Abstract
Summary
T follicular helper (Tfh) cells help development of antibody responses via Interleukin-21 (IL-21). Here we show that activated human dendritic cells (DCs) induced naïve CD4+ T cells to become IL-21-producing Tfh-like cells through IL-12. CD4+ T cells primed with IL-12 induced B cells to produce immunoglobulins in a fashion dependent on IL-21 and inducible costimulator (ICOS), thus sharing fundamental characteristics with Tfh cells. The induction of Tfh-like cells by activated DCs was inhibited by neutralizing IL-12. IL-12 induced two different IL-21-producers: IL-21+IFN-γ+T-bet+ Th1 cells and IL-21+IFN-γ-T-bet- non-Th1 cells, in a manner dependent on signal transducer and activator of transcription (STAT)4. IL-12 also regulated IL-21 secretion by memory CD4+ T cells. Thus, IL-12 produced by activated DCs regulates antibody responses via developing IL-21-producing Tfh-like cells, and inducing IL-21 secretion from memory CD4+ T cells. These data suggest that the developmental pathway of Tfh cells differs between mice and humans, which have considerable implications for vaccine development.
DCs sense microbial invasion and mobilize immune system effectors (Banchereau and Steinman, 1998; Pulendran et al., 2008; Reis e Sousa, 2004; Shortman and Liu, 2002). Upon recognition of signals derived from the innate immune system and/or microbial components, DCs migrate to secondary lymphoid organs, i.e., spleen and lymph nodes, where they mature and launch adaptive immunity. In particular, DC subsets play a central role in the induction of distinct subsets of T cells (Klechevsky et al., 2008; Ueno et al., 2007), which produce different sets of cytokines necessary to clear distinct types of microbes. T helper (Th) 1 cells secrete Interferon (IFN)-γ which allows for the control of intracellular microbes, Th2 cells secrete Interleukin (IL)-4 which mediates immunity against extracellular parasites (Mosmann and Coffman, 1989), and Th17 cells secrete IL-17A and IL-22 which control extracellular bacteria (Bettelli et al., 2007; Ouyang et al., 2008). DCs produce soluble factors or express cell surface molecules that regulate the fate of T cells. For example, IL-12-secreting DCs potently promote the development of Th1 cells (Trinchieri, 2003). On the contrary, DCs lacking IL-12 secretion (Langenkamp et al., 2000; Pulendran et al., 2001), particularly those expressing OX40-ligand (Ito et al., 2005), promote Th2 responses.
Recently, a subset of CD4+ T cells, T follicular helper (Tfh) cells, originally found in germinal centers (GCs) of secondary lymphoid organs (Breitfeld et al., 2000; Campbell et al., 2001; Kim et al., 2001; Schaerli et al., 2000), has been established as a critical cell compartment specialized for the help of B cell responses (Fazilleau et al., 2009; King et al., 2008; Vinuesa et al., 2005). Tfh cells express chemokine (C-X-C motif) receptor 5 (CXCR5), and migrate into B cell follicle in response to its ligand, CXCL13, which is produced by follicular DCs (Cyster et al., 2000; Gunn et al., 1998). Together with activated B cells and follicular DCs, Tfh cells constitute germinal centers (GC), where B cells undergo isotype switching and somatic hypermutation. This step permits the selection of high-affinity B cells in GCs, and leads to the generation of B cell memory (Allen et al., 2007; MacLennan, 1994). Tfh cells provide help to B cells through several factors, including CD40 ligand (CD40L) (Banchereau et al., 1994) and ICOS (Hutloff et al., 1999). In particular, Tfh cells secrete the cytokine IL-21 (Bryant et al., 2007), which drives the growth, differentiation, and isotype switching of B cells (Kuchen et al., 2007; Spolski and Leonard, 2008). Furthermore, substantial evidence shows that Tfh cells at extrafollicular sites also help B cell differentiation into plasma cells in an IL-21-dependent fashion (King et al., 2008; Odegard et al., 2008). However, the mechanism whereby human DCs induce such IL-21-producing Tfh cells is unknown. IL-21 itself provides a positive feedback loop to CD4+ T cells and induces human (Caprioli et al., 2008) and mouse (Korn et al., 2007; Nurieva et al., 2007; Suto et al., 2008; Vogelzang et al., 2008; Wei et al., 2007) naïve CD4+ T cells to secrete more IL-21. The critical involvement of IL-21 for the induction of Tfh cells in vivo was recently demonstrated in mouse model (Nurieva et al., 2008; Vogelzang et al., 2008). However, antigen presenting cells (APCs) including DCs or naïve CD4+ T cells do not secrete IL-21, therefore raising the question about the mechanism whereby APCs trigger the differentiation of IL-21 producing CD4+ T cells. Recently, IL-6 has been shown to induce mouse CD4+ T cells to secrete IL-21 (Dienz et al., 2009; Zhou et al., 2007), but whether human CD4+ T cells share the same pathway is unknown.
In this study, we demonstrate that human DCs instruct naïve CD4+ T cells to become IL-21-producing Tfh-like cells through the secretion of IL-12, thus revealing another substantial difference in the immune systems of mice and humans (Mestas and Hughes, 2004). The nomenclature of “Tfh-like” cells is discussed later.
RESULTS
Activated DCs induce naïve CD4+ T cells to produce IL-21
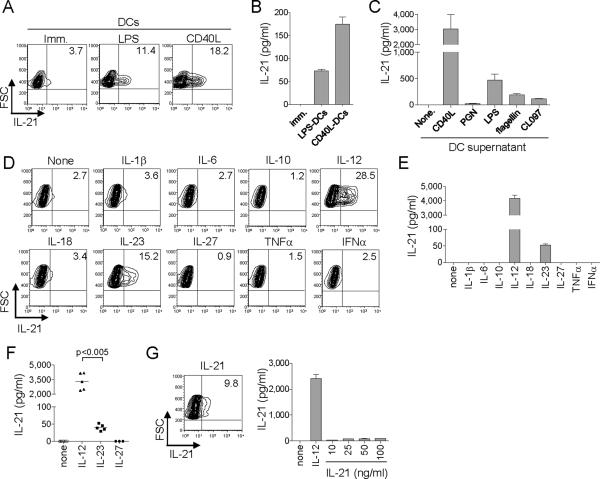
We first examined whether human DCs were able to induce naïve CD4+ T cells to differentiate into CD4+ T cells secreting IL-21. DCs were generated by culturing monocytes with GM-CSF and IL-4 for 6 d, and activated for 6 h with LPS or CD40L-transfected L cells. DCs were subsequently cultured (1.3 × 103 cells/well) with allogeneic naïve CD4+ T cells (4 × 104 cells/well). At day 7, CD4+ T cells were restimulated with PMA and ionomycin for 6 h in the presence of brefeldin A to assess intracytoplasmic IL-21 expression. Very low amounts of intracytoplasmic IL-21 were detected in a small fraction of CD4+ T cells primed with resting DCs (Figure 1A. 4.7 ± 2.7% of activated CD4+ T cells. Mean ± s.e.m. n=3). In contrast, DCs exposed to LPS or CD40L induced a larger fraction of CD4+ T cells to express IL-21 (Figure 1A. 14.8 ± 1.6%, and 22.5 ± 0.8% of activated CD4+ T cells, respectively. Mean ± s.e.m. n=3). Note that the expression of IL-21 was much higher on T cells that have been primed with activated DCs than in T cells primed with immature DCs (Figure 1A). Accordingly, CD4+ T cells primed with activated DCs, but not with immature DCs, secreted high amounts of IL-21 when re-stimulated for 24 h with anti-CD3 + anti-CD28 (Figure 1B).
Figure 1. IL-12 potently induces naïve CD4+ T cells to secrete IL-21.
(A) Human naïve CD4+ T cells cultured with allogeneic DCs stimulated with LPS or CD40L were re-stimulated at day 7 with PMA + ionomycin to test intracellular IL-21 expression. Gated to FSChi activated CD4+ T cells. A representative out of three experiments.
(B) Activated CD4+ T cells sorted at day 7 were re-stimulated with CD3 + CD28 mAbs 24 h to measure IL-21 secretion.
(C) Naïve CD4+ T cells were stimulated by CD3 + CD28 mAbs with the supernatants of DCs activated by CD40L or TLR-ligands. IL-21 production from activated CD4+ T cells as in (B).
(D) Naïve CD4+ T cells stimulated with CD3 + CD28 mAbs in the presence of the indicated cytokines and re-stimulated at day 7 with PMA + ionomycin for intracellular IL-21 expression. A representative out of three experiments.
(E, F) IL-21 secretion from activated CD4+ T cells. Experiments performed with cells from five different donors are shown in (F). Paired t-test.
(G) Expression of IL-21 in naïve CD4+ T cells primed with IL-21 (30 ng/ml) as in (D) (left). IL-21 secretion from the activated CD4+ T cells (right). A representative out of three experiments.
Next we examined whether the induction of IL-21-producing CD4+ T cells was due to factor(s) secreted by activated DCs. Thus, supernatants were collected from DCs stimulated for 48 h with CD40L or various TLR-ligands, including peptidoglycan (PGN, TLR2 ligand), LPS (TLR4 ligand), flagellin (TLR5 ligand), and CL097 (TLR7 and 8 ligand). Supernatants from activated DC cultures were added to naïve CD4+ T cells cultured on plate-bound anti-CD3 + anti-CD28. After 7 d, the cells were restimulated with anti-CD3 + anti-CD28 and production of IL-21 was measured. Although supernatants from non-activated DCs did not induce naïve CD4+ T cells to produce IL-21, supernatants from activated DCs induced IL-21 secretion (Figure 1C). The degree of induction varied with the DC activation signal. CD40L-activated DCs were the most potent (IL-21: 3020 ± 970 pg/ml from 5 × 104 cells. Mean ± s.d. n=3). LPS was the most potent TLR-ligand (470 ± 120 pg/ml), followed by flagellin (190 ± 21 pg/ml) and CL097 (110 ± 14 pg/ml). Supernatants from DCs activated with PGN were poor at developing IL-21-producing CD4+ T cells (26 ± 2 pg/ml).
Thus, DCs activated through TLRs or CD40 secrete soluble factors that induce naïve CD4+ T cells to produce IL-21.
IL-12 induces naïve CD4+ T cells to secrete IL-21
To identify the cytokine(s) that induce IL-21 in naïve CD4+ T cells, naïve CD4+ T cells were stimulated for 7 d with plate-bound anti-CD3 + anti-CD28 in the presence of cytokines (10 ng/ml, except IFN-α at 500 IU/ml) known to be produced by DCs (de Saint-Vis et al., 1998). IL-1β, IL-6, IL-10, IL-18, IL-27, TNF-α, and IFN-α did not induce CD4+ T cells able to express (Figure 1D), or secrete IL-21 (Figure 1E). However, IL-12 and to a minor extent IL-23 promoted the development of CD4+ T cells able to produce IL-21. Upon re-activation through CD3 + CD28, CD4+ T cells primed with IL-12 were able to secrete nanogram amounts of IL-21 (3.3 ± 0.4 ng/ml in a culture of 5 × 104 cells/200 μl, Mean ± s.e.m. n=5), whereas those primed with IL-23 secreted only picogram amounts (40 ± 4 pg/ml, Mean ± s.e.m. n=5. Figure 1F). IL-27, another IL-12 family cytokine, did not promote any IL-21 production. As reported earlier (Caprioli et al., 2008), IL-21 induced naïve CD4+ T cells to secrete IL-21 at some extent, though when compared to IL-12, the effect was marginal at all examined concentrations (10-100 ng/ml) (Figure 1G). Of note, IL-12 also induced IL-21-producing CD4+ T cells in cultures with serum free medium (X-VIVO15, data not shown), indicating that the induction of IL-21-producing CD4+ T cells by IL-12 does not require serum components.
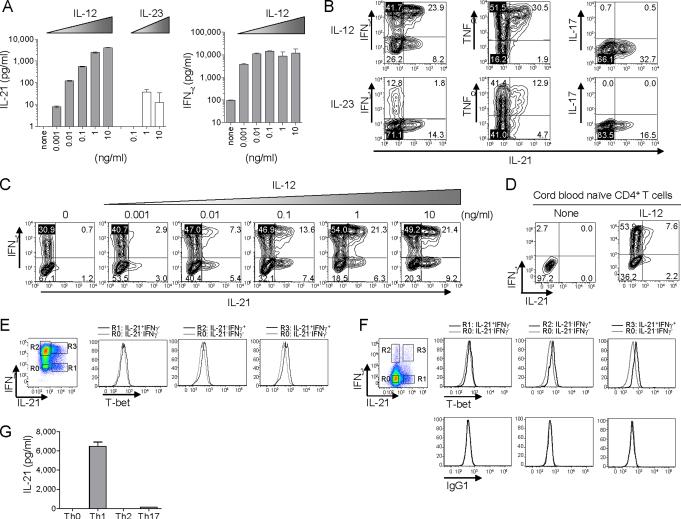
Titration studies revealed that as little as 1 pg/ml of IL-12 allowed the generation of T cells capable of secreting measurable amounts of IL-21 (Figure 2A). In contrast, much larger amounts of IL-23 were required to induce CD4+ T cells capable of producing measurable amounts of IL-21. IL-21 or IL-23 did not act in synergy with IL-12, as neither cytokines enhanced IL-21 secretion by CD4+ T cells primed with suboptimal IL-12 (100 pg/ml. Figure S1).
Figure 2. IL-12 induces two distinct types of IL-21-producers.
(A) Naïve CD4+ T cells were stimulated with CD3 + CD28 mAbs for 7 d in the presence of titrated amounts of IL-12 or IL-23. IL-21 and IFN-γ secretion upon 24 h CD3 + CD28 restimulation. A representative out of two experiments.
(B) Naïve CD4+ T cells stimulated with CD3 + CD28 mAbs in the presence of 10 ng/ml IL-12 or IL-23 were re-stimulated at day 7 with PMA + ionomycin to test intracellular cytokine expression. Gated to FSChi activated CD4+ T cells. A representative out of five experiments.
(C) Intracellular cytokine expression in CD4+ T cells primed with titrated amounts of IL-12. After re-stimulation with PMA + ionomycin. A representative out of two experiments.
(D) Highly purified cord blood naïve CD4+ T cells were stimulated for 7 d with CD3 + CD28 mAbs ± IL-12. Intracellular IL-21 and IFN-γ expression upon stimulation with PMA and ionomycin. A representative out of three experiments.
(E) Expression of T-bet and intracytoplasmic cytokines was analyzed in CD4+ T cells primed with IL-12, after re-stimulation with PMA + ionomycin. The levels of T-bet expression in IL-21+IFN-γ- (R1), IL-21-IFN-γ+ (R2) and IL-21+IFN-γ+ (R3) cells were compared to that in IL-21-IFN-γ- cells (R0).
(F) Expression of T-bet and intracytoplasmic cytokines was analyzed in tonsillar CD4+ T cells stimulated with PMA + ionomycin.
(G) Naïve CD4+ T cells were cultured for 7 d with CD3 + CD28 mAbs under Th0, Th1, Th2, or Th17-skewing conditions. IL-21 secretion upon 24 h CD3 + CD28 restimulation from activated CD4+ T cells.
Thus, IL-12 potently induces the development of IL-21-producing CD4+ T cells.
IL-12 induces two different types of IL-21-producing CD4+ T cells
IL-12 is a potent inducer of Th1 cells (Trinchieri, 2003), and accordingly induced naïve CD4+ T cells to secrete IFN-γ (Figure 2A). Indeed, 73 ± 2% of IL-21-positive CD4+ T cells induced by IL-12 (10 ng/ml) co-expressed IFN-γ (Mean ± s.e.m. n=6. Figure 2B). However, IL-12 also promoted in a dose dependent manner the development of IL-21-expressing CD4+ T cells that lacked IFN-γ expression (Figure 2C). Importantly, IL-12 induced both IFN-γ+IL-21+ and IFN-γ-IL-21+ cells from highly purified cord blood naïve CD4+ T cells (Figure 2D). To determine the commitment to the Th1 pathway, the expression of T-bet, a Th1-associated transcriptional factor, was analyzed. Although IFN-γ+IL-21+ CD4+ T cells as well as IFN-γ+IL-21- expressed T-bet, IFN-γ-IL-21+ CD4+ T cells lacked T-bet expression (Figure 2E). Thus, IFN-γ+IL-21+ cells induced by IL-12 are committed to the Th1 pathway. Notably, these two different IL-21-producers were also present in blood and tonsils (Figure S2). Furthermore, consistent with the IL-12-induced CD4+ T cells, tonsillar IFN-γ+IL-21+ CD4+ T cells expressed T-bet, whereas IFN-γ-IL-21+ cells did not (Figure 2F).
All the IL-21-positive CD4+ T cells induced by IL-12 co-expressed TNF-α (Figure 2B). IL-21-positive CD4+ T cells induced by IL-12 did not co-express IL-17A. IL-23 also induced IL-21-positive CD4+ T cells co-expressing TNF-α, but preferentially induced IFN-γ-IL-21+ non-Th1 cells, which lacked T-bet expression (data not shown). IL-21-positive CD4+ T cells induced by IL-23 did not co-express IL-17A.
The observation that IL-12 potently induces IL-21-producers prompted us to determine whether naïve CD4+ T cells cultured under Th2 and Th17-skewing conditions would become IL-21-producers as well. Naïve CD4+ T cells were cultured for 7 d with CD3 + CD28 mAbs in the presence of no cytokine (Th0), IL-12 + anti-IL-4 (Th1), IL-4 + anti-IL-12 (Th2), or IL-23 + IL-1β + IL-6 (Th17), and the secretion of IL-21 by activated CD4+ T cells was analyzed. T cells polarized under Th1 conditions secreted far more IL-21 than those polarized under Th17 conditions (Figure 2G). Naïve CD4+ T cells cultured under Th0 and Th2 conditions did not secrete detectable amounts of IL-21.
Naïve CD4+ T cells primed with IL-12 induce B cells to produce Igs through IL-21
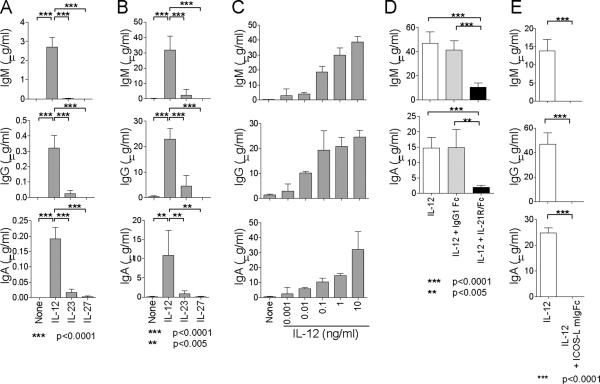
Inasmuch as Tfh cells help B cell immunoglobulin (Ig) production through IL-21 (Bryant et al., 2007), we next examined whether naïve CD4+ T cells stimulated with IL-12 would share this property. Thus, naïve CD4+ T cells primed in the presence of IL-12 or other cytokines were sorted, and cocultured (4 × 104 cells/well) in the presence of CpG (TLR9 ligand) with autologous blood IgD+CD27- naïve B cells (4 × 104 cells/well) pre-activated with anti-IgM. Staphylococcal enterotoxin B (SEB), a superantigen, was added to induce T-B interactions, and Igs were measured in supernatants at day 14. CD4+ T cells primed without cytokine failed to induce naïve B cells to secrete Igs (Figure 3A, none). In contrast, CD4+ T cells primed with IL-12 induced naïve B cells to secrete Igs, including IgM (2.7 ± 0.5 μg/ml, Mean ± s.e.m. n=6.), IgG (0.32 ± 0.08 μg/ml), and IgA (0.19 ± 0.04 μg/ml). Naïve B cells co-cultured with CD4+ T cells primed with IL-23 produced much lower amounts of Igs (IgM: 0.02 ± 0.02 μg/ml, IgG 0.02 ± 0.02 μg/ml, IgA 0.02 ± 0.01 μg/ml. Mean ± s.e.m. n=6). CD4+ T cells primed with IL-27 barely induced Ig production. Consistent results were obtained with cells collected from three different donors (Figure S3). Interaction between T cells and B cells was required for optimal B cell help, as B cells produced less Igs in the absence of SEB (Figure S4). Similarly, naïve CD4+ T cells primed with IL-12 induced much higher amounts of Ig production from memory B cells than CD4+ T cells primed with IL-23 (Figure 3B, IL-12: IgM 31.9 ± 3.7 μg/ml, IgG 23.0 ± 1.7 μg/ml, IgA 10.8 ± 2.6 μg/ml. IL-23: IgM 2.3 ± 1.6 μg/ml, IgG 4.5 ± 1.7 μg/ml, IgA 0.9 ± 0.3 μg/ml. Mean ± s.e.m. n=6). Furthermore, IL-12 promoted the development of Tfh-like cells in a dose-dependent manner, as CD4+ T cells primed with higher amounts of IL-12 induced more Ig secretion from B cells (Figure 3C).
Figure 3. CD4+ T cells primed in the presence of IL-12 help B cells.
(A) Naive B cells pre-activated with anti-IgM were cultured in the presence of CpG and SEB with autologous CD4+ T cells primed with the indicated cytokines. Ig titers in the supernatants were measured at day 14. Mean ± s.e.m. n=6. Unpaired two-tailed t-test. A representative out of three experiments.
(B) Memory B cells were cultured in the presence of SEB with autologous CD4+ T cells primed with the indicated cytokines. Ig levels at day 14. Mean ± s.e.m. n=6. Unpaired two-tailed t-test. A representative out of three experiments.
(C) Ig secretion from memory B cells cultured with CD4+ T cells primed with titrated amounts of IL-12. A representative out of two experiments.
(D) IL-21R-Fc was added to block IL-21 to the co-cultures of memory B cells and CD4+ T cells primed with IL-12. Ig levels at day 14. Mean ± s.e.m. n=6. Unpaired two-tailed t-test. A representative out of three experiments.
(E) Blocking of ICOS by ICOS-L-mIgFc during T-B cultures. Ig levels at day 14. Mean ± s.e.m. n=6. Unpaired two-tailed t-test. A representative out of three experiments.
To determine whether CD4+ T cells primed in the presence of IL-12 help B cells through IL-21, soluble IL-21 receptor-Fc chimeric protein was added to cultures of T and B cells. Blocking IL-21 potently inhibited B cells to secrete Igs (Figure 3D). Conversely, the addition of IL-21 into the cultures of CD4+ T cells primed in the absence of cytokines potently induced B cells to secrete Igs (Figure S5). Addition of ICOS-L-mIgFc protein totally abrogated Ig secretion from B cells, indicating that Ig secretion from B cells was dependent on ICOS expressed on activated CD4+ T cells (Figure 3E). ICOS-ICOS-L interaction appears to be critical for the cognate interaction between B and T cells, as ICOS-L-mIgFc protein abrogated their cluster formation in the culture, and resulted in the B cell death (data not shown). As shown previously, IL-12 induced the expression of ICOS on CD4+ T cells (Wassink et al., 2004), at higher amounts than IL-23 or IL-27 (Figure S6). Of note, IL-12 did not affect CXCR5 expression on activated naïve CD4+ T cells (Figure S7). Naïve CD4+ T cells primed in the presence of any tested cytokines did not express CD57, a molecule expressed by a fraction of Tfh cells in the GC (Figure S7).
Thus, CD4+ T cells primed with IL-12 share a fundamental property of Tfh cells, which is to help B cells through IL-21 and via the ICOS-ICOS-L interaction.
Bacteria-activated DCs induce IL-21-producing CD4+ T cells through IL-12
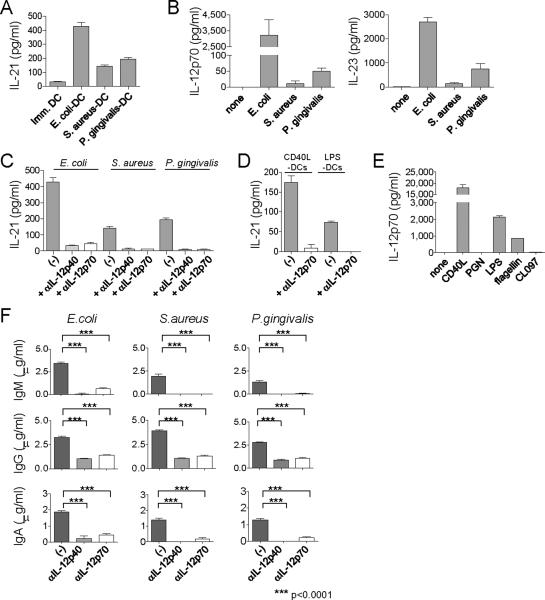
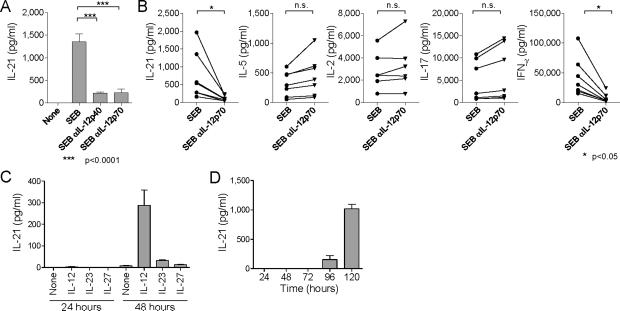
We next examined whether DCs exposed to bacteria induce naïve CD4+ T cells to produce IL-21. DCs were incubated for 6 h with heat-killed bacteria including E. coli (gram negative), S. aureus (gram positive), and P. gingivalis (gram positive), and then cultured with allogeneic naive CD4+ T cells. Activated CD4+ T cells sorted at day 7 were re-stimulated with CD3 + CD28 mAbs to measure IL-21 secretion. CD4+ T cells primed with E. coli-activated DCs secreted more IL-21 than those primed with unstimulated DCs (Figure 4A). DCs stimulated with S. aureus or P. gingivalis were also able to induce IL-21 production by CD4+ T cells though less potently than did E. coli-activated DCs. Indeed, E. coli-activated DCs secreted far more IL-12p70 and IL-23 than S. aureus and P. gingivalis-activated DCs (Figure 4B). Addition of IL-12p40 blocking mAb, which inhibits both IL-12 and IL-23, to the co-cultures of E.coli-activated DCs and T cells inhibited IL-21 secretion by CD4+ T cells (Figure 4C). Notably, addition of IL-12p70 blocking mAb, which inhibits IL-12 alone, was sufficient to inhibit IL-21 secretion (85 ± 5% inhibition by IL-12p70 blocking. Mean ± s.e.m, n=5.). Blocking of IFN-α, IFN-γ, IL-1β, or TNF-α did not inhibit IL-21 secretion (Figure S8). IL-12p70 blocking also resulted in a substantial decrease of IL-21 secretion by CD4+ T cells stimulated with DCs exposed to S. aureus (87 ± 3% decrease, n=4), or P. gingivalis (80 ± 9% decrease, n=4). Overall, anti-IL-12p40 and anti-IL-12p70 were equally potent at inhibiting the induction of IL-21-producing CD4+ T cells (Figure S9). These results indicate that the induction of IL-21-producers by bacteria-activated DCs was mainly mediated by IL-12. This pathway was also shared by DCs stimulated with CD40L and LPS (Figure 4D). As expected, CD40L-stimulated DCs, whose supernatant induced naïve CD4+ T cells to secrete the highest amounts of IL-21 (Figure 1C), produced the largest amount of IL-12p70 (Figure 4E). Although LPS induced DCs to produce the highest amounts of IL-12p70 among examined TLR-ligands, PGN barely induced DCs to secrete IL-12. This was consistent with the observation that the culture supernatant of PGN-stimulated DCs poorly induced CD4+ T cells to secrete IL-21 (Figure 1C).
Figure 4. DCs exposed to bacteria induce IL-21-producing Tfh-like cells through IL-12.
(A) IL-21 secretion from naïve CD4+ T cells cultured with allogeneic DCs exposed to heat-killed bacteria. A representative out of five experiments.
(B) IL-12 and IL-23 secretion from DCs exposed to heat-killed bacteria. A representative out of five experiments.
(C) IL-21 secretion of CD4+ T cells primed with bacteria-exposed DCs in the presence of anti IL-12p40 or IL-12p70 mAb. A representative out of five experiments.
(D) IL-21 secretion of CD4+ T cells primed with CD40L- or LPS-activated DCs in the presence of anti IL-12p70 mAb. A representative out of three experiments.
(E) IL-12 secretion from DCs exposed to CD40L or TLR-ligands. A representative out of three experiments.
(F) Naïve CD4+ T cells were cultured for 7 d with allogeneic bacteria-exposed DCs in the presence of anti IL-12p40 or IL-12p70 mAbs. Activated CD4+ T cells were sorted and cultured with autologous memory B cells, and produced Igs were analyzed. Mean ± s.e.m. n=6. Unpaired two-tailed t-test. A representative out of four experiments.
CD4+ T cells primed with bacteria-activated DCs were able to induce B cells to produce Igs (Figure 4F). The induction of Ig secretion by B cells was significantly impaired when anti-IL-12p40 was added during co-cultures of DCs and T cells. Furthermore, blocking IL-12p70 was sufficient to inhibit the development of Tfh-like cells. Thus, IL-12 secreted by DCs exposed to bacteria is critical for the differentiation of naïve CD4+ T cells into Tfh-like cells.
Thus, DCs activated by CD40L, bacteria, and their components (including TLR-ligands) induce the development of IL-21-producing Tfh-like cells through IL-12.
IL-12 increases the secretion of IL-21 by memory CD4+ T cells
We next determined whether IL-12 also controls IL-21 secretion by memory CD4+ T cells. Fresh PBMCs from healthy adults produced high amounts of IL-21 when cultured with SEB for 48 h (740 ± 250 pg/ml, Mean ± s.e.m. n=7. Figure 5A, B). Blocking IL-12p70 during the 48 h activation period resulted in a significant decrease of IL-21 secretion (110 ± 30 pg/ml. n=7. p<0.05. Figure 5B). Blocking of both IL-12 and IL-23 with anti-IL-12p40 decreased IL-21 secretion (120 ± 40 pg/ml. n=6) to a degree comparable to blocking IL-12 alone. The secretion of IFN-γ was also strongly inhibited with anti-IL-12p70, but blocking IL-12 did not alter the secretion of other cytokines including IL-2, IL-5, and IL-17A (Figure 5B). Thus, blocking IL-12 specifically inhibited the secretion of both IL-21 and IFN-γ.
Figure 5. IL-12 promotes IL-21 secretion by blood memory CD4+ T cells.
(A, B) Fresh PBMCs were stimulated with SEB in the presence of anti IL-12p40 or IL-12p70 mAb. IL-21 (A) or the indicated cytokines (B) secreted cytokines during 48 h cultures were measured. Symbols represent results from seven different donors.
(C) Memory CD4+ T cells were stimulated with CD3 + CD28 mAbs in the presence of the indicated cytokines. IL-21 secretion in the supernatants was measured at 24 and 48 h of culture.
(D) Naive CD4+ T cells were stimulated with CD3 + CD28 mAbs in the presence IL-12. IL-21 secretion in the supernatants was measured at the indicated time points.
To further confirm the IL-12-dependent induction of IL-21 secretion by memory CD4+ T cells, memory CD4+ T cells were sorted and stimulated with anti-CD3 + anti-CD28 in the presence of IL-12, IL-23 and IL-27. As shown in Figure 5C, IL-12 potently induced memory CD4+ T cells to secrete IL-21 between 24 to 48 h post-stimulation, indicating the delayed mode of action. Naïve CD4+ T cells did not secrete detectable amounts of IL-21 during the first 72 h (Figure 5D). These observations indicate that IL-12 directly acts on memory CD4+ T cells, and promotes the secretion of IL-21.
Induction of IL-21-producers by IL-12 is dependent on STAT4
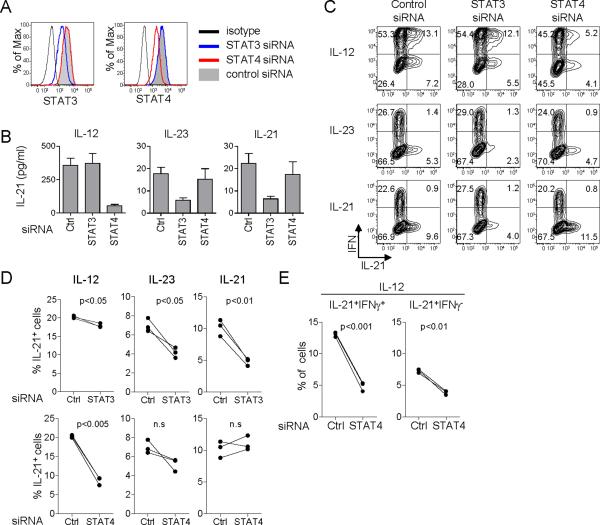
IL-12 activates multiple signal transduction pathways, including signal transducer and activator of transcription 3 (STAT3) and STAT4 (reviewed in (Watford et al., 2004)). Induction of IFN-γ in CD4+ T cells by IL-12 is largely dependent on the activation of STAT4 (Kaplan et al., 1996; Thierfelder et al., 1996). To determine the contribution of STAT3 and STAT4 for the induction of IL-21, STAT3 and STAT4 were targeted in naïve CD4+ T cells by transfecting siRNA. siRNA transfection of naïve CD4+ T cells stimulated with anti-CD3 + anti-CD28 mAbs for 5-7 d resulted in a decrease of the expression of STAT3 and STAT4 by approximately 60% at 24 h post-transfection (Figure 6A). Transfected CD4+ T cells were stimulated for 48 h with CD3 + CD28 mAbs in the presence of IL-12, IL-23 or IL-21. Activated CD4+ T cells were sorted, and then re-stimulated with CD3 + CD28 mAbs for 24 h to measure IL-21 secretion. STAT4 blocking substantially decreased IL-21 production by CD4+ T cells stimulated by IL-12, but not by CD4+ T cells stimulated by IL-23 or IL-21 (Figure 6B). In contrast, the induction of IL-21-producers in response to IL-21 was largely dependent on STAT3, an observation consistent with previous studies performed using mouse (Nurieva et al., 2008; Wei et al., 2007; Zhou et al., 2007) and human CD4+ T cells (Caprioli et al., 2008). Furthermore, IL-23 also induced IL-21-producers in a STAT3-dependent manner.
Figure 6. IL-12 induce IL-21 producers through STAT4.
(A) Naïve CD4+ T cells stimulated with CD3 + CD28 mAbs for 5-7 d were transfected with siRNA specific for STAT3 or STAT4. The levels of STAT3 and STAT4 expression at 24 h post-transfection were analyzed by flow cytometry. Cells transfected with control siRNA (gray), STAT3 siRNA (blue), and STAT4 siRNA (red) are shown. Black histogram represents staining with control isotype. Gated to AQUA-FSChi activated CD4+ T cells. A representative out of three experiments.
(B) Transfected CD4+ T cells were cultured for 48 h in the presence of IL-12, IL-23 or IL-21. Activated CD4+ T cells were then re-stimulated with CD3 + CD28 mAbs for 24 h to measure IL-21 secretion. A representative out of three experiments.
(C) After 48 h culture with IL-12, IL-23, or IL-21, the expression of IL-21 was analyzed after 6h restimulation with PMA + ionomycin. Gated to AQUA-FSChi activated CD4+ T cells. A representative out of three experiments.
(D) Percentages of IL-21-expressing CD4+ T cells in three independent experiments.
(E) Percentages of IFNγ+IL-21+ and IFNγ-IL-21+ cells in STAT4 siRNA-transfected CD4+ T cells cultured with IL-12.
The induction of IL-21-expressing CD4+ T cells was further analyzed by intracytoplasmic cytokine staining following 6 h stimulation with PMA and ionomycin. As expected, STAT4 blocking inhibited the development of IFN-γ-expressing CD4+ T cells in response to IL-12 (Figure 6C. 68 ± 5 % with control vs. 54 ± 5 % with STAT4 blocking. Mean ± s.e.m, n=3. p<0.005). Consistent with the analysis of IL-21-production, the development of IL-21-expressing CD4+ T cells by IL-12 was largely dependent on STAT4 (Figure 6C, D). Furthermore, STAT4 blocking inhibited the development of both IFN-γ+IL-21+ Th1 cells and IFN-γ-IL-21+ non-Th1 cells (Figure 6E. IFN-γ+IL-21+: 13.0 ± 0.2 % with control vs. 4.8 ± 0.4 % with STAT4 blocking. Mean ± s.e.m., n=3. p<0.001; IFN-γ-IL-21+: 7.3 ± 0.2 % with control vs. 3.8 ± 0.2 % with STAT4 blocking. p<0.01). STAT3 blocking only marginally decreased the generation of IL-21-expressing CD4+ T cells by IL-12 (Figure 6D). In contrast, The development of IL-21-expressing CD4+ T cells by IL-23 and IL-21 was confirmed to be dependent on STAT3, but not on STAT4 (Figure 6C, D).
Thus, the development of human IL-21-producing CD4+ T cells by IL-12 is largely dependent on STAT4.
DISCUSSION
Our study addresses how human DCs induce the differentiation of naïve CD4+ T cells into T cells with a capacity to help B cells through IL-21. We show that IL-12 is a key factor derived from human DCs to drive differentiation. Such IL-21-producing CD4+ T cells induced by IL-12 share fundamental characteristics with tonsillar Tfh cells, including 1) production of a large amount of IL-21, 2) capacity to help B cells in a manner dependent on IL-21 and ICOS, and 3) presence of two distinct IL-21+CD4+ T cell subsets: IFN-γ+IL-21+ and IFN-γ-IL-21+.
The definition of Tfh cells still remains controversial. As the name indicates, Tfh cells are generally defined by their location in vivo, i.e., at the follicular sites of secondary lymphoid organs. However, in a similar concept applied to other Th subsets, Tfh cells are also defined by their exclusive functions: i.e., the secretion of IL-21 and the function to help B cells through IL-21, rather than by their location. This function-based nomenclature fits well with a recent view of Tfh “subsets,” where Tfh cells are derived from different Th lineages and differently regulate B cell immunity (Fazilleau et al., 2009). Furthermore, an increasing number of studies indicates that IL-21-producing “extrafollicular” Tfh cells (King et al., 2008; Odegard et al., 2008) and pre-GC Tfh cells (Fazilleau et al., 2009) share many characteristics with GC Tfh cells. This further supports the nomenclature of Tfh cells as a T cell subset specialized for B cell help, particularly through the secretion of IL-21. As the nomenclature of “Tfh” cells has not been officially defined, we called in this manuscript the IL-21-producing CD4+ T cells with the capacity to help B cells as “Tfh-like” cells for the sake of simplicity.
Several findings are shown which together establish the significance of IL-12 in the development of IL-21-producing Tfh-like cells in humans: 1) Soluble factors secreted by activated DCs efficiently induce naïve CD4+ T cells to become cells able to secrete IL-21; 2) IL-12 is the most efficient DC-derived cytokine able to induce IL-21 in CD4+ T cells. This finding extends a previous observation showing that Th1-promoting culture condition induces IL-21 in human naïve CD4+ T cells (Volpe et al., 2008), 3) DCs activated by TLR-ligands, CD40L, and bacteria secrete IL-12, and potently induce naïve CD4+ T cells to secrete IL-21, 4) Blocking IL-12 during co-cultures of activated DCs and naïve CD4+ T cells results in a significant inhibition of IL-21 secretion from primed CD4+ T cells as well as the development of Tfh-like cells, and 5) CD4+ T cells generated in vitro by culturing naïve CD4+ T cells with IL-12 are able to help B cells to secrete Igs in a manner dependent on IL-21. Among different TLR agonists tested, TLR4 agonists (LPS and gram negative bacteria such as E. coli) were the most potent activators of DCs with respect to inducing naïve CD4+ T cells to secrete IL-21. In contrast, TLR2 agonists (PGN and gram-positive bacteria such as S. aureus, and P. gingivalis) were poor activators, which is consistent with their poor ability to induce DCs to produce IL-12 (Gerosa et al., 2008; Pulendran et al., 2001). Furthermore, IL-12 was found to act as a critical factor not only for the development of IL-21-producing CD4+ T cells but also as a factor helping memory CD4+ T cells to secrete IL-21. Thus, human DCs appear to regulate antibody responses through the secretion of IL-12.
Importantly, IL-12 induces both IFN-γ+IL-21+ Th1 cells and IFN-γ-IL-21+ non-Th1 cells from highly purified cord blood naïve CD4+ T cells. Induction of these two different IL-21-producers might be best explained by a potential heterogeneity within naïve CD4+ T cells, as recently shown by CD161-expressing naïve CD4+ T cells as progenitor of Th17 cells (Cosmi et al., 2008).
The development of IL-21-producing CD4+ T cells by IL-12 is largely dependent on STAT4. STAT4 regulates the development of both IFN-γ+IL-21+ Th1 cells and IFN-γ-IL-21+ non-Th1 cells. The contribution of STAT3 in the development of IL-21-producers by IL-12 is marginal. In contrast, IL-23 and IL-21 induce IL-21-producers in a STAT3-dependent fashion. These two cytokines mainly induce IFN-γ-IL-21+ non-Th1 cells. Thus, both STAT3 and STAT4 are involved in the induction of IL-21 in human CD4+ T cells, and distinct signaling pathways appear to induce different types of IL-21-producers. Our findings are corroborated by the demonstration that STAT3 deficient CD4+ T cells obtained from hyper IgE syndrome patients can secrete IL-21 (Milner et al., 2008).
The IL-12-IL-21 axis demonstrated herein with human cells does not appear to be shared by mouse cells, inasmuch as several studies showed that IL-12 does not induce mouse naïve CD4+ T cells to secrete IL-21 (Dienz et al., 2009; Suto et al., 2008; Wei et al., 2007). In mice, IL-6 and IL-21 have been reported as critical factors in the generation of IL-21-producing CD4+ T cells both in vitro and in vivo (Dienz et al., 2009; Korn et al., 2007; Nurieva et al., 2007; Suto et al., 2008; Zhou et al., 2007). As both IL-6 and IL-21 signal through STAT3, it has been concluded that STAT3 is critical in the generation of IL-21-producing CD4+ T cells. Indeed, STAT3 deficient mice severely impaired the development of Tfh cells (Nurieva et al., 2008; Wei et al., 2007). Thus, the distinct mechanism in the development of IL-21-producing CD4+ T cells adds to the list of differences between the human and mouse immune systems (Mestas and Hughes, 2004).
Taken together, our results indicate that IL-12, which activates cellular immunity by inducing Th1 cells, also contributes to humoral immunity indirectly through the generation of Tfh cells. Actually, IL-12 from activated DCs also acts directly on activated naïve B cells together with IL-6 to induce their differentiation into plasma cells (Dubois et al., 1998). Therefore, when DCs form the “ménage à trois” complex with antigen-specific T cells and B cells (Qi et al., 2006), IL-12 secreted from DCs may act on both CD4+ T cells and B cells to optimize the development of antibody responses (Figure S10).
The discovery of the role of IL-12 in humans for the induction and the activation of IL-21-producing Tfh cells may have a profound impact on the development of new therapies. Identifying adjuvants that induce DCs to secrete IL-12 might improve vaccines aiming at efficient induction of neutralizing antibodies. Indeed, studies with rhesus macaques have concluded that IL-12 enhances the induction of specific antibody responses in vivo when used as vaccine adjuvant, (Hirao et al., 2008; Schadeck et al., 2006; van der Meide et al., 2002). In contrast, blocking of IL-12 might be beneficial to prevent the development of autoreactive B cells in autoimmune diseases.
Experimental Procedures
Isolation of CD4+ T cells and B cells
The study was approved by the Institutional Review Boards of Baylor Health Care System. Informed consent was obtained from healthy subjects for the collection of blood apheresis samples. PBMCs were purified from apheresis blood samples obtained from adult volunteers. CD4+ T cells were first enriched by negative selection with purified CD8 (HIT8a), CD11b (LM1/2), CD11c (B-ly6), CD14 (M5E2), CD15 (W6D3), CD16 (3G8), CD19 (J4.119), CD45RO (UCHL1), CD56 (C218) and HLA-DR (B8.12.2) mAbs, and Dynabeads Pan Mouse IgG (Dynal). Naive CD4+ T cells were further purified by sorting with FACSAria (BD) as CCR7+ CD8- CD56- HLA-DR- CD45RA+ CD4+ cells. Memory CD4+ T cells were directly sorted from PBMCs as CD8- CD56- CD45RA- CD4+ cells. Cord blood naïve CD4 T cells were sorted from frozen cells (AllCells) as CCR7+ CD8- CD56-HLA-DR- CD45RA+ CD4+. Cell purity was >99%.
B cells were first enriched from apheresis PBMCs by positive selection using CD19 MicroBeads (Miltenyi). Then, naïve and memory B cells were sorted with FACSAria as IgD+ CD27- CD3- CD11c- CD14- cells and CD27+ CD3- CD11c- CD14- cells, respectively. Cell purity was >98%.
Coculture of DCs and naïve CD4 + T cells
Monocytes were isolated from PBMCs using Monocyte Isolation Kit II (Miltenyi). DCs were generated by culturing monocytes with 50 ng/ml IL-4 (R&D) and 100 ng/ml GMCSF (Leukine) At day 6, DCs were stimulated with irradiated CD40L-transfected L cells, PGN (5 μg/ml), LPS (50 ng/ml), flagellin (20 ng/ml), CL097 (imidazoquinoline compound - 5μg/ml), heat killed-Escherichia coli (108/ml), -Staphylococcus aureus (108/ml, InvivoGen), or -Porphyromonas gingivalis (108/ml, InvivoGen). After 6 h stimulation, DCs were harvested and carefully washed; DCs stimulated with CD40L-transfected L cells were sorted as CD11c+CD40L- DCs. Activated DCs (1.3 × 103 cells/well) were cultured for 7 d with allogeneic naïve CD4+ T cells (4 × 104 cells/well) in RPMI complete medium. In some experiments, 10 μg/ml anti-IL-12p40 (C8.6) or anti-IL-12p70 (M122) blocking mAbs were added to the culture. For the assessment of IL-12 secretion, DCs harvested at 6 h stimulation were cultured for another 24 h in flat bottom 96 well plates (2 × 105 cells/well), and the secreted IL-12p70 and IL-23 were measured by ELISA (eBiosciences).
Stimulation of naïve CD4+ T cells via CD3 andCD28
Naïve or memory CD4+ T cells (1 × 105 cells/well) were stimulated with plate-bound CD3 mAb (5 μg /ml, OKT3) and soluble CD28 mAb (1 μg/ml. CD28.2) in flat bottom 96 well plates in RPMI complete medium in the presence of: IL-1β, IL-4, IL-6, IL-10, IL-12, IL-18, IL-27, and TNF-α (R&D), IL-21 (BioSource), IL-23 (eBiosciences) (10 ng/ml each or at indicated concentration), or IFN-α (IFN-α2b. 500 IU/ml. Schering). In some experiments, anti-IL-4 (MP4-25D2) or anti-IL-12p70 mAbs (2 μg/ml) were added to the culture.
Intracellular staining
Naïve CD4+ T cells stimulated for 7-9 d were restimulated with PMA (25 ng/ml) and ionomycin (1 μg/ml) for 6 h in the presence of GolgiPlug (BD) for the last 4 h. Cells were then fixed and permeabilizedand the expressed cytokines in the cytoplasm were analyzed with IL-21 PE or A647 (3A3-N2), IL-17A PE (64DEC17), TNF-α APC (MAb11) and IFN-γ APC (B27) mAbs. For T-bet staining, cells were fixed/permeabilized with the Foxp3 Staining Buffer Set (eBiosciences), and stained with T-bet A488 (4B10), IL-21 PE and IFN-γ APC antibodies. In some experiments, dead cells were further excluded from the analysis by labeling with cells LIVE/DEAD fixable Aqua (Invitrogen) prior to fixation/permeabilization. Expression of each molecule was assessed in activated CD4+ T cells (FSChigh cells) with FlowJo software (TreeStar).
Cytokine secretion from activated CD4+ T cells
Activated naïve CD4+ T cells were sorted at day 7-9 as FSChigh cells (CD3 + /CD28 stimulation), or CD11clowFSChigh cells (co-culture with DCs). Sorted CD4+ T cells were restimulated with CD3 mAb and soluble CD28 mAb in 96 flat bottom well plates (5 × 104 cells/well) in Yssel media (Gemini) supplemented with 10% FBS. After 24 h, the levels of produced cytokines were assessed by Luminex.
Co-culture of T and B cells
Activated CD4+ T cells were co-cultured with autologous naïve or memory B cells (4 χ 104 cells/well each) in 96-well round-bottom plates in Yssel medium/10% FBS in the presence of endotoxin-reduced SEB (0.25 ng/ml). Naïve B cells were pre-activated for 2 h with 1 μg/ml rabbit anti-human IgM (Irvine Scientific) and CpG type B (0.5μg/ml, ODN2006, InvivoGen) was added to the culture. In some experiment ICOS-L-mIgFc (Ancell), IgG1Fc or IL-21R/Fc (R&D) were added to the culture. Igs (IgM, IgG and IgA) produced in the cultures were analyzed by ELISA at day 6 or 14.
siRNA transfection
Naïve CD4+ T cells stimulated with CD3 + CD28 mAbs for 5-7 d were transfected with siRNA using the Human T cell Nucleofector Kit and Nucleofector II device (Amaxa). siRNA to target STAT3 (s743), STAT4 (s13531) and silencer select negative control #1 siRNA (Ambion) were used at 5nM (0.5 nmol/5 χ 106 cells/transfection). Cells were transferred at 6 h post-transfection to the wells with CD3 + CD28 Abs. Cytokines (IL-12 (10ng/ml), IL-23 (10ng/ml) or IL-21 (50ng/ml)) were added to the culture 24 h post transfection, and the cytokine expression was analyzed 48 h later.
STAT3 and STAT4 staining
The expression of STAT3 and STAT4 was assessed 24 h post siRNA transfection. After labeling with LIVE/DEAD fixable Aqua, cells were incubated with BD cytofix buffer (BD) and then permeabilized with BD Phosflow Perm Buffer III (BD). Cells were then incubated with anti-STAT3 APC (BD) and anti-STAT4 (Zymed) Abs, , followed by incubation with goat anti-rabbit IgG FITC.
Statistics
The significance of the difference between groups in the experiments was evaluated by two-tailed unpaired or paired t-test. A value of p< 0.05 was considered significant.
Development of human IL-21 bead-based assay using Luminex technology
See Supplementary text.
Supplementary Material
ACKNOWLEDGMENTS
We thank E. Kowalski, S. Coquery for cell sorting; L. Walters for cell processing from apheresis blood; G. Zurawski for IL-21 generation; G. Xia and J. Connolly for technical discussion. This study was supported by U19-AI057234, R01-CA84512, R01-CA078846 (J.B.), and Baylor Health Care Foundation (H.U. and J.B.). J.B. holds the W. W. Caruth, Jr. Chair for Transplantation Immunology Research.
REFERENCES
- Allen CD, Okada T, Cyster JG. Germinal-center organization and cellular dynamics. Immunity. 2007;27:190–202. doi: 10.1016/j.immuni.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banchereau J, Bazan F, Blanchard D, Briere F, Galizzi JP, van Kooten C, Liu YJ, Rousset F, Saeland S. The CD40 antigen and its ligand. Annual review of immunology. 1994;12:881–922. doi: 10.1146/annurev.iy.12.040194.004313. [DOI] [PubMed] [Google Scholar]
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- Bettelli E, Korn T, Kuchroo VK. Th17: the third member of the effector T cell trilogy. Curr Opin Immunol. 2007;19:652–657. doi: 10.1016/j.coi.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitfeld D, Ohl L, Kremmer E, Ellwart J, Sallusto F, Lipp M, Forster R. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. J Exp Med. 2000;192:1545–1552. doi: 10.1084/jem.192.11.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant VL, Ma CS, Avery DT, Li Y, Good KL, Corcoran LM, de Waal Malefyt R, Tangye SG. Cytokine-mediated regulation of human B cell differentiation into Ig-secreting cells: predominant role of IL-21 produced by CXCR5+ T follicular helper cells. J Immunol. 2007;179:8180–8190. doi: 10.4049/jimmunol.179.12.8180. [DOI] [PubMed] [Google Scholar]
- Campbell DJ, Kim CH, Butcher EC. Separable effector T cell populations specialized for B cell help or tissue inflammation. Nat Immunol. 2001;2:876–881. doi: 10.1038/ni0901-876. [DOI] [PubMed] [Google Scholar]
- Caprioli F, Sarra M, Caruso R, Stolfi C, Fina D, Sica G, MacDonald TT, Pallone F, Monteleone G. Autocrine regulation of IL-21 production in human T lymphocytes. J Immunol. 2008;180:1800–1807. doi: 10.4049/jimmunol.180.3.1800. [DOI] [PubMed] [Google Scholar]
- Cosmi L, De Palma R, Santarlasci V, Maggi L, Capone M, Frosali F, Rodolico G, Querci V, Abbate G, Angeli R, et al. Human interleukin 17-producing cells originate from a CD161+CD4+ T cell precursor. J Exp Med. 2008;205:1903–1916. doi: 10.1084/jem.20080397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyster JG, Ansel KM, Reif K, Ekland EH, Hyman PL, Tang HL, Luther SA, Ngo VN. Follicular stromal cells and lymphocyte homing to follicles. Immunol Rev. 2000;176:181–193. doi: 10.1034/j.1600-065x.2000.00618.x. [DOI] [PubMed] [Google Scholar]
- de Saint-Vis B, Fugier-Vivier I, Massacrier C, Gaillard C, Vanbervliet B, Ait-Yahia S, Banchereau J, Liu YJ, Lebecque S, Caux C. The cytokine profile expressed by human dendritic cells is dependent on cell subtype and mode of activation. J Immunol. 1998;160:1666–1676. [PubMed] [Google Scholar]
- Dienz O, Eaton SM, Bond JP, Neveu W, Moquin D, Noubade R, Briso EM, Charland C, Leonard WJ, Ciliberto G, et al. The induction of antibody production by IL-6 is indirectly mediated by IL-21 produced by CD4+ T cells. J Exp Med. 2009;206:69–78. doi: 10.1084/jem.20081571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois B, Massacrier C, Vanbervliet B, Fayette J, Briere F, Banchereau J, Caux C. Critical role of IL-12 in dendritic cell-induced differentiation of naive B lymphocytes. J Immunol. 1998;161:2223–2231. [PubMed] [Google Scholar]
- Fazilleau N, Mark L, McHeyzer-Williams LJ, McHeyzer-Williams MG. Follicular helper T cells: lineage and location. Immunity. 2009;30:324–335. doi: 10.1016/j.immuni.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerosa F, Baldani-Guerra B, Lyakh LA, Batoni G, Esin S, Winkler-Pickett RT, Consolaro MR, De Marchi M, Giachino D, Robbiano A, et al. Differential regulation of interleukin 12 and interleukin 23 production in human dendritic cells. J Exp Med. 2008;205:1447–1461. doi: 10.1084/jem.20071450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn MD, Ngo VN, Ansel KM, Ekland EH, Cyster JG, Williams LT. A B-cell-homing chemokine made in lymphoid follicles activates Burkitt's lymphoma receptor-1. Nature. 1998;391:799–803. doi: 10.1038/35876. [DOI] [PubMed] [Google Scholar]
- Hirao LA, Wu L, Khan AS, Hokey DA, Yan J, Dai A, Betts MR, Draghia-Akli R, Weiner DB. Combined effects of IL-12 and electroporation enhances the potency of DNA vaccination in macaques. Vaccine. 2008 doi: 10.1016/j.vaccine.2008.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutloff A, Dittrich AM, Beier KC, Eljaschewitsch B, Kraft R, Anagnostopoulos I, Kroczek RA. ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature. 1999;397:263–266. doi: 10.1038/16717. [DOI] [PubMed] [Google Scholar]
- Ito T, Wang YH, Duramad O, Hori T, Delespesse GJ, Watanabe N, Qin FX, Yao Z, Cao W, Liu YJ. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J Exp Med. 2005;202:1213–1223. doi: 10.1084/jem.20051135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan MH, Sun YL, Hoey T, Grusby MJ. Impaired IL-12 responses and enhanced development of Th2 cells in Stat4-deficient mice. Nature. 1996;382:174–177. doi: 10.1038/382174a0. [DOI] [PubMed] [Google Scholar]
- Kim CH, Rott LS, Clark-Lewis I, Campbell DJ, Wu L, Butcher EC. Subspecialization of CXCR5+ T cells: B helper activity is focused in a germinal center-localized subset of CXCR5+ T cells. J Exp Med. 2001;193:1373–1381. doi: 10.1084/jem.193.12.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King C, Tangye SG, Mackay CR. T follicular helper (TFH) cells in normal and dysregulated immune responses. Annual review of immunology. 2008;26:741–766. doi: 10.1146/annurev.immunol.26.021607.090344. [DOI] [PubMed] [Google Scholar]
- Klechevsky E, Morita R, Liu M, Cao Y, Coquery S, Thompson-Snipes L, Briere F, Chaussabel D, Zurawski G, Palucka AK, et al. Functional specializations of human epidermal langerhans cells and CD14+ dermal dendritic cells. Immunity. 2008;29:497–510. doi: 10.1016/j.immuni.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn T, Bettelli E, Gao W, Awasthi A, Jager A, Strom TB, Oukka M, Kuchroo VK. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchen S, Robbins R, Sims GP, Sheng C, Phillips TM, Lipsky PE, Ettinger R. Essential role of IL-21 in B cell activation, expansion, and plasma cell generation during CD4+ T cell-B cell collaboration. J Immunol. 2007;179:5886–5896. doi: 10.4049/jimmunol.179.9.5886. [DOI] [PubMed] [Google Scholar]
- Langenkamp A, Messi M, Lanzavecchia A, Sallusto F. Kinetics of dendritic cell activation: impact on priming of TH1, TH2 and nonpolarized T cells. Nat Immunol. 2000;1:311–316. doi: 10.1038/79758. [DOI] [PubMed] [Google Scholar]
- MacLennan IC. Germinal centers. Annual review of immunology. 1994;12:117–139. doi: 10.1146/annurev.iy.12.040194.001001. [DOI] [PubMed] [Google Scholar]
- Mestas J, Hughes CC. Of mice and not men: differences between mouse and human immunology. J Immunol. 2004;172:2731–2738. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- Milner JD, Brenchley JM, Laurence A, Freeman AF, Hill BJ, Elias KM, Kanno Y, Spalding C, Elloumi HZ, Paulson ML, et al. Impaired T(H)17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature. 2008;452:773–776. doi: 10.1038/nature06764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annual review of immunology. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- Nurieva R, Yang XO, Martinez G, Zhang Y, Panopoulos AD, Ma L, Schluns K, Tian Q, Watowich SS, Jetten AM, Dong C. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448:480–483. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- Nurieva RI, Chung Y, Hwang D, Yang XO, Kang HS, Ma L, Wang YH, Watowich SS, Jetten AM, Tian Q, Dong C. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity. 2008;29:138–149. doi: 10.1016/j.immuni.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odegard JM, Marks BR, DiPlacido LD, Poholek AC, Kono DH, Dong C, Flavell RA, Craft J. ICOS-dependent extrafollicular helper T cells elicit IgG production via IL-21 in systemic autoimmunity. J Exp Med. 2008;205:2873–2886. doi: 10.1084/jem.20080840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang W, Kolls JK, Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity. 2008;28:454–467. doi: 10.1016/j.immuni.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulendran B, Kumar P, Cutler CW, Mohamadzadeh M, Van Dyke T, Banchereau J. Lipopolysaccharides from distinct pathogens induce different classes of immune responses in vivo. J Immunol. 2001;167:5067–5076. doi: 10.4049/jimmunol.167.9.5067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulendran B, Tang H, Denning TL. Division of labor, plasticity, and crosstalk between dendritic cell subsets. Curr Opin Immunol. 2008;20:61–67. doi: 10.1016/j.coi.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi H, Egen JG, Huang AY, Germain RN. Extrafollicular activation of lymph node B cells by antigen-bearing dendritic cells. Science. 2006;312:1672–1676. doi: 10.1126/science.1125703. [DOI] [PubMed] [Google Scholar]
- Reis e Sousa C. Toll-like receptors and dendritic cells: for whom the bug tolls. Semin Immunol. 2004;16:27–34. doi: 10.1016/j.smim.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Schadeck EB, Sidhu M, Egan MA, Chong SY, Piacente P, Masood A, Garcia-Hand D, Cappello S, Roopchand V, Megati S, et al. A dose sparing effect by plasmid encoded IL-12 adjuvant on a SIVgag-plasmid DNA vaccine in rhesus macaques. Vaccine. 2006;24:4677–4687. doi: 10.1016/j.vaccine.2005.10.035. [DOI] [PubMed] [Google Scholar]
- Schaerli P, Willimann K, Lang AB, Lipp M, Loetscher P, Moser B. CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. J Exp Med. 2000;192:1553–1562. doi: 10.1084/jem.192.11.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shortman K, Liu YJ. Mouse and human dendritic cell subtypes. Nature Rev Immunol. 2002;2:151–161. doi: 10.1038/nri746. [DOI] [PubMed] [Google Scholar]
- Spolski R, Leonard WJ. Interleukin-21: basic biology and implications for cancer and autoimmunity. Annual review of immunology. 2008;26:57–79. doi: 10.1146/annurev.immunol.26.021607.090316. [DOI] [PubMed] [Google Scholar]
- Suto A, Kashiwakuma D, Kagami S, Hirose K, Watanabe N, Yokote K, Saito Y, Nakayama T, Grusby MJ, Iwamoto I, Nakajima H. Development and characterization of IL-21-producing CD4+ T cells. J Exp Med. 2008;205:1369–1379. doi: 10.1084/jem.20072057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thierfelder WE, van Deursen JM, Yamamoto K, Tripp RA, Sarawar SR, Carson RT, Sangster MY, Vignali DA, Doherty PC, Grosveld GC, Ihle JN. Requirement for Stat4 in interleukin-12-mediated responses of natural killer and T cells. Nature. 1996;382:171–174. doi: 10.1038/382171a0. [DOI] [PubMed] [Google Scholar]
- Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- Ueno H, Klechevsky E, Morita R, Aspord C, Cao T, Matsui T, Di Pucchio T, Connolly J, Fay JW, Pascual V, et al. Dendritic cell subsets in health and disease. Immunol Rev. 2007;219:118–142. doi: 10.1111/j.1600-065X.2007.00551.x. [DOI] [PubMed] [Google Scholar]
- van der Meide PH, Villinger F, Ansari AA, Groenestein RJ, de Labie MC, van den Hout YJ, Koornstra WH, Bogers WM, Heeney JL. Stimulation of both humoral and cellular immune responses to HIV-1 gp120 by interleukin-12 in Rhesus macaques. Vaccine. 2002;20:2296–2302. doi: 10.1016/s0264-410x(02)00101-9. [DOI] [PubMed] [Google Scholar]
- Vinuesa CG, Tangye SG, Moser B, Mackay CR. Follicular B helper T cells in antibody responses and autoimmunity. Nat Rev Immunol. 2005;5:853–865. doi: 10.1038/nri1714. [DOI] [PubMed] [Google Scholar]
- Vogelzang A, McGuire HM, Yu D, Sprent J, Mackay CR, King C. A fundamental role for interleukin-21 in the generation of T follicular helper cells. Immunity. 2008;29:127–137. doi: 10.1016/j.immuni.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Volpe E, Servant N, Zollinger R, Bogiatzi SI, Hupe P, Barillot E, Soumelis V. A critical function for transforming growth factor-beta, interleukin 23 and proinflammatory cytokines in driving and modulating human T(H)-17 responses. Nat Immunol. 2008;9:650–657. doi: 10.1038/ni.1613. [DOI] [PubMed] [Google Scholar]
- Wassink L, Vieira PL, Smits HH, Kingsbury GA, Coyle AJ, Kapsenberg ML, Wierenga EA. ICOS expression by activated human Th cells is enhanced by IL-12 and IL-23: increased ICOS expression enhances the effector function of both Th1 and Th2 cells. J Immunol. 2004;173:1779–1786. doi: 10.4049/jimmunol.173.3.1779. [DOI] [PubMed] [Google Scholar]
- Watford WT, Hissong BD, Bream JH, Kanno Y, Muul L, O'Shea JJ. Signaling by IL-12 and IL-23 and the immunoregulatory roles of STAT4. Immunol Rev. 2004;202:139–156. doi: 10.1111/j.0105-2896.2004.00211.x. [DOI] [PubMed] [Google Scholar]
- Wei L, Laurence A, Elias KM, O'Shea JJ. IL-21 is produced by Th17 cells and drives IL-17 production in a STAT3-dependent manner. J Biol Chem. 2007;282:34605–34610. doi: 10.1074/jbc.M705100200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Ivanov, Spolski R, Min R, Shenderov K, Egawa T, Levy DE, Leonard WJ, Littman DR. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8:967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.