Abstract
Context
Sudden cardiac death (SCD) after myocardial infarction (MI) has not recently been assessed in the community. Post-MI risk stratification for SCD commonly relies on baseline characteristics and little is known about the relationship between recurrent ischemia or heart failure (HF) and SCD.
Objective
To evaluate the risk of SCD after MI and the impact of recurrent ischemia and HF on SCD.
Design, setting, and participants
2,997 Olmsted County residents experiencing an MI between 1979 and 2005.
Main outcome measures
SCD defined as out-of-hospital death due to coronary disease. Observed survival free of SCD compared to that expected in Olmsted County.
Results
During a median follow-up of 4.7 years (25th–75th percentile 1.6–7.1, date of last follow-up 02-29-2008), 1,160 deaths occurred, 282 (24%) SCD. The 30-day cumulative incidence of SCD was 1.2% (95% confidence interval [CI] 0.8–1.6%). Thereafter, the rate of SCD was constant at 1.2%/year yielding a 5-year cumulative incidence of 6.9% (95% CI 5.9% to 7.9%). The 30-day incidence of SCD was 4-fold higher than expected (standardized mortality ratio=4.2, 95% CI 2.9 to 5.8). In the year thereafter, the risk of SCD was lower than expected (standardized mortality ratio=0.66, 95% CI 0.50 to 0.85). The risk of SCD declined over time (hazard ratio=0.62, 95% CI 0.44 to 0.88 for MIs in 1997–2005 compared to 1979–1987; p =0.03). Recurrent events, ischemia (n=842) or HF (n=365), occurred in 2,080 patients. After adjustment for baseline characteristics, recurrent ischemia was not associated with SCD (hazard ratio=1.26, 95% CI 0.96 to 1.65; p=0.09), while HF markedly increased the risk of SCD (hazard ratio= 4.20, 95% CI 3.10 to 5.69; p<0.001)
Conclusions
The risk of SCD is highest during the first month after MI and declined over time. SCD is independently associated with HF but not with recurrent ischemia.
INTRODUCTION
Sudden cardiac death (SCD) is a devastating complication of myocardial infarction (MI).1 Long-term population studies outlining the incidence of SCD after MI in the community are decades old.2–6 Since the publication of these reports, the in-hospital mortality after MI has decreased substantially because of evidence-based therapies, yielding a growing number of MI survivors potentially at risk for SCD.7–11 Incidence and predictors of post-MI SCD have recently been examined in large clinical trials12–14 and among patients from tertiary care centers.15–18 However, these selected patients may not be representative of patients in the community.19 Thus, in an era when the long-term prognosis after MI has greatly improved, community-based data reflecting the contemporary risk of post-MI SCD are lacking.
Determining the risk of SCD after an MI remains challenging.20 Implantable cardioverter-defibrillators (ICD) decrease mortality in patients with reduced ejection fraction. However, most patients receiving an ICD for primary prevention of SCD do not use the devices to terminate cardiac tachyarrhythmias,21 at least not during the period of follow-up of most trials. Further, recent data from the Defibrillator in Acute Myocardial Infarction (DINAMIT)22 and the Multicenter Automatic Defibrillator Implantation Trial (MADIT)-II,23 show that ICD placement less than 40 days after a MI does not reduce mortality, suggesting that post-MI SCD risk is time-dependent. 24, 25 One issue with current risk prediction approaches for SCD is that predictions based solely on characteristics assessed at baseline, shortly after the MI, may be insufficient. This leads to our hypothesis, as yet untested, that inter-current events, such as recurrent ischemia and heart failure (HF) occurring after the index MI increase the risk of SCD.
The objectives of the present investigation were to address the aforementioned gaps in knowledge within a population-based surveillance study of all MI patients in Olmsted County, Minnesota, between 1979 and 2005. Specifically, we set out to define the burden of SCD post-MI in the community, to examine if it changed over time, and to assess its relationship with inter-current ischemia and HF after MI.
METHODS
Study setting
Epidemiologic research is possible in Olmsted County, Minnesota, because the county is relatively isolated from other urban centers and nearly all medical care is delivered to local residents by a small number of providers. Except for the fact that a higher proportion of the working population is employed in the health care industry, the characteristics of the population of Olmsted County are similar to those of white persons in the United States (www.census.gov). All medical care providers, including the Mayo Medical Center, the Olmsted Medical Group and its affiliated Olmsted Community Hospital, employ a unit medical record system. Thus, detailed information on all inpatient, outpatient and emergency department visits and all laboratory results, pathology reports, correspondence and records of physician visits to nursing homes or private homes are kept in one place. Since the early 1960s, extensive indices based on clinical or histological diagnosis, surgical procedures and billing data have also been kept for all providers of health care under the auspices of the Rochester Epidemiology Project.26 This allows linkage of information from essentially all sources of heath care available to and used by the residents of Olmsted County and provides a unique infrastructure for analyzing disease determinants and outcomes.
Assembling the MI cohort
Methods used in the identification of patients with incident MI have been published before.26 Briefly, lists of patients discharged from Olmsted County hospitals between 1979 and 2005 with a diagnosis compatible with MI were obtained from the Rochester Epidemiology Project index of diagnoses. The target International Classification of Diseases, Ninth Revision codes were 410 (acute MI), 411 (other acute and subacute forms of ischemic heart disease), 412 (old MI), 413 (angina pectoris) and 414 (other forms of ischemic heart disease). All events coded as 410, a 50% random sample of codes 411, and a 10% random sample of codes 412, 413 and 414 were reviewed. The sampling fractions were similar to those used in other studies.27 Minnesota law requires obtaining general research authorization from every patient before the release of any information from medical records for research purposes and as this study did not include patient contact, no specific consent was necessary.
To ascertain the incident MI status, medical records were reviewed by abstractors and data on cardiac pain, cardiac biomarkers, and electrocardiogram results were obtained. All electrocardiograms (ECG) were interpreted according to the Minnesota code.28 Standard criteria were applied to assign a MI diagnosis based on these data. Information on the reliability of these criteria has been published before.11
Clinical data and inter-current cardiac events
Baseline demographic and clinical characteristics including hypertension, hyperlipidemia, smoking status, diabetes mellitus, obesity, family history of coronary heart disease, Killip class and other comorbidities at the time of index MI were recorded from physicians’ clinical notes. Total comorbidity burden was summarized by the Charlson comorbidity index.29
The first episodes of inter-current cardiac events that occurred after the incident MI were recorded. Heart failure was diagnosed using the Framingham Heart Study criteria requiring the simultaneous presence of at least 2 major criteria (i.e. paroxysmal nocturnal dyspnea or orthopnea, neck vein distension, rales, cardiomegaly, acute pulmonary edema, S3 gallop, venous pressure ≥16 cm of water, hepatojugular reflux) or 1 major criterion with 2 minor criteria (i.e. ankle edema, night cough, dyspnea upon exertion, hepatomegaly, pleural effusion, vital capacity decreased by 1/3 from maximum, heart rate ≥120 beats/min).30 Recurrent ischemia was defined as hospitalization for recurrent MI or unstable angina using the physicians’ diagnosis.31, 32
Follow-up and ascertainment of sudden death
All patients were followed-up until death or the date of the last follow-up as ascertained by reviewing the entire community medical records. The final date of follow up was 2/29/2008. All death certificate data obtained from the Minnesota Department of Health were searched to identify individuals with age >25 years who were listed as residents of Olmsted County at the time of death. Information on age and sex of the decedent and the date, site and underlying cause of death, as determined by the state nosologist, was collected. Since 1968, all death certificates issued in Minnesota have described the site of death. Out-of-hospital deaths were defined as those occurring outside of acute-care or long-term-care hospitals, including deaths occurring in emergency departments, private homes, public places, nursing or boarding-care homes, and infirmaries, as well as deaths among persons declared dead on arrival at a hospital. Sudden cardiac death was defined as out-of-hospital deaths whose primary cause of death was classified as coronary heart disease on the death certificate (International Classification of Diseases, Ninth Revision codes 410–414). This definition, previously validated in the Olmsted County population, provides a robust positive predictive value for SCD due to coronary heart disease and occurring within 24 hours of the onset of symptoms.33
Statistical analysis
Data are presented as frequency or as mean ± standard deviation. Patients who died in hospital and could not by design meet criteria for SCD were not included. The analysis thus pertained to hospital survivors. Characteristics of patients who died suddenly were compared to those who survived or died of other causes with proportional hazards regression. Survival free of SCD was analyzed while treating deaths from other causes as a competing risk and survival free of recurrent ischemic events and HF was analyzed treating all deaths as a competing risk.34 The risk of SCD after MI was compared to the risk of SCD in the general population of Olmsted County matched on age and sex and expressed as a standardized mortality ratio.
Predictors of SCD were assessed by Cox proportional hazards regression, with recurrent ischemic events and HF treated as time-dependent covariates. HF that occurred after hospitalization for MI but before dismissal was included as an intercurrent event. In the model, patients were treated as being at risk for SCD after hospital dismissal. Interactions between year of MI and all predictor variables were tested. Only the year*age interaction was found to be significant. The proportional hazards assumption was assessed using the Schoenfeld residuals and found to be valid. Additive hazard models were used to assess the absolute increment in SCD risk associated with inter-current events. Differences in the baseline hazard were observed before and after 30 days after hospital dismissal, so time epochs were created for the first 30 days post-MI and yearly thereafter. The additive model was fit in terms of event rate per epoch. A p value < 0.05 was considered statistically significant. Analyses were done with SAS version 8.2 (SAS Institute Inc., Cary, North Carolina) and S-PLUS version 8.0.1 (Insightful Corp, Seattle, Washington). The Mayo Clinic and Olmsted Medical Center Review Boards reviewed and approved all aspects of the study.
RESULTS
The myocardial infarction incidence cohort
Between 1979 and 2005, 3,296 incident MIs occurred in Olmsted County; 299 died in-hospital leaving 2997 hospital survivors as the study population. At the time of index MI, these patients were 67±14 years old and 59% were men; cardiovascular risk factors were prevalent and 59% of the patients had at least one comorbid condition. Most cases in this community cohort presented electrocardiographically as non ST segment elevation MIs (Table 1). Approximately half were treated with reperfusion/revascularization with 1156 (39%) undergoing percutaneous coronary intervention, 282 (9%) undergoing coronary artery bypass grafting, and 318 (11%) treated with thrombolytics.
Table 1.
Baseline clinical characteristics associated with sudden death
| No. missing | All patients (n=2997) |
Patients with sudden death (n=282) |
Patients without sudden death (n=2715) | Hazard ratio (95% confidence intervals) |
p-value | |
|---|---|---|---|---|---|---|
| Age (years, mean ± SD) | 0 | 67±14 | 75±14 | 66±14 | 1.08 (1.07 – 1.09) | <0.001 |
| Female, n (%) | 0 | 1230 (41) | 148 (52) | 1082 (40) | 1.90 (1.50 – 2.40) | <0.001 |
| Hypertension, n (%) | 0 | 1695 (57) | 186 (66) | 1509 (56) | 2.10 (1.64 – 2.70) | <0.001 |
| Hyperlipidemia, n (%) | 0 | 1169 (39) | 72 (26) | 1097 (40) | 0.67 (0.51 – 0.88) | 0.004 |
| Diabetes mellitus, n (%) | 0 | 629 (21) | 74 (26) | 555 (20) | 1.79 (1.37 – 2.34) | <0.001 |
| Former or current smoker, n (%) | 8 | 1859 (62) | 144 (51) | 1715 (63) | 0.55 (0.44 – 0.70) | <0.001 |
| Obesity, n (%) | 0 | 859 (29) | 52 (18) | 807 (30) | 0.63 (0.46 – 0.85) | 0.002 |
| Familial coronary disease, n (%) | 130 | 607 (21) | 45 (17) | 562 (22) | 0.67 (0.49 – 0.93) | 0.015 |
| Comorbidity index | 0 | <0.001 | ||||
| 0, n (%) | 1241 (41) | 79 (28) | 1162 (43) | 1 | ||
| 1–2, n (%) | 1049 (35) | 108 (38) | 941 (35) | 2.13 (1.59 – 2.85) | ||
| ≥ 3, n (%) | 707 (24) | 95 (34) | 612 (22) | 4.60 (3.37 – 6.28) | ||
| Killip class 2–4, n (%) | 27 | 900 (30) | 141 (51) | 759 (28) | 3.05 (2.41 – 3.86) | <0.001 |
| Anterior MI, n (%) | 279 | 975 (36) | 104 (45) | 871 (35) | 1.63 (1.25 – 2.11) | <0.001 |
| ST elevation MI, n (%) | 279 | 928(34) | 85 (37) | 843 (34) | 0.98 (0.75 – 1.28) | 0.86 |
| Reperfusion/ revascularization, n (%) |
16 | 1560 (52) | 58 (21) | 1502 (56) | 0.23 (0.18 – 0.31) | <0.001 |
MI denotes myocardial infarction
Incidence of sudden death after MI
Over a median follow-up of 4.7 years (25th–75th percentile 1.6–7.1 years), 1160 deaths were enumerated, 282 (24%) of which were classified as SCD.
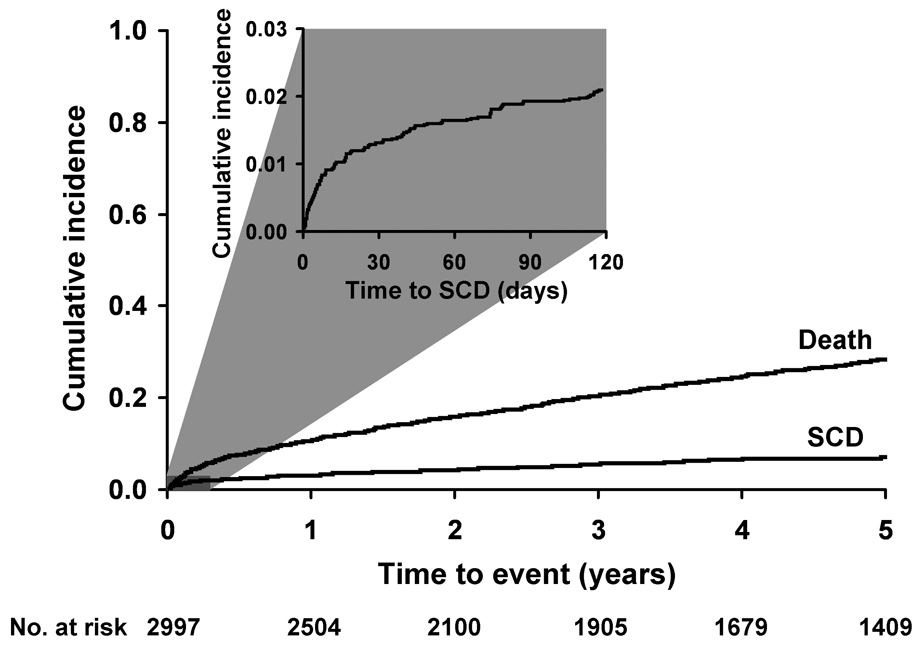
During the first 30 days after hospital dismissal, 35 SCD occurred for a cumulative incidence of 1.2% (95% confidence interval [CI] 0.8% to 1.6%) in the first month. Thereafter, the rate of SCD declined markedly to a mean annual mortality of 1.2% per year. Figure 1 shows the cumulative incidence of SCD treating other causes of death as a competing risk. At 1 year, the cumulative incidence of SCD was 3.0% (95% CI 2.4% to 3.7%). At 5 years, the cumulative incidence of SCD was 6.9% (95% CI 5.9% to 7.9%).
Figure 1.
Cumulative incidence of sudden death and all-cause mortality after myocardial infarction among residents of Olmsted County, Minnesota.
Patients who experienced SCD were older, more likely to be women and to have a history of hypertension, diabetes mellitus and other comorbidities (Table 1). They were more likely to present in a higher Killip class and with an anterior MI. Patients who experienced SCD were less likely to have been treated with reperfusion/revascularization.
The risk of SCD after MI decreased during the 27 years of the study period. With MIs that occurred between 1979–1987 as the reference and after adjusting for age, the risk of SCD was 20% lower (hazard ratio=0.80; 95% CI 0.60 to 1.07) for MIs that occurred from 1988 to 1996 and 38% lower (hazard ratio=0.62; 95% CI 0.44 to 0.88) for MIs that occurred between 1997 and 2005 (p=0.025).
Risk of sudden death after MI compared with the general population
In the first 30 days after hospital dismissal, the 35 SCD that were enumerated represent more than a 4-fold increase in the risk of SCD when compared to the 8 SCD expected among age and sex-matched subjects within the same source population of Olmsted County (standardized mortality ratio=4.2, 95% CI 2.9 to 5.8). In the year thereafter, the risk of SCD after MI was lower than that expected in the general population (standardized mortality ratio=0.66, 95% CI 0.50 to 0.85) and remained lower than expected in the following years (Table 2).
Table 2.
Observed number of sudden deaths after myocardial infarction compared with the numbers expected in the general population of Olmsted County
| Time after myocardial infarction | Observed sudden deaths after myocardial infarction | Rate (% per person-year) | Expected sudden deaths † | Standardized mortality ratio (95% CI) |
|---|---|---|---|---|
| 0–30 days | 35 | 14.8 | 8.4 | 4.18 (2.91–5.81) |
| Year 1* | 59 | 2.2 | 89.4 | 0.66 (0.50–0.85) |
| Year 2* | 30 | 1.3 | 73.5 | 0.41 (0.28–0.58) |
| Year 3* | 29 | 1.5 | 65.9 | 0.44 (0.30–0.63) |
| Year 4* | 21 | 1.2 | 59.6 | 0.35 (0.22–0.54) |
These numbers are those expected in the age and sex-matched population from Olmsted County
Year intervals are every 365 days after the first 30 days.
Inter-current events and sudden death
Inter-current cardiac events occurred frequently after MI (2,080 of the 2,997 patients; 69%). A total of 842 patients had recurrent ischemia alone, 365 had HF alone and 873 had both events. Among the patients who experienced both events, the events occurred within 30 days of each other in 378 (43%) patients.
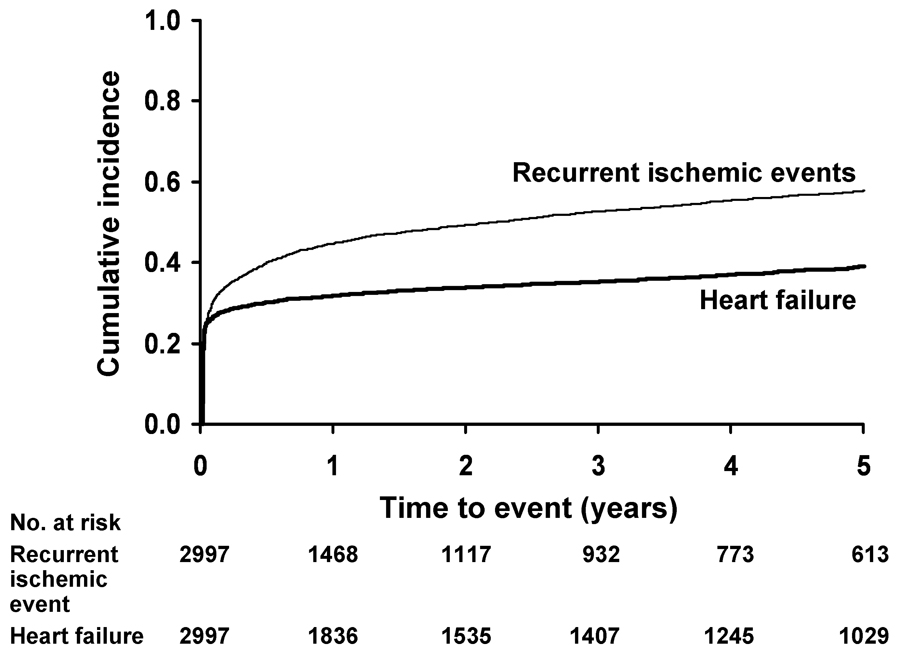
For recurrent ischemia, the 30-day cumulative incidence was 28% (95% CI 26% to 29%) and the 1-year cumulative incidence was 42% (95% CI 41% to 44%) (Figure 2). Beyond the first year after the MI, the cumulative incidence of recurrent ischemia exhibited a constant increase with a 5-year cumulative incidence of 56% (95% CI 55% to 58%). This equates to a yearly rate of recurrent ischemia of 3.5% beyond the first year post-MI. Recurrent ischemia during follow-up was associated with an increased risk of SCD (hazard ratio=1.31, 95% CI 1.01 to 1.71; p=0.041). After adjusting for baseline clinical characteristics (age, sex, year of MI, hypertension, diabetes mellitus, comorbidity, Killip class, reperfusion, and heart failure post-MI), the association between SCD and recurrent ischemia was attenuated and became non-significant (hazard ratio =1.26, 95% CI 0.96 to 1.65; p=0.090) (Table 3).
Figure 2.
Cumulative incidence of recurrent ischemic events and heart failure after myocardial infarction among residents of Olmsted County, Minnesota.
Table 3.
Multivariable predictors of sudden death after myocardial infarction among residents of Olmsted County, Minnesota.
| Hazard ratio (95% CI) |
P value | |
|---|---|---|
| Baseline characteristics | ||
| Women | 0.75 (0.58–0.98) | 0.033 |
| Age=60 | 0.003* | |
| 1979–1987 | 1 | |
| 1988–1996 | 0.93 (0.56–1.53) | |
| 1997–2005 | 0.35 (0.17–0.74) | |
| Age=70 | ||
| 1979–1987 | 1 | |
| 1988–1996 | 1.10 (0.78–1.56) | |
| 1997–2005 | 0.59 (0.35–0.98) | |
| Age=80 | ||
| 1979–1987 | 1 | |
| 1988–1996 | 1.30 (0.93–1.83) | |
| 1997–2005 | 0.97 (0.65–1.46) | |
| Hypertension | 1.24 (0.95–1.63) | 0.12 |
| Diabetes mellitus | 1.09 (0.80–1.48) | 0.60 |
| Comorbidity Index 1–2 | 1.10 (0.80–1.52) | 0.018 |
| Comorbidity Index ≥ 3 | 1.66 (1.12–2.45) | |
| Killip class 2–4 | 1.35 (1.04–1.75) | 0.022 |
| Reperfusion/revascularization | 0.39 (0.29–0.54) | <0.001 |
| Follow-up events | ||
| Recurrent ischemic event | 1.26 (0.96–1.65) | 0.090 |
| Heart failure | 4.20 (3.10–5.69) | <0.001 |
P value for age*year interaction
For HF, the 30-day cumulative incidence was 26% (95% CI 24% to 27%) and at 5 years it was 38% (95% CI 37% to 40%) (Figure 2). This equates to a yearly HF rate of 2.4% beyond the first 30 days post-MI. HF exhibited a strong adverse association with SCD (hazard ratio=8.1, 95% CI 6.2 to 10.6; p<0.001), which remained strong after adjustment for baseline clinical characteristics (age, sex, year of MI, hypertension, diabetes mellitus, comorbidity, Killip class, reperfusion, and recurrent ischemia post-MI) (hazard ratio=4.20, 95% CI 3.10 to 5.69; p<0.001) (Table 3). Expressed in terms of absolute risk and comparing to patients who did not experience HF during follow-up, the absolute risk of SCD for patients with HF was, on average, 2.5% (95% CI 1.9% to 3.1%; p<0.001) higher in the first 30 days after MI, and in each year thereafter. Given the aforementioned temporal decline in the risk of SCD, we then examined the absolute risks of SCD according to the presence or absence of HF by time period. Considering 75-year-old patients experiencing an MI in 1979–1987 as an example, the absolute risk of SCD for those with HF was 4.3% (95% CI 3.6% to 5.1%) within the first 30 days after MI and in each year thereafter compared to an absolute risk of SCD of 1.9% (95% CI 1.3% to 2.4%) among those who did not experience HF. For 75-year-old patients experiencing an MI in 1988–1996, the absolute risk of SCD was 3.7% (95% CI 3.0% to 4.4%) and 1.2% (95% CI 0.7% to 1.6%) for those experiencing and not experiencing heart failure, respectively. Finally, for 75-year-old patients experiencing an MI during the most recent time period (1997–2005), the absolute risk of SCD was 2.4% (95% CI 1.7% to 3.2%) and 0% (95% CI −0.7% to 0.6%) depending on the occurrence or non-occurrence of heart failure, respectively.
Ancillary analyses
For the 1204 MIs occurring in 1988–1998, left ventricular systolic function was assessed among 693 (58%) patients within 30 days after index MI. Of these, 337 (49%) had left ventricular ejection fraction below 50%. When left ventricular ejection fraction was included in the multivariable analysis, there was no change in the association between SCD and inter-current cardiac events (recurrent ischemia hazard ratio=1.60, 95% CI 0.90 to 2.82; p=0.11); HF hazard ratio=3.64, 95% CI 1.71 to 7.75; p<0.001). In particular, the strength of the association between HF and SCD remained strong.
Fifty patients had defibrillators implanted before their date of last follow-up. All analyses were repeated while excluding these patients and yielded similar results.
DISCUSSION
The present data, which represent the experience of a large, community-based incidence cohort, indicate that the risk of SCD after MI is the highest during the first month, when it markedly exceeds that noted in the general population. Thereafter, however, among 30-day survivors, the risk of SCD declines markedly to 1.2% per year, lower than that expected in the general population. Further, the risk of SCD post-MI has declined by more than 40% over the past quarter of a century, a decline that predates the widespread use of defibrillators but parallels drastic changes in medical therapy for acute MI including reperfusion and secondary prevention. While the risk of SCD beyond the first 30 days is low, it is markedly increased by the occurrence of HF during follow-up, which underscores the importance of continued surveillance of post-MI patients and the dynamic nature of risk stratification.
Sudden death after myocardial infarction
The rather sparse community data on the incidence of SCD after MI largely predate the widespread use of evidence-based treatment for MI. In the Framingham Heart Study, the 5-year cumulative incidence of SCD was approximately 7% 3 using a conservative definition of SCD whereby only deaths within one hour of symptom onset were included. In the Multicenter Post Infarction Program, conducted in the 1980s, SCDs were more frequent (3.6% per year).5 More recently, in a cohort study of MI patients discharged from several Canadian medical centers, SCD occurred at a rate of 1.9% per year, a figure consistent with the present data (15). On the other hand, post-MI SCD was less common (< 1% per year) among patients discharged after optimal treatment including revascularization and medical therapy in single-center European studies.15, 17 These differences across studies likely reflect multiple factors including differences in definitions, differences in time periods when studies were conducted, and differences between community experiences and single-center studies. Methodological issues notwithstanding, taken collectively, these reports indicate that beyond the first month post-MI, SCD is a rather infrequent albeit devastating complication. The present data augment previous reports by indicating that the risk of SCD after MI has markedly declined over time and by providing a reference framework on the occurrence of SCD among age and sex matched subjects from the same source population. After the initial month after the MI event, the rate of SCD becomes less than expected in the general population, most likely reflecting the well-known survivor bias effect, which is amplified as the duration of follow-up increases. With regards to the temporal decline in SCD after MI, there was prior indirect evidence that this might be occurring. Indeed, whereas approximately 40%–50% of all post-MI deaths were due to SCD in studies prior to the 1980s,5, 6 this proportion has decreased to 20%–30% in more contemporary cohorts including the present one.15, 17, 18 However, historical comparisons across cohorts are fraught with challenges as source populations often differ markedly, which can in turn explain differences in outcomes. The present study, using the same population and robust, standardized methods of MI ascertainment which have remained constant during the study period, directly demonstrates a profound reduction in the occurrence of SCD after MI in one single population under rigorous surveillance. Importantly, the practice of implanting defibrillators is unlikely to have impacted the present findings as their use was minimal in this cohort.
The risk of SCD after MI is the highest during the first 30 days as was underscored by data from the Valsartan in Acute Myocardial Infarction Trial (VALIANT) 13 and the Home Automated External Defibrillator Trial (HAT).35 Both of these trials focused on high risk groups and indicated that the occurrence of SCD among 30-day MI survivors was approximately 1% per year. The present community data extend these findings by indicating that in a community-based cohort, the risk of SCD among all patients with MI is analogous to that of the highest risk groups in these trials, is highest early after the MI and declines markedly thereafter. While these data may come across as seemingly at odds with the efficacy of implantable cardioverter-defibrillators as reported in most randomized trials, interpreting the results of these trials is challenging as recently underscored.36
Inter-current cardiac events
Inter-current cardiac events are common after MI and remain common even in contemporary cohorts.32, 37–40 The frequent occurrence of HF is of particular concern given its adverse impact on the occurrence of SCD, which is far greater than and independent of risk factors that can be measured at the time of the index MI. In a recent report from the Multicenter Automatic Defibrillator Implantation Trial (MADIT)-II, participants randomized to ICD were studied to assess the effects of inter-current cardiac events on appropriate ICD discharges for ventricular tachycardia or fibrillation.41 After adjustment for numerous factors including left ventricular ejection fraction, New York Heart Association functional class, medications and laboratory results, HF and recurrent ischemia were associated with a 2.5 and 1.5 times higher risk of device therapy, respectively.41 The findings presented herein support and amplify such previous reports by indicating that, in a community-based cohort, cardiac events during follow-up are associated with a marked increase in the risk of SCD, particularly for heart failure, which increases the risk of SCD by a 4-fold factor. This increase in the risk related to the occurrence of heart failure cannot be fully interpreted without its integration within the appraisal of the absolute risk of SCD, which is particularly high during the first month after MI. Indeed, understanding the excess risk conferred by heart failure requires integrating its relative risk with the absolute risk of the SCD at a given point in time during follow-up.42 Thus, clinicians should be particularly concerned about the adverse impact of heart failure on SCD when heart failure occurs early during follow-up.
Strengths and limitations
The racial and ethnic composition of Olmsted County may limit the generalization of these data to groups under-represented in the population. While no single community can completely represent the nation as a whole, studies of chronic diseases in Olmsted County indicate that results from the county can be extrapolated to a large part of the population. Left ventricular function was not uniformly assessed in all patients, consistent with current practice.43–46 However, an ancillary analysis restricted to persons with measurement of left ventricular function showed similar results. Herein, we defined SCD as out-of-hospital deaths whose primary cause of death was coronary heart disease 33. The validity of this method is quite robust for SCD due to coronary disease occurring within 24 hours of symptom onset and this definition is analogous to that used by others 13. In our experience, using one hour as the time frame to determine the sudden nature of death could not be implemented with an acceptable accuracy. To the extent that subjects would have sought care prior to death, the presence of clinical non-fatal conditions would have been taken into account in the analysis. It is conceivable that out-of-hospital deaths occurring after MI may be more likely to be coded as cardiac in origin. This limitation is shared by all studies addressing this subject. Importantly, the temporal trends in SCD post-MI is not affected by definitional issues as the definition of SCD remained constant during the study period.
Our study also has a number of important strengths. The internal validity of the present data is quite robust as our ascertainment identified all consecutive incident MIs in the community evaluated according to rigorous validation criteria which remained constant over time. Indeed, the present data represents the comprehensive experience of a community for more than 2 decades during a time period that is minimally affected by the trials of defibrillator implant post-MI such that the present results are not influenced by this evolving practice. The availability of rich clinical follow-up for non-fatal clinical events that occur after the initial hospitalization is a distinct feature of the present community study as most other MI registries47, 48 and surveillance studies9, 10, 49 seldom include post-hospital clinical follow-up. This unique strength enabled us to integrate inter-current clinical events post-MI in the prediction of SCD, which has important direct clinical implications for risk stratification. Indeed, these data underscore the dynamic nature of risk stratification and the crucial importance of clinicians reassessing patients’ risks after MI if they develop HF. Finally, the innovative statistical methodology applied herein, which builds on extensive experience with survival analysis,50 allowed us to report on the absolute risk after MI, an important yet seldom reported element of risk stratification.42, 51, 52
To this end, the present data document that in contemporary times, the risk of SCD after MI in the absence of inter-current HF is quite low, which can help in decision making for primary prevention of SCD among post-MI patients.
CONCLUSION
In the community, the risk of SCD is the highest during the first month after MI, when it markedly exceeds that noted in the general population. Among 30-day survivors, the risk of SCD declines rapidly but is markedly increased by the occurrence of HF during follow-up. This underscores the importance of continued surveillance of post-MI patients and the dynamic nature of risk stratification. Moreover, the risk of SCD after MI has declined substantially over the past quarter of a century, before the widespread use of defibrillators, which underscore the importance of evidence-based therapy for acute MI including reperfusion and secondary prevention.
ACKNOWLEDGEMENTS
This study was supported by grants from the Public Health Service and the National Institutes of Health (AR30582, R01 HL 59205 and R01 HL 72435). Dr. Roger is an Established Investigator of the American Heart Association. Dr. Adabag is supported, in part, by VA Clinical Science R&D Service (Grant no. 04S-CRCOE 001), Washington, DC. The funding organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
The corresponding author had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
REFERENCES
- 1.Huikuri HV, Castellanos A, Myerburg RJ. Sudden death due to cardiac arrhythmias. N Engl J Med. 2001 Nov 15;345(20):1473–1482. doi: 10.1056/NEJMra000650. [DOI] [PubMed] [Google Scholar]
- 2.Risk stratification and survival after myocardial infarction. N Engl J Med. 1983 Aug 11;309(6):331–336. doi: 10.1056/NEJM198308113090602. [DOI] [PubMed] [Google Scholar]
- 3.Berger CJ, Murabito JM, Evans JC, Anderson KM, Levy D. Prognosis after first myocardial infarction. Comparison of Q-wave and non-Q-wave myocardial infarction in the Framingham Heart Study. Jama. 1992 Sep 23–30;268(12):1545–1551. doi: 10.1001/jama.268.12.1545. [DOI] [PubMed] [Google Scholar]
- 4.Kannel WB, Sorlie P, McNamara PM. Prognosis after initial myocardial infarction: the Framingham study. Am J Cardiol. 1979 Jul;44(1):53–59. doi: 10.1016/0002-9149(79)90250-9. [DOI] [PubMed] [Google Scholar]
- 5.Marcus FI, Cobb LA, Edwards JE, et al. Mechanism of death and prevalence of myocardial ischemic symptoms in the terminal event after acute myocardial infarction. Am J Cardiol. 1988 Jan 1;61(1):8–15. doi: 10.1016/0002-9149(88)91295-7. [DOI] [PubMed] [Google Scholar]
- 6.Mukharji J, Rude RE, Poole WK, et al. Risk factors for sudden death after acute myocardial infarction: two-year follow-up. Am J Cardiol. 1984 Jul 1;54(1):31–36. doi: 10.1016/0002-9149(84)90299-6. [DOI] [PubMed] [Google Scholar]
- 7.Fox CS, Evans JC, Larson MG, Kannel WB, Levy D. Temporal trends in coronary heart disease mortality and sudden cardiac death from 1950 to 1999: the Framingham Heart Study. Circulation. 2004 Aug 3;110(5):522–527. doi: 10.1161/01.CIR.0000136993.34344.41. [DOI] [PubMed] [Google Scholar]
- 8.Gerber Y, Jacobsen SJ, Frye RL, Weston SA, Killian JM, Roger VL. Secular trends in deaths from cardiovascular diseases: a 25-year community study. Circulation. 2006 May 16;113(19):2285–2292. doi: 10.1161/CIRCULATIONAHA.105.590463. [DOI] [PubMed] [Google Scholar]
- 9.Goldberg RJ, Gorak EJ, Yarzebski J, et al. A communitywide perspective of sex differences and temporal trends in the incidence and survival rates after acute myocardial infarction and out-of-hospital deaths caused by coronary heart disease. Circulation. 1993 Jun;87(6):1947–1953. doi: 10.1161/01.cir.87.6.1947. [DOI] [PubMed] [Google Scholar]
- 10.McGovern PG, Pankow JS, Shahar E, et al. Recent trends in acute coronary heart disease--mortality, morbidity, medical care, and risk factors. The Minnesota Heart Survey Investigators. N Engl J Med. 1996 Apr 4;334(14):884–890. doi: 10.1056/NEJM199604043341403. [DOI] [PubMed] [Google Scholar]
- 11.Roger VL, Jacobsen SJ, Weston SA, et al. Trends in the incidence and survival of patients with hospitalized myocardial infarction, Olmsted County, Minnesota, 1979 to 1994. Ann Intern Med. 2002 Mar 5;136(5):341–348. doi: 10.7326/0003-4819-136-5-200203050-00005. [DOI] [PubMed] [Google Scholar]
- 12.Abildstrom SZ, Rask-Madsen C, Ottesen MM, et al. Impact of age and sex on sudden cardiovascular death following myocardial infarction. Heart. 2002 Dec;88(6):573–578. doi: 10.1136/heart.88.6.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Solomon SD, Zelenkofske S, McMurray JJ, et al. Sudden death in patients with myocardial infarction and left ventricular dysfunction, heart failure, or both. N Engl J Med. 2005 Jun 23;352(25):2581–2588. doi: 10.1056/NEJMoa043938. [DOI] [PubMed] [Google Scholar]
- 14.Yap YG, Duong T, Bland M, et al. Temporal trends on the risk of arrhythmic vs. non-arrhythmic deaths in high-risk patients after myocardial infarction: a combined analysis from multicentre trials. Eur Heart J. 2005 Jul;26(14):1385–1393. doi: 10.1093/eurheartj/ehi268. [DOI] [PubMed] [Google Scholar]
- 15.Huikuri HV, Tapanainen JM, Lindgren K, et al. Prediction of sudden cardiac death after myocardial infarction in the beta-blocking era. J Am Coll Cardiol. 2003 Aug 20;42(4):652–658. doi: 10.1016/s0735-1097(03)00783-6. [DOI] [PubMed] [Google Scholar]
- 16.Jordaens L, Tavernier R. Determinants of sudden death after discharge from hospital for myocardial infarction in the thrombolytic era. Eur Heart J. 2001 Jul;22(14):1214–1225. doi: 10.1053/euhj.2000.2464. [DOI] [PubMed] [Google Scholar]
- 17.Makikallio TH, Barthel P, Schneider R, et al. Frequency of sudden cardiac death among acute myocardial infarction survivors with optimized medical and revascularization therapy. Am J Cardiol. 2006 Feb 15;97(4):480–484. doi: 10.1016/j.amjcard.2005.09.077. [DOI] [PubMed] [Google Scholar]
- 18.Rouleau JL, Talajic M, Sussex B, et al. Myocardial infarction patients in the 1990s--their risk factors, stratification and survival in Canada: the Canadian Assessment of Myocardial Infarction (CAMI) Study. J Am Coll Cardiol. 1996 Apr;27(5):1119–1127. doi: 10.1016/0735-1097(95)00599-4. [DOI] [PubMed] [Google Scholar]
- 19.Steg PG, Lopez-Sendon J, Lopez de Sa E, et al. External validity of clinical trials in acute myocardial infarction. Arch Intern Med. 2007 Jan 8;167(1):68–73. doi: 10.1001/archinte.167.1.68. [DOI] [PubMed] [Google Scholar]
- 20.Hohnloser SH, Gersh BJ. Changing late prognosis of acute myocardial infarction: impact on management of ventricular arrhythmias in the era of reperfusion and the implantable cardioverter-defibrillator. Circulation. 2003 Feb 25;107(7):941–946. doi: 10.1161/01.cir.0000054211.00668.9b. [DOI] [PubMed] [Google Scholar]
- 21.Moss AJ, Zareba W, Hall WJ, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002 Mar 21;346(12):877–883. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 22.Hohnloser SH, Kuck KH, Dorian P, et al. Prophylactic use of an implantable cardioverter-defibrillator after acute myocardial infarction. N Engl J Med. 2004 Dec 9;351(24):2481–2488. doi: 10.1056/NEJMoa041489. [DOI] [PubMed] [Google Scholar]
- 23.Wilber DJ, Zareba W, Hall WJ, et al. Time dependence of mortality risk and defibrillator benefit after myocardial infarction. Circulation. 2004 Mar 9;109(9):1082–1084. doi: 10.1161/01.CIR.0000121328.12536.07. [DOI] [PubMed] [Google Scholar]
- 24.Myerburg RJ, Kessler KM, Castellanos A. Sudden cardiac death. Structure, function, and time-dependence of risk. Circulation. 1992 Jan;85(1 Suppl):I2–I10. [PubMed] [Google Scholar]
- 25.Salukhe TV, Dimopoulos K, Sutton R, Coats AJ, Piepoli M, Francis DP. Life-years gained from defibrillator implantation: markedly nonlinear increase during 3 years of follow-up and its implications. Circulation. 2004 Apr 20;109(15):1848–1853. doi: 10.1161/01.CIR.0000125522.10053.29. [DOI] [PubMed] [Google Scholar]
- 26.Roger VL, Killian J, Henkel M, et al. Coronary disease surveillance in Olmsted County objectives and methodology. J Clin Epidemiol. 2002 Jun;55(6):593–601. doi: 10.1016/s0895-4356(02)00390-6. [DOI] [PubMed] [Google Scholar]
- 27.White AD, Folsom AR, Chambless LE, et al. Community surveillance of coronary heart disease in the Atherosclerosis Risk in Communities (ARIC) Study: methods and initial two years' experience. J Clin Epidemiol. 1996 Feb;49(2):223–233. doi: 10.1016/0895-4356(95)00041-0. [DOI] [PubMed] [Google Scholar]
- 28.Prineas R, Crow R, Blackbur H. The Minnesota Code Manual of Electrocardiographic Findings: Standards and Procedures for Measurements and Classification. Liettleton, MA: Wright-PSG; 1982. [Google Scholar]
- 29.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 30.Ho KK, Pinsky JL, Kannel WB, Levy D. The epidemiology of heart failure: the Framingham Study. J Am Coll Cardiol. 1993 Oct;22(4 Suppl A):6A–13A. doi: 10.1016/0735-1097(93)90455-a. [DOI] [PubMed] [Google Scholar]
- 31.Goraya TY, Jacobsen SJ, Kottke TE, Frye RL, Weston SA, Roger VL. Coronary heart disease death and sudden cardiac death: a 20-year population-based study. Am J Epidemiol. 2003 May 1;157(9):763–770. doi: 10.1093/aje/kwg057. [DOI] [PubMed] [Google Scholar]
- 32.Jokhadar M, Jacobsen SJ, Reeder GS, Weston SA, Roger VL. Sudden death and recurrent ischemic events after myocardial infarction in the community. Am J Epidemiol. 2004 Jun 1;159(11):1040–1046. doi: 10.1093/aje/kwh147. [DOI] [PubMed] [Google Scholar]
- 33.Goraya TY, Jacobsen SJ, Belau PG, Weston SA, Kottke TE, Roger VL. Validation of death certificate diagnosis of out-of-hospital coronary heart disease deaths in Olmsted County, Minnesota. Mayo Clin Proc. 2000 Jul;75(7):681–687. doi: 10.4065/75.7.681. [DOI] [PubMed] [Google Scholar]
- 34.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999 Mar 30;18(6):695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 35.Bardy GH, Lee KL, Mark DB, et al. Home use of automated external defibrillators for sudden cardiac arrest. N Engl J Med. 2008 Apr 24;358(17):1793–1804. doi: 10.1056/NEJMoa0801651. [DOI] [PubMed] [Google Scholar]
- 36.Bunch TJ, Hohnloser SH, Gersh BJ. Mechanisms of sudden cardiac death in myocardial infarction survivors: insights from the randomized trials of implantable cardioverter-defibrillators. Circulation. 2007 May 8;115(18):2451–2457. doi: 10.1161/CIRCULATIONAHA.106.683235. [DOI] [PubMed] [Google Scholar]
- 37.Guidry UC, Evans JC, Larson MG, Wilson PW, Murabito JM, Levy D. Temporal trends in event rates after Q-wave myocardial infarction: the Framingham Heart Study. Circulation. 1999 Nov 16;100(20):2054–2059. doi: 10.1161/01.cir.100.20.2054. [DOI] [PubMed] [Google Scholar]
- 38.Hellermann JP, Goraya TY, Jacobsen SJ, et al. Incidence of heart failure after myocardial infarction: is it changing over time? Am J Epidemiol. 2003 Jun 15;157(12):1101–1107. doi: 10.1093/aje/kwg078. [DOI] [PubMed] [Google Scholar]
- 39.Bursi F, Weston SA, Killian JM, Gabriel SE, Jacobsen SJ, Roger VL. C-reactive protein and heart failure after myocardial infarction in the community. Am J Med. 2007 Jul;120(7):616–622. doi: 10.1016/j.amjmed.2006.07.039. [DOI] [PubMed] [Google Scholar]
- 40.Spencer FA, Meyer TE, Goldberg RJ, et al. Twenty year trends (1975–1995) in the incidence, in-hospital and long-term death rates associated with heart failure complicating acute myocardial infarction: a community-wide perspective. J Am Coll Cardiol. 1999;34(5):1378–1387. doi: 10.1016/s0735-1097(99)00390-3. [DOI] [PubMed] [Google Scholar]
- 41.Singh JP, Hall WJ, McNitt S, et al. Factors influencing appropriate firing of the implanted defibrillator for ventricular tachycardia/fibrillation: findings from the Multicenter Automatic Defibrillator Implantation Trial II (MADIT-II) J Am Coll Cardiol. 2005 Nov 1;46(9):1712–1720. doi: 10.1016/j.jacc.2005.05.088. [DOI] [PubMed] [Google Scholar]
- 42.Myerburg RJ, Mitrani R, Interian A, Jr, Castellanos A. Interpretation of outcomes of antiarrhythmic clinical trials: design features and population impact. Circulation. 1998 Apr 21;97(15):1514–1521. doi: 10.1161/01.cir.97.15.1514. [DOI] [PubMed] [Google Scholar]
- 43.Devlin G, Anderson FA, Heald S, et al. Management and outcomes of lower risk patients presenting with acute coronary syndromes in a multinational observational registry. Heart. 2005 Nov;91(11):1394–1399. doi: 10.1136/hrt.2004.054007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fox KA, Anderson FA, Jr, Dabbous OH, et al. Intervention in acute coronary syndromes: do patients undergo intervention on the basis of their risk characteristics? The Global Registry of Acute Coronary Events (GRACE) Heart. 2007 Feb;93(2):177–182. doi: 10.1136/hrt.2005.084830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vaccarino V, Rathore SS, Wenger NK, et al. Sex and racial differences in the management of acute myocardial infarction, 1994 through 2002. N Engl J Med. 2005 Aug 18;353(7):671–682. doi: 10.1056/NEJMsa032214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wiviott SD, Morrow DA, Frederick PD, Antman EM, Braunwald E. Application of the Thrombolysis in Myocardial Infarction risk index in non-ST-segment elevation myocardial infarction: evaluation of patients in the National Registry of Myocardial Infarction. J Am Coll Cardiol. 2006 Apr 18;47(8):1553–1558. doi: 10.1016/j.jacc.2005.11.075. [DOI] [PubMed] [Google Scholar]
- 47.Rogers WJ, Canto JG, Lambrew CT, et al. Temporal trends in the treatment of over 1.5 million patients with myocardial infarction in the US from 1990 through 1999: the National Registry of Myocardial Infarction 1, 2 and 3. J Am Coll Cardiol. 2000 Dec;36(7):2056–2063. doi: 10.1016/s0735-1097(00)00996-7. [DOI] [PubMed] [Google Scholar]
- 48.Steg PG, Goldberg RJ, Gore JM, et al. Baseline characteristics, management practices, and in-hospital outcomes of patients hospitalized with acute coronary syndromes in the Global Registry of Acute Coronary Events (GRACE) Am J Cardiol. 2002 Aug 15;90(4):358–363. doi: 10.1016/s0002-9149(02)02489-x. [DOI] [PubMed] [Google Scholar]
- 49.Watkins S, Thiemann D, Coresh J, Powe N, Folsom AR, Rosamond W. Fourteen-year (1987 to 2000) trends in the attack rates of, therapy for, and mortality from non-ST-elevation acute coronary syndromes in four United States communities. Am J Cardiol. 2005 Nov 15;96(10):1349–1355. doi: 10.1016/j.amjcard.2005.07.037. [DOI] [PubMed] [Google Scholar]
- 50.Gandy A, Therneau TM, Aalen OO. Global tests in the additive hazards regression model. Stat Med. 2008 Mar 15;27(6):831–844. doi: 10.1002/sim.2972. [DOI] [PubMed] [Google Scholar]
- 51.Lloyd-Jones DM, Tian L. Predicting cardiovascular risk: so what do we do now? Arch Intern Med. 2006 Jul 10;166(13):1342–1344. doi: 10.1001/archinte.166.13.1342. [DOI] [PubMed] [Google Scholar]
- 52.Pencina MJ, D'Agostino RB, Sr, D'Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008 Jan 30;27(2):157–172. doi: 10.1002/sim.2929. discussion 207–112. [DOI] [PubMed] [Google Scholar]