Abstract
Alcohol abuse predisposes the host to bacterial infections. In response to bacterial infection, the bone marrow hematopoietic activity shifts toward granulocyte production which is critical for enhancing host defense. This study investigated the hematopoietic precursor cell response to bacteremia and how alcohol affects this response. Acute alcohol intoxication was induced in Balb/c mice 30 min prior to initiation of Escherichia coli bacteremia. Bacteremia caused a significant increase in the number of bone marrow lineage(lin3)-c-kit+Sca-1+ cells. Marrow lin-c-kit+Sca-1+ cells isolated from bacteremic mice showed an increase in CFU-GM activity compared to controls. In addition to enhanced proliferation of lin-c-kit+Sca-1+ cells as reflected by BrdU incorporation, phenotypic inversion of lin-c-kit+Sca-1- cells primarily accounted for the rapid increase in marrow lin-c-kit+Sca-1+ cells following bacteremia. Bacteremia increased plasma concentration of TNF-α. Culture of marrow lin-c-kit+Sca-1- cells with recombinant murine TNF-α for 24 h caused a dose dependent increase in conversion of these cells to lin-c-kit+Sca-1+ cells. Sca-1 mRNA expression by the cultured cells was also up-regulated following TNF-α stimulation. Acute alcohol intoxication inhibited the increase in the number of lin-c-kit+Sca-1+ cells in the bone marrow after E. coli infection. Alcohol impeded the increase in BrdU incorporation into marrow lin-c-kit+Sca-1+ cells in response to bacteremia. Alcohol also suppressed the plasma TNF-α response to bacteremia and inhibited TNF-α-induced phenotypic inversion of lin-c-kit+Sca-1- cells in vitro. These data show that alcohol inhibits the hematopoietic precursor cell response to bacteremia which may serve as one mechanism underlying the impaired host defense in alcohol abusers with severe bacterial infections.
This is an author-produced version of a manuscript accepted for publication in The Journal of Immunology (The JI). The American Association of Immunologists, Inc. (AAI), publisher of The JI, holds the copyright to this manuscript. This version of the manuscript has not yet been copyedited or subjected to editorial proofreading by The JI; hence, it may differ from the final version published in The JI (online and in print). AAI (The JI) is not liable for errors or omissions in this author-produced version of the manuscript or in any version derived from it by the U.S. National Institutes of Health or any other third party. The final, citable version of record can be found at www.jimmunol.org.
Keywords: rodent, bacterial, cell activation, hematopoiesis, inflammation
Introduction
Hematopoiesis in normal hosts is tightly regulated in order to maintain daily blood cell turnover among different lineages (1, 2). During bacterial infection, however, the equilibrium of hematopoiesis is altered, whereby granulopoiesis becomes predominant with inhibition of other lineage (lymphoid and erythroid) development (3-8). Since granulocytes [neutrophils or polymorphonuclear leukocytes (PMNs)] constitute the first line of phagocytic defense in the systemic circulation, this enhancement of PMN production is critically important for eliminating invading pathogens. Failure to develop an adequate granulopoietic response to infection results in increased morbidity and mortality (9, 10). It is well known that all types of blood cells are derived from hematopoietic stem cells. These primitive precursors reside in the bone marrow and possess the unique capacity for self renewal as well as the potential to differentiate into mature blood cells (1). At the present time, knowledge about the functional adjustment of these cells towards lineage commitment during the granulopoietic response to bacterial infection remains limited.
Alcohol abuse is a major risk factor for developing bacterial infections. Alcoholic patients with bacterial infection are characterized by severe symptoms, frequent complications, and poor outcomes (11-15). These patients often present with granulocytopenia, which is an indicator of increased mortality (16). Excessive alcohol consumption has been shown to injure the bone marrow (17-20). Exposure of bone marrow cells to alcohol at concentrations commonly observed in intoxicated patients suppresses granulocyte colony formation (21). Currently, the mechanisms underlying alcohol-induced impairment of the granulopoietic response to bacterial infection remain to be elucidated.
In recent years, flow cytometry has been extensively used for phenotypic analysis of hematopoietic cells based on their specific surface markers. Studies have shown that in C57BL/6 mouse strain, bone marrow lineage (lin)-c-kit+Sca-1+ cells are highly purified (or enriched) hematopoietic stem cells, while lin-c-kit+Sca-1- cell population primarily contains myeloid progenitors (22, 23). In Balb/c and FVB/N mouse strains, CFU-spleen (CFU-S) cells express high levels of the Sca-1 antigen (24).
During bacterial infection, the infected tissues produce large amounts of cytokines including tumor necrosis factor-α (TNF-α), granulocyte colony-stimulating factor (G-CSF), and CXC chemokines [interleukin-8 (IL-8) in humans and keratinocyte-derived chemokine (KC) in mice] (25-28). These mediators are potent stimulants for inflammation, granulopoiesis, and mobilization of hematopoietic precursor cells from the bone marrow into the systemic circulation (29-31). Studies have shown that the number of hematopoietic precursor cells is increased in the systemic circulation in hosts challenged with bacterial components (32). Although these mobilized hematopoietic precursor cells appear to play a role in the process of tissue repair (32-35) and immunosurveillance (36), the relationship between the release of hematopoietic precursor cells from the bone marrow and the changes in the marrow pool of these precursors as well as its effect on the granulopoietic response to bacterial infection remains to be determined. Furthermore, there are no published data examining the effects of alcohol on the alterations of hematopoietic precursor cells during bacterial infection. Our current study was conducted on a murine model of bacteremia in the presence of acute alcohol intoxication. The purpose of this investigation is to define the effects of alcohol intoxication on the hematopoietic precursor cell response to bacterial infection.
Materials and Methods
Animals
Male Balb/c mice (7 to 10 weeks old; Charles River, Wilmington, MA) with a body weight of 22.7±0.1 g were maintained on a standard laboratory diet and were housed in a specific pathogen free facility with a 12-h light/dark cycle. Acute alcohol intoxication was induced in mice by intraperitoneal (i.p.) injection of 20% alcohol in pyrogen-free saline at a dose of 5 g/kg. The blood alcohol levels were 119.7 ± 1.3 mM, 106.3 ± 1.5 mM, 87.7 ± 3.6 mM, and 48.4 ± 3.5 mM, respectively, at 45 min, 90 min, 3 h, and 6 h post alcohol administration (n = 5 at each time point). Control mice were i.p. injected with an equal volume of saline. Thirty min later, mice were challenged intravenously (i.v.) with either 1×106 or 5×107 colony-forming units (CFUs) of live E. coli (E11775 from the American Type Culture Collection, Rockville, MD; in 50 μl of saline/mouse) or recombinant murine TNF-α (1μg or 105 U in 50 μl of saline/mouse, Biosource International, Inc., Camarillo, CA) via penile vein injection under isoflurane anesthesia. Control mice were i.v. injected with an equal volume of vehicle. The animals were sacrificed at scheduled time points after the i.v. challenge as indicated in each figure legend. In a subgroup of mice challenged with intravenous saline or 1×106 E. coli, 5-bromo-2-deoxyuridine (BrdU, 1 mg in 100 μl of PBS/mouse, BD PharMingen, San Diego, CA) was administered i.v. at the same time when mice were challenged with saline or bacteria. Upon sacrifice, a heparinized blood sample was obtained by cardiac puncture. White blood cells (WBCs) were quantified under a light microscope with a hemacytometer. Blood smears were prepared on slides. Wright-Giemsa stain was used to perform differential WBC counts. Plasma was separated and stored at -80°C. Peripheral blood mononuclear cells (PBMCs) were isolated using Lympholyte-Mammal density separation medium (Cedarlane, Homby, Ontario, Canada) and protocols provided by the manufacturer. Femurs and tibias were collected and bone marrow cells were flushed out with a total volume of 2 ml PBS containing 2% bovine serum albumin (BSA, HyClone Laboratories, Logan, UT) through a 23-gauge needle. Bone marrow cells were filtered through a 70 micron nylon mesh (Sefar America INC. Kansas City, MO). Erythrocytes in isolated PBMCs and bone marrow cell samples were lysed with Purescript® RBC lysis solution (Gentra Systems, Valencia, CA). After washing twice with PBS containing 2% BSA, the remaining nucleated cells were quantified under a light microscope with a hemacytometer. The experiments described here were performed in adherence to the National Institutes of Health guidelines on the use of experimental animals. Approval of the Animal Care and Use Committee of Louisiana State University Health Sciences Center was obtained prior to initiating these experiments.
Culture of bacteria
For each experiment, frozen stock cultures of E. coli were added to tryptic soy broth and incubated for 18 h at 37°C in an orbital shaker. Bacteria were collected and washed twice with PBS. A suspension of bacteria in PBS at a concentration of 1× 109 CFUs/ml was prepared based on its optical density at 600 nm. Actual numbers of viable bacteria were verified by standard plate counts of the bacterial suspensions on MacConkey agar plates following overnight incubation at 37°C.
Bacterial numbers were determined in the blood and tissue samples. Liver and spleen samples were collected and homogenized with 9 volumes of PBS using sterilized glass homogenizers driven by a NSI-12 Fractional Horsepower Motor (Bodine Electric Co., Chicago, IL). Serial 1:10 dilutions of blood or tissue homogenates were prepared and each sample dilution was cultured (100 μl sample suspension) on MacConkey agar plates in triplicate. Bacterial colonies were quantified following overnight incubation of the plates at 37°C.
Flow cytometric analysis
Nucleated bone marrow cells or isolated PBMCs suspended in RPMI-1640 (Invitrogen, Grand Island, NY) containing 2% fetal calf serum (2 × 106 cells in 100 μl medium) were added with a mixed panel of biotinylated anti-mouse lineage markers [10 μg/mL of each antibody against CD3e (clone 145-2C11), CD45R/B220 (clone RA3-6B2), CD11b/CD18 (Mac-1, clone M1/70), Gr-1 (Ly-6G/Ly-6C, clone RB6-8C5), or TER 119 (clone TER-119)] or isotype control antibodies (clones A19-3, R35-95, A95-1) (BD PharMingen). Following incubation for 15 min at 4°C, PE-conjugated streptavidin (10 μg/mL) and 10 μg/mL of each fluorchrome conjugated anti-mouse c-kit (CD117, clone 2B8), Sca-1 (Ly-6A/E, clone D7), and CD34 (clone RAM34) (BD PharMingen), or the matched isotype control antibodies (clones A95-1 and R35-95) were added into the incubation system. The samples were further incubated in the dark for 15 min at 4°C. The cells were then washed with cold PBS. For measuring BrdU incorporation, the cells were further processed using a BD BrdU Flow Kit (BD PharMingen, San Diego, CA). At the end of the staining procedure, cells were suspended in 0.5 ml of PBS containing 1% paraformaldehyde. Analysis of cell phenotypes and BrdU incorporation was performed on a FACSAria or a LSR-II flow cytometer with FACSDiva software (Becton Dickinson, San Jose, CA). In each sample, 300,000 cells were acquired for analysis.
Sorting of bone marrow lin-c-kit+Sca-1- and lin-c-kit+Sca-1+ cells
Pooled nucleated bone marrow cells (∼1 ×108 cells) were suspended in StemSpan serum-free medium (StemCell Technologies, Vancouver, BC, Canada). Staining procedure for cell surface makers was similar as described above, except that the amount of antibodies used for each sample was increased proportionally. Sorting of marrow lin-c-kit+Sca-1- and lin-c-kit+Sca-1+ cells was performed on the FACSAria flow cytometer with FACSDiva software. The purity of sorted cell population was 97-100%.
In vitro culture of bone marrow lin-c-kit+Sca-1- cells
For phenotypic analysis, sorted marrow lin-c-kit+Sca-1- cells from normal mice were plated into a 96-well tissue culture plate with 5 × 104 cells per well in a total volume of 100 μl StemSpan serum-free medium. For determination of Sca-1 mRNA expression, sorted marrow lin-c-kit+Sca-1- cells from normal mice were plated into a 12-well tissue culture plate with 1 × 106 cells per well in a total volume of 1 ml StemSpan serum-free medium. The cells were cultured without or with recombinant murine TNF-α (Biosource International, Inc., Camarillo, CA) in the absence and presence of different concentrations of alcohol. The cells were cultured at 37°C in an atmosphere of 5% CO2 and the corresponding alcohol environments (37) for 24 h. At the end of culture, cells were stained with fluorchrome-conjugated anti-mouse c-kit and Sca-1 antibodies. Flow cytometric analysis of live (propidium iodide negative) cells was conducted on a LSR-II flow cytometer with FACSDiva software (Becton Dickinson, San Jose, CA). For determination of Sca-1 mRNA expression, total RNA was extracted using an RNeasy Plus Mini kit (Qiagen, Valencia, CA) and procedures provided by the manufacturer.
Preparation of Sca-1 RNA standard and real-time RT-PCR determination of Sca-1 mRNA
A loopful of cloned E. coli (Cat No. MGC-6188, ATCC, Manassas, VA) carrying murine Sca-1 gene in the pCMV-SPORT6 vector was cultured in 100 ml of LB broth containing ampicillin (10 mg/100 ml) at 37°C on an orbital shaker for 18 h. Plasmid DNA was purified using a Qiagen Plasmid Midi Kit (Qiagen) and procedures provided by the manufacturer. Sca-1 RNA was prepared by in vitro transcription of purified plasmid DNA using a RiboMAX™ Large Scale RNA Production Systems – T7 kit (Promega Co. Madison, WI). Purification was performed using the RNase-Free Bio-Rad Micro Bio-Spin Columns P-30 Tris (Bio-Rad Laboratories, Hercules, CA). The standard RNA was quantified spectrophotometrically at 260 nm and tested for DNA contamination using real-time RT-PCR in the absence of reverse transcriptase. The residual DNA copies in the prepared RNA standard were less than 1/50,000. The standard RNA was stored in aliquots at -80 °C until assay. For each subsequent real-time quantitative RT-PCR assay, Sca-1 and 18S ribosomal RNA (Taqman Applied Biosystems, Foster City, CA) standard curves (ranging from 102 to 109 copies of Sca-1 RNA per reaction and 10-5 to 101 ng of 18S ribosomal RNA per μl of reaction volume) were generated by serial dilution of stock standard RNA aliquots.
Real-time RT-PCR determination of Sca-1 mRNA expression was performed using a protocol as described previously (38). The amplification primers and probes used for determination of Sca-1 expression are listed as follows:
Forward primer 5′-GTTTGCTGATTCTTCTTGTGGCCC
Reverse primer 5′-ACTGCTGCCTCCTGAGTAACAC
Probe 5′-AGCTCAGGGACTGGAGTGT TACCAGTGCT
This set of primers and probe was designed using Primer Express software (PE Applied Biosystems) The primers and probes for detection of 18S ribosomal RNA were purchased from Taqman Applied Biosystems. The Sca-1 mRNA quantity in each sample was determined by comparing its cycle threshold (CT) number with those of Sca-1 RNA standard curve. Sca-1 mRNA value was then normalized to the content of 18S rRNA in each sample. The results are expressed as copies mRNA/ng rRNA.
Colony forming unit (CFU) assay
CFU assay of sorted bone marrow lin-c-kit+Sca-1+ cells was performed by culturing the cells on Methocult GF M3434 and Methocult GF M3534 media (StemCell Technologies, Vancouver, Canada), respectively. One milliliter of Methocult GF M3434 or Methocult™ GF M3534 media containing 100 sorted bone marrow lin-c-kit+Sca-1+ cells was plated to a 35 mm Nunclon™ dish (Nunc, Rodkilde, Denmark). Each sample was cultured in triplicate for seven days at 37°C in an atmosphere of 5% CO2. Colonies containing 50 or more cells were then enumerated.
Luminex assay and ELISA measurement of plasma cytokines
Plasma concentrations of cytokines including TNF-α, interferon-γ (IFN-γ), interleukin(IL)-1α, IL-1β, IL-3, IL-6, G-CSF, granulocyte-monocyte colony-stimulating factor (GM-CSF), KC, fibroblast growth factor (FGF), and vascular endothelial growth factor (VEGF) were measured using a Mouse Cytokine Multi-Plex kit (Biosource International, Camarillo, CA). All luminex assays were performed on the Bio-Plex™ Protein Array System (Bio-Rad Laboratories). Plasma concentrations of stem cell factor (SCF), thrombopoietin (TPO), and insulin-like growth factor-II (IGF-II) were measured using the Quantikine Mouse SCF and TPO ELISA Kits and the DuoSet Mouse IGF-II ELISA Kit (R&D Systems Inc., Minneapolis, MN).
Statistical analysis
Data are presented as mean ± SEM. The sample size is indicated in the legend of each figure. Statistical analyses of data were conducted using unpaired Student t test (for comparison between two groups) and one-way analysis of variance followed by Student-Newman-Keuls test or Proc Mixed (SAS, 2004) 2-way analysis of variance (for comparisons among multiple groups). Differences were considered statistically significant at P < 0.05.
Results
Alteration of hematopoietic precursor cell populations in the bone marrow
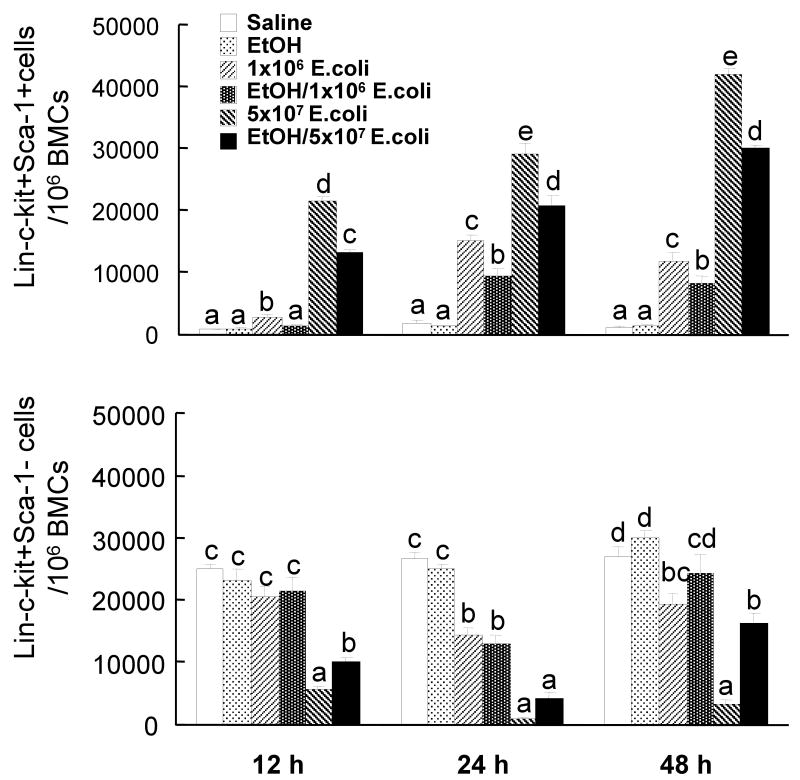
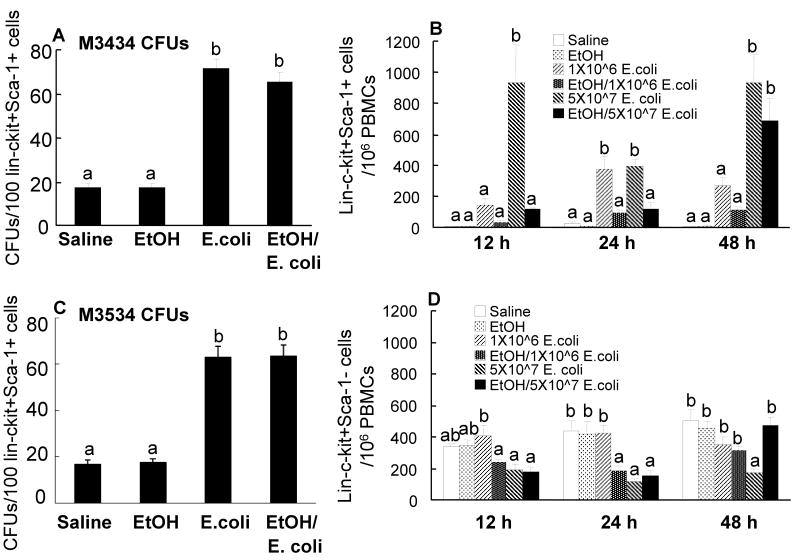
In order to examine the effects of alcohol intoxication on changes in marrow hematopoietic precursor cells following systemic infection with E. coli, bone marrow cells were analyzed by flow cytometry based on their phenotypic surface markers. As mentioned previously, mouse bone marrow lin-c-kit+Sca-1+ cells are highly purified hematopoietic stem cells, while lin-c-kit+Sca-1- cells are primarily myeloid progenitors (22). In contrast to human hematopoietic stem cells that are highly enriched in CD34+ cell population in the bone marrow, mouse long-term repopulating hematopoietic stem cells are CD34 low/negative (39). As shown in figures 1 and 2, the lin-c-kit+Sca-1+ cell population in the bone marrow of control mice was very small. Similar observations have been reported previously in C57BL/6 mice (22). By 12 h post E. coli infection, the number of lin-c-kit+Sca-1+ cells in the bone marrow was markedly increased and this increase was sustained until 48 h following bacteremia. The increase in marrow lin-c-kit+Sca-1+ cell population was more pronounced in mice receiving 5×107 CFUs of E. coli compared to those challenged with lower dose of bacteria. The changes in the number of lin-c-kit+Sca-1+CD34- cells paralleled that of the lin-c-kit+Sca-1+ cell population in the bone marrow in response to E. coli bacteremia (data not shown). Acute alcohol intoxication did not alter the number of lin-c-kit+Sca-1+ cells in the bone marrow of saline treated mice. However, the increases in the numbers of marrow lin-c-kit+Sca-1+ cells following E. coli infection were significantly attenuated by acute alcohol intoxication (Figures 1 and 2).
Figure 1.
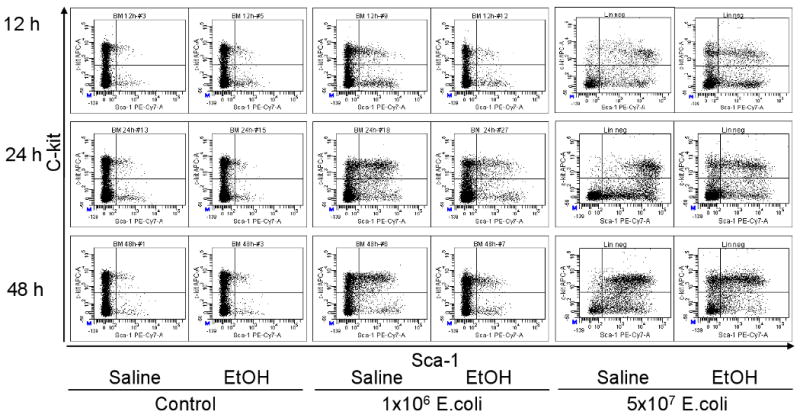
Representative dot plots of c-kit-APC vs. Sca-1-PE-Cy7 of lineage negative bone marrow cells. Saline: mice received i.p. saline; EtOH: mice received i.p. alcohol; Control: mice received i.v. saline; 1×106 E. coli: mice received i.v. 1×106 E. coli; 5×107 E. coli: mice received i.v. 5×107 E. coli.
Figure 2.
Changes in lin-c-kit+Sca1+ and lin-c-kit+Sca-1- cells in the bone marrow. Saline: mice received i.p. saline plus i.v saline; EtOH: mice received i.p. alcohol plus i.v. saline; 1×106 E. coli: mice received i.p. saline plus i.v. 1×106 E. coli; EtOH/1×106 E. coli: mice received i.p. alcohol plus i.v. 1×106 E. coli; 5×107 E. coli: mice received i.p. saline plus i.v. 5×107 E. coli; EtOH/5×107 E. coli: mice received i.p. alcohol plus i.v. 5×107 E. coli. Data are mean ± SEM (n = 4∼7). Bars with different letters (a – d) at the same time point in each panel are statistically different (p < 0.05).
In contrast to this increase in the lin-c-kit+Sca-1+ population, the number of lin-c-kit+Sca-1- cells was decreased following E. coli infection. This cell population decreased to approximately 23% of the control value at 12 h post i.v. challenge with 5×107 E. coli whereas the decrease was delayed in mice receiving the lower dose of E. coli. By 24 h after infection, the number of bone marrow lin-c-kit+Sca-1- cells was reduced by both doses of E. coli compared to uninfected mice and this decrease was sustained through 48 h of the infection. Acute alcohol intoxication attenuated the reduction of marrow lin-c-kit+Sca-1 cell population in animals at 12 h and 48 h after i.v. challenge with 5×107 E. coli.
Alteration of cell BrdU incorporation
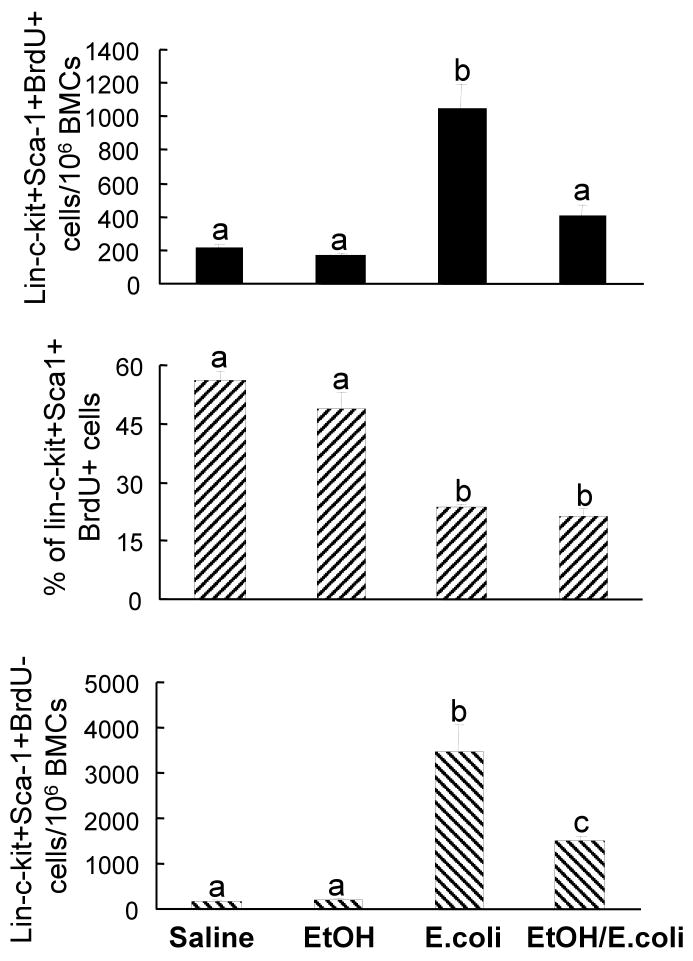
In order to understand the mechanisms underlying the alcohol-induced inhibition of the increase in marrow lin-c-kit+Sca-1+ cells following bacteremia, the in vivo BrdU incorporation technique was utilized to determine the effects of alcohol on hematopoietic precursor cell proliferation in the bone marrow. At 24 h after i.v challenge with 1×106 E. coli, the absolute number of lin-c-kit+Sca-1+BrdU+ cells in bone marrow was increased compared to the controls, although the percentage of BrdU+ cells in the marrow lin-c-kit+Sca-1+ cell population was significantly reduced compared to those of control mice (Figure 3). Alcohol intoxication suppressed the increase in the absolute number of lin-c-kit+Sca-1+BrdU+ cells in the bone marrow of mice with bacteremia. However, alcohol intoxication did not affect the percentage of BrdU+ cells in the marrow lin-c-kit+Sca-1+ cell population following E. coli infection. Similar to changes in the absolute number of marrow lin-c-kit+Sca-1+BrdU+ cells, E. coli infection also caused a marked increase in the absolute number of lin-c-kit+Sca-1+BrdU- cells in the bone marrow. Acute alcohol intoxication inhibited this increase in the number of marrow lin-c-kit+Sca-1+BrdU- cells. Neither bacteremia nor alcohol intoxication affected BrdU incorporation into marrow lin-c-kit+Sca-1- cells (Data not shown).
Figure 3.
BrdU incorporation into bone marrow lin-ckit+Sca1+ cells. Saline: mice received i.p. saline plus i.v. saline; EtOH: mice received i.p. alcohol plus i.v. saline; E. coli: mice received i.p. saline plus i.v. 1×106 E. coli; EtOH/E. coli: mice received i.p. alcohol plus i.v. 1×106 E. coli. Data are mean ± SEM (n = 5). Bars with different letters (a – c) in each panel are statistically different (p < 0.05).
The plasma cytokine response
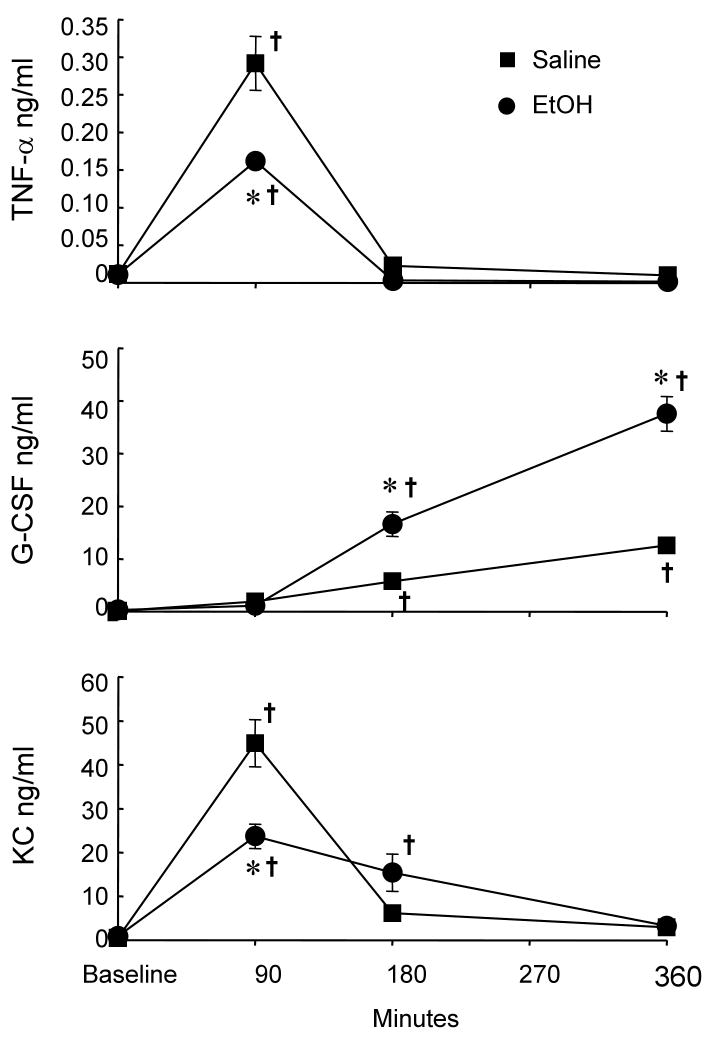
Cytokines and growth factors are important mediators that regulate hematopoietic activity in the bone marrow. Therefore, we determined the alterations of related cytokine and growth factor levels in the systemic circulation during the early stage of E. coli infection. At 90 min post i.v. challenge with 1×106 E. coli, the plasma concentration of TNF-α was significantly increased compared to the baseline level (Figure 4). This increase in plasma level of TNF-α was inhibited in mice with acute alcohol intoxication. At 3 h and 6 h following bacteremia, the plasma concentration of TNF-α returned to the baseline level in mice both without and with alcohol intoxication. Plasma concentrations of G-CSF were not altered at 90 min following bacteremia in comparison to the baseline values in mice without or with alcohol intoxication. However, G-CSF levels in the plasma were markedly increased above the baseline value at 3 h and 6 h following bacteremia. This increase in plasma concentration of G-CSF was even higher in alcohol-intoxicated mice at these two time points. Similar to the alteration of plasma TNF-α level, plasma KC concentration was significantly increased at 90 min following E. coli infection. Alcohol intoxication inhibited the increase in plasma KC level at this time point of bacteremia. The increase in plasma KC concentration declined at 3 h after E. coli infection in mice without or with alcohol intoxication. Toward 6 h post E. coli infection, plasma KC levels in mice both without and with alcohol intoxication returned to the baseline values. Plasma levels of IL-1β in mice without or with alcohol intoxication were increased minimally but with statistical significance at 3 h post E. coli infection (data not shown). In addition, plasma GM-CSF levels also showed a minimal but statistically significant increase at both 3 h and 6 h following bacteremia in mice without or with alcohol intoxication (data not shown). The plasma levels of all other cytokines and growth factors determined were not altered following intravenous challenge with 1×106 E. coli at all three time points monitored (data not shown).
Figure 4.
Plasma cytokine levels post i.v. challenge with 1×106 E. coli. The baseline level of each cytokine in saline or alcohol treated group is the pooled values of the corresponding cytokine in animals treated with saline or alcohol without bacterial challenge at different time points. Data are mean ± SEM (n = 5). *: p < 0.05 compared to the Saline group at the same time point; †: p < 0.05 compared to the baseline value.
Inversion of phenotype following in vitro culture of lin-c-kit+Sca-1- cells
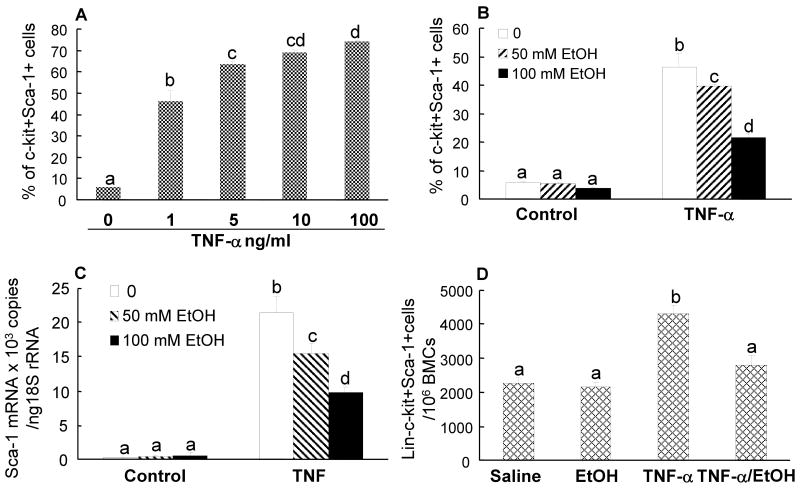
The in vivo BrdU data suggest that the observed increase in bone marrow lin-c-kit+Sca-1+ cells during bacteremia may not be primarily due to accelerated proliferation of lin-c-kit+Sca-1+ cells. Instead, a large number of cells in the expanded marrow pool of lin-c-kit+Sca-1+ cells are BrdU negative, which indicates that other mechanisms may be involved in the increase in this cell population. Since the expansion of the lin-c-kit+Sca-1+ cell population was accompanied by a significant reduction in the number of lin-c-kit+Sca-1- cells in the bone marrow following E. coli infection, we hypothesized that lin-c-kit+Sca-1- cells may invert to lin-c-kit+Sca-1+ cells during bacteremia by induction of Sca-1 expression. Figure 5A shows the phenotypic shift of isolated bone marrow lin-c-kit+Sca-1- cells following 24 h culture with recombinant murine TNF-α. Exposure of lin-c-kit+Sca-1- cells to TNF-α caused a dose-dependent increase in the number of c-kit+Sca-1+ cells in the culture system. This response of Sca-1 expression tended to plateau when TNF-α concentration exceeded 5 ng/ml. TNF-α-induced Sca-1 expression by these cells occurred at both protein and mRNA levels (Figures 5B and 5C). When lin-c-kit+Sca-1- cells were cultured with 1 ng/ml of TNF-α, alcohol caused a dose-dependent attenuation of the increase in Sca-1 expression at both protein and mRNA levels in these cells (Figures 5B and 5C). Intravenous administration of recombinant murine TNF-α also induced an increase in marrow lin-c-kit+Sca-1+ cell population (Figure 5D). Acute alcohol intoxication impaired this TNF-α induced increase in the number of marrow lin-c-kit+Sca-1 cells. We also cultured lin-c-kit+Sca-1- cells with recombinant murine G-CSF and KC. G-CSF and KC at doses up to 150 ng/ml and 100 ng/ml, respectively, did not induce Sca-1 expression by cultured lin-c-kit+Sca-1- cells (data not shown).
Figure 5.
A: TNF-α-induced in vitro inversion of lin-c-kit+Sca1- cells to lin-c-kit+Sca-1+ cells. B and C: the effects of alcohol on TNF-α (1 ng/ml) induced in vitro inversion of lin-c-kit+Sca1- cells to lin-c-kit+Sca-1+ cells and Sca-1 mRNA expression by lin-c-kit+Sca-1- cells. D: the effect of acute alcohol intoxication on the alteration of marrow lin-c-kit+Sca-1+ cell population in response to i.v. challenge with TNF-α. Data are mean ± SEM (n = 5). Bars with different letters (a – d) in each panel are statistically different (p < 0.05).
Alteration of CFU activity in bone marrow lin-c-kit+Sca-1+ cells
To test functional activity of lin-c-kit+Sca-1+ cells in the bone marrow, lin-c-kit+Sca-1+ cells sorted from control mice and mice challenged with 1×106 E. coli in the absence and presence of acute alcohol intoxication were cultured with Methocult GF M3434 and M3534 media. The Methocult GF M3434 medium supports the growth of multiple lineages including CFU-granulocyte/erythrocyte/macrophage/megakaryocyte (CFU-GEMM), CFU-granulocyte/macrophage (CFU-GM), CFU-granulocyte (CFU-G), CFU-macrophage (CFU-M), and brust-forming unit-erythroid (BFU-E), while Methocult GF M3534 only supports the growth of granulocyte and monocyte lineages including CFU-GM, CFU-G, and CFU-M. As shown in Figures 6A and 6C, lin-c-kit+Sca-1+ cells isolated from mice at 24 h post i.v. E. coli exhibited a significant increase in CFU activity when cultured on Methocult GF M3434. This increase in CFU formation resulted essentially from an increase in CFU-GM (including CFU-G and CFU-M) activity of these cells. In vivo alcohol intoxication did not show any significant effect on the CFU activity of lin-c-kit+Sca-1+ cells isolated from either control or E. coli-infected mice when the cells were cultured for 7 days in vitro in the absence of alcohol.
Figure 6.
A and C: CFU activity of bone marrow lin-ckit+Sca1+ cells in M3434 and M3534 media. Data are mean ± SEM (n = 10). Bars with different letters (a – b) in each panel are statistically different (p < 0.05). B and D: changes in lin-c-kit+Sca1+ and lin-c-kit+Sca-1- cells in the systemic circulation. Data are mean ± SEM (n = 4∼7). Bars with different letters (a – b) at the same time point in each panel are statistically different (p < 0.05).
Alteration of bone marrow release of lin-c-kit+Sca-1+ and lin-c-kit+Sca-1- cells
During bacterial infection, the increase in inflammatory mediator production may stimulate bone marrow to release hematopoietic precursor cells. Since the marrow pools of lin-c-kit+Sca-1+ and lin-c-kit+Sca-1- cells are dramatically altered following bacteremia in control and alcohol-intoxicated mice, this alteration may affect release of these cells into the systemic circulation. As shown in figure 6B, mobilization of lin-c-kit+Sca-1+ cells into the circulation was significantly increased at 12 h, 24 h, and 48 h after i.v. challenge with 5×107 E. coli. This significant increase in mobilization of lin-c-kit+Sca-1+ cells was also seen at 24 h after i.v. challenge with 1×106 E. coli. Acute alcohol intoxication profoundly suppressed the increased mobilization of lin-c-kit+Sca-1+ cells into the circulation at 12 h and 24 h following i.v. challenge with 5×107 E. coli. Alcohol intoxication also suppressed the increase in the number of lin-c-kit+Sca-1+ cells in the blood at 24 h post i.v. challenge with 1×106 E. coli. Figure 6D shows the changes in the number of blood lin-c-kit+Sca-1- cells. At both 24 h and 48 h following i.v. challenge with 5×107 E. coli, the number of lin-c-kit+Sca-1- cells in the circulation was significantly reduced. Furthermore, the number of lin-c-kit+Sca-1- cells in the blood was also reduced at 24 h following i.v. challenge with either 1×106 or 5×107 E. coli with alcohol intoxication
Alteration of blood PMN counts, tissue bacterial clearance, and animal survival
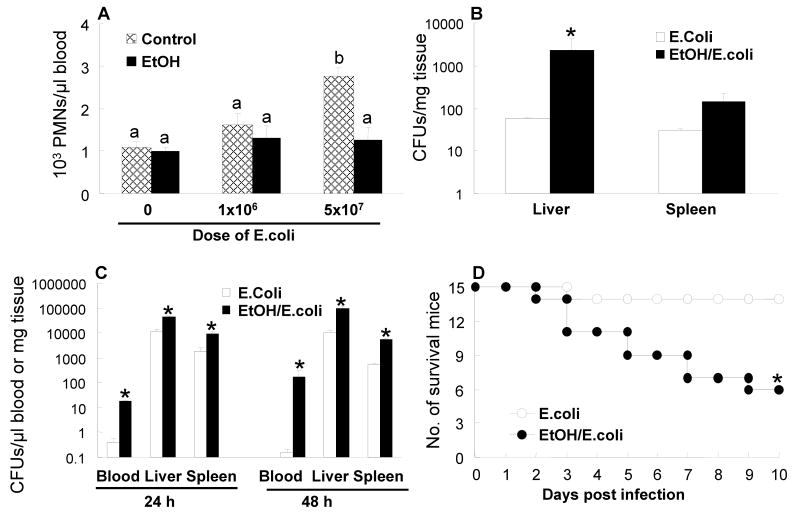
Since expansion of marrow lin-c-kit+Sca-1+ cell population during E. coli infection supports granulopoiesis, inhibition of the lin-c-kit+Sca-1+ cell response by acute alcohol intoxication may impair host defense against bacterial infection. We determined blood PMN counts following E. coli bacteremia. At 12 h of E. coli infection, no statistically significant difference of blood PMN counts was observed among all groups (data not shown). By 24 h, mice challenged with 5×107 E. coli showed a significant increase in blood PMN counts (2.6 times of the control value, Figure 7A). Acute alcohol intoxication suppressed this increase in blood PMN counts. At 48 h of the infection, blood PMN counts of mice challenged with 5×107 E. coli remained to be 1.8 times of the control value. However, this difference did not reach statistical significance (data not shown). Acute alcohol intoxication impaired bacterial clearance in our current model. Although blood bacterial cultures were negative at 24 h after i.v. challenge with 1×106 E. coli in mice both without or with alcohol intoxication, alcohol intoxicated mice showed a significantly higher level of E. coli CFUs in the liver than did un-intoxicated mice (Figure 7B). At both 24 h and 48 h following i.v. challenge with 5×107 E. coli, bacterial colonies in the blood, liver, and spleen samples were all significantly higher in mice intoxicated with alcohol than in those without alcohol treatment (Figure 7C). In addition, acute alcohol intoxication significantly increased the mortality rate of mice challenged with 5×107 E. coli (Figure 7D).
Figure 7.
A: blood PMN counts at 24 h post i.v. challenge. Data are mean ± SEM (n = 5). Bars with different letters (a – b) are statistically different (p < 0.05). B: E. coli CFU counts in cultures of the liver and spleen tissue samples at 24 h post i.v. challenge with 1×106 E. coli. Data are mean ± SEM (n = 5). *: p < 0.05 compared to E. coli group. C: E. coli CFU counts in cultures of the blood, liver, and spleen samples from mice challenged with 5×107 E. coli. Data are mean ± SEM (n = 5). *: p < 0.05 compared to E. coli group. D: changes of survival rate following i.v. challenge with 5×107 E. coli. Data are mean ± SEM (n = 5). *: p < 0.05 compared to E. coli group.
Discussion
It has long been recognized that excessive alcohol consumption predisposes the host to bacterial infections (16, 40). Alcohol is an immunosuppressive drug that impairs multiple immune defense functions (40). Clinical investigations have shown that alcoholic patients with severe bacterial infection including pneumonia and sepsis frequently present with granulocytopenia, which predicts a poor outcome (16). In response to bacterial infection, hematopoietic activity in the bone marrow is directed toward granulocyte production which is critical for enhancing host defenses against invading pathogens. Since all blood cell types are derived from bone marrow hematopoietic stem cells, we hypothesized that the functional activities of primitive hematopoietic precursor cells are modified during bacterial infection in order to reprogram them for an enhanced commitment to granulocyte production. Alcohol may exert a negative impact on this early stage of the granupoietic response. Previous studies reported by Quesenberry and colleagues show that intraperitoneal administration of Salmonella typhosa endotoxin causes a rapid decrease in the number of marrow granulocytic progenitors as assayed by in vitro colony forming cell technique (growing granulocyte and macrophage colonies in soft agar) (41). This reduction of granulocytic progenitor cells occurs within 20 min after endotoxin, reaches a nadir at 6 h, and then returns to baseline values by 48 h. In contrast, Barthlen and colleagues observed an increase in CFU-GM in the bone marrow of mice 12 h following the onset of abdominal sepsis (5). In order to further clarify changes in hematopoietic precursor cells at the pluripotent stem cell and the immediately committed progenitor cell levels, we analyzed the bone marrow cells of mice with E. coli bacteremia in the absence and presence of acute alcohol intoxication using flow cytometry based on the phenotypic cell surface markers. Our results show that at 12, 24, and 48 h post i.v. challenge with 5×107 E. coli, the number of marrow lin-c-kit+Sca-1- cells in mice both without or with alcohol intoxication was significantly reduced. In mice challenged with 1×106 E. coli, lin-c-kit+Sca-1- cells in the bone marrow were also reduced at 24 h regardless the alcohol treatment. These results are in agreement with observations in endotoxin-treated mice reported by Quesenberry and colleagues (41). Interestingly, the marrow lin-c-kit+Sca-1+ cell populations were markedly increased following E. coli infection in our current model despite the decrease in the number of lin-c-kit+Sca-1- cells. In other paralleled studies, we observed that Balb/c mice intratracheally challenged with Streptococcus pneumoniae and C57BL/6 mice intravenously challenged with live or heat-inactivated E. coli also show a rapid increase in the lin-c-kit+Sca-1+ or lin-c-kit+Sca-1+CD34- cell population in the bone marrow (unpublished data). These data suggest that expansion of the marrow lin-c-kit+Sca-1+ cell pool is likely a fundamental component of the host defense response to severe bacterial infection. Alcohol administration exerted a profound negative effect on this response. The increases in the lin-c-kit+Sca-1+ cell populations in the bone marrow at all time points measured following E. coli bacteremia were inhibited in mice with acute alcohol intoxication.
To analyze mechanisms underlying the expansion of the marrow lin-c-kit+Sca-1+ cell pool, we performed in vivo BrdU incorporation assay. The results showed that the absolute number of BrdU positive lin-c-kit+Sca-1+ cells in the bone marrow was increased at 24 h after i.v. challenge with 1×106 E. coli. This increase in BrdU incorporation suggests activation of cell proliferation in these cells, which may contribute to the observed expansion of the marrow lin-c-kit+Sca-1+ cell pool. Acute alcohol intoxication suppressed this increase in the number of BrdU positive lin-c-kit+Sca-1+ cells in the bone marrow. Obviously, the increase in lin-c-kit+Sca-1+ cell proliferation is not the sole event causing the expansion of marrow lin-c-kit+Sca-1+ cell pool. The percentage of BrdU positive cells in the marrow lin-c-kit+Sca-1+ cell population was actually reduced at 24 h post E. coli infection when compared to the controls. A significant number of the increased lin-c-kit+Sca-1+ cells in the bone marrow were BrdU negative, which suggests that mechanism(s) other than increase in cell proliferation may be involved. Acute alcohol intoxication also inhibited the increase in the number of lin-c-kit+Sca-1+BrdU- cells in the bone marrow following E. coli bacteremia.
Recent studies have shown that engraftable stem cells and progenitors exist in a reversible continuum, termed progenitor/stem cell inversions (42, 43). In an in vitro culture system, it has been observed that while traveling through cell cycle, stem cells acquire a progenitor phenotype and lose their stem cell phenotype during the late S and early G2 phases. This phenomenon is reversible by the next G1 phase. Colvin and colleagues have postulated that the progenitors and the stem cells may, in fact, be the same cell population in different reversible functional states (43). The results of this study showed that bacteremia-induced expansion of the marrow lin-c-kit+Sca-1+ cell pool was accompanied by a reduction in lin-c-kit+Sca-1- cell population in the bone marrow. A major portion of cells in the expanded marrow lin-c-kit+Sca-1+ cell pool appeared not to be derived from the proliferation of these cells. We speculate that the majority of this increased number of BrdU negative lin-c-kit+Sca-1+ cells in the bone marrow may be inverted from the pre-existing lin-c-kit+Sca-1- cells.
Cytokines produced by infected tissue are key mediators for regulation of the inflammatory response. Previously, it has been reported that TNF-α enhances Sca-1 expression by endothelial cells and IFN-γ stimulate Sca-1expression by lymphocytes (44, 45). The results of our current study showed that plasma level of TNF-α, but not IFN-γ, was significantly increased in the early stage of bacteremia. To test the potential role of TNF-α in mediating the possible phenotypic inversion of lin-c-kit+Sca-1- cells during bacteremia, lin-c-kit+Sca-1- cells isolated from the bone marrow of control mice were cultured in vitro with recombinant murine TNF-α. The results showed that TNF-α caused a dose-dependent increase in the number of c-kit+Sca-1+ cells in the culture system. TNF-α also caused a significant up-regulation of Sca-1 mRNA expression by the cultured cells. These data suggest that TNF-α is potentially responsible for inducing the phenotypic inversion of lin-c-kit+Sca-1- cells in our current model of bacteremia. Alcohol at concentrations of 50 mM and 100 mM inhibited Sca-1 protein and mRNA expression by cultured marrow lin-c-kit+Sca-1- cells following exposure to 1 ng/ml of TNF-α. Plasma concentrations of G-CSF and KC were also significantly increased following bacteremia. However, in vitro culture of isolated marrow lin-c-kit+Sca-1- cells with either recombinant murine G-CSF (150 ng/ml) or KC (100 ng/ml) did not induce any significant increase in Sca-1 expression by these cells (data not shown). In an unpublished study, administration of 50 μg/kg G-CSF did not alter the number of lin-c-kit+Sca-1+ cells in the bone marrow in mice (unpublished data). In the present study, intravenous administration of TNF-α at a dose of 1 μg or 105 U per mouse caused an increase in the number of lin-c-kit+Sca-1+ cells in the bone marrow at 24 h post TNF-α challenge. Alcohol intoxication suppressed this lin-c-kit+Sca-1+ cell response to TNF-α challenge. These observations further support that TNF-α-mediated phenotypic inversion of lin-c-kit+Sca-1- cells may contribute to the rapid expansion of the lin-c-kit+Sca-1+ cell population in the bone marrow following bacteremia. Alcohol intoxication may inhibit this phenotypic inversion by both impairing TNF-α production and suppressing the lin-c-kit+Sca-1- cell response to TNF-α.
During bacterial infection, acceleration of myeloid lineage differentiation with enhancement of granulocyte production becomes a predominant feature of hematopoiesis (3-8). In order to understand the expansion of marrow lin-c-kit+Sca-1+ cell pool in relation to the granulopoietic activity in the bone marrow following E. coli infection, lin-c-kit+Sca-1+ cells isolated from the bone marrow of mice without or with acute alcohol intoxication were cultured with Methocult GF M3434 and M3534 media. Our results showed that lin-c-kit+Sca-1+ cells of mice challenged with 1×106 E. coli displayed a significant increase in CFU-GM activity as compared to those of control animals. These data suggest that cells in the rapidly expanded lin-c-kit+Sca-1+ pool in the bone marrow following bacteremia are functionally activated for granulopoiesis. In vivo alcohol intoxication did not affect CFU activity of sorted marrow lin-c-kit+Sca-1+ cells when the cells were cultured in vitro in the absence of alcohol.
Both G-CSF and KC are potent factors mediating the bone marrow release of hematopoietic stem cells and progenitors in mice (29-31). In the present study, the plasma levels of these two cytokines were significantly increased following i.v. challenge with 1×106 E. coli. We also observed that the mobilization of lin-c-kit+Sca-1+ cells into the circulation was significantly increased during bacteremia. Although acute alcohol intoxication inhibited the plasma KC response at 90 min after E. coli infection, the plasma concentrations of these two cytokines caught up or became even higher in alcohol-intoxicated mice between 3 h and 6 h following bacteremia when compared to those of mice without alcohol intoxication. However, the bacteremia-induced increase in mobilization of lin-c-kit+Sca-1+ cells into the circulation was inhibited by alcohol intoxication at 24 h post i.v. challenge with 1×106 E. coli. The number of lin-c-kit+Sca-1- cells was also reduced in the circulation in alcohol-intoxicated mice at 24 h post i.v. challenge with 1×106 E. coli. In mice challenged with 5×107 E. coli, the alteration of lin-c-kit+Sca-1+ and lin-c-kit+Sca-1- cell populations in the systemic circulation followed a similar pattern of change as they did in the bone marrow. These data suggest that the altered lin-c-kit+Sca-1+ and lin-c-kit+Sca-1- cell pools in the bone marrow may be the major players in controlling the release of related cells from the bone marrow into the blood stream. The expanded marrow pool of lin-c-kit+Sca-1+ cells during bacteremia supported the enhancement of lin-c-kit+Sca-1+ cell mobilization into the systemic circulation. Alcohol intoxication inhibited the increase in marrow lin-c-kit+Sca-1+ population following E. coli infection. Accordingly, the mobilization of these primitive precursors into the systemic circulation was attenuated.
Our current study shows that alcohol intoxication impairs the functional adjustment of hematopoietic precursor cells in response to bacteremia. Since expansion of marrow lin-c-kit+Sca-1+ cell pool supports the enhancement of granulopoiesis during bacterial infection, inhibition of this response may compromise host defense against invading pathogens. In this light, increase in blood PMN counts following i.v. challenge with 5×107 E. coli was not seen in animals intoxicated with alcohol. Mice with acute alcohol intoxication exhibited significant increases in bacterial burden in the systemic circulation and vital organ tissues. Acute alcohol intoxication was also associated with a significant increase in animal death following i.v. challenge with 5×107 E. coli.
In summary, lin-c-kit+Sca-1+ cell population in the bone marrow is rapidly expanded in response to bacteremia. Both enhanced proliferation of lin-c-kit+Sca-1+ cells and phenotypic inversion from lin-c-kit+Sca-1- cells contribute to the observed increase in lin-c-kit+Sca-1+ cell population in the bone marrow. Cells in the expanded marrow lin-c-kit+Sca-1+ cell pool are functionally activated for granulopoiesis. In addition, mobilization of lin-c-kit+Sca-1+ cells into the systemic circulation is significantly enhanced along with the expansion of the marrow lin-c-kit+Sca-1+ cell population. Alcohol intoxication suppresses this lin-c-kit+Sca-1+ cell response by impairing both the proliferation of lin-c-kit+Sca-1+ cells and phenotypic inversion of lin-c-kit+Sca-1- cells in the bone marrow, which may serve as one mechanism underlying the impaired host defense response to severe bacterial infection in hosts excessively consuming alcohol.
Acknowledgments
We thank Amy B. Weinberg, Joseph S. Soblosky, PhD, Rhonda R. Martinez, and Jane A. Schexnayder for their expert technical assistance. The authors also thank Connie P. Porretta for her expert assistance with flow cytometric analyses and cell sorting. We thank Howard Blakesly for his help in data analysis and graphic preparation.
Footnotes
This work was supported by Public Health Service Grants AA09803, HL075161, HL073770, and HL76100.
Abbreviations used in this paper: BMCs: bone marrow cells; E. coli: Escherichia coli; KC: keratinocyte-derived chemokine; Lin: lineage.
References
- 1.Kondo M, Wagers AJ, Manz MG, Prohaska SS, Scherer DC, Beilhack GF, Shizuru JA, Weissman IL. Biology of hematopoietic stem cells and progenitors: implications for clinical application. Annu Rev Immunol. 2003;21:759–806. doi: 10.1146/annurev.immunol.21.120601.141007. [DOI] [PubMed] [Google Scholar]
- 2.Akala OO, Clarke MF. Hematopoietic stem cell self-renewal. Curr Opin Genet Dev. 2006;16:496–501. doi: 10.1016/j.gde.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 3.Ueda Y, Kondo M, Kelsoe G. Inflammation and the reciprocal production of granulocytes and lymphocytes in bone marrow. J Exp Med. 2005;201:1771–1780. doi: 10.1084/jem.20041419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hartmann DW, Entringer MA, Robinson WA, Vasil ML, Drebing CJ, Morton NJ, True L. Regulation of granulopoiesis and distribution of granulocytes in early phase of bacterial infection. J Cell Physiol. 1981;109:17–24. doi: 10.1002/jcp.1041090103. [DOI] [PubMed] [Google Scholar]
- 5.Barthlen W, Zanti N, Pfeffer K, Heidecke CD, Holzmann B, Stadler J. Impact of experimental peritonitis on bone marrow cell function. Surgery. 1999;126:41–47. doi: 10.1067/msy.1999.99060. [DOI] [PubMed] [Google Scholar]
- 6.Marsh JC, Boggs DR, Gartwright GE, Wintrobe MM. Neutrophil kinetics in acute infection. J Clin Invest. 1967;12:1943–1953. doi: 10.1172/JCI105684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Terashima T, Wiggs B, English D, Hogg JC, Van Eeden SF. Polymorphonuclear leukocyte transit times in bone marrow during streptococcal pneumonia. Am J Physiol. 1996;271:L587–L592. doi: 10.1152/ajplung.1996.271.4.L587. [DOI] [PubMed] [Google Scholar]
- 8.Cronkite EP. Analytical review of structure and regulation of hemopoiesis. Blood Cells. 1988;14:313–328. [PubMed] [Google Scholar]
- 9.Fine MJ, Smith MA, Carson CA, Mutha SS, Sankey SS, Weissfeld LA, Kapoor WN. Prognosis and outcomes of patients with community-acquired pneumonia. A meta-analysis. JAMA. 1996;275:134–141. [PubMed] [Google Scholar]
- 10.Perlino CA, Rimland D. Alcoholism, leucopenia, and pneumococcal sepsis. Am Rev Respir Dis. 1985;132:757–760. doi: 10.1164/arrd.1985.132.4.757. [DOI] [PubMed] [Google Scholar]
- 11.Ruiz M, Ewig S, Torres A, Arancibia F, Marco F, Mensa J, Sanchez M, Martinez JA. Severe community-acquired pneumonia. Risk factors and follow-up epidemiology. Am J Respir Crit Care Med. 1999;160:923–929. doi: 10.1164/ajrccm.160.3.9901107. [DOI] [PubMed] [Google Scholar]
- 12.Musher DM, Alexandraki I, Graviss EA, Yanbeiy N, Eid A, Inderias LA, Phan HM, Solomon E. Bacteremic and nonbacteremic pneumococcal pneumonia. A prospective study. Medicine (Baltimore) 2000;79:210–221. doi: 10.1097/00005792-200007000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Fernandez-Sola J, Junque A, Estruch R, Monforte R, Torres A, Urbano-Marquez A. High alcohol intake as a risk and prognostic factor for community-acquired pneumonia. Arch Intern Med. 1995;155:1649–1654. doi: 10.1001/archinte.1995.00430150137014. [DOI] [PubMed] [Google Scholar]
- 14.Hammond JM, Lyddell C, Potgieter PD, Odell J. Severe pneumococcal pneumonia complicated by massive pulmonary gangrene. Chest. 1993;104:1610–1612. doi: 10.1378/chest.104.5.1610. [DOI] [PubMed] [Google Scholar]
- 15.Saitz R, Ghali WA, Moskowitz MA. The impact of alcohol-related diagnoses on pneumonia outcomes. Arch Intern Med. 1997;157:1446–1452. [PubMed] [Google Scholar]
- 16.Macgregor RR, Loubia DB. Alcohol and infection. Curr Clin Top Infect Dis. 1997;17:291–315. [PubMed] [Google Scholar]
- 17.Heermans EH. Booze and blood: the effects of acute and chronic alcohol abuse on the hematopoietic system. Clin Lab Sci. 1998;11:229–232. [PubMed] [Google Scholar]
- 18.Ballard HS. Hematological complications of alcoholism. Alcohol Clin Exp Res. 1989;13:706–720. doi: 10.1111/j.1530-0277.1989.tb00408.x. [DOI] [PubMed] [Google Scholar]
- 19.Michot F, Gut J. Alcohol-induced bone marrow damage. A bone marrow study in alcohol-dependent individuals. Acta Haematol. 1987;78:252–257. doi: 10.1159/000205888. [DOI] [PubMed] [Google Scholar]
- 20.Ballard HS. Alcohol-associated pancytopenia with hypocellular bone marrow. Am J Clin Pathol. 1980;73:830–834. doi: 10.1093/ajcp/73.6.830. [DOI] [PubMed] [Google Scholar]
- 21.Imperia PS, Chikkappa G, Philips PG. Mechanism of inhibition of granulopoiesis by ethanol. Proc Soc Exp Biol Med. 1984;175:219–225. doi: 10.3181/00379727-175-41792. [DOI] [PubMed] [Google Scholar]
- 22.Okada S, Nakauchi H, Nagayoshi K, Nishikawa S, Miura Y, Suda T. In vivo and in vitro stem cell function of c-kit- and Sca-1-positive murine hematopoietic cells. Blood. 1992;80:3044–3050. [PubMed] [Google Scholar]
- 23.Osawa M, Nakamura K, Nishi N, Takahasi N, Tokumoto Y, Inoue H, Nakauchi H. In vivo self-renewal of c-Kit+ Sca-1+ Lin(low/-) hemapoietic stem cells. J Immunol. 1996;156:3027–3214. [PubMed] [Google Scholar]
- 24.Jurecic R, Van NT, Belmont JW. Enrichment and functional characterization of Sca-1+WGA+, lin-WGA+, lin-Sca-1+, and lin-Sca-1+WGA+ bone marrow cells from mice with an Ly-6a haplotype. Blood. 1993;82:2673–2683. [PubMed] [Google Scholar]
- 25.Osuchowski MF, Welch K, Siddiqui J, Remick DG. Circulating cytokine/inhibitor profiles reshape the understanding of the SIRS/CARS continuum in sepsis and predict mortality. J Immunol. 2006;177:1967–1974. doi: 10.4049/jimmunol.177.3.1967. [DOI] [PubMed] [Google Scholar]
- 26.Kagsbjerg P, Jones I, Viderfors T, Holmberg H. Diagnostic value of blood cytokine concentrations in acute pneumonia. Thorax. 1995;50:1253–1257. doi: 10.1136/thx.50.12.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pauksen K, Elfman L, Ulfgren AK, Venge P. Serum levels of granulocyte-colony stimulating factor (G-CSF) in bacterial and viral infections, and in atypical pneumonia. Br J Haematol. 1994;88:256–260. doi: 10.1111/j.1365-2141.1994.tb05015.x. [DOI] [PubMed] [Google Scholar]
- 28.Fujishima S, Sasaki J, Shinozawa Y, Takuma K, Kimura H, Suzuki M, Kanazawa M, Hori S, Aikawa N. Serum MIP-1 alpha and IL-8 in septic patients. Intensive Care Med. 1996;22:1169–1175. doi: 10.1007/BF01709331. [DOI] [PubMed] [Google Scholar]
- 29.Gazitt Y. Recent developments in the regulation of peripheral blood stem cell mobilization and engraftment by cytokines, chemokines and adhesion molecules. J Hematother Stem Cell Res. 2001;10:229–236. doi: 10.1089/15258160151134908. [DOI] [PubMed] [Google Scholar]
- 30.Starckx X, Van Den Steen PE, Wuyts A, Van Damme J, Opdenakker G. Neutrophil gelatinase B and chemokines in leukocytosis and stem cell mobilization. Leuk Lymphoma. 2002;43:233–241. doi: 10.1080/10428190290005982. [DOI] [PubMed] [Google Scholar]
- 31.Pelus LM, Fukuda S. Peripheral blood stem cell mobilization: the CXCR2 ligand GRObeta rapidly mobilizes hematopoietic stem cells with enhanced engraftment properties. Exp Hematol. 2006;34:1010–1020. doi: 10.1016/j.exphem.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 32.Yamada M, Kubo H, Kobayashi S, Ishizawa K, Numasaki M, Ueda S, Suzuki T, Sasaki H. Bone marrow-derived progenitor cells are important for lung repair after lipopolysaccharide-induced lung injury. J Immunol. 2004;172:1266–1272. doi: 10.4049/jimmunol.172.2.1266. [DOI] [PubMed] [Google Scholar]
- 33.Jackson KA, Majka SM, Wang H, Pocius J, Hartley CJ, Majesky MW, Entman ML, Michael LH, Hirschi KK, Goodell MA. Regeneration of ischemic cardiac muscle and vascular endothelium by adult stem cells. J Clin Invest. 2001;107:1395–1402. doi: 10.1172/JCI12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Majka SM, Jackson KA, Kienstra KA, Majesky MW, Goodell MA, Hirschi KK. Distinct progenitor populations in skeletal muscle are bone marrow derived and exhibit different cell fates during vascular regeneration. J Clin Invest. 2003;111:71–79. doi: 10.1172/JCI16157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Orlic D, Kajstura J, Chimenti S, Limana F, Jakoniuk I, Quaini F, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Mobilized bone marrow cells repair the infarcted heart, improving function and survival. Proc Natl Acad Sci U S A. 2001;98:10344–10349. doi: 10.1073/pnas.181177898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Massberg S, Schaerli P, Knezevic-Maramica I, Köllnberger M, Tubo N, Moseman EA, Huff IV, Junt T, Wagers AJ, Mazo IB, von Andrian UH. Immunosurveillance by hematopoietic progenitor cells trafficking through blood, lymph, and peripheral tissues. Cell. 2007;131:994–1008. doi: 10.1016/j.cell.2007.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Z, Bagby GJ, Stoltz D, Oliver P, Schwarzenberger PO, Kolls JK. Prolonged ethanol treatment enhances lipopolysaccharide/phorbol myristate acetate-induced tumor necrosis factor-alpha production in human monocytic cells. Alcohol Clin Exp Res. 2001;25:444–449. [PubMed] [Google Scholar]
- 38.Zhang P, Zhong Q, Bagby GJ, Nelson S. Alcohol intoxication inhibits pulmonary S100A8 and S100A9 expression in rats challenged with intratracheal lipopolysaccharide. Alcohol Clin Exp Res. 2007;31:113–121. doi: 10.1111/j.1530-0277.2006.00269.x. [DOI] [PubMed] [Google Scholar]
- 39.Osawa M, Hanada K, Hamada H, Nakauchi H. Long-term lymphohematopoietic reconsititution by a single CD34-low/negative hematopoietic stem cell. Science. 1996;273:242–245. doi: 10.1126/science.273.5272.242. [DOI] [PubMed] [Google Scholar]
- 40.Zhang P, Bagby GJ, Happel KI, Raasch CE, Nelson S. Alcohol abuse, immunosuppression, and pulmonary infection. Curr Drug Abuse Rev. 2008;1:56–67. doi: 10.2174/1874473710801010056. [DOI] [PubMed] [Google Scholar]
- 41.Quesenberry PJ, Morley A, Miller M, Rickard K, Howard D, Stohlman F., Jr Effect of endotoxin on granulopoiesis and the in vitro colony-forming cell. Blood. 1973;41:391–398. [PubMed] [Google Scholar]
- 42.Quesenberry PJ, Colvin GA, Abedi M, Lambert JF, Moore B, Demers D, Greer D, McAuliffe C, Dooner M, Lum LG, Badiavas E, Falanga V. The marrow stem cell: the continuum. Bone Marrow Transplant. 2003;32:S19–S22. doi: 10.1038/sj.bmt.1703938. [DOI] [PubMed] [Google Scholar]
- 43.Colvin GA, Lambert JF, Moore BE, Carlson JF, Dooner MS, Abedi M, Cerny J, Quesenberry PJ. Intrinsic hematopoietic stem cell/progenitor plasticity: inversions. J Cell Physiol. 2004;199:20–31. doi: 10.1002/jcp.10436. [DOI] [PubMed] [Google Scholar]
- 44.Luna G, Paez J, Cardier JE. Expression of the hematopoietic stem cell antigen Sca-1 (Ly-6A/E) in liver sinusoidal endothelial cells: Possible function of Sca-1 in endothelial cells. Stem Cell Dev. 2004;13:528–535. doi: 10.1089/scd.2004.13.528. [DOI] [PubMed] [Google Scholar]
- 45.Chen HC, Frissora F, Durbin JE, Muthusamy N. Activation induced differential regulation of stem cell antigen-1 (Ly-6A/E) expression in murine B cells. Cell Immunol. 2003;225:42–52. doi: 10.1016/j.cellimm.2003.09.006. [DOI] [PubMed] [Google Scholar]