivariable logistic regression model. No independent association was identified between impaired cognitive status and SBP (OR 1.02, 95% CI 0.99–1.06) or PP (OR 0.99, 95% CI 0.95–1.03).
Table 3 Factors associated with cognitive impairment in the final multivariable logistic regression model
DISCUSSION
Our cross-sectional study showed a linear relationship between higher DBP levels and impaired cognitive status in individuals without prior history of stroke or TIAs. This relationship persisted after adjustment for demographic characteristics, vascular risk factors, health behaviors, depressive symptoms, and antihypertensive medications. Although both higher PP and SBP values were related to cognitive impairment in unadjusted analyses, these associations were no longer significant after accounting for demographics, health behaviors, and vascular risk factors. We also found no evidence of interaction between age or race and the relationship between BP and cognitive function.
In our data set, a 10-mm Hg increment of DBP was associated with 7% higher odds of impaired cognitive status. This finding is concordant, though at an attenuated level, with an Italian cross-sectional survey24 where each 10-mm Hg increment in DBP was cross-sectionally associated with a 29% higher odds for cognitive impairment. Likewise, a similar cross-sectional relationship was previously documented in a male Swedish cohort, with each SD difference in DBP values being associated with a 45% higher likelihood of low cognitive function.25 Interestingly, another study after evaluating longitudinally the association of BP with cognitive status in the Framingham Study documented that elevated BP levels were linearly correlated to a higher likelihood of impaired cognitive functioning in individuals receiving no antihypertensive mediations.26 In contrast, the East Boston27 and the Maastricht28 Aging Studies reported no cross-sectional association between BP and cognitive function, whereas in the Indo-US Cross National Epidemiologic Study each 10-mm Hg difference in DBP or SBP was related to more than a 10% decrement in cognitive impairment.29 Moreover, higher SBP (and not DBP) levels were related to cognitive impairment in other reports.4,30 Finally, a recent report from the Women’s Health Initiative Memory Study failed to detect any longitudinal independent associations between hypertension or high BP levels with mild cognitive impairment or probable dementia in older (mean age 71 ± 4 years), cognitively intact, postmenopausal women.31 Important methodologic disparities related to the different study samples with regard to age and race, different definitions of impaired cognitive status, different BP cutoffs, and different confounders taken into account in the multivariate analyses may account for these discrepant results.
Interestingly, the Atherosclerosis Risk in Community (ARIC) investigators, who evaluated the second largest population sample of 13,840 individuals, 25% of whom were of African-American origin, documented an independent cross-sectional association between hypertension (defined as ≥160/95 mm Hg) and lower scores on cognitive tests. However, the potential linear relationships of BP variables with low cognitive function were not reported in the ARIC cohort.3 A pooled analysis of studies where BP variables were not dichotomized and were evaluated as continuous variables may provide some more definitive data to support the notion that higher BP levels are linearly associated with cognitive functioning independent of age, health behaviors, and stroke risk factors. In addition, the causal link between hypertension and cognitive impairment can be established only in well-designed and adequately powered longitudinal studies. Indeed, after completion of the 4-year follow-up data in the REGARDS data set, we aim to perform similar analyses to evaluate the reported cross-sectional associations between higher DBP and impaired cognitive status in a longitudinal fashion.
It is intriguing that DBP but not SBP (nor PP) was independently related to impaired cognitive status in our data set. Experimental studies have shown that small cerebral arterioles, which are influenced profoundly by DBP, undergo vascular atrophy progressively with age.32 Neuropathologic data indicate that elevated DBP levels accelerate this process and lead to formation of ischemic white matter lesions in subcortical areas of the brain.33 Moreover, higher DBP levels have been associated with the severity of white matter disease both cross-sectionally and longitudinally in large randomly selected samples from 2 population-based studies.34 Furthermore, higher DBP (and not SBP or PP) was an independent predictor of white matter hyperintensity progression in elderly individuals both without neuropsychiatric disease35 and with Alzheimer disease (AD).36 Interestingly, a recent longitudinal study showed that higher DBP in persons untreated for hypertension 5 years before MRI was the only BP component that predicted more atrophy of structures affected by AD, such as the hippocampus and amygdala, on brain MRI 5 years later, whereas higher severity of white matter lesions coexisted with more prominent hippocampal atrophy.37 In view of the former considerations, it may be hypothesized that diastolic hypertension accelerates arteriosclerotic changes in the brain, predisposing to arteriosclerosis and arteriolar tortuosity of small cerebral vessels. These vascular changes, incorporating medial thickening and intimal proliferation, result in a reduction of luminal diameter, which in turn causes increased resistance to flow and decline in cerebral perfusion. Such hypoperfusion may produce discrete regions of small cerebral infarctions and diffuse ischemic changes in the periventricular and deep white matter (leukoaraiosis), causing vascular cognitive impairment and also contributing to the pathogenesis of AD by destabilizing neurons and synapses.
Certain limitations of the present study need to be acknowledged. For one, the cross-sectional design of the study does not provide evidence of a causative relation between BP levels and cognitive impairment. Moreover, the interpretation of cross-sectional relationships between BP and cognitive function is problematic because cognitive impairment itself may affect BP levels through its effects on diet and weight loss. Another study limitation is the lack of imaging data, confining the ability to link BP and its control to neuropathology and cognitive deficits. Furthermore, because BP measurements were performed only in the sitting position, we were unable to assess the potential association of orthostatic hypotension with impaired cognitive status in our data set. It may also be argued that the imbalances between patients who were included and excluded from the present analyses may have influenced the documented association between DBP and impaired cognitive status. However, the older age, the higher prevalence of diabetes mellitus, and the more elevated BP variables in the group of patients who were excluded from the analysis may be attributed to the fact that all patients with prior history of stroke or TIA were not included in our final sample of 19,836. Interestingly, we documented the same independent association between higher DBP and impaired cognitive status when we repeated our multivariable logistic regression analyses after including patients with a prior history of stroke or TIA. Finally, the possibility of confounding due to central obesity (which shows a stronger relationship with cognitive function than body mass index),38,39 undetected sleep apnea, and concomitant nonstroke cardiovascular disease (such as myocardial infarction or congestive heart failure) should be acknowledged as another possible limitation of the present analyses.
ACKNOWLEDGMENT
The authors acknowledge the participating investigators and institutions for their valuable contributions: The University of Alabama at Birmingham (Study PI, Statistical and Data Coordinating Center, Survey Research Unit): George Howard, DrPH, Leslie McClure, PhD, Virginia Howard, PhD, Libby Wagner, MA, Virginia Wadley, PhD, Rodney Go, PhD, Monika Safford, MD, Ella Temple, PhD, Margaret Stewart, MSPH, J. David Rhodes, BSN; University of Vermont (Central Laboratory): Mary Cushman, MD; Wake Forest University (ECG Reading Center): Ron Prineas, MD, PhD; Alabama Neurological Institute (Stroke Validation Center, Medical Monitoring): Camilo Gomez, MD, Susana Bowling, MD; University of Arkansas for Medical Sciences (Survey Methodology): LeaVonne Pulley, PhD; University of Cincinnati (Clinical Neuroepidemiology): Brett Kissela, MD, Dawn Kleindorfer, MD; Examination Management Services, Incorporated (In-Person Visits): Andra Graham; Medical University of South Carolina (Migration Analysis Center): Daniel Lackland, DrPH; Indiana University School of Medicine (Neuropsychology Center): Frederick Unverzagt, PhD; National Institute of Neurological Disorders and Stroke, NIH (funding agency): Claudia Moy, PhD.
DISCLOSURE
Dr. Tsivgoulis, Dr. Alexandrov, Dr. Wadley, and Dr. Moy report no disclosures. Dr. Unverzagt serves as consultant for Eli Lilly and Co.; and receives research support from the NIH [R01 AG026096 (PI), U01 NR004508 (PI), R01 AG09956 (Co-PI), P30 AG10133 (Co-PI)], Indiana University [ITRAC 23-87539 (PI)], and the American Cancer Society [RSGPB04-089-01-PBP (Co-PI)]. Dr. Go receives research support from the NINDS [U01NS04588 (Coinvestigator)]. Dr. Kissela has received speaker honoraria from Boehringer-Ingelheim and Allergan; receives research support from the NIH [NIH-NINDS R-01 NS30678 (Multiple PI), NIH-NINDS R-01 NS039987 (Site PI and Member of Executive Committee), NIH-NINDS U-01 NS041588 (Member of Executive Committee)]; and has served as an expert witness in medicolegal cases related to stroke. Dr. Howard serves on a scientific advisory board for Bayer Healthcare; serves as a Section Editor for Stroke and on the editorial board of American Society of Hypertension; and receives research support from Amgen and the NIH [NINDS NS41588 (PI), NINDS NS38384 (Co-PI of Coordinating Center), NIAMS AR02247 (Co-PI), NICHD HD39939 (Co-PI), NINDS NS038529 (Coinvestigator), NHLBI HL77100 (Coinvestigator), and NINDS NS34789 (Coinvestigator)].
Address correspondence and reprint requests to Dr. Georgios Tsivgoulis, Comprehensive Stroke Center, University of Alabama at Birmingham, RWUH M226, 1530 3rd Avenue S, Birmingham, AL 35294-3280 tsivgoulisgiorg@yahoo.gr
Supported by a cooperative agreement U01 NS041588 from the National Institute of Neurological Disorders and Stroke, NIH, Department of Health and Human Services. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke or the NIH. Dr. Tsivgoulis is recipient of a neurosonology fellowship grant from the Neurology Department of Eginition Hospital, University of Athens School of Medicine, Athens, Greece.
Disclosure: Author disclosures are provided at the end of the article.
Received December 17, 2008. Accepted in final form May 22, 2009.
REFERENCES
- 1.Fratiglioni L, De Ronchi D, Agóero-Torres H. World-wide prevalence and incidence of dementia. Drugs Aging 1999;15:365–375. [DOI] [PubMed] [Google Scholar]
- 2.Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet 2005;365:217–223. [DOI] [PubMed] [Google Scholar]
- 3.Cerhan JR, Folsom AR, Mortimer JA, et al. Correlates of cognitive function in middle-aged adults. Gerontology 1998;44:95–105. [DOI] [PubMed] [Google Scholar]
- 4.Kuo HK, Sorond F, Iloputaife I, Gagnon M, Milberg W, Lipsitz LA. Effect of blood pressure on cognitive functions in elderly persons. J Gerontol A Biol Sci Med Sci 2004;59:1191–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harrington F, Saxby BK, McKeith IG, Wesnes K, Ford GA. Cognitive performance in hypertensive and normotensive older subjects. Hypertension 2000;36:1079–1082. [DOI] [PubMed] [Google Scholar]
- 6.Launer LJ, Masaki K, Petrovitch H, Foley D, Havlik RJ. The association between midlife blood pressure levels and late-life cognitive function: the Honolulu-Asia Aging Study. JAMA 1995;274:1846–1851. [PubMed] [Google Scholar]
- 7.Guo Z, Fratiglioni L, Winblad B, Viitanen M. Blood pressure and performance on the Mini-Mental State Examination in the very old: cross-sectional and longitudinal data from the Kungsholmen Project. Am J Epidemiol 1997;145:1106–1113. [DOI] [PubMed] [Google Scholar]
- 8.Morris MC, Scherr PA, Hebert LE, et al. Association between blood pressure and cognitive function in a biracial community population of older persons. Neuroepidemiology 2002;21:123–130. [DOI] [PubMed] [Google Scholar]
- 9.Waldstein SR, Giggey PP, Thayer JF, Zonderman AB. Nonlinear relations of blood pressure to cognitive function: the Baltimore Longitudinal Study of Aging. Hypertension 2005;45:374–379. [DOI] [PubMed] [Google Scholar]
- 10.Farmer ME, White LR, Abbott RD, et al. Blood pressure and cognitive performance: the Framingham Study. Am J Epidemiol 1987;126:1103–1114. [DOI] [PubMed] [Google Scholar]
- 11.Hebert LE, Scherr PA, Bennett DA, et al. Blood pressure and late life cognitive function change: a biracial longitudinal population study. Neurology 2004;62:2021–2024. [DOI] [PubMed] [Google Scholar]
- 12.Elias MF, Elias PK, Sullivan LM, Wolf PA, D’Agostino RB. Lower cognitive function in the presence of obesity and hypertension: the Framingham Heart Study. Int J Obes 2003;27:260–268. [DOI] [PubMed] [Google Scholar]
- 13.Kivipelto M, Helkala E-L, Honninen T, et al. Midlife vascular risk factors and late life cognitive impairment: a population-based study. Neurology 2001;56:1683–1689. [DOI] [PubMed] [Google Scholar]
- 14.Glynn RJ, Beckett LA, Hebert LE, Morris MC, Scherr PA, Evans DA. Current and remote blood pressure and cognitive decline. JAMA 1999;281:438–445. [DOI] [PubMed] [Google Scholar]
- 15.Triantafyllidi H, Arvaniti C, Lekakis J, et al. Cognitive impairment is related to increased arterial stiffness and microvascular damage in patients with never-treated essential hypertension. Am J Hypertens 2009;22:525–530. [DOI] [PubMed] [Google Scholar]
- 16.Qiu C, Winblad B, Viitanen M, Fratiglioni L. Pulse pressure and risk of Alzheimer disease in persons aged 75 years and older: a community based, longitudinal study. Stroke 2003;34:594–599. [DOI] [PubMed] [Google Scholar]
- 17.Freitag MH, Peila R, Masaki K, Petrovitch H, Ross GW, White LR, Launer LJ. Midlife pulse pressure and incidence of dementia: the Honolulu-Asia Aging Study. Stroke 2006;37:33–37. [DOI] [PubMed] [Google Scholar]
- 18.Howard VJ, Cushman M, Pulley L, et al. The Reasons for Geographic and Racial Differences in Stroke Study: objectives and design. Neuroepidemiology 2005;25:135–143. [DOI] [PubMed] [Google Scholar]
- 19.Morton LM, Cahill J, Hartge P. Reporting participation in epidemiologic studies: a survey of practice. Am J Epidemiol 2006;163:197–203. [DOI] [PubMed] [Google Scholar]
- 20.Folstein MF, Folstein SE, McHugh SE. Mini-Mental State: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 21.Callahan CM, Unverzagt FW, Hui SL, Perkins AJ, Hendrie HC. Six-Item Screener to identify cognitive impairment among potential subjects for clinical research. Med Care 2002;40:771–781. [DOI] [PubMed] [Google Scholar]
- 22.Melchior LA, Huba GJ, Brown VB, Reback CJ. A short depression index for women. Educ Psychol Meas 1993;53:1117–1125. [Google Scholar]
- 23.Qiu C, Winblad B, Fratiglioni L. The age-dependent relation of blood pressure to cognitive function and dementia. Lancet Neurol 2005;4:487–499. [DOI] [PubMed] [Google Scholar]
- 24.Cacciatore F, Abete P, Ferrara N, et al. The role of blood pressure in cognitive impairment in an elderly population. J Hypertens 1997;15:135–142. [DOI] [PubMed] [Google Scholar]
- 25.Kilander L, Nyman H, Boberg M, Hansson L, Lithell H. Hypertension is related to cognitive impairment: a 20-year follow-up of 999 men. Hypertension 1998;31:780–786. [DOI] [PubMed] [Google Scholar]
- 26.Elias MF, Wolf PA, D’Agostino RB, Cobb J, White LR. Untreated blood pressure level is inversely related to cognitive functioning: the Framingham Study. Am J Epidemiol 1993;138:353–364. [DOI] [PubMed] [Google Scholar]
- 27.Scherr PA, Hebert LE, Smith LA, Evans DA. Relation of blood pressure to cognitive function in elderly. Am J Epidemiol 1991;134:1303–1315. [DOI] [PubMed] [Google Scholar]
- 28.van Boxtel MP, Gaillard C, Houx PJ, Buntinx F, de Leeuw PW, Jolles J. Can the blood pressure predict cognitive task performance in a healthy population sample? J Hypertens 1997;15:1069–1076. [DOI] [PubMed] [Google Scholar]
- 29.Pandav R, Dodge HH, DeKosky ST, Ganguli M. Blood pressure and cognitive impairment in India and the United States: a crossnational epidemiological study. Arch Neurol 2003;60:1123–1128. [DOI] [PubMed] [Google Scholar]
- 30.Budge MM, de Jager C, Hogervorst E, Smith AD. Total plasma homocysteine, age, systolic blood pressure, and cognitive performance in older people. J Am Geriatr Soc 2002;50:2014–2018. [DOI] [PubMed] [Google Scholar]
- 31.Johnson KC, Margolis KL, Espeland MA, et al; Women’s Health Initiative Memory Study and Women’s Health Initiative Investigators. A prospective study of the effect of hypertension and baseline blood pressure on cognitive decline and dementia in postmenopausal women: the Women’s Health Initiative Memory Study. J Am Geriatr Soc 2008;56:1449–1458. [DOI] [PubMed] [Google Scholar]
- 32.Hajdu MA, Heistad DD, Siems JE, Baumbach GL. Effects of aging on mechanics and composition of cerebral arterioles in rats. Circ Res 1990;66:1747–1754. [DOI] [PubMed] [Google Scholar]
- 33.Englund E, Brun A, Alling C. White matter changes in dementia of Alzheimer’s type: biochemical and neuropathological correlates. Brain 1988;111:1425–1439. [DOI] [PubMed] [Google Scholar]
- 34.de Leeuw FE, de Groot JC, Oudkerk M, et al. A follow-up study of blood pressure and cerebral white matter lesions. Ann Neurol 1999;46:827–833. [DOI] [PubMed] [Google Scholar]
- 35.Schmidt R, Schmidt H, Kapeller P, Lechner A, Fazekas F. Evolution of white matter lesions. Cerebrovasc Dis 2002;13 Suppl 2:16–20. [DOI] [PubMed] [Google Scholar]
- 36.de Leeuw FE, Barkhof F, Scheltens P. Progression of cerebral white matter lesions in Alzheimer’s disease: a new window for therapy? J Neurol Neurosurg Psychiatry 2005;76:1286–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.den Heijer T, Launer LJ, Prins ND, et al. Association between blood pressure, white matter lesions, and atrophy of the medial temporal lobe. Neurology 2005;64:263–267. [DOI] [PubMed] [Google Scholar]
- 38.Jagust W, Harvey D, Mungas D, Haan M. Central obesity and the aging brain. Arch Neurol 2005;62:1545–1458. [DOI] [PubMed] [Google Scholar]
- 39.Whitmer RA, Gustafson DR, Barrett-Connor E, Haan MN, Gunderson EP, Yaffe K. Central obesity and increased risk of dementia more than three decades later. Neurology 2008;71:1057–1064. [DOI] [PubMed] [Google Scholar]


